SUMMARY
Hearing loss is frequent in intensive care patients and can be due to several causes. However, sepsis has not been examined as a possible cause. The aim of this study is to assess the influence of experimental sepsis on hearing thresholds and to evaluate pathological changes in the cochlea. The cecal ligation puncture technique was used to induce sepsis in 18 mice. Results were compared with those from 13 sham-operated and 13 untreated control mice. The hearing thresholds of the animals were evaluated with auditory evoked brainstem responses prior to the induction of sepsis and again at the peak of the disease. Immediately after the second measurement, the mice were sacrificed and the inner ears harvested and prepared for further evaluation. The cochleae were examined with light microscopy, electron microscopy and immunohistochemistry for Bax, cleaved caspase-3 and Bcl-2. The mice with sepsis showed a significant hearing loss but not the control groups. Induction of apoptosis could be shown in the supporting cells of the organ of Corti. Furthermore, excitotoxicity could be shown at the basal pole of the inner hair cells. In this murine model, sepsis leads to significant hearing impairment. The physiological alteration could be linked to apoptosis in the supporting cells of the organ of Corti and to a disturbance of the synapses of the inner hair cells.
INTRODUCTION
The incidence of sepsis is still high, with up to three cases per 1000 population per year, more than half of them requiring intensive care management (Angus et al., 2001). Multi-organ failure includes involvement of the central nervous system, peripheral nerves and skeletal muscles (Latronico and Bolton, 2011), but so far systemic sepsis has not been linked to hearing impairment. However, hearing loss has been accepted as an unappreciated phenomenon in critical care. Postulated causes range from trauma, ototoxic medications, local infections, vascular and hematologic disorders, autoimmune disease and environmental noise (Halpern et al., 1999).
Recently, our group demonstrated the involvement of the inner ear in murine cerebral malaria, a systemic inflammatory disease (Schmutzhard et al., 2010) showing apoptosis in the fibrocytes of the spiral ligament, which play an essential role in the electrolyte circulation of the cochlea, and a breakdown of the blood-labyrinth barrier (Schmutzhard et al., 2012). The fact that no malaria-typical alterations, like microhemorrhages or leukocyte sequestration, could be found in the temporal bones suggests that the pathological findings in the inner ear might be linked to the systemic inflammatory reaction.
Comparable animal models exist for severe sepsis. A well-established form is the cecal ligation puncture (CLP) model. In this approach, sepsis is induced with a ligation of the cecum and an additional standardized puncture of the ligated part (Rittirsch et al., 2009). Activation of the apoptosis cascade is a frequently observed pathological reaction in the inner ear. In a guinea pig animal model for Menière’s disease, an activation of cleaved caspase-3 (an established apoptosis detection marker) has been described in the fibrocytes of the spiral ligament and the stria vascularis (Labbé et al., 2005). In perinatal asphyxia, a cleaved caspase-3 activation has been shown in the inner and outer hair cells. Additionally, downregulation of the anti-apoptotic pathways could be demonstrated with decreased Bcl-2 labeling (Schmutzhard et al., 2009).
The upregulation of the Bcl-2 anti-apoptotic protein needs to be compared with the expression rate of the Bax protein, the pro-apoptotic antagonist of the Bcl-2 protein. A Bax/Bcl-2 ratio favoring Bax expression with additional downstream cleaved caspase-3 upregulation is a solid sign for induced apoptosis (Salakou et al., 2007).
The aim of this experiment was to study whether in the standardized CLP mouse model sepsis leads to hearing impairment. Possible pathological alterations causing such a hearing loss were looked for by light microscopy, transmission electron microscopy (TEM) and immunohistochemistry for cleaved caspase-3, Bcl-2 and Bax, thus focusing on the activation of apoptotic and anti-apoptotic pathways.
TRANSLATIONAL IMPACT.
Clinical issue
Hearing loss is known to be an unpleasant side effect of intensive care treatment. Various ototoxic factors such as medication, trauma and autoimmune response have been identified as potential causes. However, the possible effects of sepsis – a frequent cause of critical care hospitalization – on inner ear function have not yet been examined.
Results
In this prospective animal study, the effects of sepsis on the inner ear were investigated in C57BL/6 laboratory mice. Regularly hearing animals were infected using the cecal ligation puncture technique, and the hearing of the mice was assessed at the peak of the disease. Statistical evaluation revealed significant hearing loss, at all frequencies, in the mice with sepsis. Subsequent histological and immunohistochemical analysis indicated an induction of apoptosis in a specialized cell subtype of the organ of Corti, as well as glutamate excitotoxicity at the basal pole of the inner hair cell.
Implications and future directions
This study indicates, for the first time, that sepsis can be causative of hearing loss in the absence of other ototoxic influences. In light of these data, specialists should be encouraged to perform post-therapeutic hearing tests in patients suffering from sepsis syndrome. Nonetheless, this finding awaits validation in humans. Therefore, further studies, including objective hearing tests such as auditory evoked brainstem responses and otoacoustic emissions, need to be performed in patients with septicemia.
RESULTS
A total of 16 (out of 18) CLP mice were included in the evaluation. Two CLP mice died unobserved during the experiment and were excluded. One animal had an elevated pretreatment auditory brainstem response (ABR) threshold on the left ear. In this animal, only the right ear was evaluated. The sham-operated group was composed of 13 mice. This left 16 CLP mice, 13 sham mice and 13 untreated control mice for statistical evaluation.
Laboratory data, body weight and temperature
Laboratory data, weight loss, low body temperature and bacteremia indicated that sepsis had been successfully induced in CLP mice. Blood cultures for all 16 CLP mice were positive for Escherichia coli. Klebsiella oxytoca grew in the blood culture of one CLP animal. All control animals had negative blood cultures. Sufficient volumes of blood for the analysis of pH levels could be drawn in 9/16 CLP animals, 8/13 sham and in 4/13 controls. In CLP mice, the mean blood pH level was 7.15±0.08 (all values are given as mean ± s.d.), for sham animals it was 7.28±0.05 (CLP versus sham P=0.001) and for controls it was 7.39±0.07 (CLP versus control P=0.0028) (supplementary material Fig. S1A). The pretreatment body weight of the CLP mice was 21.58±1.18 g, the sham animals 20.8±1.6 g (CLP versus sham P=0.147) and the controls 21.45±1.67 g (CLP versus control P=0.895) (supplementary material Fig. S1C). The post-treatment body weight of the CLP mice was 19±1.64 g (pre-CLP versus post-CLP P<0.001), the sham animals 21.07±1.3 g (pre-sham versus post-sham P=0.521) and in controls 23.12±2.02 g (pre-control versus post-control P=0.03) (supplementary material Fig. S1D). Lactate levels were evaluated for 9/16 CLP animals, 2/13 sham animals and 4/13 controls. The lactate value for the CLP mice was 21.68±12.7 mg/dl, the sham animals 13.95±7.42 mg/dl and the controls 10.65 ±3.23 mg/dl (supplementary material Fig. S1B). Body temperature was evaluated for 16/16 CLP animals, 6/13 sham animals and 5/13 control animals. At inclusion, the values for the CLP animals ranged from 38.31±0.9°C, the sham group 38.8±1.3°C and the controls 37.8 ±0.5°C (supplementary material Fig. S1E). At sacrifice, the temperature had dropped significantly in the CLP animals to 33.19±2.13°C (CLP inclusion versus CLP sacrifice P<0.0001). No change of the temperature could be noted in the sham group with 38.2±0.8°C (sham inclusion versus sham sacrifice P=0.47) and the control animals with 38.42±0.92°C (control inclusion versus control sacrifice P=0.28) (supplementary material Fig. S1F).
ABR measurements
Pretreatment ABR thresholds could be identified in all 42 animals at sound pressure levels ranging from 15 to 80 dB (supplementary material Fig. S2). The average pretreatment thresholds of left and right ears for all 42 animals were 54.8±7.3 dB at 4 KHz, 31.6±6.3 dB at 8 KHz, 25.9±5.6 dB at 16 KHz and 46.2±15.12 dB at 32 KHz. The post-treatment threshold averages of the left and right ears were 57.7±7.67 dB at 4 KHz, 35.7±8.5 dB at 8 KHz, 27.8±7.3 dB at 16 KHz and 50.8±13.2 dB at 32 KHz. The mean pretreatment pure tone audiometry for all 42 animals was 39.6±6.8 dB (sound pressure level; SPL), the mean post-treatment pure tone audiometry was 43±6.9 dB.
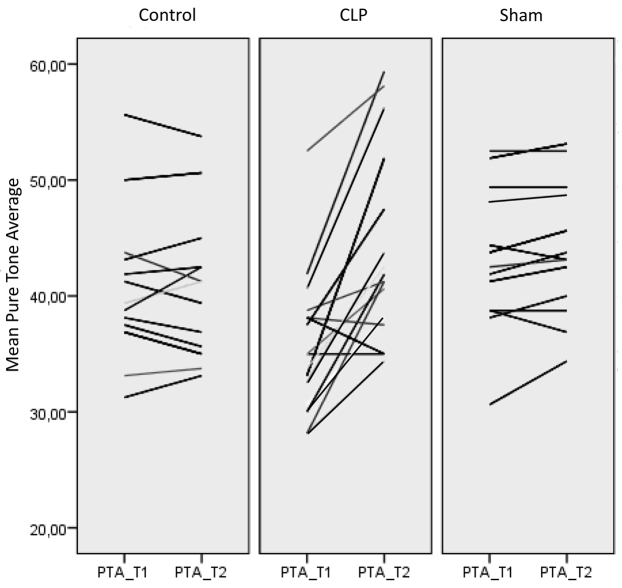
Sepsis resulted in a significant increase in ABR thresholds (i.e. hearing loss), although ABR thresholds remained stable in sham and control animals (Fig. 1). Adjusted for pretreatment ABR thresholds, the mean post-treatment pure tone audiometry estimated marginal mean for CLP animals was 47.43 dB [95% confidence interval (CI) 45.12 dB to 49.7 dB], for the sham group 40.8 dB (95% CI 38.3 dB to 43.3 dB) and for control mice 39.7 dB (95% CI 37.4 dB to 42.1 dB). Post-treatment thresholds of the CLP group differed significantly from the control group (P<0.001) and from sham-operated animals (P=0.002).
Fig. 1.
Spaghetti plot of the audiological results for the control, CLP and the sham group. All audiological results were averaged at the time-point of inclusion as pure tone average 1 (PTA_T1). The audiological results at the time-point of sacrifice were averaged as pure tone average 2 (PTA_T2). Each line in the graph stands for one individual, connecting PTA_T1 and PTA_T2. A horizontal line, as seen in the control and the sham group, indicates no hearing loss; a steep line indicates a change in the hearing threshold between the first and second measurements.
Light microscopy
Four temporal bones of mice with a hearing loss of up to 20 dB in various frequencies were further examined with light microscopy. The structural analysis revealed a well-preserved spiral ganglion in all examined temporal bones, independently of any hearing loss. Furthermore, the stria vascularis and the spiral ligament did not show any morphological alterations (supplementary material Fig. S3). The organ of Corti showed the following morphological alterations: (i) The inner hair cells showed extensive vacuolization at the basal pole, which could be correlated to hearing loss. Temporal bones of mice without hearing impairment did not show the basal vacuolization. Hair cells of mice with hearing loss of 15–20 dB showed a basal vacuolization ranging from the basal pole of the inner hair cell to the basal membrane. Hearing loss of 10 dB could be correlated to a minor basal vacuolization not reaching the basal membrane. No basal vacuolization was found in temporal bones of mice without hearing loss. (ii) The apical pole of the inner hair cells was well preserved. (iii) Except for the vacuolization at the basal pole, the outer hair cells showed an identical morphological presentation. (iv) The Deiters’ cells (supporting cells of the outer hair cells) showed an intensive staining of the cytoplasm, a rough configuration of the cell and a chromatin condensation with nuclear shrinkage. (v) Similar cell morphology could be shown in the Claudius’ and Hensen’s cells.
These pathological alterations could be found exclusively in temporal bones of mice with hearing loss (supplementary material Fig. S3A–D). Two sham animals were further evaluated with light microscopy and did not show the basal vacuolization at the inner hair cells. The supporting cells, the outer hair cells, the stria vascularis, the spiral ligament and the spiral ganglion cells did not reveal any pathological alterations (supplementary material Fig. S3E–H).
Three animals of the control group were further evaluated with light microscopy. No hearing impairment had been detected in these animals. The spiral ganglion cells and the stria vascularis were well preserved. The organ of Corti showed regular inner and outer hair cells. No vacuolization was seen at the basal pole of the inner hair cells. The Deiters’, Claudius’ and Hensen’s cells (supporting cells of the organ of Corti) showed a smooth configuration and euchromatin-containing nuclei.
Transmission electron microscopy
TEM was performed on four temporal bones of septic mice with hearing loss and two sham animals without hearing loss. Examination of the supporting cells revealed the following pathological alterations: Firstly, the Deiters’ cells of the hearing-impaired animals showed a cellular shrinking and chromatin condensation (Fig. 2A,B). Secondly, examination of the Claudius’ cells revealed a cellular shrinking and chromatin condensation. The membranes (cellular, nuclear) of the examined temporal bones were well preserved.
Fig. 2.
TEM of the inner hair cells and supporting cells. (A) Overview of the organ of Corti of a CLP mouse with hearing loss, focusing on the supporting cells. The outer hair cells appear normal. A Deiters’ cell has pathological alterations. (B) Close-up view of a Deiters’ cell with reduction in the cellular volume and chromatin condensation (asterix). (C) High magnification of a CLP mouse inner hair cell. The basal membrane of the cell is well preserved. The vacuolization (arrows) is due to a swelling of the afferent nerve fibers, with membrane disruption and loss of cytoplasmic content. (D) Higher magnification of a basal swelling, which is marked with an asterix in C. The vacuole contains a membrane remnant (arrow). Furthermore, on the corresponding inner hair cell side a synaptic ribbon can be seen (bold arrow). (E) Organ of Corti from a sham animal. The basal pole of the inner hair cell does not show any vacuolization. The Deiters’ cell shows a regular configuration with euchromatin-containing nucleus (arrow). (F) Close-up view of the inner hair cell shown in E. At the basal pole, regular afferent nerve fibers with intact membranes and cytoplasmatic content can be seen (asterix). OHC, outer hair cell; IHC, inner hair cell; DC, Deiters’ cells.
The inner hair cells of septic mice with hearing impairment showed a well-preserved basal pole. The vacuolization could be connected to the afferent nerve fibers. The dendrites showed a massive swelling, with membrane disruption and loss of cytoplasmic content (Fig. 2C,D). The sham animals did not show any basal vacuolization, which was similar to the findings from light microscopy (Fig. 2E,F).
Cleaved caspase-3 reaction
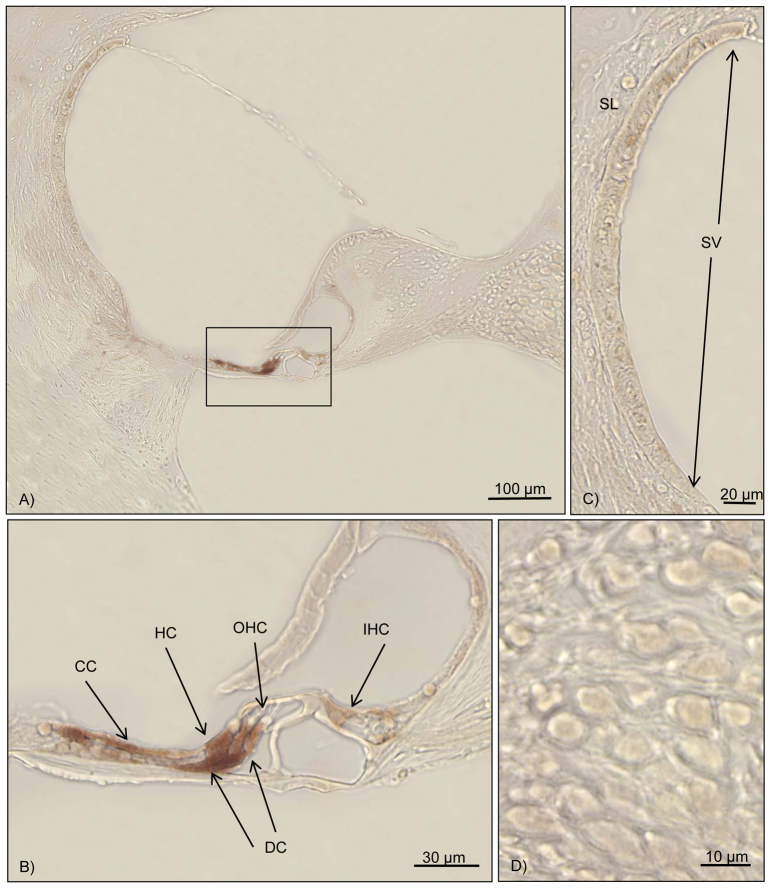
Seven cochleae from septic mice with varying levels of hearing loss were randomly included into the immunohistochemical analysis. Distinct staining for the cleaved caspase-3 was seen in the supporting cells of the cochlea (Deiters’ cells, Claudius’ cells and Hensen’s cells) (Table 1; Fig. 3B). Animals without hearing loss did not show any reaction or only a very mild staining in the supporting cells of the cochlea (Table 1). The immunohistochemical evaluation of three control animals without any hearing loss did not reveal any immunolabeling for cleaved caspase-3 (Table 1).
Table 1.
Immunohistochemical results for cleaved caspase-3
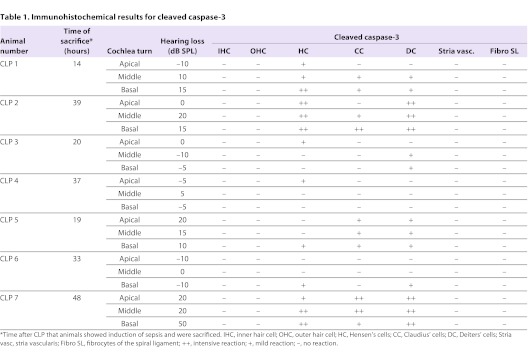
Fig. 3.
Immunohistochemistry for cleaved caspase-3 in a hearing-impaired animal. Brown staining marks cleaved caspase-3 activity. (A) Overview of the cochlea duct at the middle turn. The box marks the organ of Corti. (B) High magnification of the organ of Corti. Positive labeling for cleaved caspase-3 can be detected in Deiters’ cells, Hensen’s cells and Claudius’ cells. No staining can be detected in the inner and outer hair cells. (C) High magnification of the stria vascularis and the spiral ligament. No reaction can be detected. (D) Close-up of the spiral ganglion cells. No labeling can be visualized. OHC, outer hair cell; IHC, inner hair cell; CC, Claudius’ cells; DC, Deiters’ cells; HC, Hensen’s cells; SL, spiral ligament; SV, stria vascularis.
Bcl-2 and Bax reaction
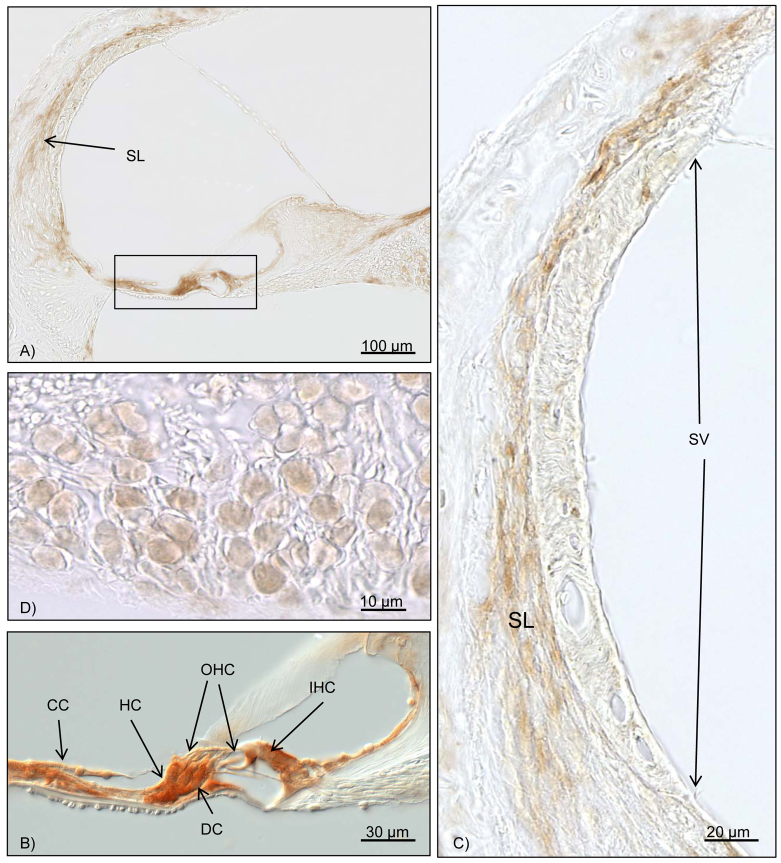
Seven cochleae from septic mice with varying levels of hearing loss were randomly included in the immunohistochemical Bcl-2 evaluation. An intensive reaction could be detected in the fibrocytes of the spiral ligament. Furthermore, positive immunolabeling for Bcl-2 could be detected in the supporting cells of the organ of Corti. No immunohistochemical reaction could be noted in the inner and outer hair cells. The spiral ganglion cells labeled positive for Bcl-2 (Table 2; Fig. 4A–D). The cochleae of three control animals did not show any positive reaction for Bcl-2.
Table 2.
Immunohistochemical results for Bax and Bcl-2
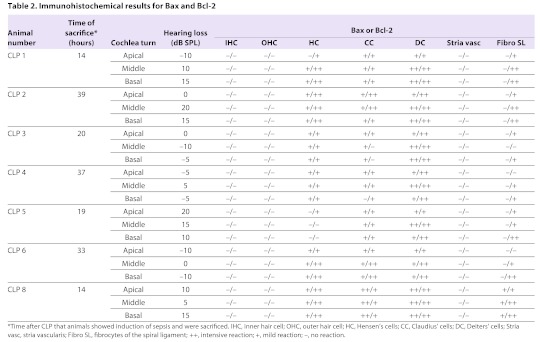
Fig. 4.
Immunohistochemical results for Bcl-2. Brown staining marks Bcl-2 activity. (A) Overview of the cochlea duct at the middle turn. The box marks the organ of Corti, with intensive Bcl-2 staining. Further reactivity can be seen in the spiral ligament. (B) Close-up of the organ of Corti. With the differential interference contrast mode, the inner and outer hair cells do not show any Bcl-2 activity. High Bcl-2 activation is seen for the Deiters’ cells, Hensen’s cells, Claudius’ cells and other supporting cells. (C) High magnification of the stria vascularis and the spiral ligament. Upregulation of Bcl-2 in the fibrocytes of the spiral ligament can be noted. No reaction is found in the stria vascularis. (D) Bcl-2 activation can be seen in the spiral ganglion cells. OHC, outer hair cell; IHC, inner hair cell; CC, Claudius’ cells; DC, Deiters’ cells; HC, Hensen’s cells; SL, spiral ligament; SV, stria vascularis.
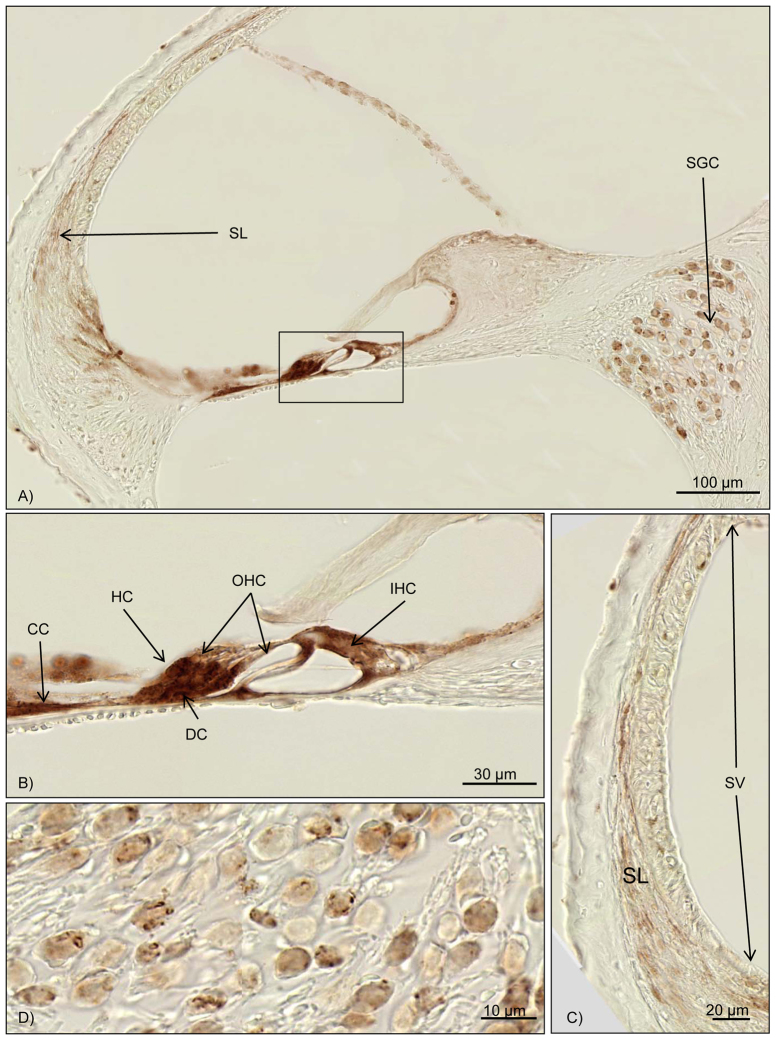
Bax immunohistochemistry was performed for all CLP mice. An intensive upregulation of Bax could be seen in the supporting cells of the organ of Corti, comparable with the Bcl-2 expression (Table 2; Fig. 5A,B). One temporal bone did show a mild Bax reaction in the stria vascularis and the fibrocytes of the spiral ligament (Table 2; Fig. 5C,D). The remaining six cochleae did not show a Bax reaction in these two structures (Table 2).
Fig. 5.
Immunohistochemical results for Bax. Brown staining marks Bax activity. (A) Overview of the cochlea duct at the middle turn. The box marks the organ of Corti, with intensive Bax staining. Further reactivity can be seen in the spiral ligament and the spiral ganglion cells. (B) Close-up of the organ of Corti. High Bax-2 activation is seen for Deiters’ cells, Hensen’s cells, Claudius’ cells and other supporting cells. (C) High magnification of the stria vascularis and the spiral ligament. Upregulation of Bax in the fibrocytes of the spiral ligament can be noted. No reaction is found in the stria vascularis. (D) Bax activation can be seen in the spiral ganglion cells. OHC, outer hair cell; IHC, inner hair cell; CC, Claudius’ cells; DC, Deiters’ cells; HC, Hensen’s cells; SGC, spiral ganglion cells; SL, spiral ligament; SV, stria vascularis.
DISCUSSION
An involvement of the inner ear in severe sepsis syndrome has only been reported in a single case of sepsis with Capnocytophaga canimorsus following a dog bite (Widlund and Duberg, 2010). In our study, a significant hearing loss could be demonstrated in mice suffering from sepsis experimentally induced with the cecal ligation puncture technique. Furthermore, the morphological and immunohistochemical evaluation revealed an induction of apoptosis in the supporting cells of the organ of Corti and glutamate excitotoxicity at the basal pole of the inner hair cells as key pathophysiological mechanisms potentially explaining the functional impairment. No hearing loss and no pathological alterations could be demonstrated in control animals.
The blood cultures of the CLP group showed a bacteremia with E. coli and K. oxytoca. No bacteria could be grown in the control or sham animals. proving the ‘successful induction of sepsis’ in the CLP group.
Blood examinations showed pH levels ranging from 7.0 to 7.26 and lactate levels ranging from 5.6 to 41.1 mg/dl in the CLP group. In the control and sham groups pH was 7.21–7.47 and lactate 7.2–19.2 mg/dl. Lactate acidosis is a typical pathological finding in sepsis syndrome (Eissa et al., 2010), thus confirming the disease in the CLP mouse group.
The first ABR measurement showed regular hearing thresholds for C57 BL/6J mice younger than 3 months (Zheng et al., 1999). The mean hearing threshold in the septic mice deteriorated by an average of 8.1 dB SPL, in contrast to no change in the control group and an average loss of 0.76 dB SPL in the sham mice. Adjusting for differences in the first measurement, the hearing loss between the first and the second measurements in the sepsis group was significantly different (CLP versus control P<0.001, CLP versus sham P=0.002). No difference was detected between the first and the second measurements for the control and the sham group. This finding clearly supports the hypothesis that sepsis leads to a significant hearing impairment. So far, no experimental data exists evaluating the inner ear in sepsis syndrome. Another severe systemic inflammatory disease, severe malaria, has been linked to hearing loss (Schmutzhard et al., 2010). No malaria-typical alterations have been detected in the temporal bones of hearing-impaired malaria mice, suggesting that the hearing impairment results from the general inflammatory reaction (Schmutzhard et al., 2011). Thus, the detection of a significant hearing loss in sepsis is well in line with this experimental background.
The question of whether the observed significant hearing impairment in mortally sick mice is transient or permanent damage cannot be answered with this study. In order to show a permanent hearing loss in mice with sepsis further studies using sepsis models with a lower mortality rate need to be applied.
Light microscopy showed a well-preserved cochlea with regular spiral ganglion cells, stria vascularis and sensory epithelium. The supporting cells in the organ of Corti showed morphological alterations, such as dark cytoplasm indicating high protein synthesis accompanied by apoptotic activity, cell shrinkage and a chromatin condensation with shrunken nuclei. These alterations could be detected in Deiters’ cells, Hensen’s cells and Claudius’ cells – these supporting cells of the organ of Corti are involved in potassium recirculation. A high potassium concentration in the endolymph is responsible for the endolymphatic potential, which is needed for sensory transduction in the inner and outer hair cells. Therefore, a lack of potassium in the endolymph results in a malfunction of the cochlea (Kikuchi et al., 2000). Electron microscopy of cells involved with potassium recycling revealed cell shrinkage, with folding of the plasma membrane and chromatin condensation. These morphological alterations are known to be typical morphological hallmarks of apoptosis (Häcker, 2000). These typical morphological findings support the notion that apoptosis may be the primary cause of hearing impairment. Damage of the membranes (cell, nuclear) as typically seen in necrosis could not be observed. Therefore, necrosis is unlikely to play an important role in the pathophysiology of this hearing impairment.
A further interesting finding is the vacuolization on the basal pole of the inner hair cells. The vacuolization could be linked to hearing loss in the CLP mice. Furthermore, the intensity of the vacuolization was associated with the severity of the hearing loss. No such pathological alteration could be found in the control and sham animals. Electron microscopy found the vacuolization to be linked to a massive swelling of the radial dendrites. These alterations have been reported to be induced by excessive AMPA, kainite and glutamate exposure. Furthermore, the identical pathological alteration has been shown in ischemia- and noise-induced hearing loss, which could be linked to an excitotoxic pathway (Ruel et al., 2007). With these pathological results in the CLP animals, excitotoxicity of glutamate at the radial dendrites is a further possible pathomechanism of the observed hearing loss.
We applied immunohistochemical labeling for activated caspase-3 as a crucial executioner of apoptosis and a reliable method of detecting apoptosis, which could be visualized in Deiters’ cells, Claudius’ cells and Hensen’s cells (i.e. the supporting cells of the organ of Corti). The positive labeling for cleaved caspase-3 was exclusively detected in the hearing-impaired animals, whereas almost no labeling could be shown in the CLP mice without hearing impairment. The positively labeled temporal bones showed differences in the intensity of the labeling: mice with hearing impairment and suffering from sepsis for 2 days showed a more intensive reaction with cleaved caspase-3, whereas only mild staining could be detected in mice being sacrificed within less than 24 hours after sepsis induction. Similar time dependency of the upregulation of cleaved caspase-3 in the CLP model has been previously described in intestinal cells (Coopersmith et al., 2002a).
The induction of apoptosis in the CLP model is a well-described effect in various organs, such as gastrointestinal cells, hepatocytes and lung epithelial cells (Coopersmith et al., 2002b; Kim et al., 2000; Mutunga et al., 2001). Kafa and colleagues suspected that the sepsis-associated encephalopathy was caused by an activation of the apoptosis cascade (Kafa et al., 2010). The inner ear belongs to the same intracranial blood circuit as the brain, and the blood-labyrinth barrier of the inner ear has a micro-anatomical structure comparable to the blood-brain barrier (Choi and Kim, 2008). Therefore, a similar reaction of the inner ear as that described in the brain can be expected. Our findings of apoptosis in the fibrocytes of the spiral ligament and a breakdown of the blood-labyrinth barrier as pathological mechanisms of the hearing loss in the malaria mouse model support this hypothesis. In malaria, the systemic inflammatory reaction (comparable with the sepsis inflammatory response) has been suspected to cause these pathological alterations (Schmutzhard et al., 2012). Surprisingly, apoptosis in the CLP inner ears cannot be found in the fibrocytes of the spiral ligament as described in the malaria inner ears (Schmutzhard et al., 2012). The mice with sepsis, however, showed an induction of apoptosis in the supporting cells (Deiters’, Hensen’s and Claudius’ cells). In an animal model of Menière’s disease, the induction of apoptosis in these supporting cells could be associated with oxidative stress, a well-known and obviously more relevant reaction in sepsis (Labbé et al., 2005; Wang et al., 2012).
The third focus of this study was the anti-apoptotic activity. Bcl-2 is an accepted anti-apoptotic protein (Pfannenstiel and Praetorius, 2008). Immunohistochemical labeling for Bcl-2 in the temporal bones of the CLP mice revealed an anti-apoptotic activity in the supporting cells and the fibrocytes of the spiral ligament. The intensity of this upregulation could not be linked to the level of hearing loss. Comparing the cleaved caspase-3 labeling and the Bcl-2 labeling in the supporting cells, a coexpression of the anti-apoptotic activity and the pro-apoptotic activity could be found. The upregulation of Bcl-2 is often observed simultaneously with an upregulation of the Bax protein, the pro-apototic antagonist of the Bcl-2 family proteins. Simultaneous labeling for both Bax and Bcl-2 in the supporting cells strongly supports a pro-apoptotic cell activity. This is further supported by the observed cleaved caspase-3 activation, which is mediated by the Bax protein (Salakou et al., 2007).
In contrast to this finding, the anti-apoptotic activity was upregulated in the fibrocytes of the spiral ligament. Bcl-2 overexpression is known to lead to a downregulation of cleaved caspase-3 (Coopersmith et al., 2002a). This explains the lack of upregulation in the fibrocytes of the spiral ligament, as described in the malaria mouse model (Coopersmith et al., 2002b). These findings are further supported by a lack of Bax activity in the fibrocytes of the spiral ligament.
The final cause of the above described pathological alterations is not fully understood. On the one hand, a disturbance of the microcirculation as seen in sepsis encephalopathy could affect the inner ear perfusion, resulting in an ischemic stimulus that could account for the swelling of the radial dendrites (Taccone et al., 2010). On the other hand, the induction of apoptosis in various organs has been observed as a further pathophysiological mechanism in multi-organ dysfunction (Iwata et al., 2011), rendering an induction of apoptosis in the inner ear likely. Further studies on mice that survive CLP sepsis are necessary to evaluate the long-term effect of these pathological alterations.
These results show that sepsis induced by CLP leads to a significant hearing loss in mice. Furthermore, the functional deficit could be linked on one hand to an activation of apoptosis in the supporting cells of the organ of Corti, disrupting the potassium circulation in the inner ear, and on the other hand to glutamate excitotoxicity at the radial dendrites on the base of the inner hair cells (Kikuchi et al., 2000; Ruel et al., 2007). To verify the effect of systemic sepsis on the human inner ear, prospective evaluation of the hearing capacity of patients with sepsis is urgently necessary. Nevertheless, this animal experiment should alert professionals to the possibility that sepsis might directly lead to a significant hearing impairment.
MATERIALS AND METHODS
The animal studies conformed to the Austrian guidelines for the care and use of laboratory animals and were approved by the Austrian Ministry Science with the reference number BMWF-66.011/0082-II/3b/2011.
Study design
Forty-four 2-month-old C57BL/6 mice (Charles River, Sulzfeld, Germany) were used in this study. An initial objective hearing test was performed with auditory evoked brainstem responses (ABR). Animals with thresholds below 40 dB at 16 kHz were eligible. Sepsis was induced in 18 mice with cecal ligation puncture; 13 mice underwent sham surgery (laparotomy, no ligation, no puncture) and 13 mice served as control (identical housing and treatment, but no intervention). The course of sepsis was monitored for signs of prostration, loss of temperature (Brooks et al., 2007) and weight loss (Xiao et al., 2006) at 4-hour intervals. Body temperature below 34°C, severe prostration and a significant weight loss indicated ‘successful’ induction of sepsis and the animals were sacrificed at this point of time, immediately after the second ABR measurement. The control animals were sacrificed after 96 hours.
Immediately thereafter, blood samples for lactate and pH determination and for culture were obtained by cardiac puncture. If cardiac puncture was not successful within 60 seconds, the procedure was stopped in order to preserve the vulnerable inner ear structures. Animals were then sacrificed and both inner ears harvested within 6 minutes.
Prior to the audiological evaluation of the ABR data, eight randomly selected sepsis inner ears and three controls were analyzed morphologically and immunohistochemically. The left side was examined by semi-thin sectioning and TEM. The right side was examined with immunohistochemistry for cleaved caspase-3, Bax and Bcl-2.
Cecal ligation puncture mouse model
The mice were deeply anesthetized with intraperitoneal ketamine hydrochloride (Graeub®, Senden-Bösensell, Germany) (67.5 mg/kg body weight), xylazine hydrochloride (Bayer®, Leverkusen, Germany) (5.4 mg/kg body weight) and atropine sulfate (Nycomed®, Linz, Austria) (0.085 mg/kg). After fixation of the animal, the fur of the belly was trimmed and disinfected with 90% alcohol. A median incision was done and the linea alba was incised. The cecum was mobilized and ligated with a 4.0 Vicyl suture (Ethicon, Norderstedt, Germany) including approximately three-quarters of the cecum in order to induce a high-grade sepsis with an anticipated mortality of 100% after 4 days (Rittirsch et al., 2009). The perforation, saving the blood vessels, was done with a 21 gauge needle, exposing small droplets of feces on both perforation sides. The cecum was repositioned and the incision closed in layers with 4.0 Vicryl sutures. Afterwards, the animals were rescued with a subcutaneous injection of 37°C warm sodium chloride (1 ml/20 g body weight). After the procedure, the animals were kept on a 37°C warm plate with free access to unlimited food and water. Two hours after the intervention, the 4-hourly periodical assessment of body weight, body temperature and grade of prostration started (Rittirsch et al., 2009).
Auditory evoked brain stem responses
Anesthesia was performed with an intraperitoneal injection of ketamine hydrochloride (67.5 mg/kg body weight), xylazine hydrochloride (5.4 mg/kg body weight) and atropine sulfate (0.085 mg/kg). The ABR measurement (PNS 2, Tübingen, Germany) was done in an electrically shielded sound attenuated chamber for the frequencies 4, 8, 16 and 32 kHz. The potentials were recorded via three subcutaneous needle electrodes, which were placed at the vertex (positive electrode), the bulla (negative electrode) and the ipsilateral leg (ground electrode). The stimuli started at 90 dB and gradually decreased from 90 dB to 5 dB. The ABR thresholds are determined as the minimum stimulation level that produces a clearly recognizable potential. Electrical signals were averaged over 64 repetitions of stimulus pairs. The ABR results were evaluated by two independent ear, nose and throat specialists in a blinded manner.
Specimen preparation
Immediately after sacrifice, both cochleae were harvested and dissected within 6 minutes. The round and the oval windows were opened and fixed with submersion fixation and subsequently rinsed with fixans. The left cochleae were fixed with Karnovsky’s fixative (2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4) for semi-thin sectioning and TEM. The right cochleae were treated with 4% paraformaldehyde in phosphate buffered saline (PBS), pH 7.4, for further immunohistochemistry. Decalcification was performed with an 8-hour exposure of tissue in 20% EDTA (Titriplex III; Merck, Darmstadt, Germany) in PBS, pH 7.4, at 37°C.
After being thoroughly washed in PBS, Karnovsky-fixed cochleae were embedded in Embed 812 Kit© (Electron Microscopy Science, Hatfield, PA) according to the recommended procedure. Semi-thin sections (1 μm) were cut on a Leica Ultracut microtome (Wetzlar, Germany) and then stained with Toluidine Blue at 60°C for light microscopic evaluation. Paraformaldehyde-fixed specimens were cryoembedded following a published protocol (Coleman et al., 2009). For immunohistochemical examination, cryoembedded serial sections (10 μm) were cut on a cryostat (LEICA CM 3050), mounted on glass slides (SuperFrost®Plus, Menzel-Gläser, Braunschweig, Germany), air-dried for 1 hour and stored at −20°C.
Laboratory examination
Immediately before sacrifice ∼0.7 ml of blood was drawn by cardiac puncture. Approximately 300 μl was used to measure lactate and pH levels (Blood Gas Analyzer Rapidlab 865 Siemens®, Munich, Germany).
The remaining blood was cultured. The BacT/ALERT* Culture Media – Pediatric FAN bioMérieux France was used as culture vessel. The analysis of the bacteriaemia was done with BacT/ALERT® 3D Microbial Detection System bioMérieux (Craponne, France).
Light microscopy
The semithin sections (1 μm) and the immunohistochemical slides were examined using an Image-pro® 6.0 analysis system (Media Cybernetics®, Silver Spring, MD) linked to a 3 CCD color video camera (Sony DXC-950P, Tokyo, Japan) on an Olympus BX 50 light microscope (Olympus, Tokyo, Japan).
Transmission electron microscopy
TEM evaluation was done with the block surface method. Ultrathin sections (90 nm) were cut with a Leica UC6 microtome and transferred to pioloform F (polyvinylacetate)-coated (1.5% pioloform in chloroform) slot grids. Staining was performed by an automated system (Leica EM Stain) with uranyl acetate (5 g/l; 30 minutes) and lead citrate (5 g/l, 50 minutes) at 25°C. The ultrathin sections were examined with a Philips CM 120 transmission electron microscope (FEI, Eindhoven, The Netherlands) equipped with a MORADA digital camera (Olympus SIS, Münster, Germany). The software Olympus TEM Imaging Platform was used.
Immunohistochemistry
Immunohistochemistry was performed on frozen sections in a Ventana Roche Discovery XT Immunostainer (Mannheim, Germany) according to the DAB-MAP discovery research standard procedure. After incubating the sections with primary antibodies [cleaved caspase-3 1:400 (Asp175), Cell Signaling Technology, Danvers, MA; Bcl-2 (N-19), sc-492 1:200 Santa Cruz Biotechnology, Santa Cruz, CA; and Bax (P-19) rabbit polyclonal IgG492 1:50 Santa Cruz Biotechnology] for 1 hour at 37°C, a biotinylated donkey anti-rabbit IgG secondary antibody (1:400, Jackson ImmunoResearch 711-065-152) was applied for 30 minutes at room temperature. The detection was achieved using the DAB-MAP Detection Kit (Ventana 760-124) according to the diaminobenzidine development method followed by lightly counterstaining with hematoxylin (Ventana 760–2021) for 4 minutes. Sections were then manually dehydrated, cleared in xylene and cover-slipped. The immunohistochemical staining reaction was referred to positive controls (tonsil) that were added to each experiment. In addition, for control purposes, representative sections were processed in the same way as previously described by substituting them for isotype matching immunoglobulins (1:200, rabbit polyclonal IgG, Abcam, ab27478).
The sections were evaluated independently by two blinded authors in a semi-quantitative manner: ++ intensive reaction, + mild reaction, – no reaction.
Statistical evaluation
ABR measurements were performed in each animal before treatment at the start (T1) and after the treatment at the end (T2) of the observation period. Pure tone average (PTA) was calculated as the mean of the left and right ear ABR-thresholds for the frequencies 4, 8, 16 and 32 kHz for each animal. To test treatment effects (CLP versus sham or control), one-way analysis of covariance was used with post-treatment PTA (PTA_T2) as the outcome variable adjusted for pretreatment PTA (PTA_T1) as a covariate. Homogeneity of slopes was assessed graphically and by testing the interaction term treatment*PTA_T1 in a preliminary model. Covariate adjusted marginal means of PTA at T2 with 95% confidence intervals are provided.
Supplementary Material
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
J.S. did the conceptual work, established the CLP model, evaluated the ABR and the histological slices and wrote the manuscript. R.G., C.P. and T.S. prepared the immunohistochemical slices. M.J.F.B. and M.B. prepared the electron microscopic slices and did the electron microscopy. M.F. made the blood cultures. P.L. and H.R. calculated the statistics. M.H. measured the ABRs. A.S.-F. was instrumental in helping with the conceptual work, evaluated the ABR and the histological slices and helped with the manuscript
FUNDING
The work has been supported by the intramural funding program of the Medical University Innsbruck (MUI) for young scientists, MUI-Start [project number 2012032018].
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.011205/-/DC1
REFERENCES
- Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001). Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- Brooks H. F., Osabutey C. K., Moss R. F., Andrews P. L., Davies D. C. (2007). Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metab. Brain Dis. 22, 353–373 [DOI] [PubMed] [Google Scholar]
- Choi Y. K., Kim K. W. (2008). Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 41, 345–352 [DOI] [PubMed] [Google Scholar]
- Coleman B., Rickard N. A., de Silva M. G., Shepherd R. K. (2009). A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. J. Neurosci. Methods 176, 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopersmith C. M., Chang K. C., Swanson P. E., Tinsley K. W., Stromberg P. E., Buchman T. G., Karl I. E., Hotchkiss R. S. (2002a). Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit. Care Med. 30, 195–201 [DOI] [PubMed] [Google Scholar]
- Coopersmith C. M., Stromberg P. E., Dunne W. M., Davis C. G., Amiot D. M., 2nd, Buchman T. G., Karl I. E., Hotchkiss R. S. (2002b). Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287, 1716–1721 [DOI] [PubMed] [Google Scholar]
- Eissa D., Carton E. G., Buggy D. J. (2010). Anaesthetic management of patients with severe sepsis. Br. J. Anaesth. 105, 734–743 [DOI] [PubMed] [Google Scholar]
- Häcker G. (2000). The morphology of apoptosis. Cell Tissue Res. 301, 5–17 [DOI] [PubMed] [Google Scholar]
- Halpern N. A., Pastores S. M., Price J. B., Alicea M. (1999). Hearing loss in critical care: an unappreciated phenomenon. Crit. Care Med. 27, 211–219 [DOI] [PubMed] [Google Scholar]
- Iwata A., de Claro R. A., Morgan-Stevenson V. L., Tupper J. C., Schwartz B. R., Liu L., Zhu X., Jordan K. C., Winn R. K., Harlan J. M. (2011). Extracellular administration of BCL2 protein reduces apoptosis and improves survival in a murine model of sepsis. PLoS ONE 6, e14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafa I. M., Uysal M., Bakirci S., Ayberk Kurt M. (2010). Sepsis induces apoptotic cell death in different regions of the brain in a rat model of sepsis. Acta Neurobiol. Exp. (Warsz.) 70, 246–260 [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Kimura R. S., Paul D. L., Takasaka T., Adams J. C. (2000). Gap junction systems in the mammalian cochlea. Brain Res. Brain Res. Rev. 32, 163–166 [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Kim T. H., Chung H. T., Talanian R. V., Yin X. M., Billiar T. R. (2000). Nitric oxide prevents tumor necrosis factor alpha-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology 32, 770–778 [DOI] [PubMed] [Google Scholar]
- Labbé D., Teranishi M. A., Hess A., Bloch W., Michel O. (2005). Activation of caspase-3 is associated with oxidative stress in the hydropic guinea pig cochlea. Hear. Res. 202, 21–27 [DOI] [PubMed] [Google Scholar]
- Latronico N., Bolton C. F. (2011). Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 10, 931–941 [DOI] [PubMed] [Google Scholar]
- Mutunga M., Fulton B., Bullock R., Batchelor A., Gascoigne A., Gillespie J. I., Baudouin S. V. (2001). Circulating endothelial cells in patients with septic shock. Am. J. Respir. Crit. Care Med. 163, 195–200 [DOI] [PubMed] [Google Scholar]
- Pfannenstiel S., Praetorius M. (2008). [Protection and regeneration of sensory epithelia of the inner ear]. HNO 56, 13–20 [DOI] [PubMed] [Google Scholar]
- Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009). Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J., Wang J., Rebillard G., Eybalin M., Lloyd R., Pujol R., Puel J. L. (2007). Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear. Res. 227, 19–27 [DOI] [PubMed] [Google Scholar]
- Salakou S., Kardamakis D., Tsamandas A. C., Zolota V., Apostolakis E., Tzelepi V., Papathanasopoulos P., Bonikos D. S., Papapetropoulos T., Petsas T., et al. (2007). Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo 21, 123–132 [PubMed] [Google Scholar]
- Schmutzhard J., Glueckert R., Sergi C., Schwentner I., Abraham I., Schrott-Fischer A. (2009). Does perinatal asphyxia induce apoptosis in the inner ear? Hear. Res. 250, 1–9 [DOI] [PubMed] [Google Scholar]
- Schmutzhard J., Kositz C. H., Lackner P., Dietmann A., Fischer M., Glueckert R., Reindl M., Stephan K., Riechelmann H., Schrott-Fischer A., et al. (2010). Murine malaria is associated with significant hearing impairment. Malar. J. 9, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutzhard J., Kositz C. H., Lackner P., Pritz C., Glueckert R., Fischer M., Schmutzhard E., Schrott-Fischer A. (2011). Murine cerebral malaria: histopathology and ICAM 1 immunohistochemistry of the inner ear. Trop. Med. Int. Health 16, 914–922 [DOI] [PubMed] [Google Scholar]
- Schmutzhard J., Kositz C. H., Glueckert R., Schmutzhard E., Schrott-Fischer A., Lackner P. (2012). Apoptosis of the fibrocytes type 1 in the spiral ligament and blood labyrinth barrier disturbance cause hearing impairment in murine cerebral malaria. Malar. J. 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccone F. S., Su F., Pierrakos C., He X., James S., Dewitte O., Vincent J. L., De Backer D. (2010). Cerebral microcirculation is impaired during sepsis: an experimental study. Crit. Care 14, R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Holthoff J. H., Seely K. A., Pathak E., Spencer H. J., 3rd, Gokden N., Mayeux P. R. (2012). Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am. J. Pathol. 180, 505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund M., Duberg A. S. (2010). [Permanent hearing loss following dog bite. Capnocytophaga canimorsus caused severe infection with sepsis]. Lakartidningen 107, 1771–1773 [PubMed] [Google Scholar]
- Xiao H., Siddiqui J., Remick D. G. (2006). Mechanisms of mortality in early and late sepsis. Infect. Immun. 74, 5227–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q. Y., Johnson K. R., Erway L. C. (1999). Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 130, 94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.