Abstract
The Y-box Binding Protein-1 (YB-1) is a highly conserved oncogenic transcription/translation factor that is expressed in cancers affecting adults and children. It is now believed that YB-1 plays a causal role in the development of cancer given recent work showing that its expression drives the tumorigenesis in the mammary gland. In human breast cancers, YB-1 is associated with rapidly proliferating tumors that are highly aggressive. Moreover, expression of YB-1 promotes the growth of breast cancer cell lines both in monolayer and anchorage independent conditions. The involvement of YB-1 in breast cancer pathogenesis has made it a putative therapeutic target; however, the mechanism(s) that regulate YB-1 are poorly understood. This review first describes the oncogenic properties of YB-1 in cancer. It also highlights the importance of YB-1 in hardwiring signal transduction pathways to the regulation of genes involved in the development of cancer.
Keywords: YB-1, cancer, phosphorylation, signal transduction
General Background of YB-1
YB-1 is an oncogenic transcription/translation factor that leads to the development of cancer. It controls the oncogenome by shuttling between the cytoplasm and the nucleus. In the cytoplasm, YB-1 acts as a translation factor that regulates the expression of oncogenes by transporting mRNA to polysomes (Soop et al. 2003). It is also intimately involved in the translational machinery (Ashizuka et al. 2002) where YB-1 governs whether or not oncogenic mRNA’s will ultimately be translated (Evdokimova et al. 2006). Upon nuclear translocation, YB-1 regulates transcription by binding directly to genes involved in tumor growth and drug resistance. YB-1 also associates with other transcription factors, such as p53 (Okamoto T et al. 2000), AP-1 (Lasham et al. 2000), Smad 3 (Higashi et al. 2003) and p300 (Higashi et al. 2003) to indirectly regulate gene expression. The nuclear functions of YB-1 also include a role in DNA replication (En-Nia et al. 2004), repair (Marenstein et al. 2001) and mRNA transport into the cytoplasm (Soop et al. 2003). Thus, YB-1 is involved in many aspects of gene expression control that lead to tumor cell growth and drug resistance (Figure 1). YB-1 was first isolated as a transcription factor that bound to the promoter of the major histocompatibility complex class II (MHC Class II) suggesting that it could theoretically obscure immune cells from destroying cancer cells. At the same time, another group identified a DNA binding protein that interacted with the epidermal growth factor (EGFR) enhancer and Her-2 promoter (Sakura et al. 1988). Soon after, YB-1/DbpB was reported to bind to CT-rich elements in the C-myc promoter (Kolluri and Kinniburgh, 1991). From the early studies on YB-1, one can envisage that this transcription factor could facilitate the development of cancer in part by escaping immunosurveillance and at the same time inducing pro-survival pathways such as EGFR, Her-2 and c-Myc.
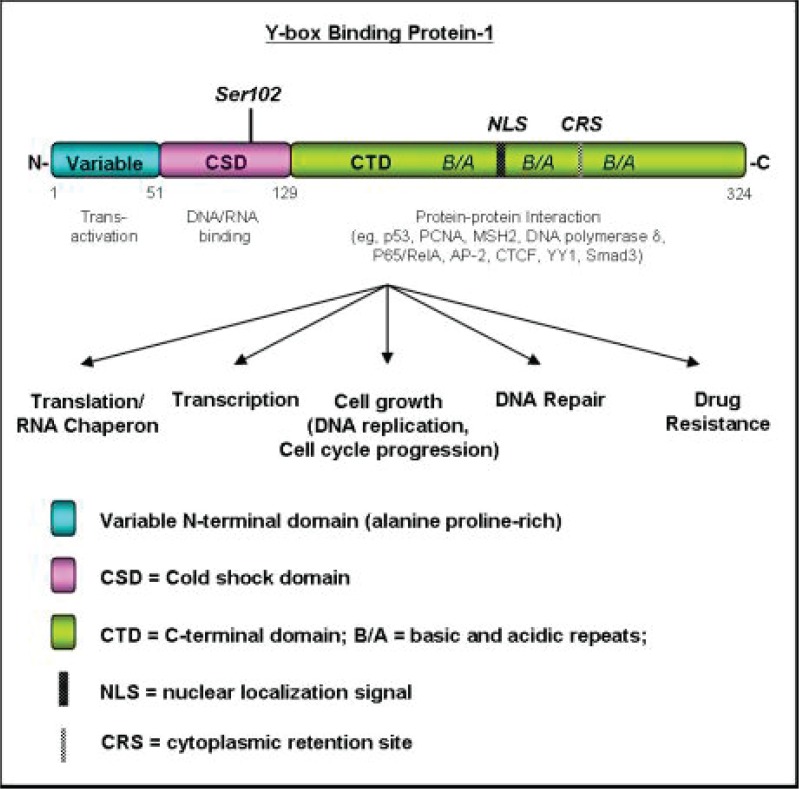
Figure 1.
The Structure and Functions of YB-1. YB-1 is made up of the N-terminal, cold shock (CSD) and C-terminal domains (CTD). These domains have unique functions. The N-terminal is necessary for transactivation whereas the CSD is important for RNA/DNA binding. Most of the characterized protein:protein interactions occur on the CTD. The CSD and CTD also work together to facilitate nuclear trafficking. Cellular trafficking is furthermore regulated by the nuclear localization signal in the C-terminal domain as well as the cytoplasmic retention signal also located in this region of the protein.
At the protein level, YB-1 consists of three domains; a non-conserved variable N-terminal domain, a highly conserved cold-shock domain (CSD), and a C-terminal domain (CTD) (Kohno et al. 2003) (Figure 1). The N-terminal domain, which is rich in alanine and proline residues, is thought to be involved in transactivation (Kohno et al. 2003). The CSD binds oligonucleotides including RNA, double stranded DNA, and single stranded DNA (Didier et al. 1988; Hasegawa et al. 1991; Kolluri et al. 1992; Bouvet et al. 1995; MacDonald et al. 1995). It also contains two conserved RNP (ribonucleoprotein particles) motifs (Graumann and Marahuel, 1996) thought to be important in the transport and translational control of mRNA. The CTD of YB-1 contains alternating regions of basic and acidic residues, termed charged zipper or B/A repeats, and is suggested to mediate protein-protein interactions (Tafuri and Wolffe, 1992; Bouvet et al. 1995; Swamynathan et al. 1998; Nambiar et al. 1998). Proteins shown to interact with YB-1 include proliferating cell nuclear antigen (PCNA), MutS homologue 2 (MSH2), and DNA polymerase δ (Ise et al. 1999; Gaudreault et al. 2004). The alternating blocks of basic and acidic residues are also common features of proteins that bind to ribonucleoprotein complexes to shuttle between the cytoplasm and the nucleus (Ranjan et al. 1993). Indeed, noncanonical nuclear localization signals (NLS) and a cytoplasmic retention site (CRS) were identified in the CTD to control YB-1 cellular localization (Bader et al. 2005). Reportedly the CSD also co-operates with the CTD to facilitate nuclear trafficking (Jurchott et al. 2003). These studies contribute to our overall understanding of how the structure of YB-1 dictates the function. It is now known that RNA/DNA binding (Klokz et al. 2002) and protein:protein interactions are controlled by specific YB-1 domains (Figure 1; Kohno et al. 2003; Raffetseder et al. 2003; Shnyreva et al. 2000).
How does YB-1 play a role in cancer?
YB-1 has been shown to be associated with many malignancies including colorectal carcinomas (Shibao et al. 1999), prostate cancer (Gimenez-Bonafe et al. 2004), osteosarcoma (Oda et al. 1998), ovarian serous adenocarcinoma (Kamura et al. 1999; Yahata et al. 2002), lung cancer (Shibahara et al. 2001; Gu et al. 2001), synovial sarcoma (Oda et al. 2003) and breast cancer (Bargou et al. 1997; Janz et al. 2002; Rubinstein et al. 2002; Huang et al. 2005; Wu et al. 2006). Much less is known about the role of YB-1 in childhood cancers. The first study of its kind will be published this year showing that YB-1 is a feature of pediatric glioblastoma multiforme (GBM) (Faury et al. 2007). This was discovered initially by comparing gene expression profiles from pediatric and adult GBM. In this study, we determined that YB-1 is highly expressed in pediatric GBM whereas the frequency of this in adult GBM was far less. Our laboratory has also gone on to study a pediatric tumor tissue microarray that includes multiple solid tumors types from children. We have further identified very high levels of YB-1 in 44% of Ewings sarcomas (n = 12/21 cases, unpublished). Interestingly YB-1 is exclusively expressed in the nucleus (unpublished). Given the expression of YB-1 in so many tumor types it seems obvious that it must play an important role in the development of cancer. It has been shown in a transgenic mouse model that YB-1 induces mammary tumor formation with 100% genetic penetrance (Bergmann et al. 2005). Close examination of the mouse mammary tumors revealed a high content of binucleate cells, most of which were tetraploid. There was also a high degree of chromosomal instability. It was speculated that YB-1 may promote breast tumor formation and/or growth by accelerating cell cycle progression and escaping DNA damage check points (Bergmann et al. 2005). Whether or not YB-1 plays a causative role in the development of other types of cancer is unknown at this time.
Independent of whether YB-1 is instrumental in the development of cancer it certainly plays an important role in mediating the growth of malignant cells. The first direct evidence for a role of YB-1 in cell proliferation came from gene knock down experiments where the loss of one allele in the chicken lymphocytic cells inhibited growth by >70% (Swamynathan et al. 2002). The growth and survival of cancer cells are also dependent upon YB-1. For example, melanoma, adenocarcinoma, hepatoma, fibrosarcoma and colon cancer cells die when YB-1 is knocked out with antisense (Swamynathan et al. 2002). Recently, we used siRNAs to inhibit the expression of YB-1, resulting in a 48% reduction in breast cell growth in monolayer (Wu et al. 2006) and under anchorage independent conditions (unpublished). Furthermore, our lab demonstrated that YB-1, but not the mutant YB-1 in which S102 is changed to alanine (YB-1A102), enhances the growth of breast cancer cells both in monolayer and anchorage-independent conditions (Sutherland et al. 2005). It was further revealed that YB-1 co-expresses with two epidermal growth factor receptors, EGFR and Her-2, and that YB-1, but not YB-1A102, up-regulates the expression of these receptors. Since EGFR and Her-2 are two important markers for breast carcinoma, it was proposed that YB-1 promotes breast cancer growth by stimulating the expression of EGFR and Her-2 (Wu et al. 2006). In further support of this observation, the expression of YB-1 in immortalized breast epithelial cells causes enhanced cell growth, which correlates with the induction of EGFR (Berquin et al. 2005). It can therefore be generally stated that YB-1 is essential for the growth of breast cancer cells. In further support of this, it was discovered by several groups that YB-1 is over-expressed in breast carcinoma and it is associated with tumor aggressiveness, relapse and poor survival (Bargou et al. 1997; Rubinstein et al. 2002; Janz et al. 2002, Wu et al. 2006).
Much of the work on YB-1 in cancer is centered around its role as a transcription factor while less is known about how this factor affects translation. As a transcription factor, YB-1 binds to the inverted CCAAT element in the Y-box (Didier et al. 1988; Goldsmith et al. 1993; Norman et al. 2001; Jürchott et al. 2003). YB-1 is also able to recognize Y-boxes without the consensus inverted CCAAT box (Mertens et al. 1997; Higashi et al. 2003; Lasham et al. 2003), and sequences flanking the Y-box have been suggested to contribute to YB-1:DNA interactions (Didier et al. 1988; Norman et al. 2001). YB-1 up-regulates the transcription of CYCLIN A (Jürchott et al. 2003), CYCLIN B1 (Jürchott et al. 2003), TOPOISOMERASE II α (Shibao et al. 1999), and DNA POLYMERASE α (En-Nia et al. 2005), implying that YB-1 enhances cell growth possibly by promoting both cell cycle progression and DNA replication. It was further demonstrated by Swamynathan et al. that cells with targeted disruption of one allele of YB-1 displayed defects in cell cycle and reduction in cell growth (Swamynathan et al. 2002). A role for YB-1 in DNA replication, on the other hand, is not as clear. Although it has been suggested that YB-1 interacts with the proliferating cell nuclear antigen (PCNA; Ise et al. 1999) and DNA polymerase δ (Gaudreault et al. 2004), whether YB-1 is part of the DNA replication holoenzyme needs further investigation.
Due to its role in cell growth, YB-1 is implicated in cancer pathogenesis. In addition to inducing the growth-promoting genes described above, YB-1 was shown to control the expression of phosphatase PTP1B (Fukada et al. 2003), matrix metalloproteinase-2 (MMP-2) (Mertens et al. 1997; Mertens et al. 2002), matrix metalloproteinase-12 (MMP-12) (Samuel et al. 2005), collagen α 1(I) (Norman et al. 2001), and collagen α 2(I) (Higashi et al. 2003; Dooley et al. 2006), all of which are involved in cell adhesion, motility, invasion and thus metastasis. The transcriptional activity of YB-1 on MMP-2 was demonstrated to be dependent on the interaction with two other transcription factors, namely the activating protein-2 (AP-2) and p53 (Mertens et al. 2002). Furthermore, YB-1 promotes the expression of several players in drug resistance, including p-glycoprotein encoded by the multi-drug resistance gene (MDR1) (Goldsmith et al. 1993; Stein et al. 2001), multi-drug resistance-related protein-1 (MRP 1) (Stein et al. 2001), as well as the major vault protein (MVP) (Stein et al. 2005). The ability to regulate genes related to metastasis and drug resistance suggests that YB-1 may be a marker for tumor aggressiveness and may predict poor response to chemotherapies.
Does the expression of YB-1 change the way cancer cells respond to therapy?
Overcoming drug resistance is key in treatment of many chronic diseases including malignancies. The ATP-binding cassette (ABC) transporters are a family of membrane proteins that are responsible for efflux of a variety of compounds out of the cell in an energy dependent manner. Although this mechanism is protective in terms of reducing toxins within cells, it also causes drug resistance by eliminating clinically useful drugs. Multidrug resistance-1 (MDR-1) gene codes for p-glycoprotein (an ABC transporter) believed to be responsible for multidrug resistance in cells (Choudhuri et al. 2006). The MDR-1 gene is often over-expressed after chemotherapy, either via gene activation or gene amplification, in various human tumors. The MDR-1 gene over-expression is observed in various malignancies including breast cancer, ovarian cancer, osteosarcoma, and synovial sarcoma. YB-1 is involved in MDR-1 gene activation in response to genotoxic stress (Ohga et al. 1998). Antisense to YB-1 sensitizes cells to cisplatin, mitomycin C and UV radiation, but not to vincristine, doxorubicin, campothecin, or etoposide (Ohga et al. 1996). Moreover, mouse embryonic stem cells expressing heterozygous YB-1 are hypersensitive to cisplatin and mitomycin C, suggesting that YB-1 has a protective capacity against cytotoxic effects of DNA damaging agents, demonstrating the involvement of YB-1 in drug resistance (Shibahara et al. 2004). Furthermore, YB-1 binds to cisplatin-damaged DNA and interacts with PCNA, a protein involved in DNA repair. Thus YB-1 may be important in DNA repair or in directing the response of the cells to DNA damage. It has also been proposed that cisplatin may activate MDR1 gene expression by stimulating YB-1 to gain access to the MDR-1 promoter (Ise et al. 1999). YB-1 is therefore considered to be important in mediating drug resistance.
Several studies demonstrate that the expression of YB-1 is particularly prevalent in hormone-dependent cancers such as those arising in the breast, ovary and prostate. A common theme between these studies is that YB-1 is notably found in the nuclear compartment and that it promotes drug resistance presumably by inducing genes that render cancer cells resistant to therapy. YB-1 positively regulates transcription of MDR-1, and in a number of malignancies YB-1 levels are closely associated with the expression of p-glycoprotein. In breast cancer, nuclear localization of YB-1 is associated with MDR-1 gene expression and drug resistance, while cytoplasmic localization of YB-1 is associated with drug sensitivity (Bargou et al.1997). The chemotherapy failure in breast cancer patients is associated with resistance, either acquired or intrinsic (primary resistance), of tumor cells. Furthermore, high levels of YB-1 protein expression are significantly associated with poor patient outcome. Breast cancer patients receiving post-operative chemotherapy with a high YB-1 expression had a 66% relapse rate after 5 years, compared to 0% relapse rate in those with low YB-1 expression (Janz et al. 2002). Therefore, early identification of patients at high risk for relapse is important and it would be of great clinical significance to have markers to predict which patients would respond to chemotherapy (i.e. chemosensitivity markers). In addition, aggressive breast cancer tumors that are both ER negative and lymph node positive are associated with high YB-1 expression. Breast cancer patients that were on a chemotherapy regimen containing anthracycline, a p-glycoprotein substrate, developed recurrence and had higher YB-1 levels compared to those treated with regimens containing non- p-glycoprotein substrate regimens (cyclophosphamide/methotrexate/5-Fluorouracil). Therefore YB-1 expression aside from being a marker of chemoresistance, may be clinically significant in aiding selection of the appropriate chemotherapy regimen (Huang et al. 2005). In serous ovarian carcinomas, the expression of YB-1 in the nucleus is a useful prognostic marker as the five-year survival curve for patients with positive nuclear YB-1 tumors was significantly worse than those with YB-1 negative tumors (Kamura et al. 1999). Co-expression of YB-1 and p-glycoprotein may reflect the resistance of ovarian cancer to chemotherapy and account for its unfavorable prognosis (Huang et al. 2004). Cisplatin resistant ovarian cancer cells have much higher YB-1 nuclear expression compared to drug sensitive parental cells. Therefore the expression of nuclear YB-1 is associated with acquired cisplatin resistance and might be used as a predictive marker for determining which patients with recurrent or persistent ovarian cancers will benefit from cisplatin based chemotherapy (Yahata et al. 2002). Finally, in the prostate, YB-1 over-expression, as well as, increased in p-glycoprotein levels is observed during malignant transformation. In addition to drug resistance, increased p-glycoprotein activity leads to lower androgen levels in the tumor cells leading to an adaptive response and androgen independence (Gimenez-Bonafe et al. 2004). YB-1 may therefore be of prognostic value in prostate cancer.
Similar to the examples cited in hormone-dependent malignancies, YB-1 has also been linked to drug resistance in colon, lung, muscle and thyroid cancers. Nuclear YB-1 expression in non-small cell lung cancer and squamous cell carcinoma correlated significantly with tumor size, lymph node metastasis, stage of disease, and poor prognosis compared to those with cytoplasmic YB-1 expression. This correlation was not observed in adenocarcinomas of the lung (Shibahara et al. 2001). YB-1 has been shown to be elevated in colorectal carcinomas, but there is no correlation with MDR-1 expression (Shibao et al. 1999). In synovial sarcoma, nuclear expression of YB-1 protein is associated with p-glycoprotein expression, and is an independent prognostic factor (Oda et al. 2003). Also, YB-1 may be a prognostic marker for multidrug resistance in osteosarcoma as its nuclear localization is associated with the expression of p-glycoprotein (Oda et al. 1998). In contrast to the abovementioned carcinomas with predominantly nuclear YB-1 over-expression, it is mainly located in the cytoplasm of anaplastic thyroid carcinoma. This suggests a role in transformation by regulating translation as well as transcription (Ito et al. 2003). Interestingly, anaplastic carcinomas also more frequently express the p-glycoprotein compared to differentiated carcinomas of the thyroid and benign adenomas, once again signifying the association between YB-1and MDR-1 expression (Sugawara 1994; Sugawara 1995). Collectively these studies indicate that YB-1 is likely to be very important in promoting the expression of a milieu of genes involved in drug resistance which could explain why its expression is so firmly associated with relapse and poor overall survival rates.
Why is YB-1 over-expressed in cancer?
From the onset it should be stated that little is known about the mechanism(s) behind the over-expression of YB-1 in cancer. One could postulate that this occurs due to gene amplification. YB-1 resides on chromosome 1p34, and genetic alterations in 1p are found in lung, colon and breast (Henderson et al. 2005). Upon closer inspection, fine mapping of 1p34 from lung cancer cell lines reveals that the region commonly amplified is at 1p34.2, the loci for myc (Kim et al. 2006) whereas YB-1 resides at 1p34.1. Similarly, amplifications are reported in lung cancer at 1p34.3 to 1p34.4 (Henderson et al. 2005) but again not at the YB-1 loci. Likewise, we find that YB-1 is not amplified in breast cancers using comparative genomic hybridization (submitted). Furthermore, there is no evidence for gene amplification at 1p34 (Shadeo et al. 2005) from breast cancer cell lines that are known to express YB-1. Thus, while YB-1 is highly expressed in breast cancer it does not appear to be due to a gain in copy number. Alternatively, it could be due to transcriptional activation.
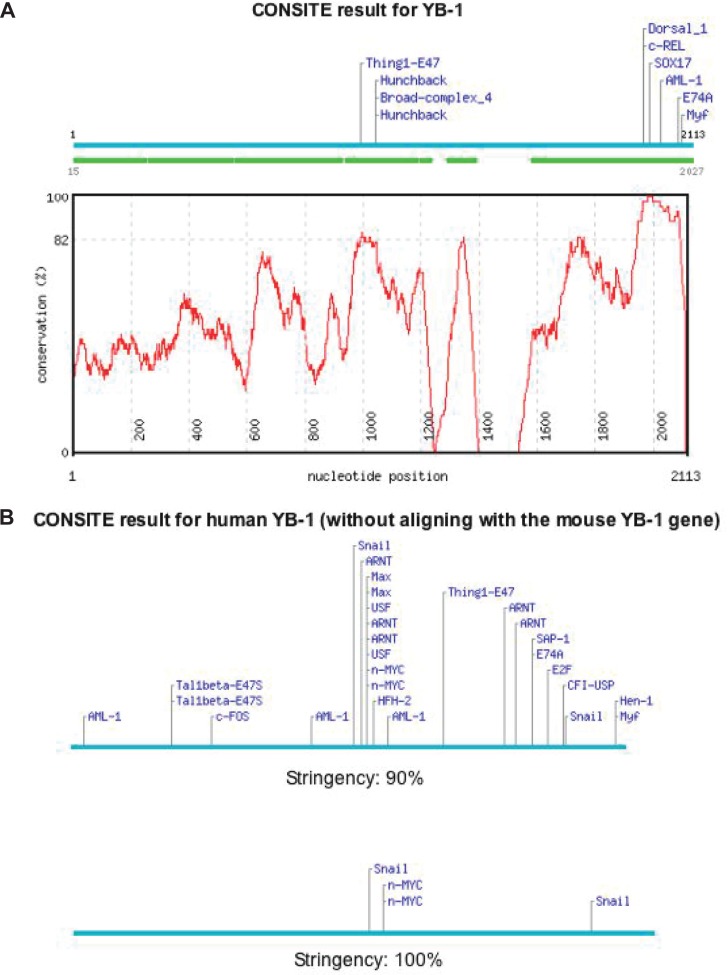
To date, very few studies have addressed the transcriptional regulation of the YB-1 promoter. In one report, multiple E-boxes and GF-boxes on the YB-1 gene promoter are bound by c-Myc, which interacts with p73 to regulate YB-1 expression (Uramoto et al. 2002). To further explore potential regulatory elements on the YB-1 promoter we retrieved the first -2kB from the UCSC genome browser (http://genome.ucsc.edu/; YBX1 RefSeq NM 004559) and analyzed it by CONSITE (http://mordor.cgb.ki.se/cgi-bin/CONSITE/consite). A particular strength of using CONSITE to predict regulatory elements is that it integrates promoters across species to generate the most plausible candidates (Sandelin et al. 2004). Because YB-1 is so highly conserved across species, we felt this was a good approach. When the YB-1 promoter (−2kB region) is compared across species, with a 90% stringency cut-off, multiple binding sites are revealed such as AML-1, rel and hunchback. If only the human YB-1 promoter is taken into consideration, it appears that there are several candidates worthy of mention including MAX, ARNT, and FOS using 90% as the cut-off. When the stringency is increased to 100% only N-Myc and Snail are predicted regulatory sites on the YB-1 promoter (Figure 2B). All three members of the Myc family, C, N and L-Myc, play important roles in the regulation of cell growth through their action as transcriptional activators when dimerized with MAX. The Myc:MAX complex binds to E-boxes (CA(T/C)GTG) in the promoter of many genes (Claassen et al. 1999; Watson et al. 2002). It is thought that N-Myc and C-Myc are redundant proteins differing only in the pattern of expression. C-myc is expressed in both embryonic and adult tissues whereas N-myc expression is predominantly restricted to embryonic tissues that do not express c-myc (Hurlin, 2005). Following this, a wide range of cancers such as Burkitt’s lymphoma (Dave et al. 2006), prostate cancer (Fleming et al. 1986) and breast cancer (Rodriguez-Pinilla et al. 2007) involve c-myc dysregulation whether this is through constitutive expression following chromosomal translocation, amplification or over-expression. In contrast, N-Myc is predominantly associated with neuroblastoma and other tumors of embryonal origin (Schuldiner et al. 2001; Hurlin, 2005) with approximately 25% of the former presenting with over-expressed or amplified N-myc. Increased copy number is particularly associated with poor prognosis and rapid tumor progression (Brodeur et al. 1989; Brodeur, 2003). In addition to Myc, Snail may also regulate the expression of YB-1 based on our analyses. Snail belongs to a superfamily of zinc-finger transcriptional repressors that bind to a six base sequence, CAGGTG, known as the E-box (Batlle et al. 2000). It is involved in cell movement during embryogenesis but more importantly when discussing cancer Snail has been implicated in promoting epithelial-mesenchymal transition. It is thought that this phenotype arises from the suppression of E-cadherin by Snail resulting in the loss of contact between neighbouring cells (Batlle et al. 2000; Cano et al. 2000). Work by Moody et al. has demonstrated that Snail over-expression can promote recurrence of primary breast tumors in mice and is associated with decreased patient disease free survival time in all patient groups (Moody et al. 2005). While N-Myc and Snail are potential leads to explain how YB-1 is regulated in cancer they are still at this time speculative. Not withstanding, mechanisms that control YB-1 expression remain largely unknown.
Figure 2.
Putative YB-1 regulatory sites were predicted using CONSITE. (A) The YB-1 promoter (−2kB) was evaluated for potential regulatory elements using CONSITE against all conserved species where the stringency was set at 90%. (B) Alternatively, if only the human database was used, at a 90% cut-off, additional regulatory elements were identified. Finally, evaluating the human database with 100% stringency revealed that N-Myc and Snail binding sites were present.
Once YB-1 is induced in cancer, it opens the question as to how it is post-translationally regulated. Many transcription factors are indeed regulated by signal transduction. Thus, does YB-1 then take the marching orders RTK’s to promote tumor growth and/or resistance to therapy?
How does signal transduction hardwire YB-1 to the genome?
There is no doubt that deregulation of YB-1 results in breast tumor pathogenesis; however, the underlying mechanisms remain unclear. In order to gain more insight into the post-translational regulation of YB-1, our lab took a bioinformatic approach using Motif Scanner to examine putative proteins that may phosphorylate and thus affect the activity of YB-1 (Table 1). As shown in Table 1, many of the proteins that may phosphorylate YB-1 are players in the cancer growth and survival signaling pathways. To focus on how YB-1 may be activated in cancer cells, we will discuss proteins or kinases that are in 1) PI3K/Akt, 2) Ras/MAPK and 3) PKC signaling cascades as these pathways are implicated in the pathogenesis and/or growth regulation of cancer.
Table 1.
Putative proteins that may phosphorylate YB-1 at Ser, Thr, and Tyr suggested by the Motif Scanning Prediction Tool.
| YB-1 Domain | Residue | Pitative Regulatory Protein | Percentile1 |
|---|---|---|---|
| AP-rich N-Term | Ser 3 | Casein Kinase 2 | 3.764 |
| Thr 7 | DNA PK | 0.846 | |
| ATM kinase | 4.202 | ||
| Ser 21 | GSK3 Kinase2 | 3.336 | |
| Thr 29 | PKC δ | 0.806 | |
| Ser 32 | Casein Kinase 1 | 4.315 | |
| Ser 36 | Erk 1 Kinase 2 | 3.248 | |
| cold shock domain | Thr 62 | PKC δ | 2.773 |
| PKC α/β/γ | 4.452 | ||
| PKC θ | 4.964 | ||
| Thr 80 | DNA PK | 2.965 | |
| Thr 89 | Calmodulin dependent kinase2 | 2.786 | |
| PKC θ | 4.964 | ||
| Ser 102 | Akt kinase3 | 2.703 | |
| Ser 102 | PKC ε | 3.527 | |
| Glu 107 | PDZ (nNOS)class 3 | 1.003 | |
| Thr 108 | DNAPK | 3.529 | |
| Casein kinase 2 | 3.764 | ||
| c-term | Tyr 162 | Grb2 SH2 | 2.274 |
| Ser 167 | ATM kinase | 4.812 | |
| Ser 176 | 14-3-3 | 1.029 | |
| Casein kinase 2 | 2.023 | ||
| Tyr 197 | Shc PTB | 3.945 | |
| p85 SH2 | 4.884 | ||
| Tyr 208 | Abl SH2 | 0.292 | |
| Crk SH2 | 1.209 | ||
| Itk SH2 | 2.124 | ||
| Nck SH2 | 2.092 | ||
| Grb2 SH2 | 2.983 | ||
| Thr 271 | DNA PK | 1.761 | |
| ATM kinase | 2.342 | ||
| Tyr 287 | Shc PTB | 3.170 | |
| Ser 314 | Casein kinase 2 | 2.775 |
Given by the Motif scanning prediction tool, the percentile tells how the protein ranks by comparing with all vertebrate proteins in Swiss-Prot, the sequence surrounding that site, and the solvent accessibility at that position.
Phosphorylation of YB-1 by GSK3 and ERK2 has been demonstrated by Coles et al. 2005.
Phosphorylation of YB-1 by Akt has been shown by Sutherland et al. 2005 and Evdokimova et al. 2006.
PI3K/Akt and YB-1
Several components of the PI3K/Akt signaling pathway may be responsible for YB-1 phos-phorylation as shown in Table 1. In the first case, we observed that YB-1 could potentially be phosphorylated by Akt at S102. This became of great interest to us given that the PI3K/Akt pathway is frequently deregulated or constitutively activated in cancer. Activated Akt is often expressed in breast cancer but not normal breast epithelial cells (Kucab et al. 2004). It is also able to transform breast epithelial cells into malignant phenotypes (Zhao et al. 2003; Zhang et al. 2003). Co-expression of constitutively active Akt1 and mutant polyomavirus T antigen resulted in mammary tumor formation in nude mice (Hutchinson et al. 2001). Exogenous expression of Akt1 enhanced breast cancer cell growth both in monolayer and soft agar assays (Ahmad et al. 1999), illustrating the ability of Akt to promote tumor progression. Moreover, clinical studies have demonstrated a relationship between Akt and relapse, distant metastasis (Perez-Tenorio et al. 2002; Schmitz et al. 2004), and poor prognosis (Bellacosa et al. 1995). By screening primary tumor tissue micro-arrays, we found that activated or phosphorylated Akt (p-Akt) was associated with YB-1 in breast cancer (Sutherland et al. 2005). We then demonstrated that Akt phosphorylates YB-1 at S102 in the CSD, by in vitro GST pull-down experiments and kinase assays (Sutherland et al. 2005). It was subsequently shown that IGF-1 stimulation led to YB-1(S102) phosphorylation in NIH3T3 cells. Wortmannin, which is a PI3K inhibitor, fully blocked phosphorylation of YB-1 (Evdokimova et al. 2006). More recently, studies in ovarian cancer demonstrate that inhibiting the PI3K/Akt pathways with LY294002 suppresses nuclear translocation in vitro and in mice (Baski et al. 2006). This is in contrast to the study in which YB-1 suppresses Akt-mediated oncogenic transformation of chicken embryo fibroblasts by inhibiting protein synthesis (Bader et al. 2003; Bader et al. 2005). The interpretation of this study would indicate that YB-1 serves as a tumor suppressor gene, which is contrary to the amassed studies indicating that it is rather an oncogene. We suspect that the Akt/YB-1 relationship in avian mesenchymal cells may be different from how this complex works in epithelial cancer cells.
Phosphorylation of YB-1 by Akt could affect this transcription factor by altering nuclear trafficking, DNA binding and/or translation. Inhibition of YB-1(S102) phosphorylation by site directed mutagenesis leads to an attenuation of nuclear translocation (Sutherland et al. 2005). This could explain why treating ovarian cancer cells with wortmannin or an integrin-linked kinase inhibitor, both of which inhibit activation of Akt, suppressed the nuclear trafficking of YB-1 (Baski et al. 2006). Therefore, nuclear translocation of YB-1 may be controlled by S102 phosphorylation via the PI3K/Akt pathway. YB-1 was shown to have a noncanonical NLS as well as a cytoplasmic retention site (CRS) in the C-terminal domain (Bader et al. 2005). It has been suggested by Jürchott et al. that both the C-terminus and the CSD are involved in YB-1 nuclear shuttling (Jürchott et al. 2003). It seems reasonable to postulate that S102 in the CSD cooperates with NLS and/or CRS in YB-1 nuclear trafficking. For example, phosphorylation of S102 may induce YB-1 conformational change, which masks the CRS and/or reveals the NLS. A second possibility is that phosphoryation affects DNA binding. Our lab found that while YB-1 bound to the first −2 kB of the EGFR promoter, YB-1(A102) was not able to interact with the first −1 kB promoter region (Wu et al. 2006). One may argue that disruption of S102 blocked nuclear translocation of YB-1 thus prevented the binding of YB-1 to the EGFR promoter. However upon careful inspection, mutation of S102 to alanine did not completely block nuclear shuttling of YB-1 (Sutherland et al. 2005). In fact, there was still approximately 50% of YB-1(A102) detected in the nucleus. Abolishment of S102 almost fully prevented Flag:YB-1(A102) from binding to the −1 kb of EGFR promoter (Wu et al. 2006). Therefore, we suggest that in addition to nuclear translocation, S102 is also important for enabling YB-1 to bind to DNA. A third possibility is that phosphorylation promotes translation of the oncogenome. It was recently demonstrated that phosphorylation of YB-1 at S102 by Akt prevented YB-1 from binding to the capped 5′ terminus of mRNA thereby promoting the translation of oncogenes such as IGF-1, VEGF and FOS (Evdokimova et al. 2006). It has also been shown that release of YB-1 from mRNA results in nuclear localization (Stenina et al. 2001). Thus, YB-1 phosphorylation by Akt allows latent oncogenic transcripts to enter into the translational machinery (Evdokimova et al. 2006). This further disputes the idea that YB-1 is somehow working as a tumor suppressor gene (Bader et al. 2003). Thus, Akt is the first kinase to be shown to regulate YB-1 post-translationally and its consequences have broad implications in the regulation of gene expression in cancer cells.
The motif scanner also predicted that the p85 subunit of PI3K may be able to phosphorylate YB-1 at Tyr197 of the C-terminal domain, possibly to alter the interaction between YB-1 and other proteins. Curiously, Tyr197 is located within the NLS (residue 183–202) (Bader et al. 2005) of YB-1, which raises the question whether YB-1 subcellular localization is affected by phosphorylation at Tyr197 by PI3K. This could explain why the PI3K inhibitor wortmannin completely abolished YB-1 phosphorylation induced by IGF-1 stimulation, whereas mutation of S102 to alanine was not able to fully prevent YB-1 phosphorylation (Evdokimova et al. 2006). The glycogen synthase kinase 3 (GSK3), which is negatively regulated by Akt (Diehl et al. 1998), could also potentially phosphorylate YB-1 at Ser21 in the N-terminal domain and affect the transcriptional activity of YB-1 (Table 1). It has been demonstrated by Coles et al. that GSK3β phosphorylated the N-terminus of YB-1, enhancing the ability of YB-1 to repress the vascular endothelial growth factor (VEGF) promoter (Coles et al. 2005). This study also implies the involvement of Akt/GSK3β/YB-1 in tumor angiogenesis. Taken together, it is expected that YB-1 phosphorylation via PI3K/Akt signaling would exert a profound effect on tumor progression.
Ras/MAPK and YB-1
Since MAPK/ERK signaling is often elevated in malignancies including breast cancer (Sivaraman et al. 1997; El-Ashry et al. 1997; Coutts and Murphy 1998; Donovan et al. 2001), it is motivating to examine whether components of this pathway also regulate YB-1 phosphorylation. Based on the data generated from the Motif Scanner, Shc and Grb2 may phosphorylate YB-1 at several tyrosine residues in the C-terminal domain. These are both adaptor proteins that transduce signals from receptor tyrosine kinases to MAPK. Moreover, the Ser36 residue located in the transactivating N-terminal domain of YB-1 may be a target for the extracellular-signal-regulated kinase 1 (ERK1), which is activated by MAPK in response to growth factor stimulation resulting in cell proliferation and survival (Chang and Karin 2001, Sebolt-Leopold et al. 2004). In a study conducted by Coles et al. it was demonstrated that ERK2 phosphorylated YB-1 in the N-terminal domain, promoting the binding of YB-1 to the VEGF promoter and VEGF expression (Coles et al. 2005). The discovery that ERK2 phosphorylates YB-1 leading to VEGF expression again implies that YB-1 also plays a role in angiogenesis to enhance the micro-environment for tumor growth. Since a recent report overrode the conventional idea that ERK1 and ERK2 play similar functions (Vantaggiato et al. 2006), one should validate whether ERK1 also phosphorylates YB-1, leading to VEGF expression.
PKC and YB-1
Many isoforms of protein kinase C (PKC) are predicted by Motif Scanner to regulate YB-1. For example, the classical PKCs (α, β, and γ), whose activation is dependent on Ca2+ and diacylglycerol (DAG) (Nishizuka et al. 1992; Nishizuka et al. 1995; Zugaza et al. 1996; Paolucci et al. 1999), may phosphorylate Thr62 in the CSD of YB-1. The novel PKCs (δ, ε, θ), which are activated by DAG (Nishizuka et al. 1992; Nishizuka et al. 1995; Zugaza et al. 1996; Paolucci et al. 1999), could potentially phosphorylate both the N-terminal domain and the CSD of YB-1. In clinical studies, PKC activities have been shown to be elevated in breast tumor tissues (O’Brian et al. 1989; Gordge et al. 1996). Cell-model based and in vitro studies also demonstrated the relationship between different PKC isoforms and cancers. It has been shown by several studies that increased PKCα is associated with increased motility and invasion of cancer cells while inhibition of PKCα reverses the phenotype (Engers et al. 2000; Masur et al. 2001; Parsons et al. 2002; Podar et al. 2002; Koivunen et al. 2004). PKCβ is suggested to have similar functions as PKCα in cancer cells and is thought to enhance invasion and proliferation of cancer cells (Schwartz et al. 1993; Sauma et al. 1996; Xia et al. 1996; Murray et al. 1999; Yoshiji et al. 1999; Jiang et al. 2004; Zhang et al. 2004). PKCε also seems to stimulate tumor development and metastasis (Akita, 2002). PKCδ, on the other hand, is thought to promote apoptosis and is thus suggested to be a tumor suppressor (Majumder et al. 2000; Sun et al. 2000; Basu et al. 2001; Blass et al. 2002; Johnson et al. 2002; Ren et al. 2002; Abbas et al. 2004; Jiang et al. 2004). Although PKCδ is considered a tumor suppressor, it was reported that PKCδ is increased in highly metastatic mammary tumor cell lines (Kiley et al. 1999). Interestingly, PKC has been suggested to play a role in the development of estrogen receptor negative (ER(−)) breast cancers. For instance, studies have illustrated that ER(−) breast cancer cell lines such as MDA-MB-231 and MDA-MB-435 express high level of PKCα (Borner et al. 1987; Platet et al. 1998). Moreover, ectopic expression of PKCα caused MCF-7 cells to become ER(−). Importantly, expression of PKCα has been associated with tamoxifen resistance in breast cancer patients (Tonetti et al. 2003). It should be pointed out that the expression of YB-1 is also associated with ER(−) breast cancers (Wu et al. 2006). Although there is currently no data demonstrating a functional link between PKC and YB-1, it would be intriguing to investigate whether this potential pathway cooperates to alter the ER status and thus increase breast cancer aggressiveness.
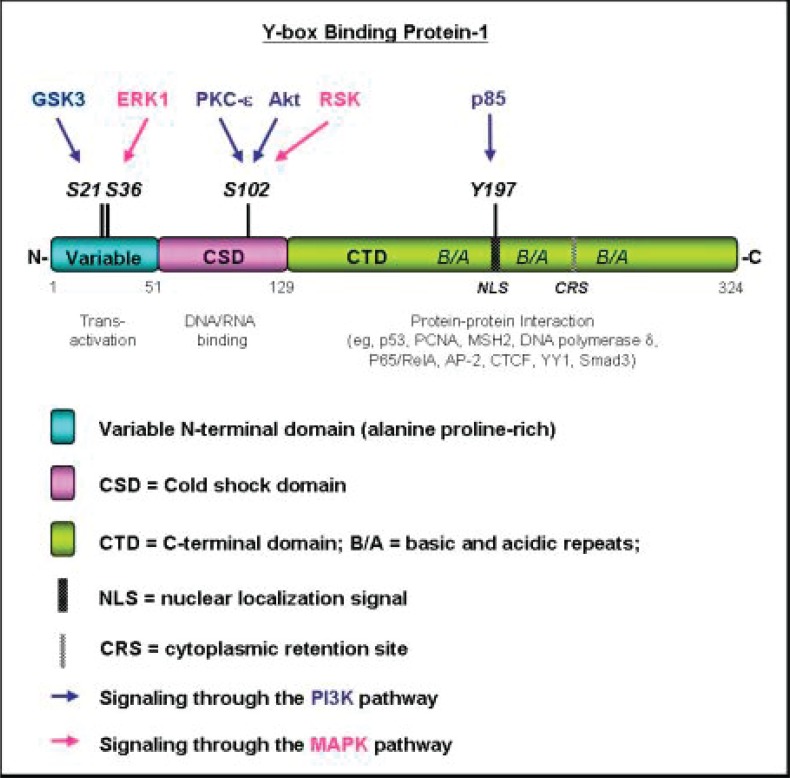
In light of the possible phosphoryation sites on YB-1, which could be mediated by the PI3K and MAP kinase pathways, we turn now to the question of how this could impact its function (Figure 3). Our initial analysis using Motif Scanner revealed that YB-1 could be potentially phosphorylated by Akt at S102, which we went on to show impacted nuclear trafficking (Sutherland et al. 2005) and more profoundly DNA binding (Wu et al. 2006). It was subsequently reported that S102 phosphorylation also decreases YB-1’s ability to act as a transcriptional repressor (Evdokimova et al. 2006). It should be pointed out that PKC and RSK also have the potential to phosphorylate S102 given that they also recognize the RxRxxS/T binding site on proteins. Additionally, we suggest that GSK and ERK1 have the potential to phosphorylate the transactivating domain at S21 and S36 respectively. Finally, the p85 subunit of PI3K could phosphory-late YB-1 at Y197, which is located in the nuclear localization signal therefore cellular trafficking may be affected (Figure 3).
Figure 3.
Summary of where signaling pathways potentially phosphorylate YB-1 and how these post-translational changes could impact its function. In this review, we have highlighted the fact that YB-1 is phosphorylated by Akt at S102 which occurs in the CSD. The consequence of S102 phosphorylation is a change in DNA binding. It also impact YB-1’s ability to regulate translation. Because PKC and RSK also have the potential to phosphorylate S102 it is conceivable that they too could alter YB-1’s transcriptional and translational capacities. GSK and ERK1 have the potential to phosphorylate YB-1 at S21 and S36 respectively, which could alter YB-1’s ability to transactivate. Alternatively the p85 subunit of PI3K could regulate YB-1 by phosphorylating Y197, which resides within the nuclear localization sequence. It is therefore possible that Pi3K could phosphorylate YB-1 and thereby stimulate nuclear trafficking.
Is YB-1 a molecular target for cancer therapy?
The expression of YB-1 in aggressive types of cancer and the evidence for a role in cell proliferation calls into question its potential as therapeutic target for treatment. There are numerous signal transduction inhibitors (STI’s) that could inhibit YB-1 indirectly by blocking the activity of upstream components of, for example, the PI3K pathway (Granville et al. 2006). Akt inhibitors could potentially be used to inhibit YB-1 activity by suppressing S102 phosphorylation. Likewise, preventing Akt activation through phosphoinositide-dependent kinase-1 inhibition (PDK-1) (Kubab et al. 2005; Crowder et al. 2005) is also a possible way of ultimately suppressing YB-1’s function. The PDK-1 inhibitor OSU03012 is an excellent candidate because it inhibits Akt activation (Kucab et al. 2004), does not result in overt toxicity in mice and it can be given orally. Rapamycin, a clinically approved immunosuppressant that inhibits the mammalian target of rapamycin (mTOR) pathway, has recently been shown to reduce the level of phosphorylated YB-1 (Evdokimova et al. 2006) and could therefore suppress the growth promoting effects. Alternatively, direct inhibition of molecules such as YB-1 is now possible either by designing small molecule inhibitors or developing molecular techniques so that they can be used in the clinic. By knocking down YB-1 using siRNA we previously reported that the growth of breast cancer cells is inhibited by 48% (Wu et al., 2006). This is a promising result that could translate into the clinic by silencing YB-1 using either antisense or small interfering RNA’s. Clinical trials are underway using antisense to inhibit BCL-2 (Chi et al. 2001; Tolcher et al. 2005) and clusterin (OGX-011) (Miyake et al. 2000; Chi et al. 2005; So et al. 2005). There are now many examples of how small interfering RNA’s can be used to slow the growth of cancer cells although the clinical development of this technology is still emerging. Recently, (shRNA) short hairpin RNA’s targeting survivin were expressed in a lentiviral vector (Jiang et al. 2006) and tested in a model of oral squamous cell carcinoma where this target is highly expressed. The loss of survivin using siRNA sensitized the cells to chemotherapy in vitro and inhibited tumor growth in mice (Jiang et al. 2006). The first clinical trials using siRNA have begun, however they are not yet being applied in the field of oncology, rather to silence the vascular endothelial growth factor (VEGF) in age-related macular degeneration (Grunweller et al. 2005). It is therefore within reach that shRNA could be used to treat other diseases such as cancer. We propose therefore that tumor growth could be inhibited by disrupting YB-1 either directly or indirectly using STI’s.
Taking a completely different approach, one might consider using the expression of YB-1 to drive the replication of oncolytic viruses as a way of treating cancer. It has been known for some time that YB-1 facilitates the replication of adenoviruses (Holm et al. 2002), which then can be used to kill tumor cells (Holm et al. 2004; Glockzin et al. 2006). A recent study has shown that infecting drug resistant cells with a YB-1 associated adenovirus can resensitize the cancer cells to chemotherapeutic agents therefore providing opportunities to use combination therapy or “Mutually Synergistic Therapy” (Mantwill et al. 2006) and prolong the period of time in which some patients may be treated. The expression of YB-1 in basal-like and Her-2 over-expressing breast cancers (submitted data) also provides an excellent opportunity for using oncolytic viruses for therapy. While these gene-based approaches are promising they are still limited by bioavailability, formulations, safety and the expense of making the products. As it is unlikely a single agent will provide us with the most effective treatment it may also be beneficial to assess rational combination therapies like the one described above. As we have discussed here there are multiple opportunities to inhibit the function of YB-1 or even use YB-1 activity to treat cancer.
Concluding Remark
While the role(s) of YB-1 have been extensively studied in cancer, the upstream signaling that regulates this fascinating oncoprotein remains unclear. The Motif Scanner serves as a useful preliminary tool to select putative kinases that may phosphorylate YB-1. To date only the relationship between YB-1 and PI3K/Akt pathway in breast cancer has been established (Sutherland et al. 2005; Evdokimova et al. 2006). Inhibiting the PI3K/Akt and thus YB-1 has been suggested to be a novel way to treat breast cancer (Wu 2006 et al.) as well as many other types of malignancies where this pathway is known to be activated. The evidence presented herein suggested that YB-1 may be regulated by multiple signaling cascades, many of which are hyper-activated in cancer. We therefore envisage that YB-1 hardwires aberrant signaling pathways to induce expression of the oncogenome through transcriptional and well as translational control (overview, Figure 4). It is now known that YB-1 is expressed in such a wide range of cancers affecting adults and children. Moreover, tumor cells depend upon YB-1 for growth; therefore it could be an excellent molecular target for cancer therapy.
Figure 4.
Schematic of the multiple functions of YB-1 in cancer cells. Signal transduction is initiated by growth factor such as IGF-1 and cytokines leading to the activation of kinases that could potentially phosphorylate YB-1. It is generally thought that YB-1 is phosphorylated by kinases such as AKT in the cytoplasm leading to nuclear trafficking and DNA binding. The phosphorylation of YB-1 can also alter its role in translation initiation, mRNA splicing and/or transport. In the nucleus, YB-1 binds to multiple genes involved in tumor cell growth by directly binding to inverted CAAT boxes. It also indirectly induces the expression of oncogenes by coupling to other transcription factors such as AP-1 and p53. YB-1 can thus induce the expression of oncogenes through transcriptional as well as translational control.
Acknowledgments
Funding for this review was made possible through a grant to SED from the Canadian Breast Cancer Research Alliance and the National Cancer Institute of Canada.
References
- Abbas T, White D, Hui L, et al. Inhibition of human p53 basal transcription by down-regulation of protein kinase Cdelta. J. Biol. Chem. 2004;279(11):9970–7. doi: 10.1074/jbc.M306979200. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Singh N, Glazer R. Role of Akt1 in 17β-estradiol- and insulin-like growth factor 1 (IGF-1)-dependent proliferation and prevention of apoptosis in MCF-7 breast carcinoma cells. Biochem Pharm. 1999;58:425–430. doi: 10.1016/s0006-2952(99)00125-2. [DOI] [PubMed] [Google Scholar]
- Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J. Biochem, (Tokyo) 2002;132(6):847–52. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- Ashizuka M, Fukuda T, Nakamura T, Shirasuna K, Iwai K, Izumi H, Kohno K, Kuwano K, Uchiumi T. Novel translational control through an iron-responsive element by interaction of multifunctional protein YB-1 and IRP2. Mol. Cell. Biol. 2002 Sep.22(18):675–6383. doi: 10.1128/MCB.22.18.6375-6383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AG, Felts KA, Jiang N, et al. box-binding protein 1 induces resistance to oncogenic transformation by the phosphati-dylinositol 3-kinase pathway. Proc. Natl. Acad. Sci., U.S.A. 2003;100(21):12384–9. doi: 10.1073/pnas.2135336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AG, Kang S, Zhao L, et al. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer. 2005;5(12):921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- Bargou RC, Jurchott K, Wagener C, et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 1997;3(4):447–50. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- Baski Y, Hosoi F, Oda Y, et al. Akt-dependent nuclear localization of Y-box binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene. 2006 doi: 10.1038/sj.onc.1210084. in press. [DOI] [PubMed] [Google Scholar]
- Basu A, Woolard MD, Johnson CL, et al. Involvement of protein kinase C-delta in DNA damage-induced apoptosis. Cell Death Differ. 2001;8(9):899–908. doi: 10.1038/sj.cdd.4400885. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, et al. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell. Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer. 1995;64(4):280–5. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- Bergmann S, Royer-Pokora B, Fietze E, et al. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005;65(10):4078–87. doi: 10.1158/0008-5472.CAN-04-4056. [DOI] [PubMed] [Google Scholar]
- Berquin I, Pang B, Dziubinski ML, et al. Y-box-binding protein confers EGF independence to mammary epithelial cells. Oncogene. 2005;24(19):3177–3186. doi: 10.1038/sj.onc.1208504. [DOI] [PubMed] [Google Scholar]
- Blass M, Kronfeld I, Kazimirsky G, et al. Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol. Cell. Biol. 2002;22(1):182–95. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C, Wyss R, Regazzi R, et al. Immunological quantitation of phospholipid/Ca2+-dependent protein kinase of human mammary carcinoma cells: inverse relationship to estrogen receptors. Int. J. Cancer. 1987;40(3):344–8. doi: 10.1002/ijc.2910400310. [DOI] [PubMed] [Google Scholar]
- Bouvet P, Matsumoto K, Wolffe AP. Sequence-specific RNA recognition by the Xenopus Y.-box proteins. An essential role for the cold shock domain. J. Biol. Chem. 1995;270(47):28297–303. doi: 10.1074/jbc.270.47.28297. [DOI] [PubMed] [Google Scholar]
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- Brodeur GM, Fong CT. Molecular biology and genetics of human neuroblastoma. Cancer genet and cytogenet. 1989;41(2):153–74. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moneno MA, Rodrigo I, et al. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell. Biol. 2000;2:74–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chi KN, Gleave ME, Klasa R, et al. A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clin. Cancer Res. 2001;7(12):3920–7. [PubMed] [Google Scholar]
- Chi KN, Eisenhauer E, Fazli L, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2′-methoxyethyl anti-sense oligonucleotide to clusterin, in patients with localized prostate cancer. J. Natl. Cancer Inst. 2005;97(17):1287–96. doi: 10.1093/jnci/dji252. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006;25(4):231–59. doi: 10.1080/10915810600746023. Review. [DOI] [PubMed] [Google Scholar]
- Claassen GF, Hann SF. Myc-mediated transformation: the repression connection. Oncogene. 1999;18:2925–33. doi: 10.1038/sj.onc.1202747. [DOI] [PubMed] [Google Scholar]
- Coles LS, Lambrusco L, Burrows J, et al. Phosphorylation of cold shock domain/Y-box proteins by ERK2 and GSK3beta and repression of the human VEGF promoter. FEBS Lett. 2005;579(24):5372–8. doi: 10.1016/j.febslet.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Coutts AS, Murphy LC. Elevated mitogen-activated protein kinase activity in estrogen-nonresponsive human breast cancer cells. Cancer Res. 1998;58(18):4071–4. [PubMed] [Google Scholar]
- Crowder RJ, Ellis MJ. Treating breast cancer through novel inhibitors of the phosphatidylinositol 3’-kinase pathway. Breast Cancer Res. 2005;7(5):212–214. doi: 10.1186/bcr1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s Lymphoma. New. Engl. J. Med. 2006;354(23):2431–42. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- Didier DK, Schiffenbauer J, Woulfe SL, et al. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y. box. Proc. Natl. Acad. Sci. U.S.A. 1988;85(19):7322–6. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, et al. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes., Dev. 1998;12(22):3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JC, Milic A, Slingerland JM. Constitutive MEK/MAPK activation leads to p27(Kip1) deregulation and antiestrogen resistance in human breast cancer cells. J. Biol. Chem. 2001;276(44):40888–95. doi: 10.1074/jbc.M106448200. [DOI] [PubMed] [Google Scholar]
- Dooley S, Said HM, Gressner AM, Floege J, En-Nia A, Mertens PR. Y-box bprotein is the critical mediator of antifibrotic interferon-gamma effects. J. Biol. Chem. 2006;28(3):1784–1795. doi: 10.1074/jbc.M510215200. [DOI] [PubMed] [Google Scholar]
- El-Ashry D, Miller DL, Kharbanda S, et al. Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene. 1997;15(4):423–35. doi: 10.1038/sj.onc.1201198. [DOI] [PubMed] [Google Scholar]
- Engers R, Mrzyk S, Springer E, et al. Protein kinase C in human renal cell carcinomas: role in invasion and differential isoenzyme expression. Br. J. Cancer. 2000;82(5):1063–9. doi: 10.1054/bjoc.1999.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- En-Nia A, Yilmaz E, Klinge U, et al. Transcription factor YB-1 mediates DNA polymerase alpha gene expression. J. Biol. Chem. 2005;280(9):7702–11. doi: 10.1074/jbc.M413353200. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Ovchinnikov LP, Sorensen PH. Y-box binding protein 1: providing a new angle on translational regulation. 2006 Jun;5(11):1143–1147. doi: 10.4161/cc.5.11.2784. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Ruzanov P, Anglesio MS, et al. Akt-mediated YB-1 phosphorylation activates translation of silent mRNA species. Mol. Cell. Biol. 2006;26(1):277–92. doi: 10.1128/MCB.26.1.277-292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faury D, Natel A, Dunn SE, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma. J Clin Oncol. 2007;25(10):1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- Fleming WH, Hamel A, MacDonald R, et al. Expression of the c-Myc protooncogene in human prostatic carcinoma and benign prostatic hyperplasia. Cancer Res. 1986;46(3):1535–8. [PubMed] [Google Scholar]
- Fukada T, Tonks NK. Identification of YB-1 as a regulator of PT.P.1B expression: implications for regulation of insulin and cytokine signaling. EMBO J. 2003;22(3):479–93. doi: 10.1093/emboj/cdg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault I, Guay D, Lebel M. YB-1 promotes strand separation in vitro of duplex DNA containing either mispaired bases or cisplatin modifications, exhibits endonucleolytic activities and binds several DNA repair proteins. Nucleic Acids Res. 2004;32(1):316–27. doi: 10.1093/nar/gkh170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Bonafe P, Fedoruk MN, Whitmore TG, et al. YB-1 is upregulated during prostate cancer tumor progression and increases P.-glycoprotein activity. Prostate. 2004;59(3):337–49. doi: 10.1002/pros.20023. [DOI] [PubMed] [Google Scholar]
- Glockzin G, Mantwill K, Jurchott K, et al. Characterization of the recombinant adenovirus vector AdYB-1: implications for oncolytic vector development. J. Virol. 2006;80(8):3904–11. doi: 10.1128/JVI.80.8.3904-3911.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith ME, Madden MJ, Morrow CS, et al. A Y.-box consensus sequence is required for basal expression of the human multidrug resistance (mdr1) gene. J. Biol. Chem. 1993;268(8):5856–60. [PubMed] [Google Scholar]
- Gordge PC, Hulme MJ, Clegg RA, et al. Elevation of protein kinase A and protein kinase C activities in malignant as compared with normal human breast tissue. Eur. J. Cancer. 1996;32A(12):2120–6. doi: 10.1016/s0959-8049(96)00255-9. [DOI] [PubMed] [Google Scholar]
- Granville CA, Memmott RM, Gills JJ, et al. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/Mammalian target of rapamycin pathway. Clin. Cancer Res. 2006;12(3):679–89. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- Graumann P, Marahiel MA. A case of convergent evolution of nucleic acid binding modules. Bioessays. 1996;18(4):309–15. doi: 10.1002/bies.950180409. [DOI] [PubMed] [Google Scholar]
- Grunweller A, Hartmann RK. RNA interference as a gene-specific approach for molecular medicine. Curr. Med. Chem. 2005;12(26):3143–61. doi: 10.2174/092986705774933489. Review. [DOI] [PubMed] [Google Scholar]
- Gu C, Oyama T, Osaki T, et al. Expression of Y. box-binding protein-1 correlates with DNA topoisomerase IIalpha and proliferating cell nuclear antigen expression in lung cancer. Anticancer Res. 2001;21(4A):2357–62. [PubMed] [Google Scholar]
- Hasegawa SL, Doetsch PW, Hamilton KK, et al. DNA binding properties of YB-1 and dbpA: binding to double-stranded, single-stranded, and abasic site containing DNAs. Nucleic Acids Res. 1991;19(18):4915–20. doi: 10.1093/nar/19.18.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Coe BP, Lee EH, et al. Genomic and gene expression profiling of minute alterations of chromosome arm 1p in small-cell lung carcinoma cells. Br. J. Cancer. 2005;92(8):1553–60. doi: 10.1038/sj.bjc.6602452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Inagaki Y, Suzuki N, et al. Y-box-binding protein YB-1 mediates transcriptional repression of human alpha 2(I) collagen gene expression by interferon-gamma. J. Biol. Chem. 2003;278(7):5156–62. doi: 10.1074/jbc.M208724200. [DOI] [PubMed] [Google Scholar]
- Holm PS, Bergmann S, Jurchott K, et al. YB-1 relocates to the nucleus in adenovirus-infected cells and facilitates viral replication by inducing E2 gene expression through the E2 late promoter. J. Biol. Chem. 2002;277(12):10427–34. doi: 10.1074/jbc.M106955200. [DOI] [PubMed] [Google Scholar]
- Holm PS, Lage H, Bergmann S, et al. Multidrug-resistant cancer cells facilitate E1-independent adenoviral replication: impact for cancer gene therapy. Cancer Res. 2004;64(1):322–8. doi: 10.1158/0008-5472.can-0482-2. [DOI] [PubMed] [Google Scholar]
- Huang J, Tan PH, Li KB, et al. Y-box binding protein, YB-1, as a marker of tumor aggressiveness and response to adjuvant chemotherapy in breast cancer. Int. J. Oncol. 2005;26(3):607–13. [PubMed] [Google Scholar]
- Huang X, Ushijima K, Komai K, et al. Co-expression of Y box-binding protein-1 and P-glycoprotein as a prognostic marker for survival in epithelial ovarian cancer. Gynecol. Oncol. 2004;93(2):287–91. doi: 10.1016/j.ygyno.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Hurlin PJ. N-Myc functions in transcription and development. Birth Defects Res. 2005;75:340–52. doi: 10.1002/bdrc.20059. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Jin J, Cardiff RD, et al. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol. Cell. Biol. 2001;21(6):2203–12. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise T, Nagatani G, Imamura T, et al. Transcription factor Y.-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 1999;59(2):342–6. [PubMed] [Google Scholar]
- Ito Y, Yoshida H, Shibahara K, et al. Y-box binding protein expression in thyroid neoplasms: its linkage with anaplastic transformation. Pathol. Int. 2003;53(7):429–33. doi: 10.1046/j.1440-1827.2003.01494.x. [DOI] [PubMed] [Google Scholar]
- Janz M, Harbeck N, Dettmar P, et al. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int. J. Cancer. 2002;97(3):278–82. doi: 10.1002/ijc.1610. [DOI] [PubMed] [Google Scholar]
- Jiang G, Li J, Zeng Z, et al. Lentivirus-mediated gene therapy by suppressing survivin in BALB/c nude mice bearing oral squamous cell carcinoma. Cancer, Biol. Ther. 2006;5(4):435–40. doi: 10.4161/cbt.5.4.2542. [DOI] [PubMed] [Google Scholar]
- Jiang XH, Tu SP, Cui JT, et al. Antisense targeting protein kinase C alpha and beta1 inhibits gastric carcinogenesis. Cancer Res. 2004;64(16):5787–94. doi: 10.1158/0008-5472.CAN-03-1172. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Lu D, Huang J, et al. Regulation of p53 stabilization by DNA damage and protein kinase C. Mol. Cancer, Ther. 2002;1(10):861–7. [PubMed] [Google Scholar]
- Jürchott K, Bergmann S, Stein U, et al. YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J. Biol. Chem. 2003;278(30):27988–96. doi: 10.1074/jbc.M212966200. [DOI] [PubMed] [Google Scholar]
- Kamura T, Yahata H, Amada S, et al. Is nuclear expression of Y. box-binding protein-1 a new prognostic factor in ovarian serous adenocarcinoma? Cancer. 1999;85(11):2450–4. doi: 10.1002/(sici)1097-0142(19990601)85:11<2450::aid-cncr21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kiley SC, Clark KJ, Duddy SK, et al. Increased protein kinase C delta in mammary tumor cells: relationship to transformation and metastatic progression. Oncogene. 1999;18(48):6748–57. doi: 10.1038/sj.onc.1203101. [DOI] [PubMed] [Google Scholar]
- Kim YH, Girard L, Giacomini CP, et al. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of mycfamily gene amplification. Oncogene. 2006 Jan.25(1):130–8. doi: 10.1038/sj.onc.1208997. [DOI] [PubMed] [Google Scholar]
- Klokz CPAM, Spronk CAEM, Lasonder E, Hoffmann A, Vuister GW, Grzesiek S, Hilbers CW. The solution structure and DNA-binding properties of the cold-shock domain of the human Y-box protein, YB-1. J. Mol. Biol. 2002;316:317–326. doi: 10.1006/jmbi.2001.5334. [DOI] [PubMed] [Google Scholar]
- Kohno K. p73 Interacts with c-Myc to regulate Y-box-binding protein-1 expression. J. Biol. Chem. 2002;277(35):31694–702. doi: 10.1074/jbc.M200266200. [DOI] [PubMed] [Google Scholar]
- Kohno K, Izumi H, Uchiumi T, et al. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25(7):691–8. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Aaltonen V, Koskela S, et al. Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res. 2004;64(16):5693–701. doi: 10.1158/0008-5472.CAN-03-3511. 15. [DOI] [PubMed] [Google Scholar]
- Kolluri R, Kinniburgh AJ. Full length cDNA sequence encoding a nuclease-sensitive element DNA binding protein. Nucleic Acids Res. 1991;19(17):4771. doi: 10.1093/nar/19.17.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri R, Torrey TA, Kinniburgh AJ. A CT promoter element binding protein: definition of a double-strand and a novel single-strand DNA binding motif. Nucleic Acids Res. 1992;20(1):111–6. doi: 10.1093/nar/20.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucab J, Lee C, Chen CS, et al. Celecoxib analogues disrupt Akt signaling, which is commonly activated in primary breast tumours. Breast Cancer Res. 2005;7(5):796–807. doi: 10.1186/bcr1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasham A, Moloney S, Hale T, et al. The Y.-box-binding protein, YB1, is a potential negative regulator of the p53 tumor suppressor. J. Biol. Chem. 2003;278(37):35516–23. doi: 10.1074/jbc.M303920200. [DOI] [PubMed] [Google Scholar]
- Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 2006;19(2):264–71. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- MacDonald GH, Itoh-Lindstrom Y, Ting JP. The transcriptional regulatory protein, YB-1, promotes single-stranded regions in the DRA promoter. J. Biol. Chem. 1995;270(8):3527–33. doi: 10.1074/jbc.270.8.3527. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, et al. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J. Biol. Chem. 2000;275(29):21793–6. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- Mantwill K, Köhler-Vargas N, Bernshausen A, et al. Inhibition of the Multidrug-resistant phenotype by targeting YB-1 with a conditionally oncolytic adenovirus: Implications for combinatorial treatment regimen with chemotherapeutic agents. Cancer Res. 2006;66(14):7195–202. doi: 10.1158/0008-5472.CAN-05-2339. [DOI] [PubMed] [Google Scholar]
- Masur K, Lang K, Niggemann B, et al. High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol. Biol. Cell. 2001;12(7):1973–82. doi: 10.1091/mbc.12.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens PR, Harendza S, Pollock AS, et al. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1. J. Biol. Chem. 1997;272(36):22905–12. doi: 10.1074/jbc.272.36.22905. [DOI] [PubMed] [Google Scholar]
- Mertens PR, Steinmann K, Alfonso-Jaume MA, et al. Combinatorial interactions of p53, activating protein-2, and YB-1 with a single enhancer element regulate gelatinase A expression in neoplastic cells. J. Biol. Chem. 2002;277(28):24875–82. doi: 10.1074/jbc.M200445200. [DOI] [PubMed] [Google Scholar]
- Miyake H, Monia BP, Gleave ME. Inhibition of progression to androgen-independence by combined adjuvant treatment with antisense BCL-XL and antisense Bcl-2 oligonucleotides plus taxol after castration in the Shionogi tumor model. Int. J. Cancer. 2000;86(6):855–62. doi: 10.1002/(sici)1097-0215(20000615)86:6<855::aid-ijc15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Moody SE, Perez D, Pan T-C, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Murray NR, Davidson LA, Chapkin RS, et al. Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J. Cell Biol. 1999;145(4):699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar A, Swamynathan SK, Kandala JC, et al. Characterization of the DNA-binding domain of the avian Y-box protein, chkYB-2, and mutational analysis of its single-strand binding motif in the Rous sarcoma virus enhancer. J. Virol. 1998;72(2):900–9. doi: 10.1128/jvi.72.2.900-909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004;10(16):5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258(5082):607–14. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9(7):484–96. [PubMed] [Google Scholar]
- Norman JT, Lindahl GE, Shakib K, et al. The Y.-box binding protein YB-1 suppresses collagen alpha 1(I) gene transcription via an evolutionarily conserved regulatory element in the proximal promoter. J. Biol. Chem. 2001;276(32):29880–90. doi: 10.1074/jbc.M103145200. [DOI] [PubMed] [Google Scholar]
- O’Brian C, Vogel VG, Singletary SE, et al. Elevated protein kinase C expression in human breast tumor biopsies relative to normal breast tissue. Cancer Res. 1989;49(12):3215–7. [PubMed] [Google Scholar]
- Oda Y, Ohishi Y, Saito T, et al. Nuclear expression of Y.-box-binding protein-1 correlates with P.-glycoprotein and topoisomerase II alpha expression, and with poor prognosis in synovial sarcoma. J. Pathol. 2003;199(2):251–8. doi: 10.1002/path.1282. [DOI] [PubMed] [Google Scholar]
- Oda Y, Sakamoto A, Shinohara N, et al. Nuclear expression of YB-1 protein correlates with P.-glycoprotein expression in human osteosarcoma. Clin. Cancer Res. 1998;4(9):2273–7. [PubMed] [Google Scholar]
- Ohga T, Koike K, Ono M, et al. Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 1996;56(18):4224–8. [PubMed] [Google Scholar]
- Ohga T, Uchiumi T, Makino Y, et al. Direct involvement of the Y-box binding protein YB-1 in genotoxic stress-induced activation of the human multidrug resistance 1 gene. J. Biol. Chem. 1998;273(11):5997–6000. doi: 10.1074/jbc.273.11.5997. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Izumi H, Imamura T, Takano H, Ise T, Uchiumi T, Kuwanto M, Kohno K. Direct interaction of p53 with the Y-box binding protein, YB-1: A mechanism for regulation of gene expression. Oncogene. 2000 Dec 14;19(54):6194–6202. doi: 10.1038/sj.onc.1204029. [DOI] [PubMed] [Google Scholar]
- Paolucci L, Rozengurt E. Protein kinase D in small cell lung cancer cells: rapid activation through protein kinase C. Cancer Res. 1999;59(3):572–7. [PubMed] [Google Scholar]
- Parsons M, Keppler MD, Kline A, et al. Site-directed perturbation of protein kinase C- integrin interaction blocks carcinoma cell chemotaxis. Mol. Cell. Biol. 2002;22(16):5897–911. doi: 10.1128/MCB.22.16.5897-5911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Tenorio G, Stal O, Southeast Sweden Breast Cancer Group Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br. J. Cancer. 2002;86(4):540–5. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou C, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Platet N, Prevostel C, Derocq D, et al. Breast cancer cell invasiveness: correlation with protein kinase C activity and differential regulation by phorbol ester in estrogen receptor-positive and -negative cells. Int. J. Cancer. 1998;75(5):750–6. doi: 10.1002/(sici)1097-0215(19980302)75:5<750::aid-ijc14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Podar K, Tai YT, Lin BK, et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J. Biol. Chem. 2002;277(10):7875–81. doi: 10.1074/jbc.M109068200. [DOI] [PubMed] [Google Scholar]
- Raffetseder U, Frye B, Rauen T, Jurchott K, Royer HD, Jansen PL, Mertens PR. Splicing factor SRp30c interaction with Y-box protein-1 confers nuclear YB-1 shuttling and alternative splice site selection. 2003 May 16;278(20):18241–18248. doi: 10.1074/jbc.M212518200. [DOI] [PubMed] [Google Scholar]
- Ranjan M, Tafuri SR, Wolffe AP. Masking mRNA from translation in somatic cells. Genes Dev. 1993;7(9):1725–36. doi: 10.1101/gad.7.9.1725. [DOI] [PubMed] [Google Scholar]
- Ren J, Datta R, Shioya H, et al. p73 beta is regulated by protein kinase Cdelta catalytic fragment generated in the apoptotic response to DNA damage. J. Biol. Chem. 2002;277(37):33758–65. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pinilla SM, Jones RL, Lambros MBK, et al. Mycamplification in breast cancer: a chromogenic in situ hybridization study. J. Clin. Pathol. 2007 doi: 10.1136/jcp.2006.043869. epub. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein DB, Stortchevoi A, Boosalis M, et al. Overexpression of DNA-binding protein B gene product in breast cancer as detected by in vitro-generated combinatorial human immunoglobulin libraries. Cancer Res. 2002;62(17):4985–91. [PubMed] [Google Scholar]
- Sakura H, Maekawa T, Imamoto F, et al. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 1988;73(2):499–507. doi: 10.1016/0378-1119(88)90514-8. [DOI] [PubMed] [Google Scholar]
- Samuel S, Twizere JC, Bernstein LR. YB-1 represses AP1-dependent gene transactivation and interacts with an AP-1 DNA sequence. Biochem, J. 2005;388:921–8. doi: 10.1042/BJ20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acid Res. 2004;1(32):W249–252. doi: 10.1093/nar/gkh372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauma S, Yan Z, Ohno S, et al. Protein kinase C beta 1 and protein kinase C beta 2 activate p57 mitogen-activated protein kinase and block differentiation in colon carcinoma cells. Cell Growth Differ. 1996;7(5):587–94. [PubMed] [Google Scholar]
- Schmitz KJ, Otterbach F, Callies R, et al. Prognostic relevance of activated Akt kinase in node-negative breast cancer: a clinicopathological study of 99 cases. Mod. Pathol. 2004;17(1):15–21. doi: 10.1038/modpathol.3800002. [DOI] [PubMed] [Google Scholar]
- Schuldiner O, Benvenisty N. A DNA microarray screen for genes involved in c-myc and n-myc oncogenesis in human tumors. Oncogene. 2001;20:4984–94. doi: 10.1038/sj.onc.1204459. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Jiang J, Kelsen D, et al. Protein kinase C: a novel target for inhibiting gastric cancer cell invasion. J. Natl. Cancer Inst. 1993;85(5):402–7. doi: 10.1093/jnci/85.5.402. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold J, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Shadeo A, Lam WL. Comprehensive copy number profiles of breast cancer cell model genomes. Breast Cancer Res. 2006;8(1):R9. doi: 10.1186/bcr1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K, Sugio K, Osaki T, et al. Nuclear expression of the Y.-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin. Cancer, Res. 2001;7(10):3151–5. [PubMed] [Google Scholar]
- Shibahara K, Uchiumi T, Fukuda T, et al. Targeted disruption of one allele of the Y-box binding protein-1 (YB-1) gene in mouse embryonic stem cells and increased sensitivity to cisplatin and mitomycin C. Cancer Sci. 2004;95(4):348–53. doi: 10.1111/j.1349-7006.2004.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao K, Takano H, Nakayama Y, et al. Enhanced coexpression of YB-1 and DNA topoisomerase II alpha genes in human colorectal carcinomas. Int. J. Cancer. 1999;83(6):732–7. doi: 10.1002/(sici)1097-0215(19991210)83:6<732::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Shnyreva M, Schullery DS, Suzuki H, Hagaki Y, Bomsztyk K. Interactin of two multifunctional proteins: Heterogeneous nuclear ribonuceoprotein K and Y-box-binding protein. J. Biol. Chem. 2000 May 19;275(20):15498–15503. doi: 10.1074/jbc.275.20.15498. [DOI] [PubMed] [Google Scholar]
- Sivaraman VS, Wang H, Nuovo GJ, et al. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J. Clin. Invest. 1997;99(7):1478–83. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A, Sinnemann S, Huntsman D, et al. Knockdown of the cytoprotective chaperone, clusterin, chemosensitizes human breast cancer cells both in vitro and in vivo. Mol. Cancer Ther. 2005;4(12):1837–49. doi: 10.1158/1535-7163.MCT-05-0178. [DOI] [PubMed] [Google Scholar]
- Soop T, Nashchekin D, Zhao J, Sun X, Alshanova-Ericsonn AT, Bjorkroth B, Ovchinnikov L, Daneholt B. A p50-like Y-box protein with a putative translational role becomes associated with pre-mRNA concomitant with transcription. J. Cell. Sci. 2003 Apr 15;116(Pt8):1493–1503. doi: 10.1242/jcs.00353. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. PNAS. 2003;100(14):8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein U, Bergmann S, Scheffer GL, et al. YB-1 facilitates basal and 5-fluorouracil-inducible expression of the human major vault protein (MVP) gene. Oncogene. 2005;24(22):3606–18. doi: 10.1038/sj.onc.1208386. [DOI] [PubMed] [Google Scholar]
- Stein U, Jurchott K, Walther W, et al. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J. Biol. Chem. 2001;276(30):28562–9. doi: 10.1074/jbc.M100311200. [DOI] [PubMed] [Google Scholar]
- Stenina OI, Shaneyfelt KM, DiCorleto PE. Thrombin induces the release of the Y.-box protein dbpB from mRNA: a mechanism of transcriptional activation. Proc. Natl. Acad. Sci. U.S.A. 2001;98(13):7277–82. doi: 10.1073/pnas.121592298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara I, Arai T, Yamashita T, et al. Expression of multidrug resistance-associated protein (MRP) in anaplastic carcinoma of the thyroid. Cancer Lett. 1994;82(2):185–8. doi: 10.1016/0304-3835(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Sugawara I, Masunaga A, Itoyama S, et al. Expression of multidrug resistance-associated protein (MRP) in thyroid cancers. Cancer Lett. 1995;95(1–2):135–8. doi: 10.1016/0304-3835(95)03878-z. [DOI] [PubMed] [Google Scholar]
- Sun X, Wu F, Datta R, et al. Interaction between protein kinase C delta and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J. Biol. Chem. 2000;275(11):7470–3. doi: 10.1074/jbc.275.11.7470. [DOI] [PubMed] [Google Scholar]
- Sutherland BW, Kucab J, Wu J, et al. Akt phosphorylates the Y.-box binding protein 1 at S102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene. 2005;24(26):4281–92. doi: 10.1038/sj.onc.1208590. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Nambiar A, Guntaka RV. Role of single-stranded DNA regions and Y.-box proteins in transcriptional regulation of viral and cellular genes. FASEB J. 1998;12(7):515–22. doi: 10.1096/fasebj.12.7.515. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Varma BR, Weber KT, et al. Targeted disruption of one allele of the Y.-box protein gene, Chk-YB-1b, in DT40 cells results in major defects in cell cycle. Biochem., Biophys., Res., Commun. 2002;296(2):451–7. doi: 10.1016/s0006-291x(02)00875-6. [DOI] [PubMed] [Google Scholar]
- Tafuri SR, Wolffe AP. DNA binding, multimerization, and transcription stimulation by the Xenopus Y. box proteins in vitro. New Biol. 1992;4(4):349–59. [PubMed] [Google Scholar]
- Tolcher AW, Chi K, Kuhn J, et al. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin. Cancer Res. 2005;11(10):3854–61. doi: 10.1158/1078-0432.CCR-04-2145. [DOI] [PubMed] [Google Scholar]
- Tonetti DA, Morrow M, Kidwai N, et al. Elevated protein kinase C alpha expression may be predictive of tamoxifen treatment failure. Br. J. Cancer. 2003;88(9):1400–2. doi: 10.1038/sj.bjc.6600923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uramoto H, Izumi H, Ise T, et al. p73 Interacts with c-Myc to regulate Y-box-binding protein-1 expression. J. Biol. Chem. 2002;277(35):31694–702. doi: 10.1074/jbc.M200266200. [DOI] [PubMed] [Google Scholar]
- Vantaggiato C, Formentini I, Bondanza A, et al. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J. Biol. 2006;5(5):14. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Oster SK, Shago M, et al. Identifying genes regulated in a myc-dependent manner. J. Biol. Chem. 2002;277(40):36921–30. doi: 10.1074/jbc.M201493200. [DOI] [PubMed] [Google Scholar]
- Wu J, Lee C, Yokom D, et al. Disruption of the Y.-Box Binding Protein-1 Results in Suppression of the Epidermal Growth Factor Receptor and HER-2. Cancer Res. 2006;66(9):4872–9. doi: 10.1158/0008-5472.CAN-05-3561. 1. [DOI] [PubMed] [Google Scholar]
- Xia P, Aiello LP, Ishii H, et al. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J. Clin. Invest. 1996;98(9):2018–26. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata H, Kobayashi H, Kamura T, et al. Increased nuclear localization of transcription factor YB-1 in acquired cisplatin-resistant ovarian cancer. J. Cancer Res. Clin. Oncol. 2002;128(11):621–6. doi: 10.1007/s00432-002-0386-6. [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Ways DK, et al. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999;59(17):4413–8. [PubMed] [Google Scholar]
- Zhang G, He B, Weber GF. Growth factor signaling induces metastasis genes in transformed cells: molecular connection between Akt kinase and osteopontin in breast cancer. Mol. Cell. Biol. 2003;23(18):6507–6519. doi: 10.1128/MCB.23.18.6507-6519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Anastasiadis PZ, Liu Y, et al. Protein kinase C (PKC) betaII induces cell invasion through a Ras/Mek-, PKC iota/Rac 1-dependent signaling pathway. J. Biol. Chem. 2004;279(21):22118–23. doi: 10.1074/jbc.M400774200. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Gjoerup OV, Subramanian RR, et al. Human mammary epithelial cell transformation through the activation of phosphatydylinositol 3-kinase. Caner Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- Zugaza JL, Sinnett-Smith J, Van Lint J, et al. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO. J. 1996;15(22):6220–30. [PMC free article] [PubMed] [Google Scholar]