YSL4 and YSL6 are two members of the yellow stripe–like family of metal transporters in Arabidopsis. This study reveals that YSL6 is a protein of the chloroplast envelope, which together with YSL4, is required for the release of iron from the chloroplast. They are active in dedifferentiating chloroplasts of mature embryos and senescent leaves, likely protecting them from free iron toxicity.
Abstract
In most plant cell types, the chloroplast represents the largest sink for iron, which is both essential for chloroplast metabolism and prone to cause oxidative damage. Here, we show that to buffer the potentially harmful effects of iron, besides ferritins for storage, the chloroplast is equipped with specific iron transporters that respond to iron toxicity by removing iron from the chloroplast. We describe two transporters of the YELLOW STRIPE1-LIKE family from Arabidopsis thaliana, YSL4 and YSL6, which are likely to fulfill this function. Knocking out both YSL4 and YSL6 greatly reduces the plant’s ability to cope with excess iron. Biochemical and immunolocalization analyses showed that YSL6 resides in the chloroplast envelope. Elemental analysis and histochemical staining indicate that iron is trapped in the chloroplasts of the ysl4 ysl6 double mutants, which also accumulate ferritins. Also, vacuolar iron remobilization and NRAMP3/4 expression are inhibited. Furthermore, ubiquitous expression of YSL4 or YSL6 dramatically reduces plant tolerance to iron deficiency and decreases chloroplastic iron content. These data demonstrate a fundamental role for YSL4 and YSL6 in managing chloroplastic iron. YSL4 and YSL6 expression patterns support their physiological role in detoxifying iron during plastid dedifferentiation occurring in embryogenesis and senescence.
INTRODUCTION
Fe is the most common redox-active metal cofactor found in proteins. Once in the cytosol, Fe must traffic to its sites of incorporation in proteins; when Fe is present in excess, it must be sent to neutral compartments for storage. Intracellular Fe traffic is made even more complex by Fe chemistry. Indeed, iron in cells exists as ferrous Fe2+ or ferric Fe3+ iron complexed with various ligands, the nature of which depends on the chemical properties of each cell compartment and on the stability of the iron compound in a particular environment. Consequently, unique types of transporters must be recruited in cell compartments to enable the transport of specific Fe species. Basic knowledge on how Fe crosses the tonoplast membrane or the mitochondria envelope is starting to emerge, but very little is known about the mechanism that mobilizes Fe into and from the chloroplast.
In seeds, vacuoles represent one of the main storage sites for Fe (Lanquar et al., 2005; Roschzttardtz et al., 2009). Recent development of powerful methods for iron imaging have established that the Arabidopsis thaliana embryo stores a large pool of Fe in the vacuoles of particular cell types, including the endodermis in the hypocotyl/radicle axis and the cell layer directly adjacent to the vasculature in embryonic cotyledons (Roschzttardtz et al., 2009). The buildup of this vacuolar Fe store depends on the presence at the tonoplast membrane of VACUOLAR IRON TRANSPORTER1 (VIT1), which transports Fe and Mn from the cytosol into the vacuole (Kim et al., 2006). During germination, mobilization of vacuolar-stored iron is mediated by two tonoplastic transporters, NATURAL RESISTANCE ASSOCIATED MACROPHAGE PROTEIN (NRAMP) 3 and 4. An Arabidopsis germinating plantlet fully consumes its vacuolar store in 4 d, but a double nramp3 nramp4 mutant fails to remobilize the stored Fe and harbors fully loaded vacuoles (Lanquar et al., 2005; Roschzttardtz, et al., 2009). Consequently, nramp3 nramp4 mutant plants cannot provide sufficient Fe to sustain plant growth when iron supply is limiting.
The bioenergetic membranes of mitochondria and chloroplasts, where the Fe-containing proteins of the electron transfer chains of respiration and photosynthesis lie, are the main sinks for iron in plant cells. In the mitochondrion, Fe transport has been shown to involve members of the MITOCHONDRIAL SOLUTE CARRIER family, such as the MITOCHONDRIA IRON TRANSPORTER in rice (Oryza sativa; Mühlenhoff et al., 2003; Li and Kaplan, 2004; Froschauer et al., 2009; Bashir et al., 2011). More than mitochondria, plastids are high Fe-demanding organelles in plant cells, as chloroplasts contain up to 80% of the cellular Fe of leaves (Terry and Abadia, 1986). However, the mechanism by which Fe is taken up by chloroplasts remains elusive. Fe2+ movement was measured across inner envelope membranes of pea (Pisum sativum) chloroplasts and identified both Fe2+ influx and Fe2+ efflux activities energized by an electrochemical proton gradient (Shingles et al., 2002). Consistent with this, ferric iron reduction was shown to occur at the chloroplast surface, mainly mediated by the FRO7 protein (Jeong et al., 2008). FRO7 belongs to a family of eight ferric-chelate reductases in Arabidopsis, whose well-known member, FERRIC REDUCTASE OXIDASE 2 (FRO2), is located at the plasma membrane of root epidermal cells where it reduces ferric iron prior to its uptake by the high-affinity ferrous iron transporter IRT1 (reviewed in Curie and Briat, 2003). Plants lacking FRO7 show severe chlorosis in Fe-deficient conditions and have a reduced chloroplastic Fe level (Jeong et al., 2008). This indicates that chloroplasts rely at least in part on a reduction step to acquire Fe, in the same manner as root epidermal cells. None of the 15 transporters of the ZIP family, the founding member being IRT1, appears as a likely candidate for the function of Fe2+ uptake at the chloroplast surface.
Recent studies have identified several other proteins that may play a role in iron import into the chloroplast. PERMEASE IN CHLOROPLASTS1 (PIC1), a protein of the Arabidopsis chloroplast inner envelope, has been shown to functionally rescue growth of the yeast Fe uptake defective mutant fet3 fet4, as does its homolog from the cyanobacteria synechocystis (Duy et al., 2007). A pic1 knockout mutant has an albino phenotype associated with dramatic structural abnormalities of the chloroplasts, ferritin accumulation, and deregulation of many Fe homeostasis-related genes. However, there is no evidence from direct measurement of chloroplastic Fe uptake to support a role for PIC in Fe uptake in the chloroplast. PIC1 was reported to interact with a member of the Ni2+-Co2+ transporters family, referred to as NiCo, which together with PIC1, is hypothesized to act as a complex to import Fe into the chloroplast (Duy et al., 2011). Unlike FRO7, whose expression is limited to young green tissues, PIC1 expression is ubiquitous, indicating that these two genes do not participate in the same pathway of Fe entry. The chloroplastic transporter MAR1, a close homolog of the IREG/Ferroportin efflux transporters, is also of interest. MAR1 expression is enhanced by Fe deficiency and its overexpression disrupts Fe homeostasis (Conte et al., 2009). MAR1 was proposed to transport an iron chelate or an iron ligand, such as nicotianamine (NA), in chloroplasts and to opportunistically take up aminoglycoside antibiotics. Finally, the ATP-Binding Cassette (ABC) protein non-intrinsic ABC protein 14 (NAP14) is another candidate for the transport of Fe into the chloroplast (Shimoni-Shor et al., 2010). NAP14 is one of two Arabidopsis orthologs of the plasma membrane FutC protein, which takes up iron in cyanobacteria. A nap14 knockout mutant shows high levels of Fe, loss of chloroplast structures, and deregulation of the Fe homeostasis genes.
YELLOW STRIPE1-LIKE (YSL) proteins are a family of transporters of metal complexes in plants (Curie et al., 2009). The first class of YSL transporters is represented by maize (Zea mays) YS1, or its rice and barley orthologs, and mediates iron acquisition at the root surface of poaceae plants (Curie et al., 2001; Schaaf et al., 2004; Murata et al., 2006; Inoue et al., 2009). YS1 orthologs transport a complex of FeIII with a ligand belonging to a family of small plant metabolites collectively called phytosiderophores (PSs). PSs are synthesized by poaceae plants in response to iron deficiency and excreted from the root to solubilize rhizospheric iron (reviewed in Curie et al., 2009). YS1 orthologs are therefore part of the primary uptake machinery specific to poaceae plants. Another class of YSL proteins, which are found in both monocots and dicots, are involved in the mobilization within the plant of metal ions, complexed either with PS or with NA, a precursor of PS with similar affinity for metals (Benes et al., 1983). Their expression is associated with vascular tissues where they are involved in the distribution of metals between organs (Koike et al., 2004; Le Jean et al., 2005; Waters et al., 2006; Zheng et al., 2012). Arabidopsis YSL1 and YSL3 are likely to mediate the remobilization of Fe, Zn, and Cu in the form of metal-NA chelates from senescent leaves and the loading of these metals into inflorescences and seeds (DiDonato et al., 2004; Le Jean et al., 2005; Chu et al., 2010). In rice, YSL2 was suggested to mediate phloem transport of FeII-NA and MnII-NA to shoots and seeds (Koike et al., 2004; Ishimaru et al., 2010) and YSL16 was shown to load Cu-NA into the phloem sap (Zheng et al., 2012).
Here, we describe the molecular and functional characterization of two additional members of the Arabidopsis family of YSL transporters, YSL4 and YSL6. We show that unlike the other studied family members, which are plasma membrane proteins, at least one of them, YSL6, is targeted to the chloroplast envelope. A double ysl4 ysl6 knockout mutant shows reduced capacity to cope with iron excess conditions, in line with the upregulation of these two genes in response to iron excess. Measurement of iron content in isolated chloroplasts as well as imaging of iron in the cells indicated that ysl4 ysl6 plants have iron-overloaded chloroplasts, a result that is consistent with the higher abundance of ferritin in these plants. By contrast, plants engineered to express YSL4 or YSL6 ubiquitously lack iron in chloroplasts and are dramatically sensitive to iron deficiency. Collectively, the results presented here indicate that YSL4 and YSL6 participate in iron homeostasis in the chloroplast by mediating its release from and preventing its accumulation into the chloroplasts.
RESULTS
The ysl4 ysl6 Double Knockout Has Altered Iron Homeostasis
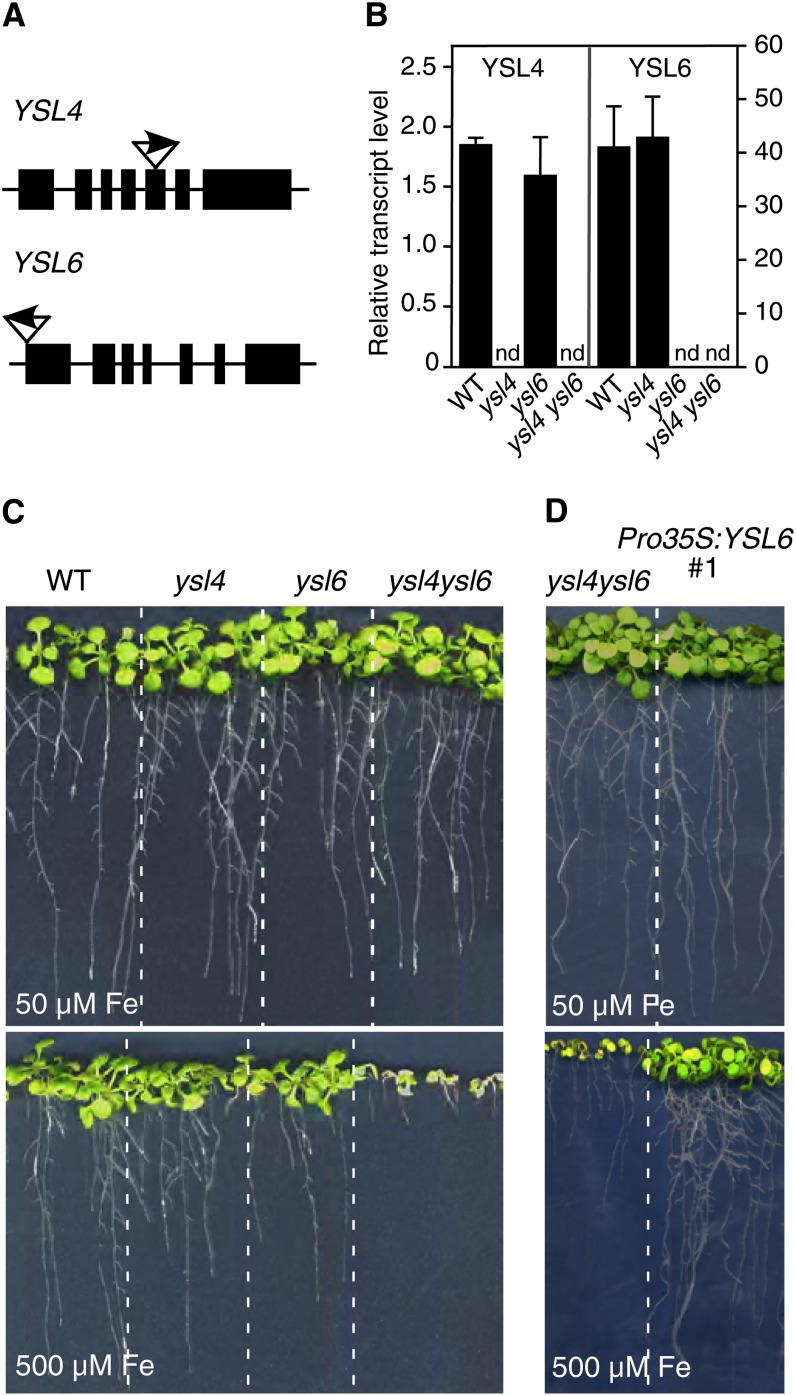
To investigate YSL4 and YSL6 functions, we used a reverse genetics approach and isolated a mutant for each gene in the available T-DNA insertion collections (Figure 1A). Single knockouts, designated ysl4-1 and ysl6-1, were genotyped and crossed to generate the double ysl4-1 ysl6-1 mutant. The lack of expression of YSL4 or YSL6 in the knockouts was confirmed by quantitative RT-PCR (Figure 1B).
Figure 1.
The Double Knockout ysl4 ysl6 Has Decreased Tolerance to Elevated Iron Levels.
(A) Position and orientation of the T-DNA insertion site for the ysl4-1 (SALK_025447) and ysl6-1 (SALK_093392) knockout mutants.
(B) Relative YSL4 and YSL6 transcript levels in wild-type, single ysl4-1 and ysl6-1, and double ysl4-1 ysl6-1 knockout plants grown for 15 d in standard 0.5× Murashige and Skoog medium, assessed by quantitative RT-PCR. nd, not detectable; WT, the wild type. Error bars represent the se of two independent experiments.
(C) Phenotype of wild-type, ysl4-1, ysl6-1, and ysl4-1 ysl6-1 plants grown for 15 d in iron-replete (50 µM) or iron-excess (500 µM) conditions.
(D) Growth restoration of ysl4-1 ysl6-1 double mutant plants complemented with a functional copy of YSL6 (Pro35S:YSL6 #1).
[See online article for color version of this figure.]
The behavior of ysl4-1 and ysl6-1 mutants was assessed in the presence of limiting or excess metal supply. The single mutants displayed no observable phenotype in response to Fe, Zn, Mn, or Cu stress (Figure 1C; see Supplemental Figure 1A online). The double ysl4 ysl6 mutant grew as wild-type plants in soil or in axenic iron-replete conditions (Figure 1C; see Supplemental Figure 2 online). By contrast, in the presence of 500 µM Fe in the medium, a condition that markedly reduced growth of the wild type, growth and primary root elongation of ysl4 ysl6 were dramatically impaired and plants harbored yellow cotyledons (Figure 1C; see Supplemental Figure 2 online). This hypersensitivity of ysl4 ysl6 plants to Fe excess was observed with 350 µM Fe (see Supplemental Figure 1B online) and was specific for Fe since it was not observed with Zn, Mn, or Cu treatments (see Supplemental Figure 1A online). Iron content measured in seeds, cotyledons, roots, and rosette leaves, regardless of the iron treatment, was unaffected in the double mutant (see Supplemental Figure 3 online). Complementation of ysl4 ysl6 with a Pro35S:YSL6 construct reverted the ysl4 ysl6 hypersensitive phenotype (Figure 1D), thereby confirming that YSL6 is required for the plant to cope with elevated levels of iron.
Expression Analysis
At the organ level, the expression of YSL6 is detected mainly in shoots, with a predominant expression in senescent leaves and in seeds, whereas YSL4 expression is ubiquitous expressed but at a comparatively low level (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi). This was confirmed by real-time RT-PCR analysis of the YSL4 and YSL6 relative transcripts levels in the main organs (see Supplemental Figure 4 online).
In order to refine the tissue localization of YSL4 and YSL6 expression, we generated transgenic Arabidopsis lines transformed with ProYSL4:GUS (β-glucuronidase) or ProYSL6:GUS constructs.
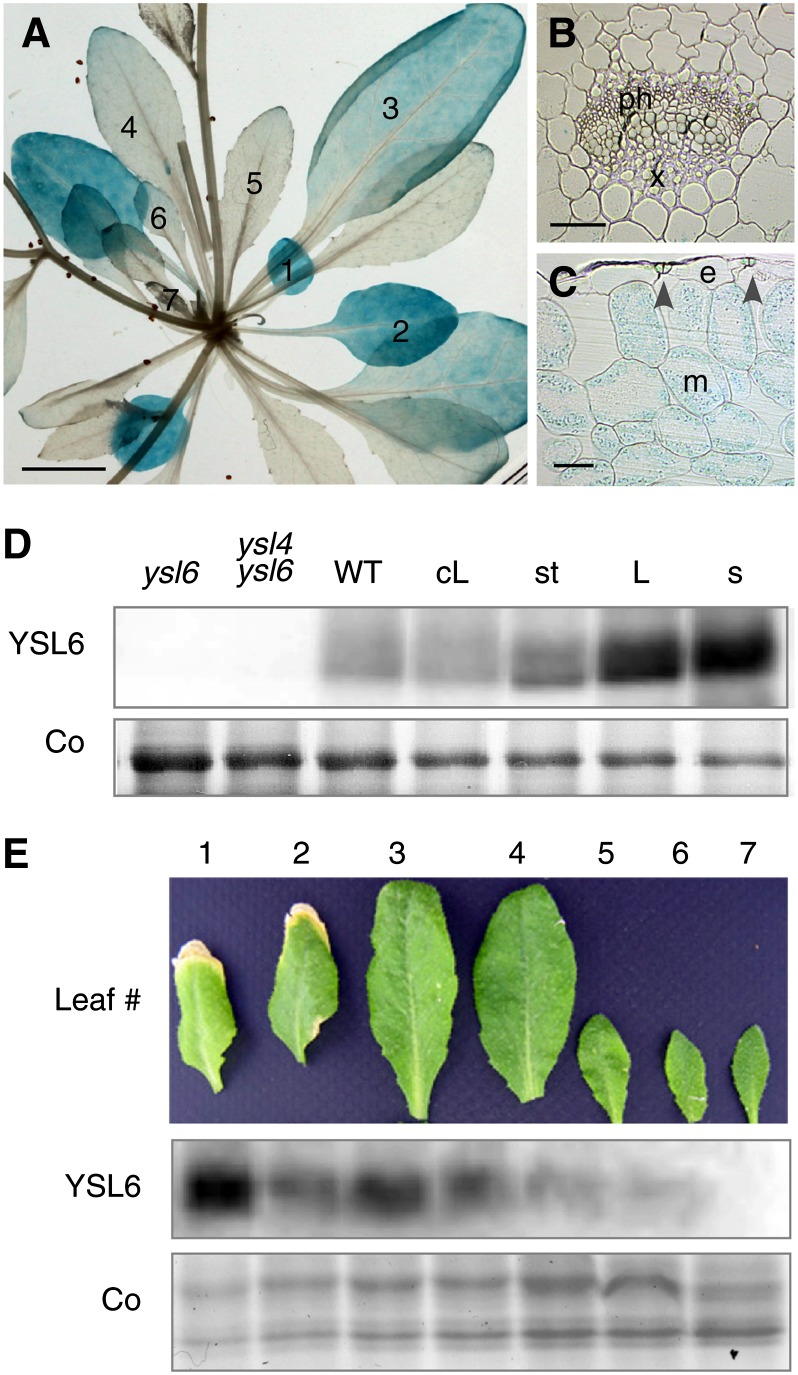
In the ProYSL6:GUS lines, GUS staining was highest in the older leaves and gradually disappeared from the 4th pair of leaves on (Figure 2A). Section across a mature leaf showed GUS activity in mesophyll cells and to a lesser extent in stomata guard cells (Figure 2C), but neither in the vasculature nor in the epidermis (Figures 2B and 2C). To confirm the presence of YSL6 in this tissue, we produced a YSL6-specific antibody targeted against two YSL6-specific peptides. Immunoblot analysis of Arabidopsis leaves detected a polypeptide at ∼60 kD in wild-type plants, a little smaller than the calculated size of YSL6 at 73.6 kD (Figure 2D). This band was absent in ysl6 or ysl4 ysl6 extracts, which confirmed the specificity of the antibody (Figure 2D). Protein accumulation was observed in rosette leaves, cauline leaves, and stem and was particularly strong in mature leaves and mature siliques (Figure 2D). Furthermore, consistent with the GUS histochemical staining observations, YSL6 protein accumulation increased with leaf age (Figure 2E).
Figure 2.
Expression of YSL6 in Shoots.
(A) to (C) Histochemical staining of GUS activity in an Arabidopsis transgenic line carrying a ProYSL6:GUS construct.
(A) Rosette of a 5-week-old soil-grown plant. Leaves are numbered from the oldest (1) to the youngest (7).
(B) and (C) Transversal section across leaf 6 showing the absence of staining in veins (B) and epidermis (C), the strong expression in mesophyll cells (C), as well as the expression in stomata ([C], arrowheads). e, epidermal cell; m, mesophyll cell; ph, phloem; x, xylem.
(D) YSL6 protein accumulation measured by immunoblot using anti-YSL6 antibodies on whole-protein extracts from shoots of 5-week-old ysl6, ysl4 ysl6, or wild-type plants as well as from cauline leaves (cL), stem (st), rosette leaves (L), and mature siliques (s). Co, Coomassie blue staining; WT, the wild type.
(E) Time-course analysis of YSL6 protein accumulation during leaf development. Signal strength increases with the age of the leaf.
Bars = 1 cm in (A) and 20 µm in (B) and (C).
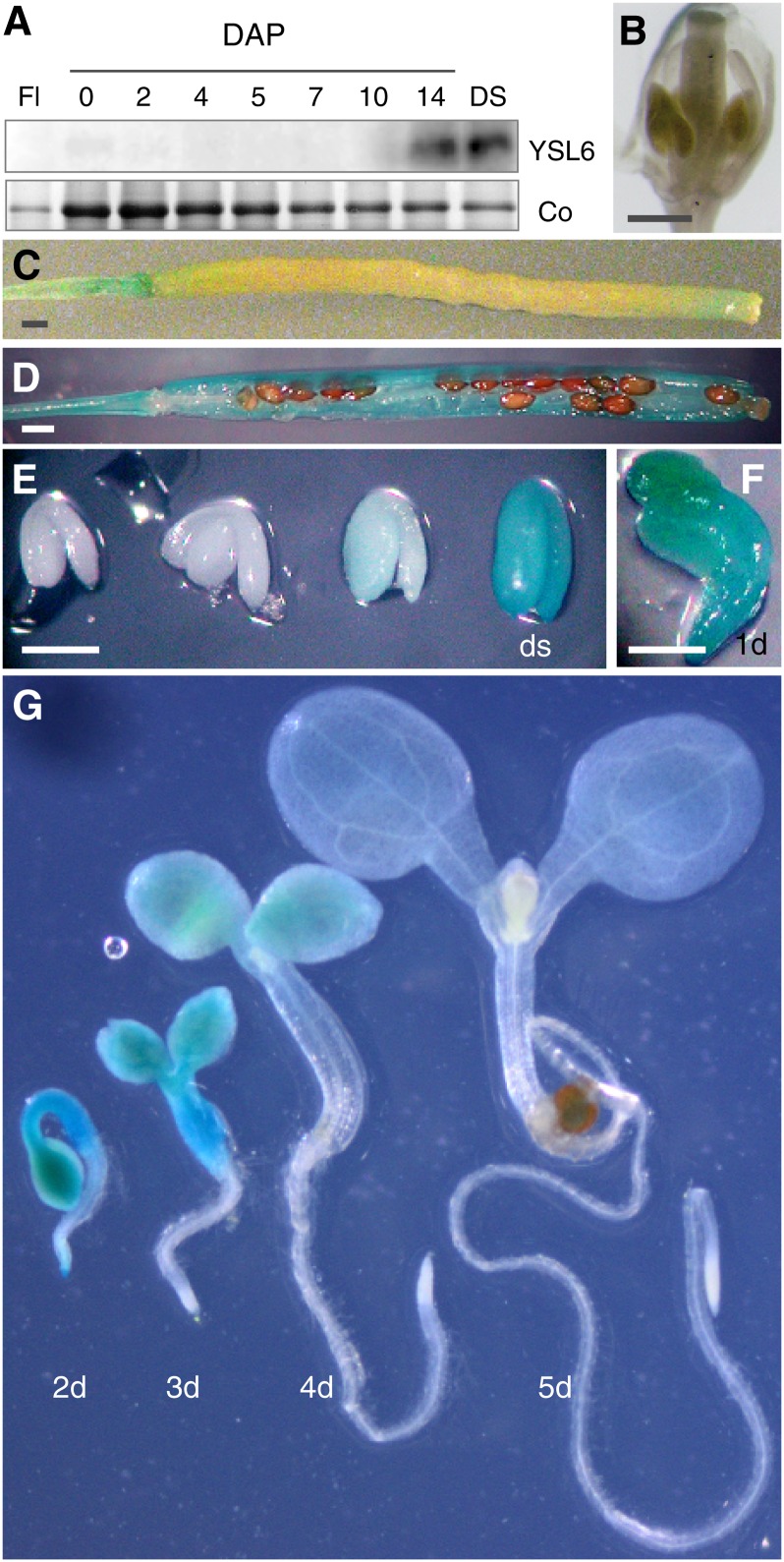
YSL6 expression was undetectable in flowers (Figures 3A and 3B) and in roots (Figure 3G). A study of YSL6 protein accumulation during fruit maturation revealed strong expression in mature siliques and in dry seed (Figure 3A). GUS staining confirmed that YSL6 promoter activity in the carpels is stronger in mature siliques (Figures 3C and 3D). During embryogenesis, the YSL6 promoter is turned on at the end of the maturation process, reaching maximal activity at the dry seed stage (Figure 3E). Upon germination, GUS staining decreased rapidly, becoming undetectable in the root by day 3 and in the cotyledons by day 5 (Figures 3F and 3G).
Figure 3.
Expression Analysis of YSL6 at the Reproductive Stage.
(A) Immunoblot analysis of YSL6 protein accumulation in flowers (Fl), siliques from 0 to 14 d after pollination (DAP), and dry seeds (DS). Co, Coomassie blue staining.
(B) to (G) Histochemical staining of GUS activity in a representative Arabidopsis transgenic line carrying a ProYSL6:GUS construct. Flower (B), young silique (C), mature silique (D), maturing embryo until the dry seed stage (ds) (E), 1-d-old germinating seedling (F), and young germinating seedlings from 2 to 5 d after imbibition (G).
Bars = 1 mm in (B) to (D) and 0.5 mm in (E) and (F).
Twenty independent transgenic lines carrying the ProYSL4:GUS construct had no detectable GUS activity (data not shown). As YSL4 transcript accumulation was 20 times lower than YSL6 (Figure 1B), YSL4 promoter activity is likely too weak to direct detectable GUS activity. Quantitative RT-PCR on the main organs revealed stronger expression in leaves (see Supplemental Figure 4 online), whereas expression databases report additional expression in developing seeds (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi).
Expression of YSL4 and YSL6 Is Controlled by Iron Status
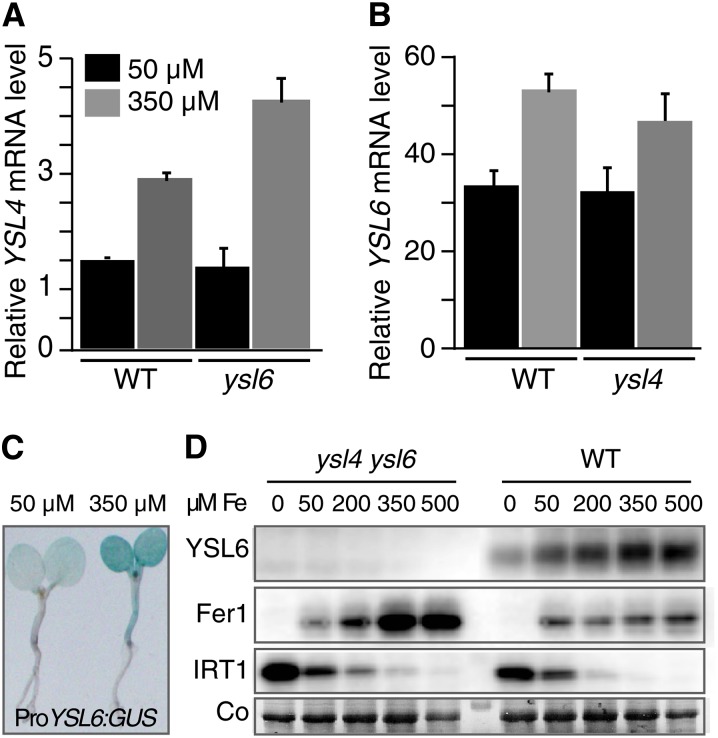
To test whether expression of YSL4 and YSL6 is modulated by iron status, we performed quantitative RT-PCR on 5-d-old plantlets grown in iron-replete (50 µM Fe) or iron-excess (350 µM Fe) conditions. YSL4 and YSL6 relative transcript abundance increased by 95 and 60%, respectively, in response to excess Fe (Figures 4A and 4B). Induction of YSL4 mRNA accumulation by Fe excess was significantly enhanced in the ysl6-1 mutant background, suggesting a compensation mechanism (Figure 4A). Such a deregulation was not observed for YSL6 in the ysl4-1 mutant (Figure 4B). Consistent with the upregulation of YSL6 by Fe supply, GUS histochemical analysis of 5-d-old ProYSL6:GUS transgenic plants showed stronger staining when germinated in the presence of 350 µM Fe (Figure 4C). YSL6 protein accumulation was monitored in response to a range of Fe concentrations in the medium. Total proteins were immunoblotted using a YSL6-specific antipeptide antibody (see Methods). A good hybridization signal was obtained with wild-type protein extracts, and the signal was absent in the ysl4 ysl6 double mutant, confirming that the antibody is specific to YSL6 (Figure 4D). YSL6 accumulation followed the pattern of the Ferritin FER1 isoform, which accumulates upon Fe overload and was opposite to the pattern of the Fe uptake transporter IRT1, which is strongly produced in response to Fe deficiency (Figure 4D). Collectively, these data have established that YSL4 and YSL6 are upregulated in conditions of high Fe supply.
Figure 4.
Regulation of YSL4 and YSL6 Expression by Iron.
(A) and (B) Relative YSL4 and YSL6 transcripts abundance determined by real-time RT-PCR in 5-d-old plants grown in iron-replete (50 µM Fe) or iron-excess (350 µM Fe) conditions. Mean values represent triplicates from two independent experiments. Error bars represent sd. WT, the wild type.
(A) YSL4 transcript level measured in wild-type or ysl6 plants showing deregulation of YSL4 in the ysl6 mutant.
(B) YSL6 transcript level measured in wild-type or ysl4 plants.
(C) Elevated iron concentration activates YSL6 promoter; 5-d-old transgenic lines expressing ProYSL6:GUS grown as in (A) and (B), were stained for GUS activity.
(D) Immunoblot analysis of YSL6 level in 5-d-old plants challenged with a range of iron concentrations. Ferritin (Fer1) and the iron transporter IRT1 are used as controls for iron overload and iron deficiency, respectively. Co, Coomassie blue staining
[See online article for color version of this figure.]
YSL6 Is a Protein of the Chloroplast Envelope
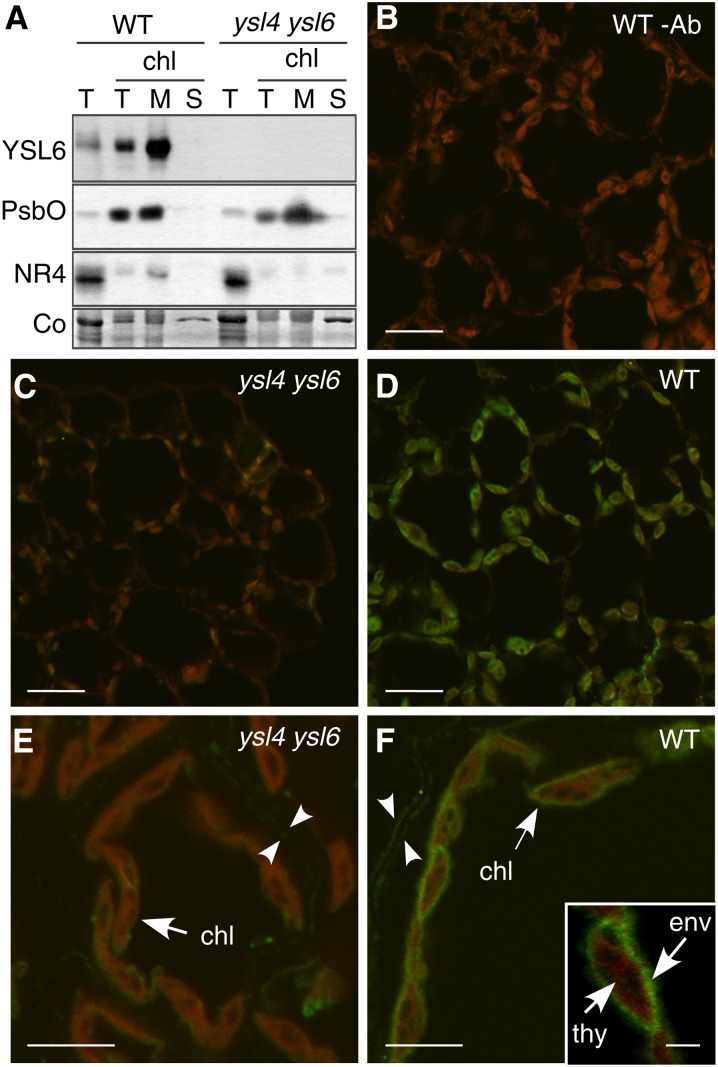
We addressed the question of the membrane localization of YSL6 using cellular protein fractionation. Like the thylakoid protein PsbO (Barber, 2003) and in contrast with the tonoplast protein NRAMP4 (Lanquar et al., 2004), YSL6 was enriched in a total extract of isolated chloroplasts and was further enriched in the microsomal fraction of these chloroplasts (Figure 5A). Therefore, unlike what was expected based on the previously reported identification of YSL4 and YSL6 in a tonoplastic proteome (Jaquinod et al., 2007), YSL6 is a protein of the chloroplast.
Figure 5.
YSL6 Is a Protein of the Chloroplast Envelope.
(A) Immunoblot analysis of YSL6 subcellular localization. Total proteins (T) were extracted from leaves of wild-type (WT) and ysl4 ysl6 plants grown in soil for 4 weeks. Chloroplasts were then isolated (chl lane T) and separated into membrane (lane M) and soluble (lane S) fractions. Antisera used are indicated to the left of the blots. NR4, NRAMP4 protein; Co, Coomassie blue staining.
(B) to (F) Immunofluorescence using anti-YSL6 antibody on leaf cross sections from 3-week-old soil-grown plants. Low magnification ([B] to [D]) and high magnification ([E] and [F]).
(B) Wild-type plant, primary anti-YLS6 antibody omitted, showing chlorophyll fluorescence.
(C) and (E) ysl4 ysl6 hybridized with the anti-YSL6 antibody, controlling anti-YSL6 antibody specificity.
(D) and (F) Wild-type leaf tissue hybridized with the anti-YSL5 antibody showing YSL6-specific signal lining up the chloroplast envelope.
Bars = 10 µm in (B) to (D), 5 µm in (E) and (F), and 2 µm in (F) inset. chl, chloroplast; env, envelope; thy, thylakoids.
To narrow down the target membrane of YSL6 in the chloroplast, we performed immunofluorescence assays on mature leaves of wild-type and ysl4 ysl6 plants using the anti-YSL6 antibody. A strong signal colocalizing with the chlorophyll fluorescence (Figure 5B) and absent in the ysl4 ysl6 mutant (Figures 5C and 5E) was observed in wild-type mature leaf tissue (Figures 5D and 5F). The YSL6 antibody labeled the circumference of the chloroplasts but not the thylakoid membranes (Figure 5F, inset). Thus, YSL6 appears to be specifically targeted to the envelope of the chloroplasts.
YSL4 and YSL6 Are Required for Iron to Exit the Chloroplasts
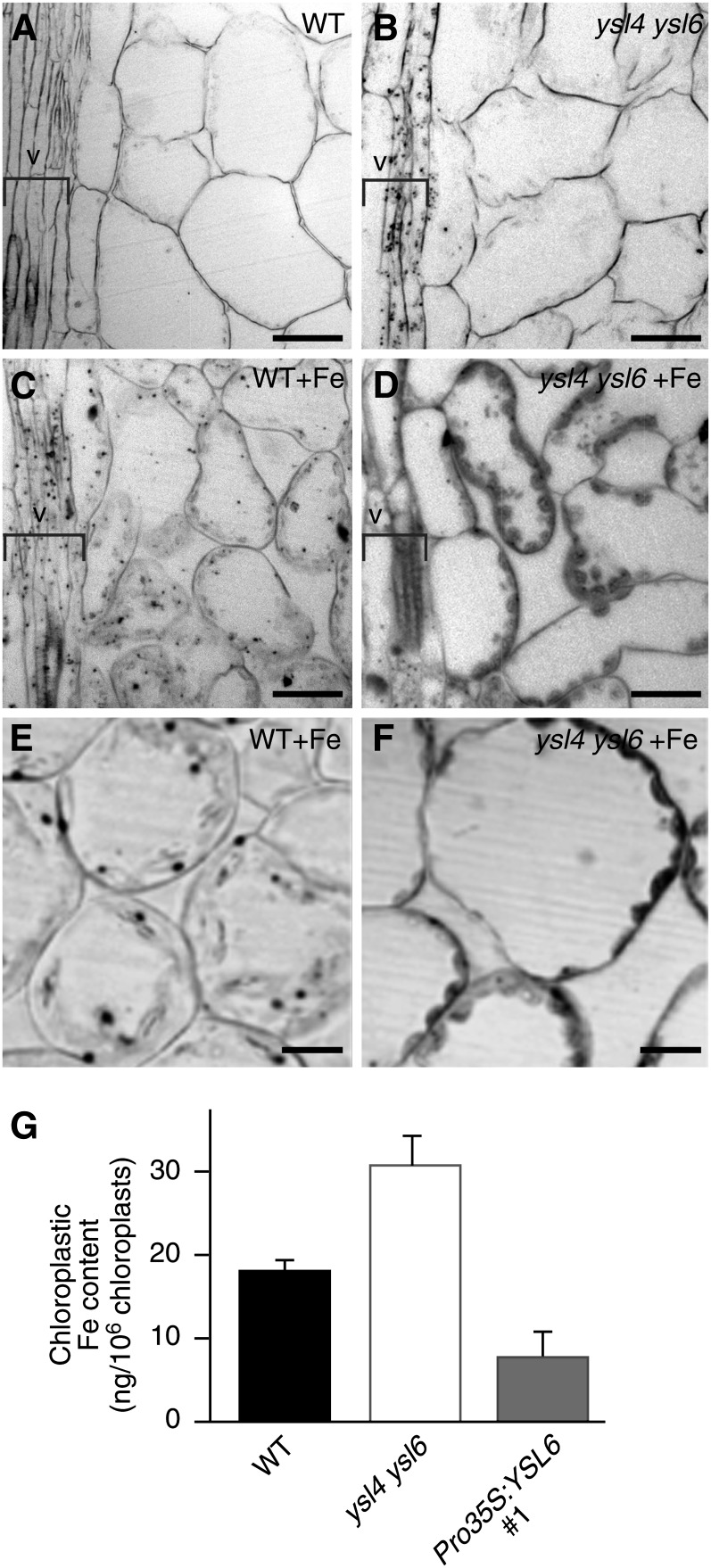
To assess whether YSL4 and YSL6 allow Fe entry into or Fe exit from the chloroplasts, we imaged iron in sections of mature leaves prepared from wild-type or ysl4 ysl6 double mutant plants, irrigated or not with 350 µM Fe using the Perls/Diamino benzidine (DAP) histochemical staining method (Roschzttardtz et al., 2009). Wild-type plants showed very little staining overall (Figure 6A) except when plants were irrigated with high iron, where dot-shaped iron-rich structures appeared in cells of the vasculature as well as in the chloroplasts of mesophyll cells (Figure 6C). These stained dots (Figure 6E) were identified as ferritin-Fe complexes as they disappear in ferritin-less mutants (H. Roschzttardtz, F. Divol, G. Conéjéro, C. Curie, and S. Mari, unpublished data). By contrast, leaves of ysl4 ysl6 plants irrigated with a standard amount of iron showed iron accumulation in the vasculature similar to that in wild-type plants treated with high iron (Figures 6B and 6C). Furthermore, when the ysl4 ysl6 plants were treated with high Fe, a dramatic increase of Fe staining was observed in the vasculature and in the mesophyll chloroplasts (Figures 6D and 6F).
Figure 6.
Iron Content Is Increased in the Chloroplasts of the ysl4 ysl6 Double Mutant.
(A) to (F) Perls/DAB staining of iron in leaf sections prepared from 3-week-old soil-grown plants irrigated with water ([A] and [B]) or 500 µM FeEDDHA (+Fe; [C] to [F]). (E) and (F) are higher magnification images of (C) and (D), respectively. Position of the veins (v) is indicated by a bracket. Bars = 20 µm in (A) to (D) and 10 µm in (E) and (F).
(A), (C), and (E) The wild type (WT).
(B), (D), and (F) ysl4 ysl6.
(G) ICP-MS measurement of iron content in isolated chloroplasts from leaves of plants grown 3 weeks in soil in short-day conditions. Values represent the mean of three measurements from one experiment representative of two biological replicates. Error bars represent sd.
This result was further confirmed by measuring iron content by inductively coupled plasma–mass spectrometry (ICP-MS) in isolated chloroplasts of wild-type and ysl4 ysl6 leaves, which showed that iron increased by 73% in the double mutant (Figure 6G).
Such a high amount of Fe in the chloroplasts of the ysl4 ysl6 plants is compatible with a role of YSL4 and YSL6 in the transport of iron at the inner chloroplast envelope from the stroma toward the cytoplasm. Moreover, this finding fits with the strong overaccumulation of ferritin observed in the ysl4 ysl6 mutant (Figure 4D) as ferritin stability is known to depend on the presence of iron.
As the YSL transporters are reportedly broad-spectrum metal transporters, we investigated the substrate specificity of YSL4 and YSL6 by analyzing the concentration of other detectable metals contained in the chloroplasts of the ysl4 ysl6 mutant. In contrast with iron, manganese concentration remained unchanged between the wild type and ysl4 ysl6 (Table 1), indicating that the transport activity by YSL4 and YSL6 is likely specific for Fe.
Table 1. Elemental Analysis of Chloroplast Content (ng/106 Chloroplasts).
| Genotype | Fe |
Mn |
||||
|---|---|---|---|---|---|---|
| Mean | sd | Fold Change | Mean | sd | Fold Change | |
| Wild type | 18.1 | 1.23 | NA | 29.0 | 2.1 | NA |
| ysl4 ysl6 | 31.5 | 3.5 | 1.74 | 26.7 | 1.8 | 0.92 |
| Pro35S:YSL6 #1 | 7.9 | 0.67 | 0.44 | 22.6 | 2.1 | 0.78 |
ICP-MS measurement in intact chloroplasts isolated from leaves of 4-week-old wild-type, ysl4 ysl6, or representative Pro35S:YSL6 line #1 grown in soil. Values represent the mean of three measurements from one experiment representative of two biological replicates. Fold change is calculated relative to the value in wild-type plants. NA, not applicable.
Ubiquitous Expression of YSL4 or YSL6 Decreases Tolerance of the Plant to Iron Deficiency
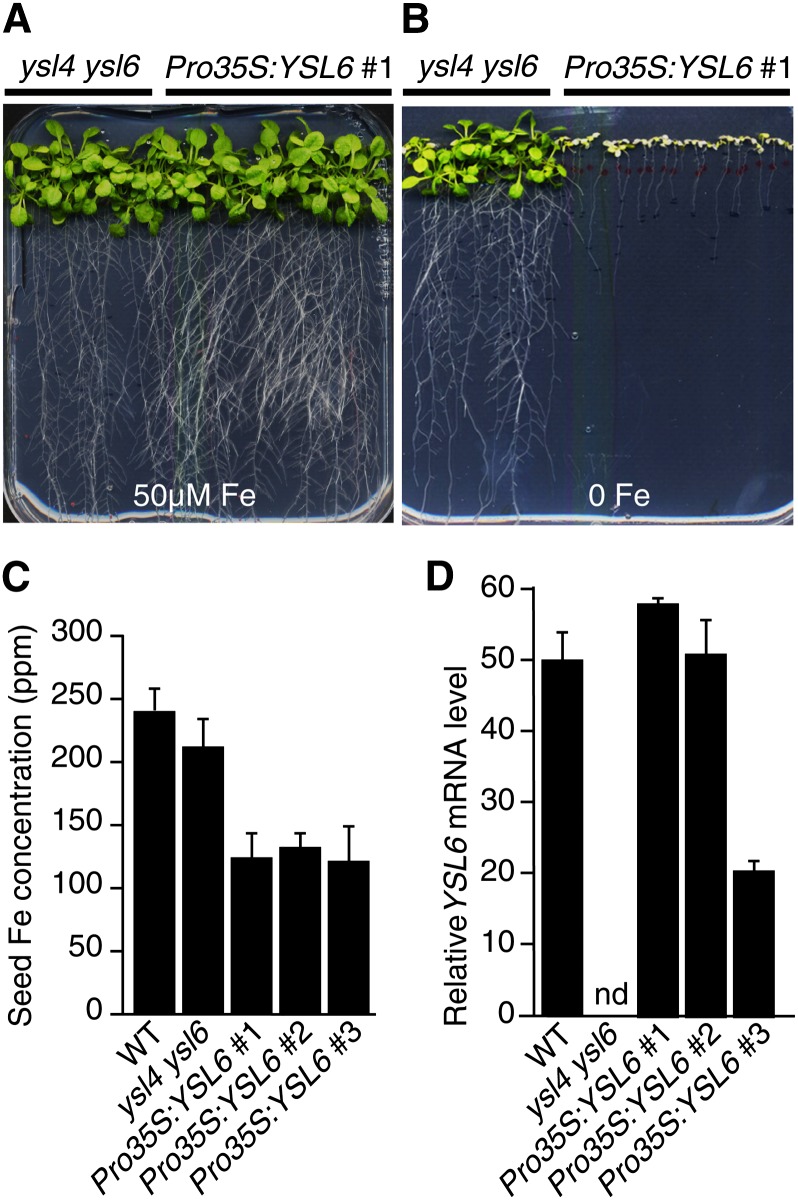
Expression of YSL6 directed by the 35S promoter did rescue ysl4 ysl6 growth defect in the presence of an excess of iron as mentioned above (Figure 1D) but did not confer hypertolerance to iron excess as could be expected from the phenotype of the ysl4 ysl6 double mutant. Interestingly, in iron-limiting conditions, growth of these Pro35S:YSL6 plants was dramatically reduced and plants were extremely chlorotic (Figure 7B). Such hypersensitivity of Pro35S:YSL6 lines was associated with a reduction of iron content in whole seeds (Figure 7C). Their growth defect in iron-limiting conditions was rescued by irrigating the mother plants with a solution of 500 µM Fe-EDDHA (see Supplemental Figure 5A online). The phenotype of the plants did not seem to result from a higher expression of YSL6 (Figure 7D) but may rather originate from the deleterious effect of its ectopic expression in normally YSL6-free tissues. Again, this finding is compatible with a function of YSL6 in the secretion of Fe from the chloroplast since increased YSL6 activity is likely to deplete iron content in the chloroplasts and impair its ability to cope with iron deficiency. This was confirmed by the measurement of iron content in isolated chloroplasts of a representative 35S:YLS6 line, which showed over 50% loss compared with the wild type and 75% compared with ysl4 ysl6 (Figure 6G, Table 1).
Figure 7.
Ubiquitous Expression of YSL6 Dramatically Decreases Plant Tolerance to Iron Deficiency and Reduces Seed Iron Content.
(A) and (B) Growth in iron-replete (A) or iron-deficient (no added iron) (B) medium of ysl4 ysl6 double mutant either complemented (Pro35S:YSL6) or not (ysl4 ysl6) with the Pro35S:YSL6 construct as described in Figure 1D.
(C) ICP-MS measurement of iron concentration in seeds of the wild type (WT), ysl4 ysl6, and three independent Pro35S:YSL6 transgenic lines that behave the same in iron deficiency. Values represent the mean of three measurements from two biological replicates. Error bars represent sd.
(D) Relative YSL6 transcript level measured by quantitative RT-PCR in the wild type, ysl4 ysl6, and Pro35S:YSL6 lines grown as in (A). nd, not detectable. Measurements were performed in triplicates in two independent experiments. Error bars represent sd.
[See online article for color version of this figure.]
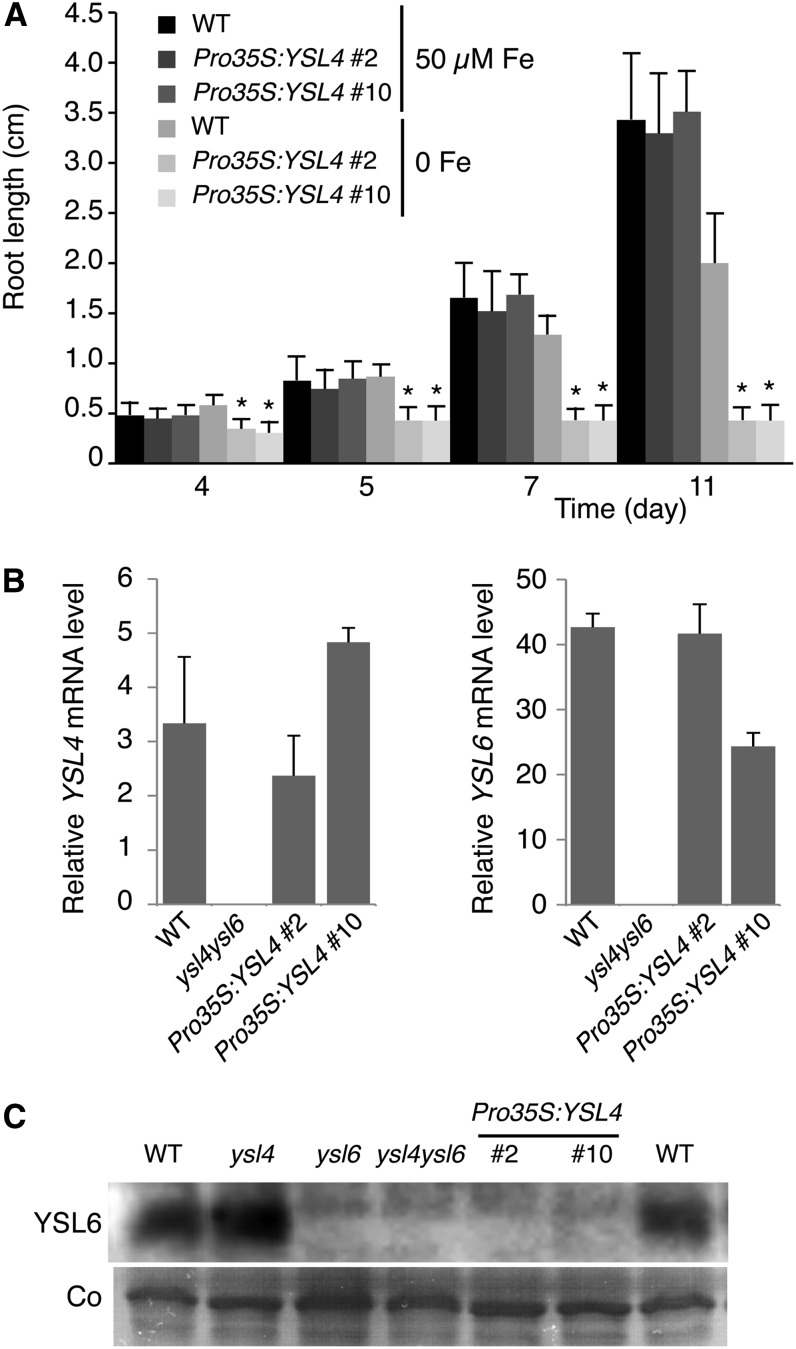
Pro35S:YSL4 lines, obtained in the single ysl4 mutant background, behaved like Pro35S:YSL6 lines (i.e., their growth was dramatically impaired when germinated in iron-deficient conditions) (Figure 8A). This phenotype did not correlate with a higher expression of YSL4 relative to the wild type (Figure 8B) and therefore may be the consequence of YSL4 ectopic expression. Moreover, 35S promoter–driven expression of YSL4 resulted in complete inhibition of YSL6 protein accumulation without affecting YSL6 transcript abundance, thus suggesting a posttranscriptional feedback inhibition of YSL6 by YSL4 ubiquitous expression (Figure 8C). This finding further supports the functional redundancy of YSL4 and YSL6 transporters and highlights the importance of YSL4 in maintaining iron homeostasis.
Figure 8.
Ubiquitous Expression of YSL4 Dramatically Decreases Plant Tolerance to Iron Deficiency and Inhibits YSL6 Synthesis.
(A) Time-course study of primary root elongation of two independent Pro35S:YSL4 lines (#2 and #10) in the ysl4 mutant background, grown in iron-replete (50 µM) or iron-deficient (no added iron) medium showing increased inhibition of primary root elongation by iron deficiency in the transgenic lines. Error bars represent sd. Asterisk indicates significant difference between wild-type (WT) and ysl4/Pro35S:YSL4 plants, P < 0.001; measurements were obtained from 25 plants from each of three separate experiments (Student’s t test).
(B) Expression of YSL4 and YSL6 genes in Pro35S:YSL4 lines. Real-time RT-PCR was performed on 7-d-old iron-replete plants from experiment (A). Measurements were made in triplicate from two independent experiments. Error bars represent sd.
(C) Ubiquitous expression of YSL4 inhibits YSL6 protein accumulation. Immunoblot analysis was performed on 7-d-old iron-replete plants from experiment (A). Total microsomal proteins were hybridized with anti-YLS6 antibody. Coomassie blue staining (Co) was used as loading control.
Role of YSL4 and YSL6 in Early Development
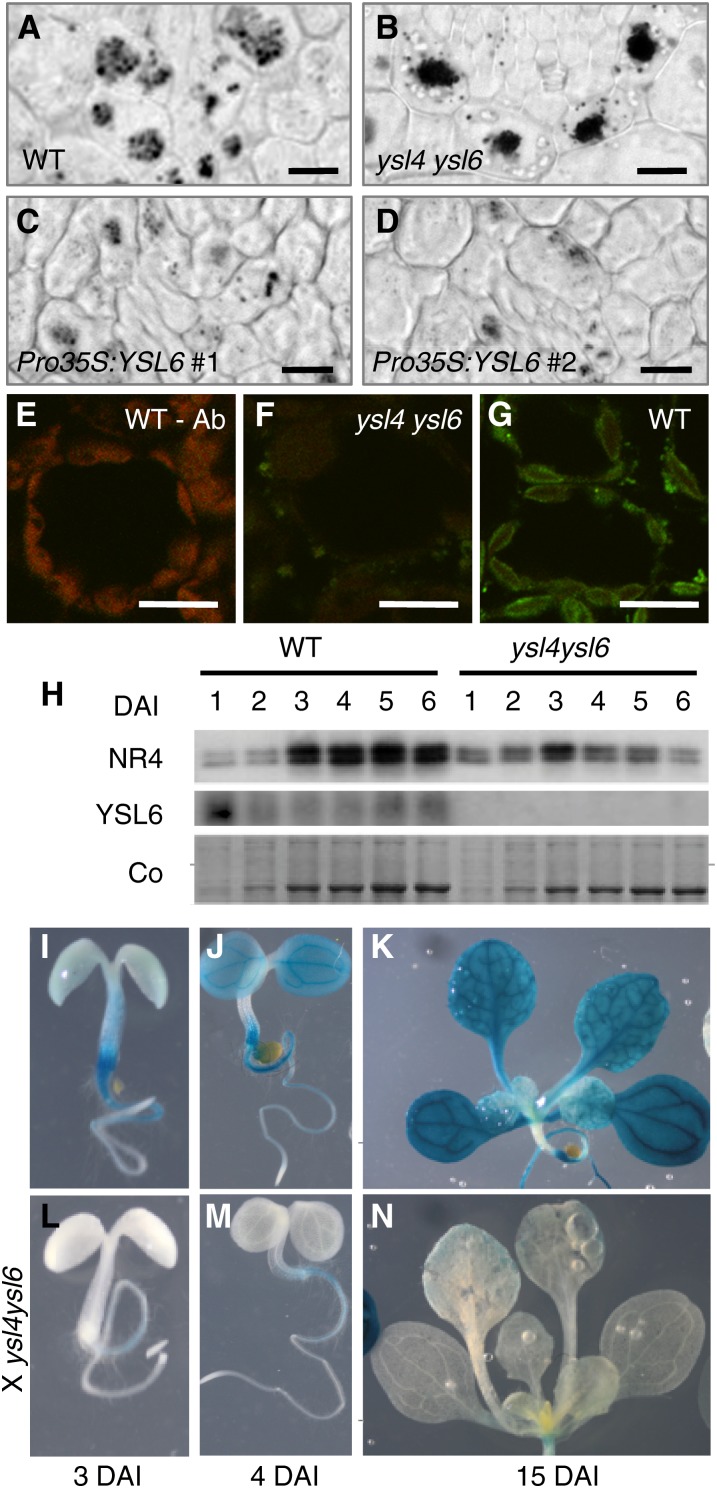
The ysl4 ysl6 hypersensitivity phenotype in iron excess appears very early during germination, which is consistent with the high expression of both genes in the embryo and very young seedlings. To understand the role of YSL4/6-mediated chloroplastic secretion of iron at this stage, we looked at iron distribution/amount in the embryos of the double mutant. In dry seed embryos, iron distribution and quantity was identical between wild-type and mutant seeds (data not shown) and showed the typical pattern of accumulation into vacuoles in a single cellular layer that surrounds vascular tissues (Roschzttardtz et al., 2009). This iron pool serves as storage to feed the plantlet during the first days of germination and, thus, rapidly decreases during the first days of germination (Lanquar et al., 2005; Roschzttardtz et al., 2009). Chloroplastic iron at this stage, though not null, is undetectable by Perls/DAB staining (Roschzttardtz et al., 2009). We then stained iron in embryos after 2 d of imbibition (Figure 9). Chloroplastic iron remained undetectable, but the fate of the vacuolar iron pool was clearly affected in the ysl4 ysl6 and in the Pro35S:YSL6 embryos. Indeed, the pattern of Fe distribution was identical among the genotypes, but the amount of iron in the vacuoles, which appears as discrete dark dots in wild-type vacuoles, was markedly increased in vacuoles of the double mutant (cf. Figures 9A and 9B). Conversely, the number of iron dots in the vacuoles was consistently lower in Pro35S:YSL6 lines (Figures 9C and 9D), supporting the view that ectopic expression of YSL6 decreases the pool of iron stored around vascular tissues in the embryo, which is likely to impair germination in conditions of iron scarcity.
Figure 9.
YSL4 and YSL6 Indirectly Control NRAMP3 and NRAMP4 and Vacuolar Iron Remobilization in the Embryo.
(A) to (D) Perls/DAB staining of iron in cotyledon thin sections from the wild type (WT) (A), ysl4 ysl6 (B), and two independent Pro35S:YSL6 lines ([C] and [D]) 2 d after imbibition. Iron is concentrated in the vacuoles of the endodermis layer.
(E) to (G) Immunofluorescence using anti-YSL6 antibody on cotyledon cross sections from 4-d-old plants.
(E) Wild-type plant, primary anti-YLS6 antibody omitted, showing chlorophyll fluorescence.
(F) ysl4 ysl6, controlling anti-YSL6 antibody specificity.
(G) The wild type showing YSL6-specific signal lining up the chloroplast envelope.
(H) Immunoblot analysis of NRAMP4 and YSL6 protein levels during germination in wild-type and ysl4 ysl6 double mutant plants. Total microsomal proteins were isolated from entire plantlets between 1 and 6 d after imbibition (DAI) and hybridized with either anti-YSL6 or anti-NRAMP4–specific antibodies. NR4, NRAMP4 protein; Co, Coomassie blue staining.
(I) to (N) Inhibition of NRAMP4 promoter activity in the absence of functional YSL4 and YSL6 genes. GUS histochemical staining of a ProNRAMP4:GUS transgenic line at 3, 4, or 15 d postimbibition, either in the wild-type ([I] to [K]) or the ysl4 ysl6 double mutant ([L] to [N]) background.
Bars = 10 µm
We next assessed the membrane localization of YSL6 in cotyledons. Anti-YSL6 immunofluorescence performed on cotyledons of wild-type plants confirmed that at this stage too, YSL6 is indeed localized in the envelope of plastids (Figures 9E to 9G). Thus, iron overaccumulation in the vacuole of the double mutant is an indirect effect of the absence of YSL4 and YSL6.
NRAMP3 and NRAMP4 are two vacuolar transporters that were shown to function in the remobilization of vacuolar iron stores during germination (Lanquar et al., 2005). Upon germination, as iron stores are used for plant growth, iron staining of the plants fades away and is undetectable after 4 d (Roschzttardtz et al., 2009). By contrast, a double nramp3 nramp4 mutant fails to empty its vacuoles and retains strong iron staining a few days after germination (Roschzttardtz et al., 2009). We found that the ysl4 ysl6 double mutant phenocopies the nramp3 nramp4 double mutant as far as iron accumulation in the germinating embryo. We therefore tested whether the lack of functional YSL4/6 impacts NRAMP3/4 expression. First, we assessed NRAMP4 protein accumulation by immunoblots. NRAMP4 accumulation, which rose 3 d after imbibition, was drastically reduced in the ysl4 ysl6 mutant (Figure 9H). Second, the effect of YSL4/6 inactivation on NRAMP3/4 expression was investigated at the tissue level. Transgenic lines expressing ProNRAMP3:GUS and ProNRAMP4:GUS constructs were crossed to the ysl4 ysl6 mutant, and GUS staining was compared between wild-type and ysl4 ysl6 backgrounds. In the wild type, the NRAMP4 promoter drove GUS expression as early as 3 d postimbibition in the hypocotyl, extending to the cotyledons and root at 4 d and to the first leaves at 15 d (Figures 9I to 9K). In the ysl4 ysl6 background however, GUS staining was almost undetectable throughout the plant (Figures 9L to 9N). The same result was obtained with ProNRAMP3:GUS lines crossed with ysl4 ysl6 (see Supplemental Figure 6 online). Collectively, these results establish that during germination, plants cope with the loss of function in both YSL4 and YSL6 by strongly inhibiting NRAMP3 and NRAMP4 expression.
DISCUSSION
YSL4/6 Act in Releasing Iron from the Chloroplast
Using reverse genetics, biochemical analyses, and Fe imaging techniques, we have shown that Arabidopsis YSL6 is a chloroplast transporter, which, together with its close homolog YSL4, is critical to prevent iron overload in chloroplasts. We isolated a double ysl4 ysl6 knockout mutant whose growth is drastically impaired in high iron environments. We attribute this phenotype to a defect of Fe efflux from the chloroplast based on the following arguments: (1) Immunoblot analysis of subcellular protein fractions and immunofluorescence assays, which have unambiguously shown that YSL6 is a protein of the chloroplast envelope (Figures 5 and 9). (2) Chloroplast iron content, estimated by Perls/DAB iron imaging of leaf tissue and elemental analysis of isolated chloroplasts, is significantly increased in the ysl4 ysl6 double mutant (Figure 6, Table 1). (3) In agreement with such a higher iron concentration in the chloroplast, ferritin abundance is increased in shoots of the ysl4 ysl6 mutant (Figure 4D). (4) Transgenic lines ubiquitously expressing YSL4 or YSL6 become dramatically intolerant to iron deficiency and, as shown for Pro35S:YSL6 plants, display a significant loss of chloroplastic Fe content (Figure 6, Table 1). Collectively, these findings argue in favor of YSL4/6 being chloroplastic transporters that enable the release of iron from the chloroplasts.
It is noteworthy that less information has been obtained on YSL4 than on YSL6. This is due largely to the extremely low expression of YSL4, which made spatial expression analyses and immunodetection studies undoable. In addition, ectopic expression of YSL4 seemed to be toxic. This was inferred from the strong inhibition of YSL6 protein accumulation observed in transgenic Pro35S:YSL4 plants (Figure 8C) and probably caused the impossibility, despite two large-scale attempts, to obtain complemented ysl4 ysl6 mutants with the same Pro35S:YSL4 construct. Although it is important to bear in mind that no formal proof exists of the chloroplast localization and function of YSL4, we believe that the data presented here support a role for YSL4 in chloroplastic iron transport for the following reasons. (1) The ysl6 phenotype only shows in the absence of YSL4, indicating that the function of YSL4 and YSL6 is at least partially redundant in the cell. This idea fits with the high sequence similarity of these two proteins, which could have identical activities or even work together as heterodimers. Such redundancy of function between YSL4 and YSL6 enables us to deduce YSL4 activity based on the knowledge obtained for YSL6. (2) Pro35S:YSL4 lines behave like Pro35S:YSL6 lines and opposite to the ysl4 ysl6 mutant regarding tolerance to iron deficiency, again supporting the view that YSL4 and YSL6 function similarly in the cell.
YSL6 Is a Noncanonical Protein of the Chloroplast Envelope
YSL6 targeting to the chloroplast was unexpected since both proteins were initially identified in a vacuolar proteomic analysis performed on Arabidopsis suspension cells (Jaquinod et al., 2007). In a more recent proteomic study of 11-d-old plantlets, YSL4 and YSL6 were identified as plasma membrane proteins (Mitra et al., 2009). Neither YSL4 nor YSL6 harbors a consensus chloroplast transit peptide at their N-terminal end. However, as much as 11 to 14% of the chloroplast proteome is composed of nuclear-encoded proteins lacking the consensus transit peptide and are likely not imported into the chloroplast via the classical Tic/Toc machinery (Zybailov et al., 2008; Armbruster et al., 2009). The lack of consensus subcellular targeting motifs in the protein sequence of YSL4 and YSL6 is further illustrated by bioinformatic predictions. Compilation of such predictions weakly suggests plasma membrane targeting for the two proteins (http://suba.plantenergy.uwa.edu.au/), while other plant membrane protein websites favor their localization in the secretory pathway (http://aramemnon.botanik.uni-koeln.de/). The latter targeting prediction retained our attention in light of the recent finding that some of the noncanonical chloroplast proteins contain signal peptides for translocation to the endoplasmic reticulum (ER) (Kleffmann et al., 2004). The ER is known to physically interact with chloroplasts, and in algae that have complex plastids, chloroplast proteins are shown to traffic through the secretory pathway (Jarvis, 2008). In plants, ER targeting of a chloroplast-located carbonic anhydrase has been reported (Villarejo et al., 2005) and was shown to ensure proper glycosylation of the protein that is required for its activity in the chloroplast (Burén et al., 2011). Noncanonical chloroplast proteins also include proteins of the outer envelope. However, unlike the inner envelope, which is highly specialized for transport, the outer envelope, which was shown biochemically to derive from the cyanobacterial outer membrane, is permeable to most ions and metabolites and hosts mainly channels (Inoue, 2011). Thus, YSL6 and possibly YSL4 are likely proteins of the chloroplast inner envelope. Proteins of the chloroplast are well-known contaminants of other subcellular protein fractions, which may explain the presence of YSL4 and YSL6 within vacuolar or plasma membrane proteomes.
YSL4 and YSL6 Represent a New Class of YSL Transporters with a Distinct Function
YSL homologs have only been described in plants. The moss Physcomitrella patens has only two YSL isoforms (Curie et al., 2009), which, very peculiarly, belong to the distinct subclass formed by Arabidopsis YSL4 and YSL6 (see Supplemental Figure 7 online). As mosses lack a vascular system (Cove et al., 2009), it might explain the absence of members from the other subclasses that are characterized by their activity in long-distance transport of metals through loading/unloading of the saps. P. patens YSLa is predicted to lie in membranes of the secretory pathway by most prediction algorithms, whereas similar to Arabidopsis YSL4 and YSL6 or rice YSL5 and YSL6, no clear targeting is predicted for P. patens YSLb. Barley (Hordeum vulgare) YSL5, another member of this subclass, was observed in internal vesicles; however, this result was obtained by fusing the protein with the green fluorescent protein (GFP), which in some instances can interfere with proper traffic of transporters to their target membrane (Zheng et al., 2011). In our own study, N and C terminus versions of YSL4-YFP and YSL6-GFP fusions, analyzed in stably transformed plants, gave inconsistent protein localization results (data not shown), but YSL6 immunofluorescence experiments consistently and uniquely labeled the chloroplasts. It is possible that, like Arabidopsis YSL4/6, moss YSLa and YSLb as well as their close higher plants relatives are all targeted to internal membranes. The idea that emerges already is that contrary to YS1 transporters that carry out iron uptake at the root surface or to the vast majority of the YSL members that seem to work in loading/unloading of the saps for long distance transport, members of this small subgroup of YSLs could all be involved in the intracellular mobilization of iron.
YSL4 and YSL6 Specifically Transport Iron
Heterologous expression of YSL4 or YSL6 in the yeast iron transport mutant fet3 fet4 failed to provide a direct assessment of their transport activity (data not shown). It is likely that, as proteins of the chloroplast envelope in plants, YSL4 and YSL6 do not localize at the plasma membrane in yeast. Based on this assumption, YSL4/6 transport activity was not assayed by expression in Xenopus laevis oocytes in this study. Unlike several other reported YSL members, we found that the spectrum of substrates that are transported by YSL4 and YSL6 is likely to be restricted to Fe. This is inferred first through the phenotype of the double ysl4 ysl6 mutant, which is specifically observed when challenging the plants with an excess of Fe but not Zn, Mn, or Cu (see Supplemental Figure 1 online), and second based on elemental analysis of chloroplast metal content showing that Fe content alone is modified in ysl4 ysl6 or Pro35S:YSL6 expressing plants (Table 1).
Measurement of ferrous iron transport at the inner envelope of pea chloroplasts has revealed the coexistence of both influx and efflux transport activities with similar affinity constants of ∼2 µM (Shingles et al., 2002). Moreover, barley chloroplasts were shown, using radiolabeled iron, to influx iron during the day and to efflux it at night (Bughio et al., 1997). It was therefore suggested that a single transporter of the chloroplast envelope could work in both directions in response to light/dark conditions. Alternately, two transporters working in opposite directions could take turns to accommodate the chloroplast demand during light and dark (Shingles et al., 2002). Until now, the molecular mechanisms by which iron is transported into and out of the chloroplast remained elusive. Candidates for Fe import into the chloroplast include the permease PIC1, which might translocate proteins that are required for iron transport in the chloroplast (Duy et al., 2007, 2011), the transporter MAR1/IREG3, which might transport an iron chelate or its ligand alone (Conte et al., 2009), or the ABC NAP14, which could be part of an iron transport complex (Shimoni-Shor et al., 2010). Our data provide strong evidence that the YSL4 and YSL6 transporters contribute to the export of iron from the chloroplast. But how is this transport energized? Transport of Fe-PS by YS1 was shown to depend on proton cotransport and membrane potential (Schaaf et al., 2004). A dependency on membrane potential was also reported for Fe uptake by chloroplast inner envelope vesicles (Shingles et al., 2002). It is tempting to speculate that eoplasts of dormant seeds or degenerative chloroplasts of senescent leaves, which harbor YSL4 and YSL6 transporters on their surface, undergo metabolic changes similar to chloroplasts at night and related to photosynthesis arrest. Dedifferentiating thylakoids are likely to release a large number of protons in the stroma, creating a proton gradient that could in turn energize Fe efflux by YSL4 and YSL6.
Consequences on Intracellular Distribution of NA
As members of the YSL family, YSL4 and YSL6 are potential transporters of metal-NA complexes. Thus, one of the implications of this work is that chloroplasts must contain NA. The presence of NA in the symplast has been reported in several instances, and immunolabeling of NA in root tips of tomato (Solanum lycopersicum) or Arabidopsis detected NA primarily in vacuoles and to a lesser extent in cytosol and mitochondria (Pich et al., 1997; Haydon et al., 2012). NA has not been detected/measured in chloroplasts. However x-ray microanalysis of leaves of the NA-less tomato mutant chloronerva (chln) has revealed the presence of electron-dense inclusions of Fe and P in the chloroplast that are distinct from ferritins and absent in wild-type plants (Becker et al., 1995). This accumulation of insoluble Fe particles in chloroplasts of chln supports the idea that in plastids too, NA maintains iron in a soluble form. It thus could be hypothesized that in the absence of a proper Fe- NA substrate in the chloroplast of chln, Fe efflux through the tomato YSL4/6 ortholog(s) cannot occur, and as a consequence, Fe builds up in plastids.
YSL4/6 Putative Function at Senescence
YSL6 is strongly expressed in senescent leaves, where we have observed that iron remains trapped in the chloroplasts. Upon senescence, photosynthesis stops and as chloroplasts degenerate, potentially harmful iron atoms are released from the thylakoid-located photosynthetic chains. It is thus possible that YSL6 acts by mediating the exit of iron atoms released in the stroma, thereby protecting plastids from oxidative damages. However, despite the fact that ysl4 ysl6 plants at this stage have an increased iron content in the chloroplasts, they do not present any macroscopic phenotype, including necrosis or yellowing of the leaf, even when senescence is induced by treatments, such as hydrogen peroxide or abscisic acid (data not shown). Like YSL6, FER1, encoding the most abundant isoform of ferritin in the leaves of Arabidopsis, is highly expressed in cauline and senescent leaves, probably as a consequence of the increase of free iron concentration in degenerating chloroplasts. The fact that ferritin abundance increased in ysl4 ysl6 may reflect a mechanism of compensation, suggesting that YSL6 and ferritin act in the same pathway. Alternately, YSL6 could participate in the remobilization of iron from the chloroplast of senescent leaves toward growing tissues and seeds. However, we found that iron content is unaffected in ysl4 ysl6 entire seeds or isolated cotyledons even when the mother plant was irrigated with iron (see Supplemental Figure 3 online). It thus remains unclear what role these proteins might have in iron homeostasis during senescence.
Crosstalk between YSL4/6 and NRAMP3/4 Transporters and between Chloroplastic and Vacuolar Iron Pools
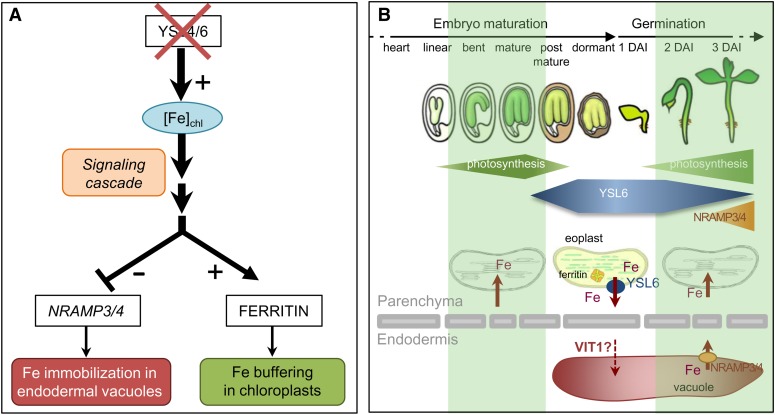
Another important territory of expression of both YSL4 and YSL6 is the developing embryo. Unlike at senescence, the absence of both YSL4 and YSL6 dramatically impacts the capability of the germinating plant to cope with iron excess. Conversely, if YSL4 or YSL6 genes are ectopically expressed in most tissues of the plant, germination becomes hypersensitive to Fe deficiency. Histochemical staining of iron in cotyledons of the ysl4 ysl6 mutant failed to detect chloroplastic iron, the abundance of which was previously shown to be below the threshold of detection using this method (Roschzttardtz et al., 2009). Instead, Fe imaging in germinated seedlings revealed an increase of vacuolar iron content in the endodermis, which constitutes the major iron pool of the seeds (Roschzttardtz et al., 2009). Two lines of evidence indicate that the increase in vacuolar iron content is likely an indirect consequence of the modification of the iron status in the embryonic chloroplasts. First, immunofluorescence analysis establishes that in cotyledons too, YSL6 protein is targeted to the envelope of chloroplasts (Figure 9). Second, expression of NRAMP3 and NRAMP4, which encode iron transporters responsible for vacuolar iron remobilization during germination, is dramatically inhibited in the ysl4 ysl6 mutant. Hence, the observed increase in vacuolar Fe content results from a lack of remobilization of vacuolar iron by NRAMP3/4 during germination rather than from a direct iron transport defect by YSL4/6 at the tonoplast envelope. A similar increase of the vacuolar iron pool was observed in embryos of the nramp3 nramp4 mutant (Roschzttardtz et al., 2009). This is consistent with the assumption that the iron pattern observed in the ysl4 ysl6 mutant is a consequence of the inhibition of NRAMP3/4. Both this result and the fact that NRAMP3/4 and YSL4/6 are both expressed in the embryo suggest a crosstalk between chloroplast and vacuolar iron pools in the embryo. If accumulation of iron in the chloroplast of ysl4 ysl6 results in switching off iron export from the vacuole, it is tempting to speculate that iron reaching the chloroplast is originating from the vacuole. Another study previously illustrated the close link that exists between chloroplast and vacuolar iron pools in seeds. Theses authors have shown that the stability of the seed ferritin isoform FER2 is lost in two genotypes that have cellular iron trapped in the vacuole, the nramp3 nramp4 mutant, and a VIT1 overexpressor line (Ravet et al., 2009). Since ferritins are stabilized by the presence of iron, an appealing explanation is that the iron is depleted in the chloroplast as a direct consequence of preventing Fe from exiting the vacuole. In both studies, however, an important caveat is that the genes considered might not always be expressed in the same cells and the balance between subcellular iron pools might respond to whole-tissue regulation rather than to direct cellular compartment interactions. Here, we show that the production of YSL4/6 and NRAMP3/4 within the embryo only partly overlaps, YSL4/6 being everywhere but around vascular tissues and NRAMP3/4 being mostly present around the veins (Lanquar et al., 2005). Thus, the feedback inhibition of NRAMP3/4 triggered by the absence of YSL4/6 must at least in part involve sensing of iron in the chloroplast of YSL4/6-producing cells and signaling to the endodermal cells to adjust iron release from the vacuolar stores (Figure 10A). The study of YSL4 and YSL6 therefore suggests the existence of an intercellular crosstalk between vacuolar and chloroplastic iron pools in the embryo.
Figure 10.
Working Model for YSL4/YSL6 Function in the Chloroplasts.
(A) Knocking out YSL4 and YSL6 reveals a putative intercellular crosstalk between vacuolar and chloroplastic iron pools in the embryo. This study shows that in the absence of both YSL4 and YSL6, iron concentration is elevated in the chloroplasts of old leaves. This situation can be extrapolated to the embryo since (1) YSL6 is present in embryonic chloroplasts, and (2) ysl4 ysl6 mutant embryos accumulate more ferritin than wild-type embryos. We thus can speculate that embryonic chloroplasts accumulate more iron in the mutant, despite chloroplastic iron being undetectable at this stage. Knocking out YSL4 and YSL6 provokes an increase of the main iron storage pool of the embryo, which is located in vacuoles of the endodermis in Arabidopsis. This is most probably due to the dramatic inhibition of NRAMP3 and NRAMP4 expression observed in the ysl4 ysl6 mutant. The pattern of expression of YSL4/6 and NRAMP3/4 genes is only poorly overlapping in the embryo, with YSL4/6 being expressed earlier and in parenchyma cells and NRAMP3/4 being expressed mostly around the veins, indicating that the crosstalk between vacuolar and chloroplastic pools is not be restricted to a cellular response. Instead, feedback inhibition of iron export from the endodermal vacuole of the mutant must involve, at least in part, sensing of the iron level in the chloroplast of parenchyma cells and signaling to the neighbor endodermis cells to adjust iron release from the vacuole.
(B) Proposed function of YSL4/YSL6 in preventing iron toxicity during early development. During maturation, the Arabidopsis embryo develops green photosynthetically active chloroplasts. At the mature stage, chloroplasts dedifferentiate into eoplasts and photosynthesis is interrupted until the beginning of germination. YSL4/6 transporter abundance is maximal during this transient phase of photosynthetic inactivity where they are likely to mediate chloroplast export of the iron released from the thylakoids. Ferritins and YSL6 are cosynthesized in the embryo at this stage and might cooperate to detoxify iron in the plastids. As iron exits the chloroplasts, it is likely sent to a neutral compartment such as the vacuole. At least in the endodermal cells, VIT1 is a good candidate to mobilize iron in the vacuole. At the beginning of germination, photosynthesis resumes, production of YSL6 is switched off, while that of NRAMP3 and NRAMP4 is turned on in order to remobilization vacuolar iron stores and ensure adequate provision of iron to the active chloroplasts. DAI, days after imbibition.
[See online article for color version of this figure.]
YSL4/6 Activity Correlates with Photosynthesis Arrest in Mature Seeds
Questions remain as to the physiological relevance of chloroplastic iron export in the embryo. Oilseed plants like Arabidopsis develop green photosynthetically active chloroplasts (Whatley, 1978; Mansfield and Briarty, 1991). At the mature seed stage, embryonic photosynthesis stops and chloroplasts dedifferentiate into plastids called eoplasts. Upon germination, eoplasts redifferentiate into chloroplasts and photosynthesis resumes. Plastid dedifferentiation in mature seed embryos, similar to chloroplast degeneration at the time of senescence, is likely to trigger a massive release of iron from the thylakoids. Time course of YSL6 protein accumulation indicates that the protein abundance is highest at the mature seed stage and decreases upon germination, corresponding exactly to the period of transient arrest of photosynthesis in the embryo (Figure 10B). The embryonic ferritin isoform, FER2, shows exactly the same timing of accumulation (Ravet et al., 2009), thus reinforcing the idea that at this stage, chloroplastic iron must be buffered. When photosynthesis resumes at the beginning of germination, YSL6 protein amounts decrease, while NRAMP3/4 protein synthesis is turned on (Figures 9), probably to assist in providing iron to the redifferentiating chloroplasts (Figure 10B). The activity of YSL4/6 becomes essential in supraoptimal iron conditions, since ysl4 ysl6 germination is exclusively altered in media containing an excess of iron. Therefore, our working hypothesis is that, at the onset of chloroplast dedifferentiation in the embryo or upon senescence, YSL4 and YSL6 act in exporting iron out of the chloroplast, either to protect plastids from the harmful effect of free iron release by photosynthetic enzymes or to redistribute iron to sink tissues. The fact that the plant depends on the presence of YSL4/6 to cope with iron excess during germination supports the view that these two transporters function in protecting the chloroplast from Fe-induced oxidative damage. YSL4/6 are coordinately produced with ferritins in mature embryos (FER2) and senescent leaves (FER1), suggesting that these proteins cooperate to detoxify free iron in plastids.
Our findings that a mechanism exists to export iron from an organelle to avoid iron-driven toxicity is novel and might operate in other organelles, in particular the metabolically active iron-rich mitochondrion. Finally, this study provides new valuable targets to reduce iron toxicity generated in strategies for iron biofortification in crops.
METHODS
Plant Material
Arabidopsis thaliana seedlings were grown in axenic conditions on plates containing half-strength Murashige and Skoog medium without Suc. Fe-citrate was added from a 50 mM stock solution into the medium. Seeds were surface sterilized and then imbibed in the dark at 4°C for 2 d. Plants were grown at 22°C in a growth chamber under a 16-h-light (150 µmol s−1 m−2)/8-h-night period.
The Arabidopsis T-DNA insertion mutants ysl4-1 and ysl6-1 were obtained from the SIGnAL collection, lines 025447 and 093392, respectively. Homozygous mutant plants were genotyped by PCR using forward primers YSL4F1 for YSL4 or YSL6F1 for YSL6 and the reverse T-DNA left border primer LB1 (see Supplemental Table 1 online).
Transformation of Arabidopsis ecotype Columbia-0, either the wild type or ysl4 ysl6 double mutant, was performed using GV303 strain of Agrobacterium tumefaciens and following the floral dip protocol (Clough and Bent, 1998). Transformed T1 plants were selected on kanamycin, and monoinsertional homozygous plants were selected in T2 based on their segregation on kanamycin.
Plasmids
The open reading frames of Arabidopsis YSL4 (At5g41000) and YSL6 (At3g27020) genes were amplified by PCR using the primers’ couples: YSL4F2 and YSL4R1, YSL6F2 and YSL6R1 (see Supplemental Table 1 online). PCR products were A-tailed and cloned into the pGEM-T Easy vector (Promega) following the manufacturer’s instructions. The pPro35S:YSL4 and pPro35S:YSL6 constructs for the overexpression/complementation in plants were obtained by subcloning the SacI-NcoI fragments obtained from the pGEM constructs into the pCHF3 vector, in translational fusion with the cauliflower mosaic virus 35S promoter.
The ProYSL4:GUS and ProYSL6:GUS constructs contain 1595 and 2000 bp, respectively, of promoter sequences located upstream of the translation initiation codon ATG. The promoter fragments were amplified by PCR from the BAC clones MEE6 (YSL4) and MOJ10 (YSL6) using the following couples of primers: YSL4F3 containing an EcoRI site, YSL4R2 containing a SacI site, YSL6F3 containing an XhoI site, and YSL6R2 containing a BamHI site.
RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA was extracted using the Trizol method (Invitrogen) following the manufacturer’s instructions. Samples were treated with DNase (Promega). Three micrograms of DNA-free RNA was used for reverse transcription by the MMLV-RT (Promega) with anchored oligo(dT23). Real-time PCR was performed using the LightCycler FastStart DNA MasterPLUS SYBR Green 1 (Roche), with the following gene-specific primers: EF1a-F and EF1a-R, YSL4F4 and YSL4R3, and YSL6F4 and YSL6R3 (see Supplemental Table 1 online).
The primer specificity was confirmed by melting curve analysis and agarose gel electrophoresis of the PCR products. Relative transcript levels (RTLs) were calculated relative to the transcript level of the constitutively expressed EF1-α A1 gene, as follows: RTL = 100 × 2(−ΔCt). The changes in cycle threshold (ΔCt) values were calculated as follows: ΔCt = Ct(target gene) – Ct(EF1-α). In all the figures, bars represent mean relative transcript levels, and error bars represent the standard error of two independent experiments.
Protein Extraction and Immunoblot Analyses
Isolation of intact chloroplasts and protein purification were performed following a procedure aimed at maximizing both purity and integrity (Hiltbrunner et al., 2001).
Total proteins were extracted by grinding 100 mg of tissue in 300 mL of extraction buffer (50 mM Tris-HCl, pH 8, 5% SDS, 5% β-mercaptoethanol, 25 mM EDTA, and 0.1% phenylmethylsulfonyl fluoride) in liquid nitrogen, followed by centrifugation at 48°C for 15 min at 14,000g. Thirty micrograms of total proteins were separated on SDS-PAGE gel and electroblotted to a Hybond-P membrane (Amersham Biosciences). Membranes were blocked overnight in 1× PBS and 0.1% Tween 20 containing 0.2% blocking reagent (Aurora protein gel blot chemiluminescence detection system; ICN Biochemicals).
The following antibodies were used: rabbit anti-YSL6 diluted 1:10,000, rabbit anti-Nramp4 diluted 1:2000 as a vacuole membrane protein marker, rabbit anti-PbsO diluted 1:10,000 as a chloroplast membrane proteins marker, rabbit anti-IRT1 diluted 1:5000, rabbit antiferritin diluted 1:20,000, and anti-rabbit conjugated to alkaline phosphatase (Invitrogen) diluted 1:20,000 as a secondary antibody. The membranes were blocked in 1% BSA. The blots were incubated 5 min in the presence of Aurora chemiluminescence substrate solution (Millipore) and revealed using a LAS 3000 imager (Fujifilm).
The YSL6 polyclonal antibody was raised in rabbit against the two synthetic peptides CSSRSRRLNLPIVTDG and MGTEIPRSAEISEALC (Eurogentec). The large bleeding was used directly in immunoblot and immunolabeling analyses. In both experiments, no signal was observed in preparations from ysl6 and ysl4 ysl6 mutants.
Immunolocalization Experiments
Leaves 2 and 3 of 3-week-old soil-grown plants and 4-d-old seedlings were vacuum infiltrated in a fixative solution containing 4% (w/v) paraformaldehyde in 10 mM PBS, pH 7.2 (7 mM NaHPO4, 3 mM NaH2PO4, 120 mM NaCl, and 2.7 mM KCl) for 1 h and incubated for 1 night in the same solution. The samples were washed with 100 mM Gly in 10 mM phosphate buffer, pH 7.5, three times, and dehydrated in successive baths of 50, 70, 95, and 100% ethanol, butanol/ethanol 1:1 (v/v), and 100% butanol for 1 night. For embedding, samples were soaked in successive 1-h baths of increasing concentrations of Safesolv (Labonord) concentrations (butanol/Safesolv 2:1, 1:2, and 0:1) and then in 1-h baths in increasing pura wax (Paraffin X-TRA; McCormick Scientific) concentrations (Safesolv/Paraplast pura wax 3:1, 1:1, and 1:3) and at least 1 night in 100% Paraplast pura wax. Eight-micrometer-thick sections were deposited on silanized-slides (DakoCytomation) and dried. Samples were then dewaxed and rehydrated following the reverse steps. Sections on slides were washed 10 min in PBS, blocked by incubating 1 h at room temperature in BSA (1% in PBS), and then incubated overnight at 4°C with anti-YSL6 polyclonal antibody (diluted at 1:500). The sections were then washed with PBS (3 × 10 min) and incubated 1 h at room temperature in the dark with anti-rabbit IgG F(ab’)2 fragment conjugated to the Alexa Fluor 488 fluorochrome (Invitrogen). After washing in PBS (3 × 10 min), the sections were mounted in Mowiol antifading solution.
Confocal Laser Scanning Microscopy
The microscopy images were obtained in the Montpellier RIO Imaging Platform with a confocal microscope (LSM 510, Meta; Carl Zeiss MicroImaging). An argon laser at 488 nm provided excitation for the Alexa 488 and chloroplast autofluorescence. The fluorescence emission signals were detected using a band-pass filter of 505 to 550 nm for GFP and a long-pass filter of 585 nm for the far-red autofluorescence of the chloroplast. Sections were observed with a Zeiss ×63 oil objective. Pictures were processed using the Zeiss LSM Image Browser software.
In Situ Perls/DAB/Hydrogen Peroxide Intensification
The samples were fixed and dehydrated as described above and then embedded in Technovit 7100 resin (Kulzer) according to the manufacturer’s instructions, and thin sections (3 µm) were cut. The sections were incubated for 45 min in Perls staining solution: 2% (v/v) HCl and 2% (w/v) K-ferrocyanide at room temperature (Roschzttardtz et al., 2009). The intensification procedure was then applied as described by Roschzttardtz et al. (2009).
Elemental Analyses
Samples were dried at 60°C for 3 d and then digested in 70% HNO3 at 120°C. Metal content was measured by ICP-MS.
GUS Histochemical Staining
The samples were fixed as described above, washed briefly in GUS assay buffer (50 mM NaPO4, 1 mM ferrocyanide, 1 mM ferricyanide, and 0.2% Triton X-100, pH 7.2), and vacuum infiltrated for 15 min in the same buffer containing 1 mM 5-bromo-4-chloro-3-indolyl-β-O-glucuronic acid. Samples were incubated for 16 h at 37°C, washed in water, and destained in successive baths of 50, 70, 95, and 100% ethanol.
For GUS histochemical staining of leaf sections, Arabidopsis plants were grown in the greenhouse during 3 weeks and stained as described before. Leaves were embedded into Technovit 7100 resin (Kulzer) according to the manufacturer’s instructions, and 4-µm sections were cut.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: Arabidopsis YSL4, At5g41000; Arabidopsis YSL6, At3g27020; rice (Oryza sativa) YSL5, Os04g32060; rice YSL6, Os04g32050; moss (Physcomitrella patens) YSLa, XP_001759545.1; and P. patens YSLb, Pp1s2_335V6.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Phenotype of Single ysl4 and ysl6 or Double ysl4 ysl6 Mutants in the Presence of High Concentrations of Metals.
Supplemental Figure 2. Inhibition of Primary Root Elongation in Iron Excess Conditions Is Exacerbated in the Double ysl4 ysl6 Mutant.
Supplemental Figure 3. Total Fe Content Is Unaffected in ysl4 ysl6 Organs.
Supplemental Figure 4. Expression Analysis of YSL4 and YSL6 in the Major Organs.
Supplemental Figure 5. Overloading of Iron during Embryo Development Restores Hypersensitivity to Iron Deficiency in Pro35S:YSL6 Lines.
Supplemental Figure 6. Inhibition of NRAMP3 Promoter Activity in the Absence of Functional YSL4 and YSL6 Genes.
Supplemental Figure 7. Sequence Alignment of the YSL Subclass Represented by Arabidopsis YSL4 and YSL6.
Supplemental Table 1. List of Gene-Specific Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank Veronique Vacchina and Ryszard Lobinsky (Institut Pluridisciplinaire de Recherche sur l′Environnement et les Matériaux, Centre National de la Recherche Scientifique, Unité Mixte de Recherche 5254, University of Pau, France) for their assistance with ICP-MS analyses. We thank Sebastien Thomine (Institut des Sciences du Vegetal, Centre National de la Recherche Scientifique, Unité Propre de Recherche 2355, Gif-sur-Yvette, France) and Frédéric Gaymard (Laboratoire de Biochimie et Physiologie Moléculaire des Plantes, Centre National de la Recherche Scientifique, Unité Mixte de Recherche 5004, Montpellier, France) for providing the anti-NRAMP4 antibody and the antiferritin antibody, respectively. Microscopy observations were made at the Montpellier Rio Imaging facility (http://www.mri.cnrs.fr/). Work was funded by the Agence Nationale pour la Recherche through grants ANR Blanc CIDS and ANR Blanc DISTRIMET, by the Centre National de la Recherche Scientifique, and by the Institut National de Recherche Agronomique. F.D. and D.C. were supported by the Agence Nationale pour la Recherche.
AUTHOR CONTRIBUTIONS
F.D. and D.C. performed the experiments. H.R. helped with histochemical staining of iron and G.C. with immunolocalization analyses. F.D., D.C., S.M., and C.C. designed the research. C.C. wrote the article.
Glossary
- PS
phytosiderophore
- GUS
β-glucuronidase
- DAB
diamino benzidine
- ICP-MS
inductively coupled plasma–mass spectrometry
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
References
- Armbruster U., Hertle A., Makarenko E., Zühlke J., Pribil M., Dietzmann A., Schliebner I., Aseeva E., Fenino E., Scharfenberg M., Voigt C., Leister D. (2009). Chloroplast proteins without cleavable transit peptides: Rare exceptions or a major constituent of the chloroplast proteome? Mol. Plant 2: 1325–1335 [DOI] [PubMed] [Google Scholar]
- Barber J. (2003). Photosystem II: The engine of life. Q. Rev. Biophys. 36: 71–89 [DOI] [PubMed] [Google Scholar]
- Bashir K., Ishimaru Y., Shimo H., Nagasaka S., Fujimoto M., Takanashi H., Tsutsumi N., An G., Nakanishi H., Nishizawa N.K. (2011). The rice mitochondrial iron transporter is essential for plant growth. Nat. Commun. 2: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R., Fritz E., Manteuffel R. (1995). Subcellular localization and characterization of excessive iron in the nicotianamine-less tomato mutant chloronerva. Plant Physiol. 108: 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes I., Schreiber K., Ripperger H., Kirsceiss A. (1983). Metal complex formation of nicotianamine, a possible phytosiderophore. Experientia 39: 261–262 [Google Scholar]
- Bughio N., Takahashi M., Yoshimura E., Nishizawa N.K., Mori S. (1997). Light-dependent iron transport into isolated barley chloroplasts. Plant Cell Physiol. 38: 101–105 [Google Scholar]
- Burén S., Ortega-Villasante C., Blanco-Rivero A., Martínez-Bernardini A., Shutova T., Shevela D., Messinger J., Bako L., Villarejo A., Samuelsson G. (2011). Importance of post-translational modifications for functionality of a chloroplast-localized carbonic anhydrase (CAH1) in Arabidopsis thaliana. PLoS ONE 6: e21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H.H., Chiecko J., Punshon T., Lanzirotti A., Lahner B., Salt D.E., Walker E.L. (2010). Successful reproduction requires the function of Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 metal-nicotianamine transporters in both vegetative and reproductive structures. Plant Physiol. 154: 197–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conte S., Stevenson D., Furner I., Lloyd A. (2009). Multiple antibiotic resistance in Arabidopsis is conferred by mutations in a chloroplast-localized transport protein. Plant Physiol. 151: 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove, D.J., Perroud, P.F., Charron, A.J., McDaniel, S.F., Khandelwal, A. and Quatrano, R.S (2009). The moss Physcomitrella patens: A novel model system for plant development and genomic studies. Cold Spring Harb. Protoc. 2009: pdb.emo115. [DOI] [PubMed]
- Curie C., Briat J.F. (2003). Iron transport and signaling in plants. Annu. Rev. Plant Biol. 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Curie C., Cassin G., Couch D., Divol F., Higuchi K., Le Jean M., Misson J., Schikora A., Czernic P., Mari S. (2009). Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. (Lond.) 103: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C., Panaviene Z., Loulergue C., Dellaporta S.L., Briat J.F., Walker E.L. (2001). Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409: 346–349 [DOI] [PubMed] [Google Scholar]
- DiDonato R.J., Jr, Roberts L.A., Sanderson T., Eisley R.B., Walker E.L. (2004). Arabidopsis Yellow Stripe-Like2 (YSL2): A metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 39: 403–414 [DOI] [PubMed] [Google Scholar]
- Duy D., Stübe R., Wanner G., Philippar K. (2011). The chloroplast permease PIC1 regulates plant growth and development by directing homeostasis and transport of iron. Plant Physiol. 155: 1709–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy D., Wanner G., Meda A.R., von Wirén N., Soll J., Philippar K. (2007). PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell 19: 986–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froschauer E.M., Schweyen R.J., Wiesenberger G. (2009). The yeast mitochondrial carrier proteins Mrs3p/Mrs4p mediate iron transport across the inner mitochondrial membrane. Biochim. Biophys. Acta 1788: 1044–1050 [DOI] [PubMed] [Google Scholar]
- Haydon M.J., Kawachi M., Wirtz M., Hillmer S., Hell R., Krämer U. (2012). Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell 24: 724–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A., Bauer J., Vidi P.A., Infanger S., Weibel P., Hohwy M., Kessler F. (2001). Targeting of an abundant cytosolic form of the protein import receptor at Toc159 to the outer chloroplast membrane. J. Cell Biol. 154: 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Kobayashi T., Nozoye T., Takahashi M., Kakei Y., Suzuki K., Nakazono M., Nakanishi H., Mori S., Nishizawa N.K. (2009). Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 284: 3470–3479 [DOI] [PubMed] [Google Scholar]
- Inoue K. (2011). Emerging roles of the chloroplast outer envelope membrane. Trends Plant Sci. 16: 550–557 [DOI] [PubMed] [Google Scholar]
- Ishimaru, Y., Masuda, H., Bashir, K., Inoue, H., Tsukamoto, T., Takahashi, M., Nakanishi, H., Aoki, N., Hirose, T., Ohsugi, R. and Nishizawa, N.K. (2010). Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 62: 379–390. [DOI] [PubMed]
- Jaquinod M., Villiers F., Kieffer-Jaquinod S., Hugouvieux V., Bruley C., Garin J., Bourguignon J. (2007). A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteomics 6: 394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P. (2008). Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol. 179: 257–285 [DOI] [PubMed] [Google Scholar]
- Jeong J., Cohu C., Kerkeb L., Pilon M., Connolly E.L., Guerinot M.L. (2008). Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions. Proc. Natl. Acad. Sci. USA 105: 10619–10624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.A., Punshon T., Lanzirotti A., Li L., Alonso J.M., Ecker J.R., Kaplan J., Guerinot M.L. (2006). Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314: 1295–1298 [DOI] [PubMed] [Google Scholar]
- Kleffmann T., Russenberger D., von Zychlinski A., Christopher W., Sjölander K., Gruissem W., Baginsky S. (2004). The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr. Biol. 14: 354–362 [DOI] [PubMed] [Google Scholar]
- Koike S., Inoue H., Mizuno D., Takahashi M., Nakanishi H., Mori S., Nishizawa N.K. (2004). OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 39: 415–424 [DOI] [PubMed] [Google Scholar]
- Lanquar V., Lelièvre F., Barbier-brygoo H., Thomine S. (2004). Regulation and function of AtNRAMP4 metal transporter protein. Soil Sci. Plant Nutr. 50: 1141–1150 [Google Scholar]
- Lanquar V., Lelièvre F., Bolte S., Hamès C., Alcon C., Neumann D., Vansuyt G., Curie C., Schröder A., Krämer U., Barbier-Brygoo H., Thomine S. (2005). Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 24: 4041–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Jean M., Schikora A., Mari S., Briat J.F., Curie C. (2005). A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. Plant J. 44: 769–782 [DOI] [PubMed] [Google Scholar]
- Li L., Kaplan J. (2004). A mitochondrial-vacuolar signaling pathway in yeast that affects iron and copper metabolism. J. Biol. Chem. 279: 33653–33661 [DOI] [PubMed] [Google Scholar]
- Mansfield S.G., Briarty L.G. (1991). Cotyledon cell development in Arabidopsis thaliana during reserve deposition. Can. J. Bot. 70: 151–164 [Google Scholar]
- Mitra S.K., Walters B.T., Clouse S.D., Goshe M.B. (2009). An efficient organic solvent based extraction method for the proteomic analysis of Arabidopsis plasma membranes. J. Proteome Res. 8: 2752–2767 [DOI] [PubMed] [Google Scholar]
- Mühlenhoff U., Stadler J.A., Richhardt N., Seubert A., Eickhorst T., Schweyen R.J., Lill R., Wiesenberger G. (2003). A specific role of the yeast mitochondrial carriers MRS3/4p in mitochondrial iron acquisition under iron-limiting conditions. J. Biol. Chem. 278: 40612–40620 [DOI] [PubMed] [Google Scholar]
- Murata Y., Ma J.F., Yamaji N., Ueno D., Nomoto K., Iwashita T. (2006). A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J. 46: 563–572 [DOI] [PubMed] [Google Scholar]
- Pich A., Hillmer S., Manteuffel R., Scholz G. (1997). First immunohistochemical localization of the endogenous Fe2+-chelator nicotianamine. J. Exp. Bot. 48: 759–767 [Google Scholar]
- Ravet K., Touraine B., Kim S.A., Cellier F., Thomine S., Guerinot M.L., Briat J.F., Gaymard F. (2009). Post-translational regulation of AtFER2 ferritin in response to intracellular iron trafficking during fruit development in Arabidopsis. Mol. Plant 2: 1095–1106 [DOI] [PubMed] [Google Scholar]
- Roschzttardtz H., Conéjéro G., Curie C., Mari S. (2009). Identification of the endodermal vacuole as the iron storage compartment in the Arabidopsis embryo. Plant Physiol. 151: 1329–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G., Ludewig U., Erenoglu B.E., Mori S., Kitahara T., von Wirén N. (2004). ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 279: 9091–9096 [DOI] [PubMed] [Google Scholar]
- Shimoni-Shor E., Hassidim M., Yuval-Naeh N., Keren N. (2010). Disruption of Nap14, a plastid-localized non-intrinsic ABC protein in Arabidopsis thaliana results in the over-accumulation of transition metals and in aberrant chloroplast structures. Plant Cell Environ. 33: 1029–1038 [DOI] [PubMed] [Google Scholar]
- Shingles R., North M., McCarty R.E. (2002). Ferrous ion transport across chloroplast inner envelope membranes. Plant Physiol. 128: 1022–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Abadia J. (1986). Function of iron in chloroplasts. J. Plant Nutr. 9: 609–646 [Google Scholar]
- Villarejo A., et al. (2005). Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat. Cell Biol. 7: 1224–1231 [DOI] [PubMed] [Google Scholar]
- Waters B.M., Chu H.H., Didonato R.J., Roberts L.A., Eisley R.B., Lahner B., Salt D.E., Walker E.L. (2006). Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 141: 1446–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley J.M. (1978). A suggested cycle of plastid developmental interrelationships. New Phytol. 80: 489–502 [Google Scholar]
- Zheng L., Fujii M., Yamaji N., Sasaki A., Yamane M., Sakurai I., Sato K., Ma J.F. (2011). Isolation and characterization of a barley yellow stripe-like gene, HvYSL5. Plant Cell Physiol. 52: 765–774 [DOI] [PubMed] [Google Scholar]
- Zheng, L., Yamaji, N., Yokosho, K., and Ma, J.F. (2012). YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 24: 3767–3782. [DOI] [PMC free article] [PubMed]
- Zybailov, B., Rutschow, H., Friso, G., Rudella, A., Emanuelsson, O., Sun, Q., and van Wijk, K.J. (2008). Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3: e1994. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.