Abstract
Objective
This study evaluated the effects of cognitive remediation for improving cognitive performance, symptoms, and psychosocial functioning in schizophrenia.
Method
A meta-analysis was conducted of 26 randomized, controlled trials of cognitive remediation in schizophrenia including 1,151 patients.
Results
Cognitive remediation was associated with significant improvements across all three outcomes, with a medium effect size for cognitive performance (0.41), a slightly lower effect size for psychosocial functioning (0.36), and a small effect size for symptoms (0.28). The effects of cognitive remediation on psychosocial functioning were significantly stronger in studies that provided adjunctive psychiatric rehabilitation than in those that provided cognitive remediation alone.
Conclusions
Cognitive remediation produces moderate improvements in cognitive performance and, when combined with psychiatric rehabilitation, also improves functional outcomes.
Cognitive impairment is a core feature of schizophrenia, with converging evidence showing that it is strongly related to functioning in areas such as work, social relationships, and independent living (1, 2). Furthermore, cognitive functioning is a robust predictor of response to psychiatric rehabilitation (i.e., systematic efforts to improve the psychosocial functioning of persons with severe mental illness) (3), including outcomes such as work, social skills, and self-care (1, 4, 5). Because of the importance of cognitive impairment in schizophrenia, it has been identified as an appropriate target for interventions (6).
Currently available pharmacological treatments have limited effects on cognition in schizophrenia (7, 8) and even less impact on community functioning (9). To address the problem of cognitive impairment in schizophrenia, a range of cognitive remediation programs has been developed and evaluated over the past 40 years. These programs employ a variety of methods, such as drill and practice exercises, teaching strategies to improve cognitive functioning, compensatory strategies to reduce the effects of persistent cognitive impairments, and group discussions.
Several reviews of research on cognitive rehabilitation in schizophrenia have been published (10–13). The general conclusions from these reviews have been that cognitive remediation leads to modest improvements in performance on neuropsychological tests but has no impact on functional outcomes. However, these reviews were limited by the relatively small number of studies that actually measured psychosocial functioning, precluding any definitive conclusions about the effects of cognitive remediation on psychosocial adjustment or the identification of program characteristics that may contribute to such effects. The rationale for cognitive remediation is chiefly predicated on its presumed effects on psychosocial functioning and improved response to rehabilitation. Therefore, a critical examination of the effects of cognitive remediation on functional outcomes is necessary in order to determine its potential role in the treatment of schizophrenia.
In recent years, the number of studies that examined psychosocial functioning has grown sufficiently to permit a closer look at the impact of cognitive remediation. We conducted a meta-analysis of controlled studies to evaluate the effects of cognitive remediation on cognitive functioning, symptoms, and functional outcomes. We also examined whether characteristics of cognitive remediation programs (e.g., hours of cognitive training), the provision of adjunctive psychiatric rehabilitation, treatment settings, patient demographics, or type of control group was related to improved outcomes. We hypothesized that cognitive remediation would improve both cognitive functioning and psychosocial adjustment. We also hypothesized that programs that provided more hours of cognitive training would have stronger effects on cognitive functioning and that adjunctive psychiatric rehabilitation would be associated with greater improvements in functional outcomes.
Method
Studies for the meta-analysis were identified by conducting MEDLINE and PsycINFO searches for English language articles published in peer-reviewed journals. The following search terms were used: cognitive training, cognitive remediation, cognitive rehabilitation, and schizophrenia. Studies meeting the following criteria were included: 1) a randomized, controlled trial of a psychosocial intervention designed to improve cognitive functioning; 2) an assessment of performance with at least one neuropsychological measure that had the potential to reflect generalization of effects rather than assessments on trained tasks only; 3) data available on either group means and standard deviations for baseline and postintervention cognitive tests or statistics from which effect sizes could be calculated; 4) a minimum of 75% of the sample reported to have schizophrenia, schizoaffective disorder, or schizophreniform disorder.
Categorization of Neuropsychological Tests
Neuropsychological tests were grouped into the following cognitive domains described by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus panel (6): attention/vigilance, speed of processing, verbal working memory, nonverbal working memory, verbal learning and memory, visual learning and memory, reasoning/problem solving, and social cognition. Each of the neuropsychological measures used in the studies meeting the inclusion criteria was assigned to one cognitive domain by consensus of the first three authors. Measures for which no consensus could be reached, that were judged to reflect more than one cognitive domain, or that the MATRICS panel deemed not sensitive to change were not included in the meta-analysis. Table 1 summarizes which neuropsychological tests were included in each cognitive domain.
TABLE 1.
Neuropsychological Assessments Included in Each Cognitive Domain
| Domain | Assessment |
|---|---|
| Attention/vigilance | Wechsler Memory Scale (WMS) information and mental control subtests |
| Search-a-Word | |
| Cancellation tasks | |
| Continuous Performance Tests | |
| Span of apprehension | |
| Labyrinth Test | |
| Sustained Attention Test | |
| Span: hits, time, and overall | |
| Preattentional processing | |
| Cross-over reaction time | |
| Cross-modal reaction time | |
| Embedded Figures Test | |
| COGLAB apprehension/masking | |
| Dichotic listening tasks | |
| Speed of processing | Trail Making Test, Parts A and B |
| WAIS, WAIS-R, or WAIS-III digit symbol subtest | |
| Stroop Test, color and word conditions | |
| Reaction time tests | |
| Letters and category fluency | |
| Verbal working memory | WAIS, WAIS-R, WAIS-III, or WMS digit span |
| WAIS-III letter-number sequencing and arithmetic subtests | |
| Digit Span Distractibility Test | |
| Other digit span tasks | |
| Trained Word Recall Task | |
| Other arithmetic tasks | |
| Sentence span | |
| Dual span | |
| Paced Auditory Serial Addition Test | |
| Nonverbal working memory | Wechsler Memory Scale—Revised (WMS-R) visual span |
| Dual span | |
| Verbal learning and memory | WMS, WMS-R, or WMS-III logical memory and verbal paired associates subtests |
| California Verbal Learning Test | |
| Rey Auditory Verbal Learning Test | |
| Hopkins Verbal Learning Test | |
| Word List Recall Task | |
| Verbal learning paradigm | |
| Denman Neuropsychological Memory Test | |
| Span-Completeness Verbal Learning Test | |
| Visual learning and memory | WMS, WMS-R, or WMS-III visual recall, visual reproduction, faces, and figural memory subtests |
| Memory for Designs Test | |
| Rey-Osterrieth Complex Figure Test | |
| Kimura recurring figures | |
| Denman Neuropsychological Memory Test | |
| Reasoning and problem solving | WAIS, WAIS-R, or WAIS-III similarities and picture arrangement subtests |
| Wechsler Intelligence Scale for Children mazes subtest | |
| Stroop Test interference condition | |
| Independent Living Scale—problem solving | |
| Gorham’s Proverbs Test and other proverb interpretation tasks | |
| Wisconsin Card Sorting Test | |
| Trail Making Test (B – A) | |
| Hinting Task | |
| Labyrinth Test | |
| Tower of Hanoi | |
| Tower of London | |
| Response inhibition | |
| Six elements | |
| Categories | |
| COGLAB card sorting test | |
| Social cognition | Social perception (Emotion Matching Test and Emotion Labeling Test) |
| Bell-Lysaker Emotion Recognition Test | |
| Social cognition | |
| Other cognitive | |
| Cognitive measures of multiple domains | Global cognitive scores |
| Mini-Mental State Examination | |
| Cognitive measures not considered sensitive to change | Peabody Picture Vocabulary Test |
| Shipley Institute of Living Scale IQ estimate | |
| WAIS-R comprehension subtest | |
| Verbal IQ | |
| Cognitive measures lacking consensus | Cognitive style |
| Hayling Sentence Completion Task | |
| Purdue Pegboard | |
| Tactile performance | |
| WMS orientation subtest | |
| Symptoms | Scale for the Assessment of Positive Symptoms |
| Scale for the Assessment of Negative Symptoms | |
| Positive and Negative Syndrome Scale | |
| Brief Psychiatric Rating Scale | |
| Holtzman Inkblot Test | |
| Paranoid Depression Scale | |
| Present State Exam | |
| Thought, language, and communication | |
| Functioning | Bay Area Functional Performance Evaluation |
| Percent “sick talk”/incoherence during the interview | |
| Life skills profile | |
| Global Assessment Scale | |
| Nurses’ Observation Scale for Inpatient Evaluation | |
| Disability Assessment Schedule | |
| Employment | |
| Social Behaviour Schedule | |
| Micro-Module Learning Test | |
| Assessment of Interpersonal Problem-Solving Skills | |
| Social adjustment | |
Calculation of Effect Sizes
Effect sizes were calculated by using posttreatment group means and standard deviations (14), pre-post difference scores, or analysis of covariance (ANCOVA) or multivariate analysis of covariance (MANCOVA) F values that covaried baseline scores on the dependent measures. Effect sizes can generally be categorized as small (0.2), medium (0.5), or large (0.8) (15). When a study reported data from multiple measures classified in the same cognitive domain, the mean of the effect sizes from those measures was used. Effect size distributions were evaluated for outliers, resulting in exclusion of results of one study from the functional outcome analyses (16).
Meta-Analytic Procedure
Meta-analyses were conducted with BioStat software (17). In order to control for study differences in sample size when mean effect sizes were computed, studies were weighted according to their inverse variance estimates. To determine whether mean effect sizes were statistically significant, the confidence interval (CI) and z transformation of the effect size were used. The homogeneity of the effect sizes across studies for each outcome domain was evaluated by computing the Q statistic (18). Then the significance level of the mean effect sizes was computed by conducting fixed-effects linear models except when the Q statistic indicated significant within-group heterogeneity, in which case we used random effects models. Moderator analyses were then conducted on those domains with significant heterogeneity, based on the Q statistic, to determine whether any participant, setting, or program variables explained variations between studies in effect sizes. These analyses were performed by clustering studies into two contrasting groups based on the moderator variable and computing the Q between and Q within statistics (18).
Moderator Variables
Several variables were considered as potential moderators of cognitive remediation. Each moderator variable was divided into two levels based on a median split. The moderator variables and levels were 1) participant characteristics: age (years) (15–37/38–50), 2) the setting (inpatient/outpatient), 3) the type of control group (active control [e.g., another intervention, such as cognitive behavior therapy or motivational interviewing]/passive control [e.g., viewing educational videos or treatment as usual]), 4) program characteristics: type of intervention (drill and practice/drill and practice plus strategy coaching or strategy coaching alone), hours of practice (determined for the overall program as well as individual cognitive domains), and the provision of adjunctive psychiatric rehabilitation (no/yes).
Some programs that provided training in social cognition employed a combination of cognitive remediation and other rehabilitation approaches, such as social skills training (19, 20), whereas others employed strictly cognitive remediation methods, such as computer-based training tasks (21). The number of hours of social cognition training was included in the total number of cognitive remediation hours only for the programs that did not combine the training with another rehabilitation approach. A variety of psychiatric rehabilitation approaches were provided in conjunction with cognitive remediation, including social skills training (20, 22), social skills/social perception training (19, 23), supported employment (24), vocational rehabilitation (25), and vocational rehabilitation and social information processing groups (26).
Results
Data from 26 studies (1,151 subjects) were included. The studies, characteristics of participants and programs, and effect sizes are displayed in Table 2. The mean sample size was 50 (SD=36, range=10–138). The mean age of the participants was 36.3 years (SD=6.0, range of means=15–47), the mean years of education was 11.8 (SD=1.0, range of means=10–13), 69% of the participants were men, and 60% were inpatients. The mean duration of cognitive remediation programs was 12.8 weeks (SD=20.9, range=1–104). Programs targeted for training an average of 2.9 cognitive domains (SD=1.6, range=1–6), whereas changes in cognitive functioning were assessed on an average of 3.1 cognitive domains (SD=1.6, range=1–6). Sixty-nine percent of the programs used a drill and practice intervention; 23% provided adjunctive psychosocial rehabilitation.
TABLE 2.
Description of Randomized, Controlled Trials of Cognitive Remediation in Schizophrenia
| Author | Sample Characteristics—Inpatient/Outpatient; Mean Age (years); Mean Education (years); % Male | Treatment group (N) | Drill and Practice/Drill and Strategy Coaching | Cognitive Remediation Program Included Psychiatric Rehabilitation | Control Group (active/passive control, N) | Hours/Weeks of Cognitive Remediation (excluding other treatment) | Cognitive Effect Size (follow-up effect size in parentheses) | Average Effect Size for Cognitive Measures | Average Effect Size for Symptom Measures | Average Effect Size for Functioning Measures |
|---|---|---|---|---|---|---|---|---|---|---|
| Wagner (1968) (45), treatment 1 | Inpatients; 44.8; not reported; 100% | Noncomputerized attention training (N=8) | Drill and practice | No | Viewing treatment group stimuli without responding (active control, N=8) | 3 hours/1 week | Visual learning memory= 0.80; reasoning and problem solving=1.40 | 1.10 | ||
| Wagner (1968) (45), treatment 2 | Inpatients; 44.8; not reported; 100% | Noncomputerized abstraction training (N=8) | Drill and practice | No | Viewing treatment group stimuli without responding (active control, N=8) | 3 hours/1 week | Visual learning memory= −1.21; reasoning and problem solving=1.12 | −0.04 | ||
| Wagner (1968) (45), treatment 3 | Inpatients; 44.8; not reported; 100% | Noncomputerized attention and abstraction training (N=16) | Drill and practice | No | Viewing treatment group stimuli without responding (active control, N=8) | 3 hours/1 week | Visual learning memory= −0.09; reasoning and problem solving=0.55 | 0.32 | ||
| Meichenbaum & Cameron (1973), includes 3-week follow-up (16) | Inpatients; 36.0; not reported; 100% | Noncomputerized training using self-talk (N=5) | Drill and practice | No | Task practice without self-talk (active control, N=5) | 4.1 hours/3 weeks | Verbal working memory= 0.77 (1.31); reasoning and problem solving= 1.26 (1.99) | 1.02 (1.65) | 1.89 (2.08) | 3.50 (3.99) |
| Benedict & Harris (1989) (46) | Inpatients; 30.3; not reported; not reported | Computerized training with advancement criteria (N=10) | Drill and practice | No | 1. Computerized training without advancement criteria (active control, N=10); 2. Treatment as usual (passive control, N=10) | 12.5 hours/8–14 weeks | Speed of processing=1.57 | 1.57 | ||
| Olbrich & Mussgay (1990) (43) | Inpatients; 30.7; 10.2; 57% | Noncomputerized training (N=15) | Drill and practice | No | Arts and crafts groups (active control, N=15) | 12 hours/3 weeks | Attention/vigilance=0.52; Verbal working memory=0.43; reasoning and problem solving=0.26 | 0.40 | 0.00 | |
| Hermanutz & Gestrich (1991) (42) | Inpatients; 31.0; 11.0; not reported | Computerized attention training (N=10) | Drill and practice | No | 1. Integrated psychological therapy focusing on cognitive, communication, and social training (active control, N=10); 2. Treatment as usual (passive control, N=10) | 7.5 hours/3–4 weeks | Treatment versus active control: attention/vigilance=0.18; treatment versus passive control: attention/vigilance= −0.09 | Treatment versus active control=0.18; treatment versus passive control= −0.09 | Treatment versus active control= 0.43; treatment versus passive control=0.24 | Treatment versus active control=−0.47; treatment versus passive control=−0.46 |
| Benedict et al. (1994) (47) | Outpatients; 38.8; 11.0; 52% | Computerized attention training (N=16) | Drill and practice | No | Treatment as usual (passive control, N=17) | 12.5 hours/3–5 weeks | Attention/vigilance=0.41; verbal learning and memory=0.13 | 0.27 | ||
| Burda et al. (1994) (48) | Inpatients; 46.6; 12.5; 97% | Computerized training using Captain’s Log (N=40) | Drill and practice | No | Treatment as usual (passive control, N=29) | 12 hours/8 weeks | Speed of processing=0.58; attention/vigilance= 0.65; verbal working memory=0.89; verbal learning and memory= 0.69; visual learning memory=−0.26 | 0.51 | ||
| Field et al. (1997) (49) | Outpatients; 28.6; not reported; 90% | Computerized training (N=5) | Drill and practice | No | Graphics-based computer games (active control, N=5) | 6 hours/3 weeks | Speed of processing=0.74; attention/vigilance= 1.08; reasoning and problem solving=0.54; other=0.00 | 0.79 | ||
| Medalia et al. (1998) (50) | Inpatients; 32.5; 10.8; 78% | Computerized attention training using orientation remedial module (N=27) | Drill and practice | No | Watching National Geographic documentaries (passive control, N=27) | 6 hours/6 weeks | Attention/vigilance=0.19 | 0.19 | 0.24 | |
| Spaulding et al. (1999) (20) | Inpatients; 35.7; 11.9; 63% | Noncomputerized training using integrated psychological therapy (N=49) | Drill and strategy coaching | Social skills training groups | Group supportive therapy emphasizing social skills (active control, N=42) | 68.3 hours/26 weeks | Speed of processing=0.02; attention/vigilance= 0.38; verbal learning and memory=−0.06; visual learning memory= −0.06; reasoning and problem solving=0.14 | 0.08 | 0.55 | 0.53 |
| Wykes et al. (1999) (27); Wykes et al. (2003) (30), 6-month follow-up | Outpatients; 38.5; 12.0; 76% | Noncomputerized training with errorless learning (N=17) | Drill and strategy coaching | No | Intensive occupational therapy (active control, N=16) | 24–40 hours/8–10 weeks | Speed of processing=0.26; verbal working memory=0.27 (0.07); nonverbal working memory= 0.06; reasoning and problem solving=0.20 (0.33) | 0.20 (0.20) | 0.59 | 0.05 |
| Medalia et al. (2000) (51), treatment 1 | Inpatients; 37.7; 11.5; 59% | Computerized problem-solving training (N=18) | Drill and strategy coaching | No | Treatment as usual (passive control, N=18) | 4.2 hours/5 weeks | Verbal learning and memory=−0.43 | −0.43 | ||
| Medalia et al. (2000) (51), treatment 2 | Inpatients; 36.5; 10.5; 59% | Computerized memory training (N=18) | Drill and practice | No | Treatment as usual (passive control, N=18) | 4.2 hours/5 weeks | Verbal learning and memory=0.39 | −0.39 | ||
| Bell et al. (2001) (26) | Outpatients in work therapy; 43.6; 13.2; 78% | Computerized training using CogReHab plus weekly social information processing group (N=31) | Drill and practice | Vocational rehabilitation and social information processing groups | Treatment as usual (passive control, N=34) | 36 hours/26 weeks | Speed of processing=0.31; verbal working memory=0.30; reasoning and problem solving=0.50; social=0.90 | 0.50 | ||
| Bell et al. (2003) (52), includes follow-up | Same as above (N=47) | Passive control, N=55 | Verbal working memory= 0.40 (0.48) | 0.40 (0.48) | ||||||
| Fiszdon et al. (2004) (53), includes follow-up | Same as above (N=45) | Passive control, N=49 | Verbal working memory= 0.53 (0.66) | 0.53 (0.66) | ||||||
| Fiszdon et al. (2005) (54) | Same as above (N=57) | Passive control, N=68 | Verbal learning and memory=0.36 | 0.36 | ||||||
| Van der Gaag (2002) (23) | Inpatients; 31.1; not reported; 64% | Noncomputerized training (N=21) | Drill and strategy coaching | Social skills/social perception training | Leisure/games group (active control, N=21) | 4 hours/11 weeks | Speed of processing= −0.02; attention/vigilance=0.09; verbal learning and memory= 0.41; visual learning memory=0.47; reasoning and problem solving=0.09; social=0.51 | 0.26 | ||
| Bellucci et al. (2002) (34) | Outpatients; 42.0; 12.6; 47% | Computerized training using Captain’s Log (N=17) | Drill and practice | No | Treatment as usual (passive control, N=17) | 8 hours/8 weeks | Speed of processing=0.56; verbal working memory=0.52; verbal learning and memory=0.49 | 0.52 | 0.32 | |
| López-Luengo & Vázquez (2003) (55) | Outpatients; 33.5; not reported; 83% | Noncomputerized training using attention process training (N=13) | Drill and practice | No | Treatment as usual (passive control, N=11) | 24 hours/43 weeks | Speed of processing=0.32; attention vigilance= 0.54; verbal working memory=0.48; verbal learning and memory= 0.57; reasoning and problem solving=0.45 | 0.53 | ||
| Hogarty et al. (2004) (19), includes 12-month follow-up | Outpatients; 37.3; not reported; 59% | Computerized training using orientation remedial module and CogReHab plus group social cognition exercises (N=67) | Drill and strategy coaching | Social skills/social perception training groups | Enriched supportive therapy including psychoeducation, illness self-management, and stress management (active control, N=54) | 75 hours/104 weeks | Speed of processing=0.83 (0.86); social=0.36 (0.66) | 0.60 (0.67) | −0.07 (0.09) | 0.37 (0.51) |
| Ueland & Rund (2005) (21); Ueland & Rund (2004) (44), 12-month follow-up | Inpatients; 15.3; not reported; 50% | Computerized and non-computerized training (N=14) | Drill and strategy coaching | No | Treatment as usual (passive control, N=12) | 30 hours/12 weeks | Attention/vigilance=0.31 (0.28); verbal working memory=0.54 (0.60); verbal learning and memory=0.33 (0.29); visual learning memory=0.11 (0.17); reasoning and problem solving=0.57 (0.31) | 0.37 (0.33) | 0.25 (0.51) | 0.31 (0.16) |
| McGurk et al. (2005) (24) | Outpatients in supported employment; 37.5; 11.2; 55% | Computerized training using Cogpack (N=23) | Drill and strategy coaching | Supported employment | Treatment as usual (passive control, N=21) | 24 hours/12 weeks | Speed of processing=0.27; verbal working memory=0.42; verbal learning and memory=0.45; reasoning and problem solving=0.18 | 0.33 | 0.45 | 1.76 |
| Sartory et al. (2005) (56) | Inpatients; 36.4; 10.3; 67% | Computerized training using Cogpack (N=21) | Drill and practice | No | Treatment as usual (passive control, N=21) | 15 hours/3 weeks | Speed of processing=0.69; verbal learning and memory=0.88 | 0.78 | ||
| Silverstein et al. (2005) (22) | Inpatients; 39.3; 10.6; 87% | Noncomputerized training using attention process training and shaping (N=18) | Drill and practice | Social skills training groups | Treatment as usual (passive control, N=13) | 18 hours/6 weeks | Attention/vigilance=0.25; verbal working memory=0.38; verbal learning and memory=0.48 | 0.37 | 0.41 | 0.68 |
| Vauth et al. (2005) (25) | Inpatients in vocational rehabilitation; 30.0; 12.5; 65% | Computerized training using Cogpack and noncomputerized training, plus cognitive adaptation therapy (N=47) | Drill and strategy coaching | Vocational rehabilitation | 1. Vocational rehabilitation and self-management training for negative symptoms (active control, N=45); 2. Treatment as usual (passive control, N=46) | 24 hours/8 weeks | Treatment versus active control; attention/vigilance=0.46; verbal learning and memory= 0.61; treatment versus passive control; attention/vigilance=0.46; verbal learning and memory=0.55; reasoning and problem solving=0.60 | Treatment versus active control= 0.54; treatment versus passive control=0.54 | Treatment versus active control= 0.44; treatment versus passive control=0.19 | Treatment versus active control=0.10; treatment versus passive control=0.46 |
| Penadés et al. (2006) (29), includes 6-month follow-up | Outpatients; 35.1; 10.2; 57% | Noncomputerized training using frontal/executive program with errorless learning (N=20) | Drill and practice | No | Cognitive behavioral therapy for psychosis (active control, N=20) | 40 hours/16 weeks | Speed of processing=0.56 (1.02); verbal working memory=0.80 (0.76); verbal learning and memory=1.19 (2.07); visual learning memory=0.75 (1.22); reasoning and problem solving=1.76 (2.05) | 1.01 (1.42) | −0.15 (−0.15) | 0.42 (0.45) |
Effects on Cognitive Performance
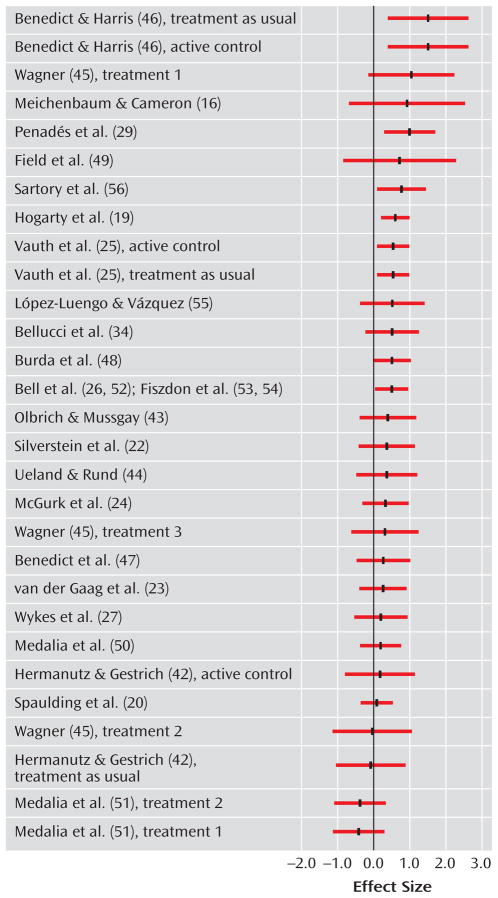
Only one study examined changes in nonverbal working memory (27), so this domain was not included in the meta-analysis. The effect sizes and related statistics for overall cognition and the other seven individual cognitive domains are provided in Table 3. In addition, the effect sizes for overall cognitive functioning for each study are depicted in Figure 1. The effect size for overall cognition was significant, as well as for six of the seven domains of cognitive performance. Most of the effects were in the medium or low-medium effect size range, indicating improved cognitive performance after cognitive remediation. The effect size for visual learning and memory was not significant (0.09).
TABLE 3.
Results of Meta-Analysis of Randomized, Controlled Trials of Cognitive Remediation in Schizophreniaa
| Outcome Domain | Effect Size | 95% CI | T Score | Subjects (N) | Cognitive Remediation Hours
|
Analysis
|
||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Hedges’s Q | df | |||||
| Global cognition | 0.41 | 0.29 to 0.52 | 6.9*** | 1,214 | 12.5 | 3–75 | 35.3 | 28 |
| Attention/vigilance | 0.41 | 0.25 to 0.57 | 5.1*** | 659 | 6.6 | 0–14 | 9.8 | 14 |
| Speed of processing | 0.48 | 0.28 to 0.69 | 5.9*** | 655 | 0.0 | 0–3 | 20.7 | 13 |
| Verbal working memory | 0.52 | 0.33 to 0.72 | 5.2*** | 428 | 0.4 | 0–8 | 3.9 | 10 |
| Verbal learning and memory | 0.39 | 0.20 to 0.58 | 5.5*** | 858 | 0.0 | 0–8 | 26.6* | 15 |
| Visual learning and memory | 0.09 | −0.26 to 0.43 | 0.6 | 424 | 0.0 | 0–3 | 14.5* | 7 |
| Reasoning/problem solving | 0.47 | 0.30 to 0.64 | 5.4*** | 564 | 3.0 | 0–32 | 21.8 | 14 |
| Social cognition | 0.54 | 0.22 to 0.88 | 3.9*** | 228 | 26.0 | 2–84 | 2.8 | 2 |
| Symptoms | 0.28 | 0.13 to 0.43 | 3.6*** | 709 | 12.2 | 14 | ||
| Functioning | 0.35 | 0.07 to 0.62 | 1.9* | 615 | 25.7** | 10 | ||
After consideration of the consistency of effect sizes across six of the seven cognitive domains and overall cognitive functioning, the clinical and theoretical significance of these moderator effects on verbal learning and memory is unclear.
p < 0.05.
p < 0.01.
p < 0.001.
FIGURE 1.
Effect Sizes for Overall Cognition in Randomized, Controlled Trials of Cognitive Remediation in Schizophrenia
Six studies also reported cognitive data at follow-up (16, 19, 21, 28–30). For these studies, the average effect size at posttreatment was 0.56 (t=4.8, df=5, p<0.001, CI=0.33–0.79; Q=3.4, df=5, n.s.), and at follow-up, it was 0.66 (t=5.7, df=5, p<0.001, CI=0.43–0.89; Q=7.8, df=5, n.s.). Similar to the results at posttreatment, cognitive remediation was associated with improved overall cognitive performance an average of 8 months later.
Hedges’s Q was significant for only one cognitive domain, verbal learning and memory, indicating significant heterogeneity in effect sizes to evaluate the effects of moderators. For this domain, a larger effect size was associated with more hours of cognitive remediation (0.57) compared with fewer hours (0.29) (Q=3.7, df=1, p<0.05) and with drill and practice (0.48) compared with drill and practice plus strategy coaching (0.23) (Q=2.0, df=1, p<0.05); hours of cognitive remediation were unrelated to program type (χ2=0.4, df=1, n.s.).
Effects on Symptoms and Functioning
Cognitive remediation was associated with a small effect size for symptoms (0.28) and between a small and a medium effect size for functioning (0.35). There was significant heterogeneity in the effect sizes for functioning (Q=25.7, df=11, p<0.01), but not for symptoms. Moderator analyses indicated that cognitive remediation resulted in stronger effect sizes for improved psychosocial functioning in studies that provided adjunctive psychiatric rehabilitation (0.47) compared to no psychiatric rehabilitation (0.05) (Q=5.5, df=1, p<0.01.), cognitive remediation programs that used drill and practice plus strategy coaching (0.62) compared to drill and practice only (0.24) (Q=4.6, df=1, p<0.05), and studies that included older (0.55) rather than younger (0.18) patients (Q=5.7, df=1, p<0.05). Program type was unrelated to age and to adjunctive psychiatric rehabilitation, but age and adjunctive psychiatric rehabilitation were significantly associated (χ2=6.7, df=1, p<0.05). Studies that provided psychiatric rehabilitation tended to serve older patients.
Discussion
The results provide support for the effects of cognitive remediation on improving cognitive functioning in schizophrenia, with effect sizes in the medium range for overall cognitive functioning (0.41) and six of the seven cognitive domains (0.39–0.54). The effects of cognitive remediation on cognitive performance were remarkably similar across the 26 studies included in the analysis despite differences in length and training methods between cognitive remediation programs, inpatient/outpatient setting, patient age, and provision of adjunctive psychiatric rehabilitation. The results indicate that cognitive remediation produced robust improvements in cognitive functioning across a variety of program and patient conditions.
The effect sizes of cognitive remediation were homogeneously distributed across studies for overall cognitive functioning and six of the seven cognitive domains, precluding the examination of moderators of treatment effects for most cognitive outcomes. Thus, contrary to our hypothesis, the number of hours programs devoted to cognitive remediation was not related to the amount of improvement in overall cognitive functioning. However, hours of training, as well as use of drill and practice rather than combined drill and practice with strategy coaching, were related to improvements in verbal learning and memory, suggesting that this domain may be more sensitive to the method and extent of cognitive remediation.
It is possible that a relatively limited amount of cognitive remediation (e.g., 5–15 hours) is sufficient to produce improved cognitive functioning and that all studies provided an adequate amount of treatment. Alternatively, the amount of cognitive remediation may not be related to immediate gains in cognitive functioning but could contribute to the retention of improvements following the termination of treatment. The impact of amount of cognitive remediation on the maintenance of treatment effects could not be evaluated in this meta-analysis because only six studies conducted follow-up assessments an average of 8 months after completion of the program. However, the mean effect size for overall cognitive performance for these studies was in the medium range (0.66), comparable in magnitude to the immediate effects of cognitive remediation. These findings provide preliminary support for the longer-term benefits of cognitive remediation on cognitive performance and point to the need for more research on the maintenance of treatment effects.
The overall effect size of cognitive remediation on improving symptoms was significant but in the small range (0.28). Previous reviews of the effects of cognitive remediation either have not examined symptoms (10, 11) or were inconclusive because of the small number of studies (12, 13). The apparently limited impact of cognitive remediation on symptoms is consistent with numerous studies showing that cognitive impairment is relatively independent of other symptoms of schizophrenia (31–33). Cognitive remediation may have some beneficial effects on symptoms by providing positive learning experiences that serve to bolster self-esteem and self-efficacy for achieving personal goals, thereby improving depression. Several studies have reported that cognitive remediation improved mood (24, 27, 34).
Cognitive remediation also had a significant effect on improving psychosocial functioning, with an average effect size of 0.35, just slightly lower than the average effect size of 0.41 for improved cognitive performance. For example, patients who participated in cognitive remediation showed greater improvements in obtaining and working competitive jobs (24, 25), the quality of and satisfaction with interpersonal relationships (19), and the ability to solve interpersonal problems (20). These findings are unique because until recently a sufficient number of studies had not measured functional outcomes from which to draw firm conclusions. The impact of cognitive remediation on improved functioning is important because the primary rationale for cognitive remediation in schizophrenia is to improve psychosocial functioning (35).
In contrast to the uniform effects across studies of cognitive remediation on overall cognitive performance and symptoms, there was significant variability in its effects on psychosocial functioning. Furthermore, as hypothesized, cognitive remediation programs that provided adjunctive psychiatric rehabilitation had significantly stronger effects on improving functional outcomes (0.47) than programs that did not (0.05). This effect is consistent with previous research showing that cognitive impairment attenuates response to psychiatric rehabilitation (1, 36, 37) and suggests that improved cognitive performance may enable some patients to benefit more from rehabilitation. The findings are also consistent with the results of a meta-analysis of integrated psychological therapy (38) in which the strongest effects on functioning were found in programs that integrated cognitive remediation and social skills training rather than programs that provided either intervention alone (39).
Cognitive remediation programs that included strategy coaching had stronger effects on functioning than programs that focused only on drill and practice. Strategy coaching typically targets memory and executive functions by teaching methods such as chunking information to facilitate recall and problem-solving skills. It is unclear whether strategy coaching is more effective because people are better able to transfer skills from the training setting into their daily lives (35) or because teaching such strategies helps patients compensate for the effects of persistent cognitive impairments on functioning (24) or both. Further research is needed to address this question.
The effects of cognitive remediation were not influenced by the nature of the control condition. Thus, simply actively or passively engaging patients in treatments designed to control for the amount of clinician contact did not appear to confer any benefit in cognitive functioning beyond the provision of usual services. These findings are consistent with the meta-analysis of the cognitive remediation-based integrated psychological therapy program (39) but differ from the psychotherapy literature, where there is ample evidence for nonspecific effects related to therapist attention (40). The mechanisms underlying the effects of cognitive remediation on improved cognitive performance, functioning, and symptoms appear to differ from those involved in psychotherapy. The results raise questions about the need to control for the amount of clinician attention given to treatment control groups in research on cognitive remediation.
So what has been learned after almost 40 years of research on cognitive remediation for schizophrenia? Although a great deal more is known about schizophrenia and its neurocognitive underpinnings and the technology for assessing and remediating cognitive impairments has evolved (e.g., most programs now employ at least some computer-based training), the effect sizes on cognitive functioning do not appear to have increased appreciably in recent years. The failure to develop more potent programs could be due to limitations imposed by the illness itself and not the fault of treatment developers. It may be argued that a similar phenomenon has occurred in the pharmacological treatment of schizophrenia, where despite the enormous investment of resources into the development of new drugs, the clinical gains in treating symptoms over the past 50 years are debatable (41).
Alternatively, the ability to improve the effectiveness of cognitive remediation may depend on attention to critical issues in research design. Two such issues deserve special consideration: the evaluation of the persistence of remediation effects on cognitive functioning and the assessment of the impact of remediation on functional outcomes. Despite the number of controlled studies of cognitive remediation, only six studies (16, 19, 21, 28–30) examined whether improvements in cognitive functioning were maintained at a posttreatment follow-up, precluding the exploration of moderators of treatment effects. The relative lack of data addressing this question may be important because different program, patient, or setting factors could influence the long-term maintenance of cognitive effects compared to short-term effects.
Similarly, only 11 studies evaluated functional outcomes (16, 19, 20, 22, 24, 25, 27, 29, 42–44), and this was the first meta-analysis to quantitatively demonstrate that cognitive remediation improved psychosocial functioning. Furthermore, the impact of cognitive remediation on functioning was moderated by several factors, including the provision of adjunctive psychiatric rehabilitation, cognitive training method, and patient age, suggesting potentially important factors for improving the impact of treatment programs. Thus, the ability to make cognitive remediation programs more effective may have been constrained by the neglect of most studies to measure the long-term effects of remediation and its impact on functional outcomes, resulting in the inability to identify moderators of treatment that could be the focus of efforts to hone and refine the intervention. Future research on cognitive remediation should routinely evaluate psychosocial functioning and the long-term effects of treatment on all outcomes of interest. In addition, research that systematically examines the interactions between cognitive remediation and psychiatric rehabilitation is warranted.
In summary, cognitive remediation was found to have consistent effects on improving cognitive performance, functioning, and symptoms. In addition, the impact of cognitive remediation on functional outcomes was significantly greater in studies that also provided psychiatric rehabilitation, suggesting that these two treatment approaches may work together in a synergistic fashion. These findings challenge the assumption that simply improving cognitive functioning in schizophrenia will spontaneously lead to better psychosocial outcomes. The results do suggest, however, that cognitive remediation may improve the response of some patients to psychiatric rehabilitation. Overall, this meta-analysis indicates that cognitive remediation may have an important role to play in improving both cognitive performance and functional outcomes in schizophrenia.
Acknowledgments
Supported by NIMH grant MH77210 and National Institute on Disability and Rehabilitation Research grant H133G050230.
Footnotes
All authors report no competing interests.
References
- 1.McGurk SR, Mueser KT. Cognitive functioning, symptoms, and work in supported employment: a review and heuristic model. Schizophr Res. 2004;70:147–174. doi: 10.1016/j.schres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Mueser KT. Cognitive functioning, social adjustment and long-term outcome in schizophrenia. In: Sharma T, Harvey P, editors. Cognition in Schizophrenia: Impairments, Importance, and Treatment Strategies. Oxford, UK: Oxford University Press; 2000. pp. 157–177. [Google Scholar]
- 3.Corrigan PW, Mueser KT, Bond GR, Drake RE, Solomon P. The Principles and Practice of Psychiatric Rehabilitation: An Empirical Approach. New York: Guilford; 2007. [Google Scholar]
- 4.Smith TE, Hull JW, Romanelli S, Fertuck E, Weiss KA. Symptoms and neurocognition as rate limiters in skills training for psychotic patients. Am J Psychiatry. 1999;156:1817–1818. doi: 10.1176/ajp.156.11.1817. [DOI] [PubMed] [Google Scholar]
- 5.Wykes T, Dunn G. Cognitive deficit and the prediction of rehabilitation success in a chronic psychiatric group. Psychol Med. 1992;22:389–398. doi: 10.1017/s0033291700030336. [DOI] [PubMed] [Google Scholar]
- 6.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Marder SR. Initiatives to promote the discovery of drugs to improve cognitive function in severe mental illness. J Clin Psychiatry. 2006;67(suppl 9):31–35. doi: 10.4088/jcp.0706e03. [DOI] [PubMed] [Google Scholar]
- 8.Rund BR, Borg NE. Cognitive deficits and cognitive training in schizophrenic patients: a review. Acta Psychiatr Scand. 1999;100:85–95. doi: 10.1111/j.1600-0447.1999.tb10829.x. [DOI] [PubMed] [Google Scholar]
- 9.Harvey PD, Green MF, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. 2004;65:361–372. [PubMed] [Google Scholar]
- 10.Krabbendam L, Aleman A. Cognitive rehabilitation in schizophrenia: a quantitative analysis of controlled studies. Psychopharmacology. 2003;169:376–382. doi: 10.1007/s00213-002-1326-5. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz MM, Moberg PJ, Gur RC, Gur RE. Approaches to cognitive remediation of neuropsychological deficits in schizophrenia: a review and meta-analysis. Neuropsychology Rev. 2001;11:197–210. doi: 10.1023/a:1012953108158. [DOI] [PubMed] [Google Scholar]
- 12.Pilling S, Bebbington P, Kuipers E, Garety P, Geddes JR, Martindale B, Orbach G, Morgan C. Psychological treatments in schizophrenia, II: meta-analyses of randomized controlled trials of social skills training and cognitive remediation. Psychol Med. 2002;32:783–791. doi: 10.1017/s0033291702005640. [DOI] [PubMed] [Google Scholar]
- 13.Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophr Bull. 2003;29:359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- 14.Thalheimer W, Cook S. How to calculate effect sizes from published research: a simplified methodology. www.work-learning.com/effect_sizes.htm.
- 15.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 16.Meichenbaum D, Cameron R. Training schizophrenics to talk to themselves: a means of developing attentional controls. Behav Ther. 1973;4:515–534. [Google Scholar]
- 17.Borenstein M, Rothstein H. Comprehensive Meta-Analysis: A Computer Program for Research Synthesis. Englewood, NJ: Biostat; 1999. [Google Scholar]
- 18.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985. [Google Scholar]
- 19.Hogarty GE, Flesher S, Ulrich RF, Carter M, Greenwald D, Pogue-Geile MF, Kechavan M, Cooley S, Di Barry AL, Garrett A, Parepally H, Zoretich R. Cognitive enhancement therapy for schizophrenia. effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61:866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- 20.Spaulding WD, Reed D, Sullivan M, Richardson C, Weiler M. Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull. 1999;25:657–676. doi: 10.1093/oxfordjournals.schbul.a033409. [DOI] [PubMed] [Google Scholar]
- 21.Ueland T, Rund BR. Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta Psychiatr Scand. 2005;111:193–201. doi: 10.1111/j.1600-0447.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein SM, Hatashita-Wong M, Solak BA, Uhlhaas P, Landa Y, Wilkniss SM, Goicochea C, Carpiniello K, Schenkel LS, Savitz A, Smith TE. Effectiveness of a two-phase cognitive rehabilitation intervention for severely impaired schizophrenia patients. Psychol Med. 2005;35:829–837. doi: 10.1017/s0033291704003356. [DOI] [PubMed] [Google Scholar]
- 23.van der Gaag M, Kern RS, van den Bosch RJ, Liberman RP. A controlled trial of cognitive remediation in schizophrenia. Schizophr Bull. 2002;28:167–176. doi: 10.1093/oxfordjournals.schbul.a006919. [DOI] [PubMed] [Google Scholar]
- 24.McGurk SR, Mueser KT, Pascaris A. Cognitive training and supported employment for persons with severe mental illness: one year results from a randomized controlled trial. Schizophr Bull. 2005;31:898–909. doi: 10.1093/schbul/sbi037. [DOI] [PubMed] [Google Scholar]
- 25.Vauth R, Corrigan PW, Clauss M, Dietl M, Dreher-Rudolph M, Stieglitz R-D, Vater R. Cognitive strategies versus self-management skills as adjunct to vocational rehabilitation. Schizophr Bull. 2005;31:55–66. doi: 10.1093/schbul/sbi013. [DOI] [PubMed] [Google Scholar]
- 26.Bell MD, Bryson G, Greig T, Corcoran C, Wexler RE. Neurocognitive enhancement therapy with work therapy. Arch Gen Psychiatry. 2001;58:763–768. doi: 10.1001/archpsyc.58.8.763. [DOI] [PubMed] [Google Scholar]
- 27.Wykes T, Reeder C, Corner J, Williams C, Everitt B. The effects of neurocognitive remediation on executive processing in patients with schizophrenia. Schizophr Bull. 1999;25:291–307. doi: 10.1093/oxfordjournals.schbul.a033379. [DOI] [PubMed] [Google Scholar]
- 28.Fiszdon JM, Whelahan H, Bryson GJ, Wexler BE, Bell MD. Cognitive training of verbal memory using a dichotic listening paradigm: impact on symptoms and cognition. Acta Psychiatr Scand. 2005;112:187–193. doi: 10.1111/j.1600-0447.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 29.Penadés R, Catalán R, Salamero M, Boget T, Puig O, Guarch J, Gastó C. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophr Res. 2006;87:323–331. doi: 10.1016/j.schres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Wykes T, Reeder C, Williams C, Corner J, Rice C, Everitt B. Are the effects of cognitive remediation therapy (CTR) durable? results from an exploratory trial in schizophrenia. Schizophr Res. 2003;61:163–174. doi: 10.1016/s0920-9964(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 31.Harvey PD, Serper MR, White L, Parrella M, McGurk SR, Moriarty PJ, Bowie C, Vadhan N, Friedman J, Davis KL. The convergence of neuropsychological testing and clinical ratings of cognitive impairment in patients with schizophrenia. Compr Psychiatry. 2001;42:306–313. doi: 10.1053/comp.2001.24587a. [DOI] [PubMed] [Google Scholar]
- 32.Liddle PF. The symptoms of chronic schizophrenia: a re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- 33.Mueser KT, Curran PJ, McHugo GJ. Factor structure of the Brief Psychiatric Rating Scale in schizophrenia. Psychol Assessment. 1997;9:196–204. [Google Scholar]
- 34.Bellucci DM, Glaberman K, Haslam N. Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophr Res. 2002;59:225–232. doi: 10.1016/s0920-9964(01)00402-9. [DOI] [PubMed] [Google Scholar]
- 35.Wykes T, Reeder C. Cognitive Remediation Therapy for Schizophrenia: Theory and Practice. London: Routledge; 2005. [Google Scholar]
- 36.Mueser KT, Bellack AS, Douglas MS, Wade JH. Prediction of social skill acquisition in schizophrenic and major affective disorder patients from memory and symptomatology. Psychiatry Res. 1991;37:281–296. doi: 10.1016/0165-1781(91)90064-v. [DOI] [PubMed] [Google Scholar]
- 37.Wykes T, Sturt E, Katz R. The prediction of rehabilitative success after three years—the use of social, symptom and cognitive variables. Br J Psychiatry. 1990;157:865–870. doi: 10.1192/bjp.157.6.865. [DOI] [PubMed] [Google Scholar]
- 38.Brenner H, Roder V, Hodel B, Kienzle N, Reed D, Liberman R. Integrated Psychological Therapy for Schizophrenic Patients. Seattle: Hogrefe & Huber; 1994. [Google Scholar]
- 39.Roder V, Mueller DR, Mueser KT, Brenner HD. Integrated psychological therapy (IPT) for schizophrenia: is it effective? Schizophr Bull. 2006;32(suppl 1):S81–S93. doi: 10.1093/schbul/sbl021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crits-Christoph P, Baranackie K, Kurcias JS, Beck AT, Carroll K, Perry K, Luborsky L, McLellan AT, Woody GE, Thompson L, Gallagher D, Zitrin C. Meta-analysis of therapist effects in psychotherapy outcome studies. Psychotherapy Res. 1991;1:81–91. [Google Scholar]
- 41.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigators: effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 42.Hermanutz M, Gestrich J. Computer-assisted attention training in schizophrenics: a comparative study. Eur Arch Psychiatry Clin Neurosci. 1991;240:282–287. doi: 10.1007/BF02189541. [DOI] [PubMed] [Google Scholar]
- 43.Olbrich R, Mussgay L. Reduction of schizophrenic deficits by cognitive training: an evaluation study. Eur Arch Psychiatry Neurol Sci. 1990;239:366–369. doi: 10.1007/BF01734543. [DOI] [PubMed] [Google Scholar]
- 44.Ueland T, Rund BR. A controlled randomized treatment study: the effects of a cognitive remediation program on adolescents with early onset psychosis. Acta Psychiatr Scand. 2004;109:70–74. doi: 10.1046/j.0001-690x.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 45.Wagner BR. The training of attending and abstracting responses in chronic schizophrenics. J Exper Res Personality. 1968;3:77–88. [Google Scholar]
- 46.Benedict RHB, Harris AE. Remediation of attention deficits in chronic schizophrenic patients: a preliminary study. Br J Clin Psychol. 1989;28:187–188. doi: 10.1111/j.2044-8260.1989.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 47.Benedict RHB, Harris AE, Markow T, McCormick JA, Nuechterlein KH, Asarnow RF. Effects of attention training on information processing in schizophrenia. Schizophr Bull. 1994;20:537–546. doi: 10.1093/schbul/20.3.537. [DOI] [PubMed] [Google Scholar]
- 48.Burda PC, Starkey TW, Dominguez F, Vera V. Computer-assisted cognitive rehabilitation of chronic psychiatric inpatients. Computers Hum Behav. 1994;10:359–368. [Google Scholar]
- 49.Field CD, Galletly C, Anderson D, Walker P. Computer-aided cognitive rehabilitation: possible application to the attentional deficit of schizophrenia: a report of negative results. Percept Mot Skills. 1997;85:995–1002. doi: 10.2466/pms.1997.85.3.995. [DOI] [PubMed] [Google Scholar]
- 50.Medalia A, Aluma M, Tryon W, Merriam AE. Effectiveness of attention training in schizophrenia. Schizophr Bull. 1998;24:147–152. doi: 10.1093/oxfordjournals.schbul.a033306. [DOI] [PubMed] [Google Scholar]
- 51.Medalia A, Dorn H, Watras-Gans S. Treating problem solving deficits on an acute psychiatric inpatient unit. Psychiatry Res. 2000;97:79–88. doi: 10.1016/s0165-1781(00)00214-6. [DOI] [PubMed] [Google Scholar]
- 52.Bell M, Bryson G, Wexler BE. Cognitive remediation of working memory deficits: durability of training effects in severely impaired and less severely impaired schizophrenia. Acta Psychiatr Scand. 2003;108:101–109. doi: 10.1034/j.1600-0447.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 53.Fiszdon JM, Bryson GJ, Wexler BE, Bell MD. Durability of cognitive remediation training in schizophrenia: performance on two memory tasks at 6-month and 12-month follow-up. Psychiatry Res. 2004;125:1–7. doi: 10.1016/j.psychres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Fiszdon JM, Cardenas AS, Bryson GJ, Bell MD. Predictors of remediation success on a trained memory task. J Nerv Ment Dis. 2005;193:602–608. doi: 10.1097/01.nmd.0000177790.23311.ba. [DOI] [PubMed] [Google Scholar]
- 55.López-Luengo B, Vázquez C. Effects of attention process training on cognitive functioning of schizophrenic patients. Psychiatry Res. 2003;119:41–53. doi: 10.1016/s0165-1781(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 56.Sartory G, Zorn C, Groetzinger G, Windgassen K. Computerized cognitive rehabilitation improves verbal learning and processing speed in schizophrenia. Schizophr Res. 2005;75:219–223. doi: 10.1016/j.schres.2004.10.004. [DOI] [PubMed] [Google Scholar]