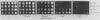Abstract
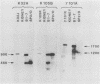
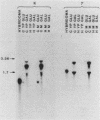
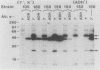
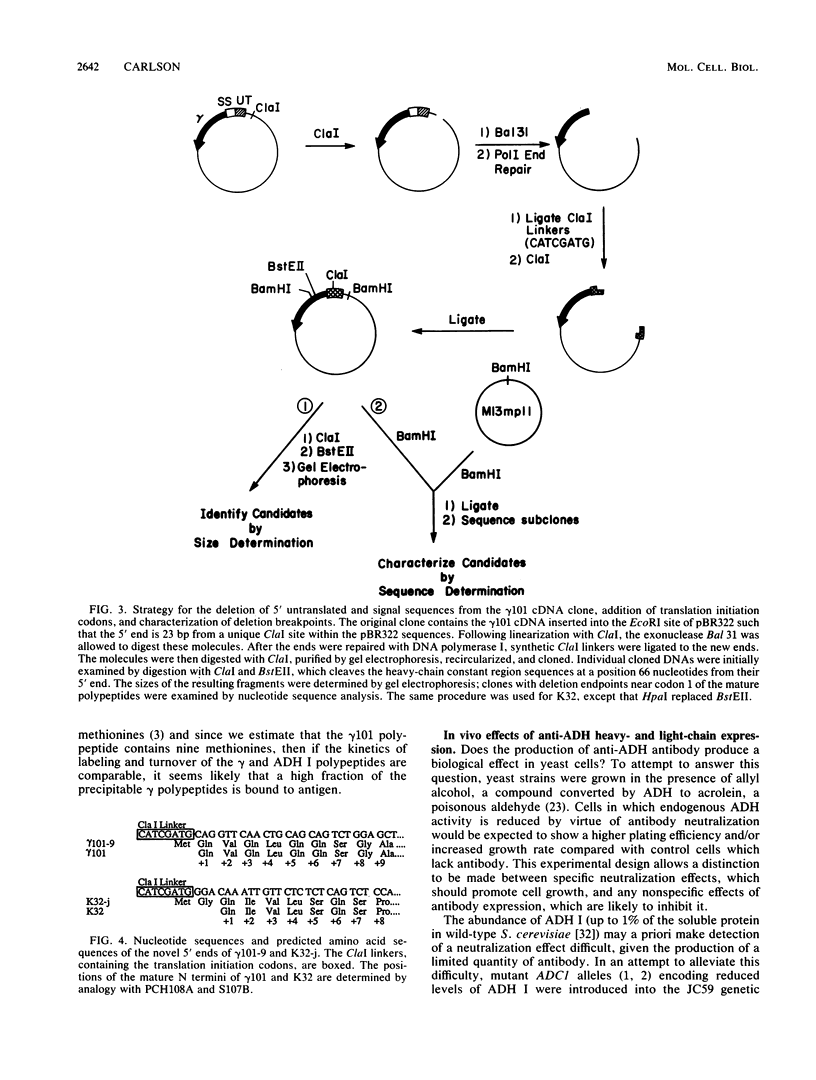
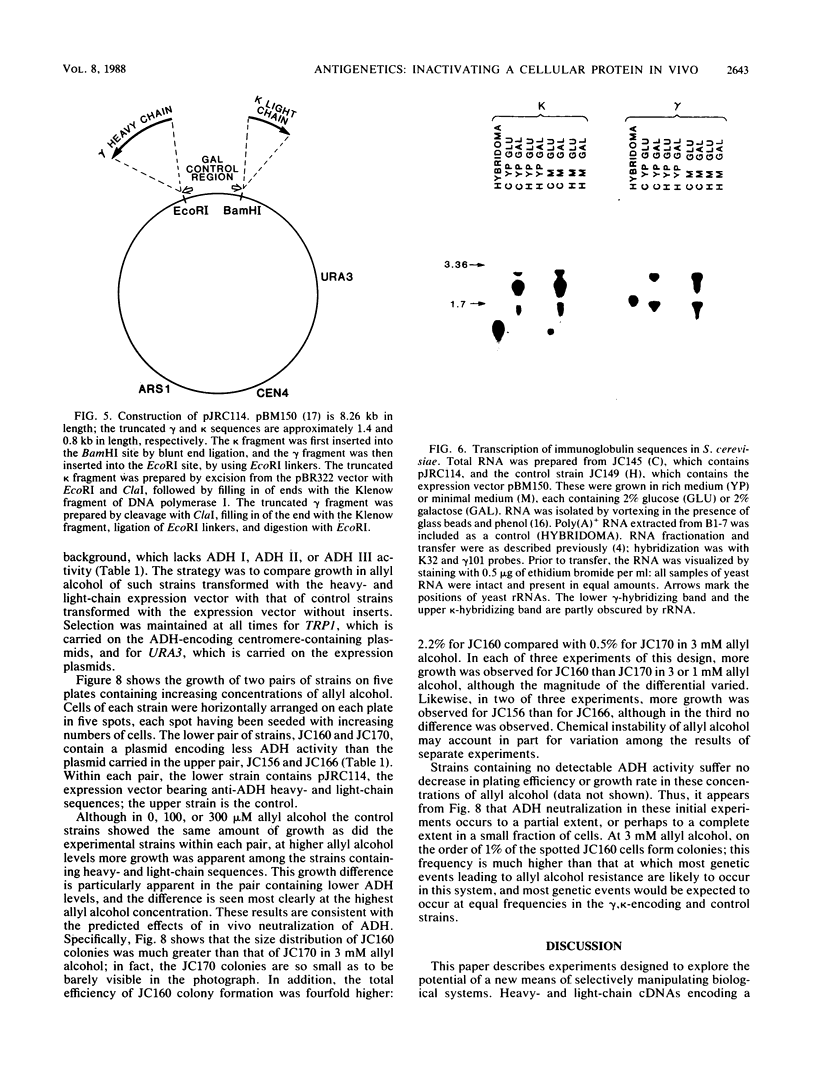
This paper presents a general means of eliminating the function of a single protein without relying on genetic alterations in its structure or level of synthesis. The strategy is based on the inducible cellular expression of neutralizing antibody to inactivate the protein selectively. The feasibility of this approach is illustrated by using alcohol dehydrogenase I (ADH I) in Saccharomyces cerevisiae as a model. Heavy- and light-chain cDNAs were isolated from a hybridoma secreting an antibody which neutralizes yeast ADH I. The cDNAs were characterized with respect to their length and identity, their signal sequences were removed, and synthetic translation initiation codons were joined to them. These truncated sequences were then inserted into an inducible expression vector and shown to be expressed as stable heavy and light chains, which assemble and bind antigen. The sequences were introduced into yeast mutants containing different levels of ADH activity, and evidence is provided that the antibodies produce limited neutralization of enzyme activity in vivo. In principle, the approach can be used for any cell type in which functional antibody can be inducibly expressed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier D. R., Sledziewski A., Young E. T. Deletion analysis identifies a region, upstream of the ADH2 gene of Saccharomyces cerevisiae, which is required for ADR1-mediated derepression. Mol Cell Biol. 1985 Jul;5(7):1743–1749. doi: 10.1128/mcb.5.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D. R., Young E. T. Characterization of a regulatory region upstream of the ADR2 locus of S. cerevisiae. Nature. 1982 Dec 23;300(5894):724–728. doi: 10.1038/300724a0. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Carlson J. R., Hogness D. S. Developmental and functional analysis of Jonah gene expression. Dev Biol. 1985 Apr;108(2):355–368. doi: 10.1016/0012-1606(85)90039-9. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Weissman I. L. Molecular tools for inactivating a yeast enzyme in vivo. Mol Cell Biol. 1988 Jun;8(6):2647–2650. doi: 10.1128/mcb.8.6.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Clark R., Wong G., Arnheim N., Milley R., McCormick F. Transient reversion of ras oncogene-induced cell transformation by antibodies specific for amino acid 12 of ras protein. Nature. 1985 Apr 18;314(6012):639–642. doi: 10.1038/314639a0. [DOI] [PubMed] [Google Scholar]
- Givol D., Zakut R., Effron K., Rechavi G., Ram D., Cohen J. B. Diversity of germ-line immunoglobulin VH genes. Nature. 1981 Jul 30;292(5822):426–430. doi: 10.1038/292426a0. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Honjo T., Obata M., Yamawaki-Katoaka Y., Kataoka T., Kawakami T., Takahashi N., Mano Y. Cloning and complete nucleotide sequence of mouse immunoglobulin gamma 1 chain gene. Cell. 1979 Oct;18(2):559–568. doi: 10.1016/0092-8674(79)90072-2. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Weissman I. L., Early P., Cole J., Hood L. Organization of kappa light chain genes in germ-line and somatic tissue. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1106–1110. doi: 10.1073/pnas.77.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Max E. E., Seidman J. G., Leder P., Scharff M. D. Two kappa immunoglobulin genes are expressed in the myeloma S107. Cell. 1981 Oct;26(1 Pt 1):57–66. doi: 10.1016/0092-8674(81)90033-7. [DOI] [PubMed] [Google Scholar]
- MEGNET R. ALKOHOLDEHYDROGENASEMUTANTEN VON SCHIZOSACCHAROMYCES POMBE. Pathol Microbiol (Basel) 1965;28:50–57. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Clarke P., Salser W. Sequence analysis of cloned cDNA encoding part of an immunoglobulin heavy chain. Nucleic Acids Res. 1979 Jul 25;6(10):3305–3321. doi: 10.1093/nar/6.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger D., Rungger-Brändle E., Chaponnier C., Gabbiani G. Intranuclear injection of anti-actin antibodies into Xenopus oocytes blocks chromosome condensation. Nature. 1979 Nov 15;282(5736):320–321. doi: 10.1038/282320a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Dickson R., Pastan I. Microinjection of anticlathrin antibodies into fibroblasts does not interfere with the receptor-mediated endocytosis of alpha2-macroglobulin. Cell. 1981 Jul;25(1):105–119. doi: 10.1016/0092-8674(81)90235-x. [DOI] [PubMed] [Google Scholar]
- Williamson V. M., Bennetzen J., Young E. T., Nasmyth K., Hall B. D. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature. 1980 Jan 10;283(5743):214–216. doi: 10.1038/283214a0. [DOI] [PubMed] [Google Scholar]
- Wills C., Jörnvall H. The two major isozymes of yeast alcohol dehydrogenase. Eur J Biochem. 1979 Sep;99(2):323–331. doi: 10.1111/j.1432-1033.1979.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Wood C. R., Boss M. A., Kenten J. H., Calvert J. E., Roberts N. A., Emtage J. S. The synthesis and in vivo assembly of functional antibodies in yeast. Nature. 1985 Apr 4;314(6010):446–449. doi: 10.1038/314446a0. [DOI] [PubMed] [Google Scholar]