Abstract
Osteoporosis and obesity are chronic disorders that are increasing in prevalence. The pathophysiology of these diseases is multifactorial and includes genetic, environmental and hormonal determinants. Long considered as distinct disorders that rarely are found in the same individual, emerging evidence from basic and clinical studies support an important interaction between adipose tissue and the skeleton. Adiposity can influence bone remodeling through three possible mechanisms including secretion of cytokines that directly target bone, adipokines that influence the central nervous system thereby changing sympathetic impulses to bone, and paracrine influences on adjacent skeletal cells. This review will focus on our current understanding of bone-fat interactions and the clinical implications of recent studies linking obesity to osteoporosis.
I. Introduction
Recent advances in our understanding of the mechanisms that maintain skeletal homeostasis have highlighted the tenet that bone metabolism is integrated with multiple organ systems including the network of adipose tissue distributed throughout the mammalian organism. Three possible mechanisms may be operative to understand how adiposity affects skeletal turnover : 1- Endocrine cytokines and growth factors released by adipocytes affect osteoblasts and osteoclasts; 2- Adipokines (e.g. leptin, adiponectin) regulate central nervous system outflow from the sympathetic nervous system, and 3- Paracrine factors elaborated by adipocytes within the bone marrow milieu influence nearby cells on the trabecular bone surface. Regarding the endocrine mediation of adipose tissue, visceral fat depots with an inflammatory element secrete cytokines including resistin, TNF-α, IL-1 and IL-6, that uncouple bone remodeling by enhancing bone resorption or suppressing bone formation. Similarly adiponectin and leptin can influence bone remodeling by endocrine actions or through their influence on hypothalamic centers that regulate sympathetic tone. The outflow of sympathetic impulses inhibits osteoblast differentiation and enhances osteoclast recruitment thereby uncoupling the bone remodeling unit. Regarding the paracrine actions of adipose tissue, bone marrow adipocytes, first identified more than a century ago, are an essential part of the bone marrow niche, and clearly are integral to both skeletal function and hematopoiesis. Moreover, the differentiation programs of osteoblasts and adipocytes share a common ancestor, the mesenchymal stem cells (MSC). Thus a new perspective about bone-fat interactions has emerged, particularly regarding skeletal metabolism. This review will center on our current knowledge of the interaction between adipose tissue and the skeleton with a focus on shared hormonal modulators and the paracrine effects of bone marrow adiposity on skeletal remodeling.
II. Hormonal Determinants between Bone and Fat
The emergence of the recent obesity epidemic has brought adipocytes to the forefront of investigations into the pathophysiology of this chronic disease. Within that framework, a paradigm shift has occurred such that adipose tissue, long considered as an inert site for energy storage, has emerged as an endocrine modulator of satiety, energy balance and pubertal development (1, 2). Additionally, disorders of adipose tissue function have recently been linked to prevalent chronic diseases such as atherosclerosis, diabetes mellitus and osteoporosis (3–6).
Adipose tissue is characterized by an abundance of adipocytes and a stromal vascular fraction that contains bipotent stem cells that can differentiate into adult adipocytes or under the appropriate conditions, pre-osteoblasts. Adipocytes have a specialized function to store fatty acids as triglycerides for future use as substrates for energy utilization by the organism. In addition these cells secrete peptides, i.e. adipokines, that act in autocrine, paracrine and endocrine pathways to influence whole body metabolic homeostasis. Adipocytes are regulated by multiple factors including the number of precursor cells, total substrate availability and hormonal influences. With respect to the latter, insulin is a critical determinant of adipocyte function by stimulating glucose uptake and inhibiting lipolysis. Insulin also enhances adipocyte differentiation and determines both adipose tissue expansion and retraction in response to nutrient availability. Not surprisingly, disorders of insulin secretion and resistance including both type I and type II diabetes, with either or low levels of circulating insulin, have been associated with changes in adipocyte structure and function. Remarkably, insulin receptors are also found on osteoblasts and through their presence, insulin can promote osteoblast differentiation. In a genetic model of a conditional deletion of insulin receptors in bone there was decreased bone mass and a remarkable metabolic phenotype (7). Taken together, both structurally and functionally, there exists a close relationship between adipocytes and osteoblasts.
One of the most important adipokines released by adipocytes in respect to metabolic homeostasis is leptin. Leptin acts by binding to leptin receptors (LRb), which in turn triggers phosphorylation of cytoplasmic tyrosine residues of LRb that initiate various signaling pathways including JAK2-STAT3, Erk1/2, and PI3K (8, 9). Leptin regulates body weight by modulating receptor-expressing neurons in the CNS, particularly within the hypothalamus and brainstem (8, 10). However, leptin has pleiotropic metabolic effects, controlling energy expenditure, locomotor activity, feeding behavior, fertility, bone mass, linear growth, adrenal activity and life span. Thus, mice with congenital absence of leptin (ob/ob mice) or leptin’s signaling receptor (db/db mice) exhibit a complex phenotype with abnormalities in several organ systems. In particular, these mice are obese but remarkably, in spite of their hormonal profile (i.e., hypogonadism and increased glucocorticoid), have high bone mass (11). Since leptin evolved coincident with the appearance of vertebrates, it has been hypothesized that the skeleton is a main leptin target (12). Supporting this hypothesis, it has been observed that mice harboring a mutation that leads to a partial gain of function in leptin signaling exhibit normal appetite, but an osteoporotic phenotype (13, 14).
Most but not all (15), evidence suggests that leptin acts centrally to inhibit the accrual of bone mass (12). One of the most convincing arguments is that a neuron-specific deletion of Lepr recapitulates the bone phenotype of ob/ob mice, whereas an osteoblast-specific one does not (12, 15). Moreover, the skeletal phenotype of ob/ob mice can be corrected by leptin administration into the third ventricle (11). The pattern of bone changes in leptin-deficient mice may be reproduced by chemical lesioning of neurons in the ventromedial hypothalamus (16). This neural damage also prevents the skeletal changes that result from central administration of leptin. Thus, emerging evidence place the hypothalamic ventromedial nucleus as the key site in the central nervous system for leptin regulation of bone mass (16). From this nucleus, sympathetic fibers transmit stimuli to bone via osteoblasts that have an abundance of the β2 adrenergic receptor (β2 AR) (16). Support for that tenet comes from genetically engineered β2 AR-disrupted mice that share the same bone phenotype with ob/ob and chemically-injured ventromedial mice. In respect to the central action of leptin, it was recently demonstrated that the leptin receptor in hypothalamic ventromedial neurons is not necessary to trigger the action of leptin (17). These data indicate that leptin probably acts at other sites of the brain to regulate metabolism. Serotoninergic neurons of the brainstem are reasonable candidates as mediators of this action, since the consequences of leptin deficiency for bone turnover and metabolism can be reversed by suppressing serotonin production in the brainstem (18). In sum, leptin, a major adipokine plays a critical role not only in regulating appetite, reproductive capacity, and energy consumption but also in bone turnover through its actions on the central nervous system
III. Bone disorders in metabolic diseases
Adipose tissue, which in normal conditions shows a high level of insulin sensitivity, is one source of insulin resistance (19, 20). The metabolic manifestations of insulin resistance include changes in several adipokines, the attraction of inflammatory macrophages, alterations in tissue remodeling and in lipolytic activity (20, 21). The hyperinsulinemia results from physiological compensation, also affects “off target” tissues (22–25). These include two major phenotypes: hyperandrogenism and hypertension (22, 23, 26). Importantly the impact of insulin resistance on bone cell function is still under investigation using both genetic models and human studies.
Notwithstanding the difficulty in defining the role of insulin resistance in skeletal remodeling, the physiologic interplay between bone and insulin has been established through recent investigations. The osteoblast is an insulin- sensitive cell, which as noted, expresses the insulin receptor (7). Genetically engineered mice lacking the insulin receptor in osteoblasts results in decreased bone formation but remarkably these mice also have increased body fat and impaired insulin sensitivity. These findings led scientists to a bone specific protein, osteocalcin. The synthesis of osteocalcin, the most abundant non-collagen peptide in the skeletal matrix, is modulated at least in part by insulin. Osteocalcin is carboxylated post-translationally on three glutamic acid residues in a vitamin K-dependent manner by the enzyme γ-carboxylase (27). The product, the γ-carboxyglutamic acid (Gla) amino acid, has the capacity to bind to calcium. On the other hand, decarboxylation decreases the hydroxyapatite-binding affinity of osteocalcin. Undercarboxylated osteocalcin that then enters the circulation regulates energy metabolism, through an increase in β-cell proliferation, insulin secretion and insulin sensitivity(7, 28, 29). Lee et al. provided the first evidence of this role by the identification of the ESP gene, which encodes the intracellular protein tyrosine phosphate OST-PTP [protein-tyrosine phosphatase 1B (PTP1B) in humans] involved in the carboxylation of osteocalcin (29).
An endocrine loop connecting bone to pancreas, where insulin promotes osteocalcin synthesis and osteocalcin in turn leads to enhanced insulin secretion, establishes a somewhat unique feed forward loop (see Figure 1,Figure 2) (12). Additionally, there is also evidence that the osteoclast is integrated into this network; insulin signaling in osteoblasts inhibits the expression of osteoprotegerin, allowing the receptor activator of nuclear factor–κB ligand to stimulate bone resorption by osteoclasts. The acidic microenvironment created by these multinucleated cells favors the decarboxylation of osteocalcin (28). It is likely that undercarboxylated osteocalcin has a greater capacity than carboxylated osteocalcin for stimulating β-cell proliferation and insulin secretion (30). Remarkably, animals lacking the insulin receptor in classic target tissues (i.e., muscle or adipocytes) do not show glucose intolerance, suggesting that other sites, such as the skeleton, are engaged in the control of glucose disposal.
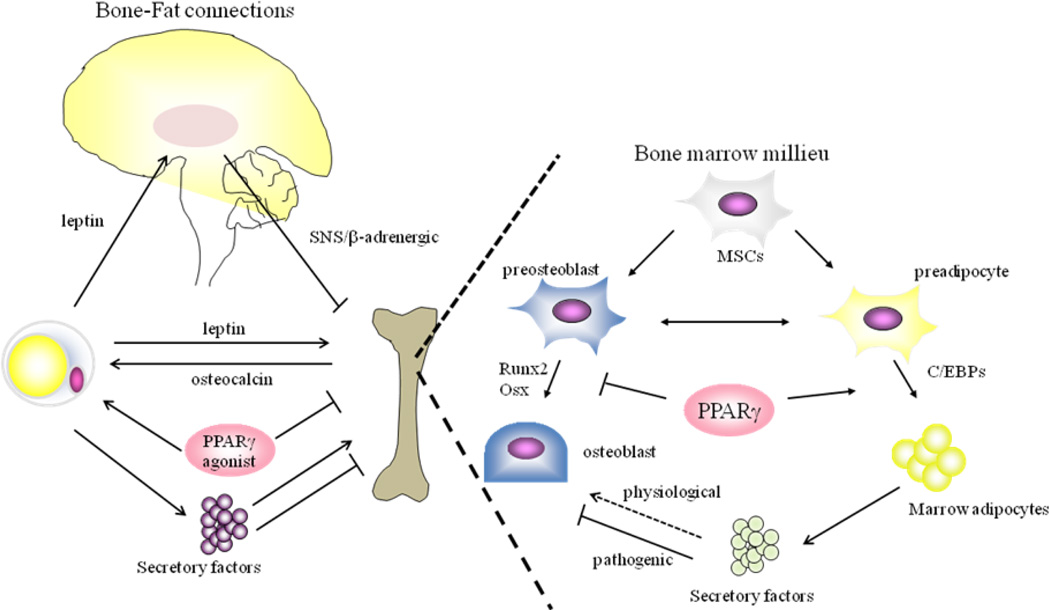
Figure 1. Schematic model of Bone-Fat connections.
Leptin regulation of bone mass includes: 1- activation of SNS/β-adrenergic signaling through hypothalamic integration, which results in bone loss, 2- direct anabolic effects on osteoblasts, although the precise mechanisms of leptin action on skeletal homeostasis remain to be elucidated. Osteocalcin produced by osteoblasts decreases fat mass in part through promoting adiponectin production in adipocytes and activation of PPARγ has been shown to cause increased adipose tissue mass and bone loss. Secretory factors produced from adipocytes, such as imflammatory cytokines, fatty acid, leptin and adiponectin, can positively or negatively regulate skeletal mass.
In a bone marrow milieu, marrow adipocytes play a pivotal role in the regulation of osteoblast function. Secretory factors including inflammatory cytokines are also produced by marrow adipocytes. These cytokines are possibly acting on osteoblasts in a paracrine manner and suppressing osteoblast function and/or differentiation in pathogenic conditions, whereas in physiological conditions, marrow adipocytes could be positively regulating bone mass, but this concept needs to be clarified. In addition, activation of PPARγ causes bone loss in part through altering the specification of MSCs towards adipogenesis and away from osteogenesis, resulting in the decreased osteoblast pool in the bone marrow microenvironment.
SNS: sympathetic nervous system, PPAR: peroxisome proliferative activated receptors, C/EBP: CCAAT/enhancer binding proteins; RUNX: runt-related transcription factor, MSC: mesenchymal stem cell,
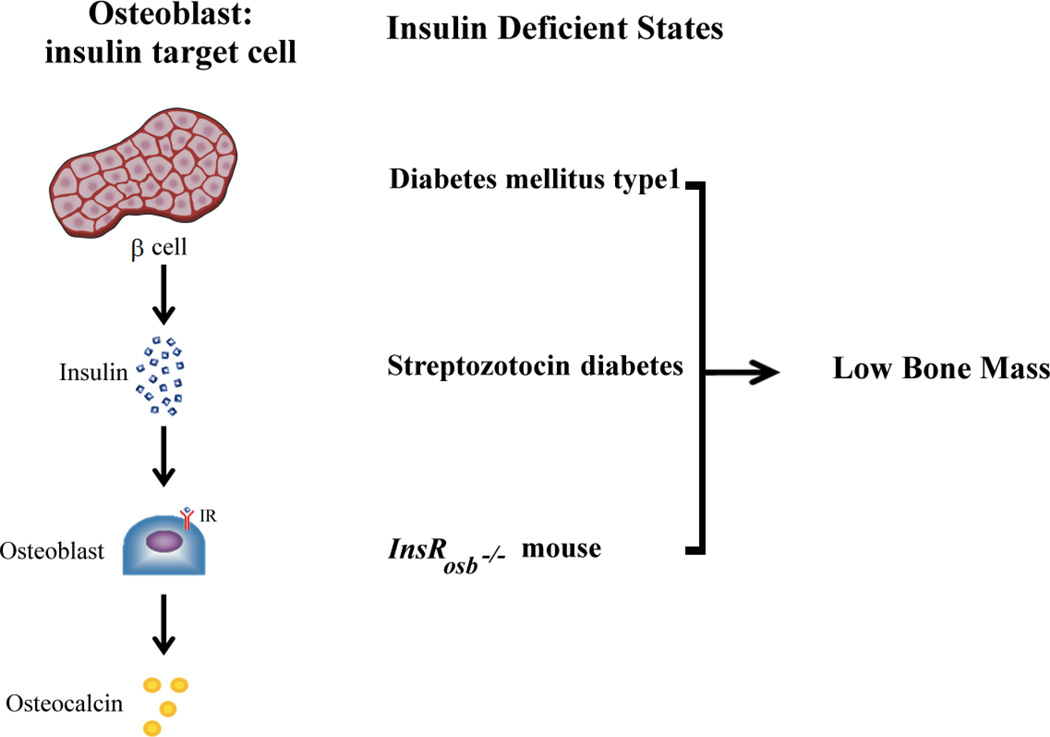
Figure 2.
Insulin regulates skeletal homeostasis in part by stimulating new bone formation and bone remodeling. Insulin deficiency or resistance leads to low bone mass and increased skeletal fragility. Enhanced bone resorption increases release of undercarboxylated osteocalcin which in turn can enhance insulin secretion and adipocyte sensitivity(7)
Further support that bone is integrated with metabolic homeostasis comes from clinical and experimental studies. Insulinopenic states such as human diabetes mellitus type 1 (31, 32) and pharmacologically induced diabetes (33) are unequivocally associated with decreased bone mass and skeletal fragility. In either model, low serum levels of osteocalcin or decreased osteocalcin expression in bone reflect impaired bone formation and a primordial defect in osteoblast function duirng states of insulin deficiency. As noted, additional evidence about bone regulation by insulin has been acquired by performing specific genetic deletion of insulin receptors in osteoblasts(7). Insulin receptor silencing led to low bone mass and was associated with increased expression of Twist2 and decreased expression of osteocalcin and Runx2. The transcriptional factor Twist2 works as a cellular inhibitor of Runx2; the latter is a key determinant in the process of osteoblast differentiation. Therefore, diabetes mellitus type 1 has a constellation of convergent factors leading to bone loss and ultimately to bone fragility (34, 35).
Obesity is associated with several major health problems and has a strong psychological impact. Hypertension, diabetes mellitus and dyslipidemia, the ominous triad associated with insulin resistance, are all closely associated with obesity and together increase significantly the risk of cardiovascular disease (36). Recent evidence suggests that independent of weight, glucose intolerance in these patients is associated with greater skeletal fragility despite near normal bone mineral density. Moreover, ultra structural analysis of insulin-resistant bones suggest there is an increase in cortical porosity which may be a major contributor to the heightened fracture risk often observed in these patients. Moderate weight loss is an efficient way to prevent the risk to develop DM in overweight and obese individuals (37), while drastic weight loss enhances the life expectancy of severely obese patients. Thus, weight loss is pursued by patients and encouraged by physicians to decrease the risk of cardiovascular disease and related disorders. However, hard tissue seems to be exquisitely sensitive to rapid decrements in body weight. For example weight loss induced by bariatric surgery, despite the improvement in insulin sensitivity and overall health, is associated with significant bone loss (38, 39). Long term studies are required to determine whether this loss is reversible with sustained weight loss and changes in anthropomorphic indices.
Not surprisingly, low body weight is also associated with changes in skeletal remodeling. Nutritional restriction in mice (40) and anorexia nervosa in humans are established causes of osteoporosis (41). Insulin sensitivity was recently investigated in patients with anorexia nervosa. Since the lack of adipose tissue in lipodystrophy is accompanied by insulin resistance, Karczewska-Kupczewska et al. evaluated insulin sensitivity by measuring glucose disposal and serum adiponectin concentration in women with anorexia nervosa (42). All three forms of adiponectin, the insulin sensitizing factor derived from adipose tissue, were increased in anorexic patients. Another study showed that, in parallel to both bone and fat depot loss, anorexic patients exhibited increased adiposity in the bone marrow niche that paralleled the increase in adiponectin and the heightened insulin sensitivity (43). Consistent with these results, it was recently observed that preadipocyte factor-1 (Pref-1) levels are increased in the circulation of anorexic women as is FGF-21 another insulin sensitizing factor (44, 45). Pref-1 is a member of the epidermal growth factor-like family of proteins and a suppressor of both adipocyte and osteoblast differentiation. Moreover, a positive correlation was observed between Pref-1 circulating concentrations and marrow fat in the proximal femur of all women in that study.
Finally, the association between insulin sensitivity/resistance and bone remodeling is a critical determinant when considering future strategies for the therapy of type II diabetes mellitus. Thiazolidenediones (TZD), the class of drugs of exogenous agonists for PPAR-γ, were introduced two decades ago (46). PPAR-γ exists in two major forms, PPAR-γ1 and PPAR-γ2. PPARγ 1 is expressed in a large range of tissues, including the liver, skeletal muscle, adipose tissue and hematopoietic cells. The expression of PPARγ 2, that contains 30 additional amino acids in its N-terminus extremity, is primarily restricted to adipocytes, stromal cells and osteoblasts. The TZDs, in general, have a complex and incompletely understood mechanism of action. The improvement in glucose metabolism is partly due to their influence on endocrine factors in adipose tissue, but, in addition, they also have independent metabolic effects. TZDs stimulate the maturation of visceral fat, and hence change the adipo-cytokine profile by adipose tissue. These agents lead to an increase in adiponectin levels, which counteracts proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and promotes beta oxidation of fatty acids via the activation of adenosine 5′-monophosphate -activated protein kinase (AMP-K)(47). The increase in beta oxidation, together with a reduction in de novo lipogenesis, reduces gluconeogenesis (48).
In the ADOPT clinical trial, which was designed to investigate the long-term efficacy of metformin, glyburide and rosiglitazone for the treatment of type II diabetes, an increased risk of fracture was detected in women, but not in men, treated with rosiglitazone compared to women treated in the other arms of this study (49). Similar results with pioglitazone were also reported in a note by Eli Lilly Canada Inc. Grey et al. reported the results of a 14-week randomized clinical trial comparing rosiglitazone (8 mg/day) to placebo in 50 post-menopausal women with normal glucose tolerance (50). In the rosiglitazone group, the decline in biochemical markers of bone formation (osteocalcin and procollagen type 1 N -telopeptide) was of the order of 10–12% compared to placebo. There was no change in serum β-CTX, the marker of bone resorption. These changes in bone turnover were accompanied by a significant 2% decline in total BMD in the rosiglitazone-treated group, even within the short time frame of this trial.
Taken together, hormonal determinants of adipose tissue can have a profound effect on skeletal remodeling. Almost certainly energy availability, which is sensed by cells that make these circulating factors, play a major role in determining how the skeleton responds to bone remodeling. In part, the cellular mechanisms mediating these changes can be traced to the bone marrow where multipotent stem cells undergo lineage allocation into either the adipocytic or osteogenic program. Those cells ultimately determine the relationship between bone and fat.
IV. Role of marrow adipocytes in skeletal metabolism
A. The transcriptional network of adipogenesis: marrow adiposity
Molecular mechanisms of adipocyte differentiation have been studied using both in vitro and in vivo models. Not surprisingly, a number of growth factors have been implicated including IGF-I, growth hormone, and insulin, emphasizing the critical connection of nutrient status with the development of adipose tissue. Adipose tissue formation is defined by integrated steps of adipocyte differentiation and maturation. These steps have been intensively studied and multiple transcription factors have been identified. The initial step of adipogenesis includes the lineage commitment of MSCs into preadipocytes, followed by the expansion of these cells. These preadipocytes in the stromal vascular fraction of adipose depots undergo a differentiation program under the tight control of multiple transcription factors including C/EBPβ/δ and PPARγ. Among these, PPARγ, a nuclear receptor and transcription factor, plays a central role in adipogenesis as supported by the fact that the loss of Pparg in mouse embryonic fibroblasts leads to a complete absence of adipogenic capacity (51). In vitro, multiple transcription factors and co-regulators have been shown to modulate the expression and function of PPARγ. For example, differentiation of 3T3-L1 cells, a well recognized cell line that is used to recapitulate adipogenesis in vitro, is regulated by the integration of several transcription factors including the C/EBP family, C/EBPβ and δ. These factors stimulate Pparg transcription by directly binding to the promoter region (52). Increased expression of Pparg activates the expression of another member of the C/EBP family, C/EBPα, which in turn enhances the expression of PPARγ. Partial loss of function of C/EBPα results in a mouse (A-Zip) with very little adipose tissue including virtually none in the bone marrow but increased bone mass. Thus, the C/EBP family is critical for the induction of PPARγ in vitro. However, in vivo adipogenesis is complex and requires other transcriptional and co-factors for PPARγ regulation in part because as demonstrated by the A-Zip mice, PPARγ expression is maintained in the adipose tissue of mice lacking C/EBPβ and/or δ (53).
The transcriptional network of marrow adipogenesis is likely governed by the same mechanisms used to regulate white adiopocyte differentiation and PPARγ is certainly critical in this process For example, streptozoticin-induced type 1 diabetic mice exhibit massive infiltration of marrow adiposity, which is antagonized by the treatment with the PPARγ antagonist, bisphenol A diglycidyl ether (BADGE) (54). Consistently, BADGE also suppresses the marrow adipogenesis induced by irradiation in mice (55). In the same vein, marrow adiposity is induced by the treatment with a PPARγ agonists (TZDs), although this effect is dependent on a type of TZD, further supporting the critical role for PPARγ in marrow fat generation.
B. Regulation of marrow stromal cell fate by PPARγ: implications for the development of osteoporosis
Adipogenesis is well recognized to be tightly linked to osteogenesis in the bone marrow milieu as demonstrated by the finding that osteoblasts and adipocytes share a common precursor; i.e.mesenchymal stem cells (MSCs) (56–58). Specification of MSC fate towards either adipocytes or osteoblasts is a fine-tuned process, and a number of lineage-specific transcription factors (such as Runx2 and osterix for osteoblasts and PPARγ2 for adipocytes) have been shown to be involved in this process (59–62). Interestingly suppression of PPARγ stimulates osteoblastogenesis and enhancement in PPARγ activity results in decreased osteogenesis, suggesting this may be a mutually exclusive process (63). These observations are also relevant in aged mice models where marrow adiposity is increased, bone mass is reduced, and there is enhanced PPARγ2 expression (64). Similarly, haploinsufficiency or a hypomorphic mutation of Pparg has been reported to have increased bone mass and reduced marrow adiposity associated with increased osteoblast number and bone formation (65, 66). Because PPARγ expression in the bone marrow increases with age, it is conceivable that PPARγ activation in the bone marrow is at least in part responsible for the age-related decrease in bone mass and increase in marrow adiposity. However the mutually exclusive hypothesis for lineage allocation has recently been challenged by findings from several groups that enhanced osteoblast activity can be found in the marrow of mice with marrow adiposity.
In addition to the pivotal role of PPARγ in lineage allocation of MSCs, mounting evidence indicates the involvement of PPARγ in osteoclast differentiation. For example, PPARγ activation has been shown to activate bone resorption in part through enhancing osteoclast differentiation by recruitment of another co-activator of PPARγ, PGC-1beta (67–70). Furthermore, the effect of PPARγ activation on osteoclastogenesis could be in part mediated by the increased expression of Rankl in an age-dependent manner (71); however, the exact role of PPARγ in osteoclastogenesis still needs to be fully delineated (72, 73). Notwithstanding, taken together, several lines of evidence highlight the pivotal role of PPARγ in the regulation of osteogenesis, adipogenesis and osteoclastogenesis, which could contribute to the pathogenesis of osteoporosis.
C. Developmental aspects of Marrow Fat in Humans
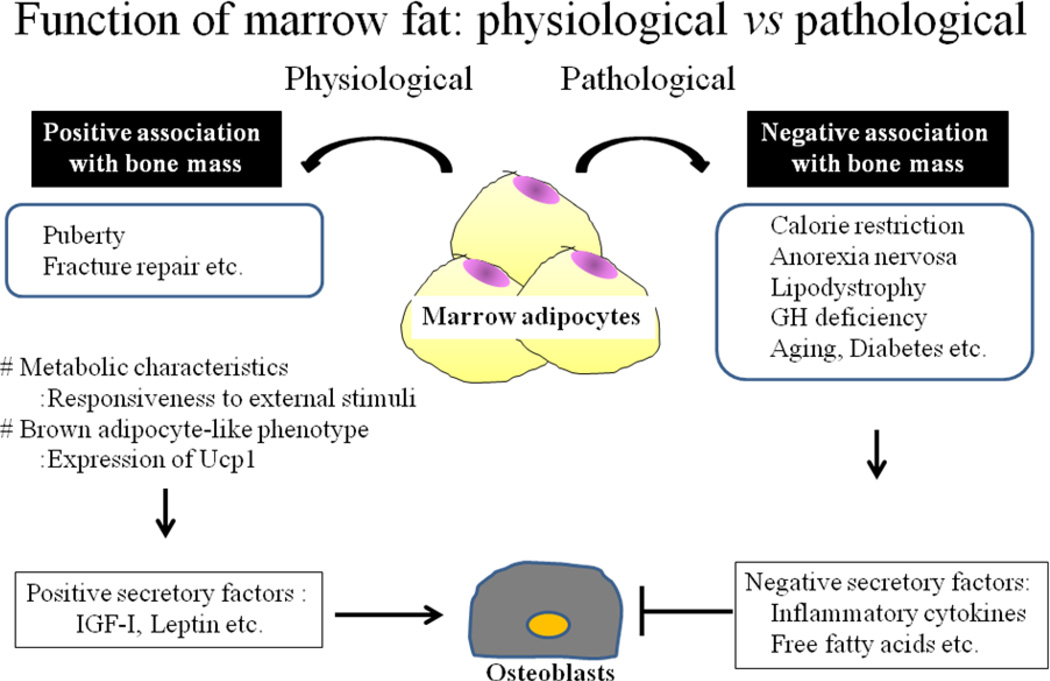
Development of marrow fat in humans is age- and context- specific. In newborns there is no marrow fat at any site and the marrow at every skeletal site is principally hematopoietic. However adipocyte number increases with age, particularly in the appendicular skeleton such that for individuals older than 30 years of age, most of the femoral marrow cavity is occupied by adipose tissue. Indeed, recent studies have indicated that more than 70% of the marrow space is occupied by fat in the appendicular skeleton of adults. This age-related increase in marrow adiposity has been associated with age-related bone loss and BMD, establishing the widely accepted tenet of the inverse correlation between these two parameters. This concept is largely true especially in pathologic conditions including postmenopausal osteoporosis, drug use (corticosteroid, thiazolidinediones (TZDs) etc.), aging, and malnutrition. However, in physiological conditions that tenet may not be true. For example, infiltration of marrow adipocytes occurs around the time of peak bone acquisition (74), supporting the hypothesis that marrow adipocytes may create a favorable skeletal microenvironment for osteoblastogenesis, thereby, maximizing bone accrual during puberty. Similarly during fracture repair and after radiation with bone marrow transplantation, adipocyte infiltration precedes osteogenic differentiation and new bone formation (Fig 3).
Figure 3. Function of marrow fat.
Marrow adipocytes produce a number of secretory factors. Such factors could have a significant role in osteoblast differentiation and/or function. In pathogenic conditions, these determinants could impact osteoblasts in a negative direction, whereas in physiological conditions these factors may have a different role from the one observed in the pathogenic conditions. There is also evidence that marrow fat is metabolically active and that genes which are characteristics to brown adipocytes are expressed in marrow adipocytes.
Importantly, the development of marrow adipocytes is strongly affected by nutritional status. Despite the similarities in terms of the transcriptional machinery used in the development of marrow adipocytes, the trigger for adipocyte formation could be different from white adipose tissue. In fact, marrow adipocyte infiltration is often observed in the clinical scenario often referred to as “fat redistribution”, in which marrow fat infiltration is associated with decrease in peripheral adipose depots. For example, HIV-related lipodystrophy causes a significant decrease in peripheral adipose tissue whereas marrow adiposity is enhanced. In that same vein, states of malnutrition including anorexia nervosa lead to an increase in marrow adiposity while there is concurrent peripheral loss of adipose depots. Importantly, over-nutrition is not a contributor to the development of marrow adiposity, suggesting that amount of peripheral fat depots is not correlated with marrow adiposity. In fact, in recent work by Gilsanz and colleagues, adolescents and young adults had significant marrow adiposity in the appendicular skeleton, but this was NOT related to subcutaneous or visceral fat depots, nor with markers of cardiovascular risk (75). However, this concept requires clarification and might be compartment-specific because there is also an evidence of a positive correlation between vertebral marrow adiposity and visceral fat mass in some scenarios (76).
V. The Function of Marrow Fat
A. Physiology
The physiologic role of adipocytes in human bone marrow is largely unknown. Marrow fat had long been regarded as a ‘filler’ for space vacated by trabecular bone loss, which is often seen in aged people and osteoporotic patients (77–80). Marrow adipogenes has thus been considered a default pathway in which MSCs enter the fat lineage because of their inability to differentiate into more complex cells such as osteoblasts or chondrocytes. However, with an extensive revision of our understanding of fat tissue as an endocrine organ, a novel concept that marrow adipocytes possess similar metabolic characteristics as some brown fat depots has emerged. Moreover, the juxtaposition of adipose tissue within the bone marrow milieu suggests that its presence may have consequences for the skeleton such that a balanced bone marrow microenvironment including marrow adipocytes is essential for normal osteogenesis(58, 81). Indeed, increased production of adipose-related factors, such as fatty acids, could positively or negatively affect metabolism in the bone marrow depending on the nature of the fatty acid and the type of receptor activation on MSCs (82). In addition, adipokines, steroids, and cytokines (83–85) can exert profound effects on neighboring marrow cells, sustaining hematopoietic and/or osteogenic processes (78, 80, 84, 85)(Fig 32).
B. “Brown-like” characteristics of marrow fat
Interestingly, expression profiling of marrow adipocytes in one mouse inbred strain with high bone mass (C3H/HeJ) has revealed the expression of genes involved in thermogenesis (e.g UCP1) and lipid metabolism, suggesting that marrow adipocytes could be metabolically active (86, 87), Rosen CJ, Unpublished observation). Furthermore, NMR spectroscopy revealed that a saturated: unsaturated fatty acid ratio in the bone marrow of that strain is nearly identical to that observed in interscapular brown adipose tissue (BAT). Similarly mice treated with rosiglitazone have enhanced marrow adiposity that has spectroscopic and genotypic characteristics identical to BAT. These lines of observations indicate that marrow adipocytes may possess “brown-like” characteristics. “Bright” (brown like) cells have recently been the focus of intense investigations particularly for investigators interested in novel treatments of obesity since these cells are generally thermogenic in nature rather than storage vehicles. These cells are found in multiple adipose depots and are activated by sympathetic tone. However, “brown-like” adipogenesis is very site and context specific. Pharmacologic interventions to convert white adipose tissue to “brown-like” are now being tested in animals with high expectations that ultimately obesity may be treatable with one of these agents. In the bone marrow, “brown-like” adipocytes could provide an energy source for neighboring cells such as osteoblasts or may simply be a means of maintaining thermoneutrality in the marrow, particularly for the appendicular skeleton (Fig. 2).
VI. Implications for osteoporosis
A. Marrow Fat and Aging
Aging is one of the risk factors for the development of osteoporosis. Age-related bone loss is characterized by the uncoupling of the bone remodeling network in which bone formation is suppressed and is associated with enhanced bone resorption. The underlying mechanisms of age-related bone loss are extremely complex and multiple factors are known to be involved in this process. Importantly, accumulating evidence clearly demonstrate that age-related bone loss is associated with increase in marrow adiposity (88). In a cross-sectional study of post-mortem iliac crest biopsies adipose tissue volume increased from 15% to 60% between 20 to 65 years old, while trabecular bone volume decreased from 26% to 16% (89). More recently, Justesen et al. reported that marrow adipose tissue increased from 40% at age 30 to 68% at 100 years old with marked decrease in bone volume (90). In addition, alteration of MSC commitment is another factor in age-related bone loss. With aging, stromal cells obtained from human bone marrow have been shown to exhibit increased number of adipogenic cells coincident with a decline in the number of osteoblastic cells. This is in part explained by the enhanced expression of PPARγ with aging in the bone marrow, thereby favoring adipogenesis of MSCs while suppressing osteogenesis. The decreased expression of growth factors involved in osteogenesis such as TGF-β/BMP, Wnt/β-catenin, and IGF-I signaling pathways also likely cause a decrease in osteoblasts with aging (64). In addition to the alteration in cell fate determination, phenotypic changes of MSCs during aging process could also be responsible for age-related bone loss. For example, impairment of cell proliferation and differentiation, as well as chromosomal instabilities of MSCs have been implicated in long-term cell culture models (91).
B. Marrow Fat and Osteoporosis
It has been recognized that those who are suffering from osteoporosis exhibit greater infiltration of marrow adipocytes compared to age-matched controls (89) (92). In vitro experiments revealed that MSCs isolated form osteoporotic patients showed enhanced adipogenic capacity either basally or during early cell differentiation compared to control subjects (56, 85, 93, 94). This may be caused by a decrease in the proliferative capacity of MSCs of osteoporotic patients and an impaired mitogenic response to IGF-1 (94, 95). Another levels of regulation could reside in the altered differentiation capacity of MSCs toward adipogenesis away from osteogenesis. For example, MSCs derived from osteoporotic donors have diminished alkaline phosphatase activity and less mineralization when maintained in the osteogenic conditions. In addition to the intrinsic characteristic of MSCs involved in cell commitment and differentiation, it is recognized that locally produced growth factors like leptin, estrogens and fatty acids may be operative in the regulation of osteoblastogenesis. For example, in vitro studies confirmed that bone marrow stromal cells were responsive to leptin such that leptin stimulates osteoblastic differentiation of stromal cells (96–98) and suppresses adipogenesis of these cells (97, 99).
Endogenous levels of estrogen also play a crucial role in the development of marrow adiposity and bone loss. Not only does uncoupling of the bone remodeling units result from a decrease in estrogen levels especially after menopause, there is also a marked increase in marrow adiposity accompanied by a decline in bone mass (90, 100). This is in part explained by the direct effect of estrogen on the cell fate decision of MSCs toward either osteoblasts or adipocytes (101, 102). Importantly, regulation of aromatase activity may provide another level of regulation of bone mass by marrow adipocyets because a significant amount of aromatase is expressed in bone marrow stromal cells (103–108). Hence, it could be possible that locally produced androgens and estrogens in marrow adipocytes can exert regulatory actions on bone marrow cells including osteogenic cells. Studies during MSC differentiation point to the potential importance of local estrogen production and action for osteogenic and adipogenic commitment (102, 103), and as a negative regulator for adipogenesis (109, 110). All these observations support the hypothesis of a threshold estradiol level for normal skeletal remodeling (111, 112), which could be attained by both appropriate endogenous aromatase activity and estrogenic precursors.
VII. Significance of Marrow Adiposity
Attempts to delineate the exact function of marrow adipocytes especially with respect to its influence on skeletal homeostasis have met with significant barriers. Clearly, marrow adipocytes are critical components for the bone marrow microenvironment and affect the physiology of neighboring cells including hematopoietic cells and osteoblasts. But to clarify the role of marrow adipocytes it will be necessary to characterize the functional features of these cells. Currently available techniques including MRI with and without spectroscopy, micro-CT and histology are useful in quantitating the amount of marrow fat, but do not provide functional information about the type of adipocyte and its role in the marrow. However, accumulating evidence from expression profiling has provided a pleiotropic feature of marrow adipocytes, in which these cells may function in a way similar to white adipocytes or brown adipocytes in a context-specific manner. This concept could be extremely plausible because marrow adiposity is likely to have a dual effect on skeletal metabolism in an age-dependent manner; bone mass and marrow adiposity increases during puberty, whereas bone mass has an inverse correlation with marrow adiposity in the elderly. If marrow adipocytes have brown-like characteristics, it is conceivable that these cells create a favorable microenvironment for osteogenesis by functioning as a source of energy for osteogenesis or as a temperature modulator. Regardless of the mechanism, the consequences of marrow adiposity as it relates to structural integrity still need to be defined. With newer cell and tissue functional studies, the possibilities of answering these questions are enhanced.
VIII. Conclusions and Clinical Correlations About the Bone-Fat Connection
There is an intimate association between bone cells and adipocytes that begin with their shared origin. Adult adipose tissue has important regulatory circuits that modulate skeletal remodeling including the direct effect of adipokines and cytokines on bone cells and the indirect effect of leptin on the sympathetic nervous system via hypothalamic nuclei. Obesity has variable effects on skeletal integrity and ultimately on future fracture risk. In individuals with increased subcutaneous fat, cortical bone mass may be enhanced due in part to loading on the outer surface of the skeleton. On the other hand, increased visceral fat has been associated with decreased trabecular bone mass. This may be due to inflammatory cytokines that are released from visceral adipocytes, which differ from subcutaneous adipocytes both in origin and in function. Thus the inherent clinical question is whether obesity is a risk factor for osteoporosis. Observational studies are conflicted although there is emerging evidence that individuals with the metabolic syndrome, and hence increased visceral fat, have a greater risk of fracture despite normal bone density (113). Similarly in the MrOs cohort, most of the males with fractures that were studied were obese suggesting a potentially important gender specific effect of adiposity on skeletal integrity (114). Further studies are needed to define the precise relationship between obesity and osteoporosis. However, recent progress has been remarkable and there are likely to be newer therapeutic advances that could target both bone and fat to reduce the risk of both osteoporosis and obesity.
References
- 1.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue -- from signalling mechanisms to clinical implications. J Intern Med. 2007;262:431–438. doi: 10.1111/j.1365-2796.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 4.Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med. 2011;270:65–75. doi: 10.1111/j.1365-2796.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 5.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Fulzele K, Riddle RC, Digirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, et al. Insulin Receptor Signaling in Osteoblasts Regulates Postnatal Bone Acquisition and Body Composition. Cell. 142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 10.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 11.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 12.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stunes AK, Westbroek I, Gordeladze JO, Gustafsson BI, Reseland JE, Syversen U. Systemic Leptin Administration in Supraphysiological Doses Maintains Bone Mineral Density and Mechanical Strength Despite Significant Weight Loss. Endocrinology. 2012 doi: 10.1210/en.2011-1848. [DOI] [PubMed] [Google Scholar]
- 16.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 17.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, Wall BM. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24:131–153. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
- 20.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muscogiuri G, Chavez AO, Gastaldelli A, Perego L, Tripathy D, Saad MJ, Velloso L, Folli F. The crosstalk between insulin and renin-angiotensin-aldosterone signaling systems and its effect on glucose metabolism and diabetes prevention. Curr Vasc Pharmacol. 2008;6:301–312. doi: 10.2174/157016108785909715. [DOI] [PubMed] [Google Scholar]
- 23.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poretsky L, Glover B, Laumas V, Kalin M, Dunaif A. The effects of experimental hyperinsulinemia on steroid secretion, ovarian [125I]insulin binding, and ovarian [125I]insulin-like growth-factor I binding in the rat. Endocrinology. 1988;122:581–585. doi: 10.1210/endo-122-2-581. [DOI] [PubMed] [Google Scholar]
- 25.Palsgaard J, Emanuelli B, Winnay JN, Sumara G, Karsenty G, Kahn CR. Crosstalk between insulin and Wnt signaling in preadipocytes: Role of Wnt Co-receptor LDL receptor related protein-5 (LRP5) J Biol Chem. 2012 doi: 10.1074/jbc.M111.337048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 27.Shea MK, Gundberg CM, Meigs JB, Dallal GE, Saltzman E, Yoshida M, Jacques PF, Booth SL. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90:1230–1235. doi: 10.3945/ajcn.2009.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferron M, Wei J, Yoshizawa T, Del Fattore A, Depinho RA, Teti A, Ducy P, Karsenty G. Insulin Signaling in Osteoblasts Integrates Bone Remodeling and Energy Metabolism. Cell. 142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullo M, Moreno-Navarrete JM, Fernandez-Real JM, Salas-Salvado J. Total and undercarboxylated osteocalcin predict changes in insulin sensitivity and beta cell function in elderly men at high cardiovascular risk. Am J Clin Nutr. 2012;95:249–255. doi: 10.3945/ajcn.111.016642. [DOI] [PubMed] [Google Scholar]
- 31.Kayath MJ, Dib SA, Vieira JG. Prevalence and magnitude of osteopenia associated with insulin-dependent diabetes mellitus. J Diabetes Complications. 1994;8:97–104. doi: 10.1016/1056-8727(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 32.Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 33.Motyl KJ, McCauley LK, McCabe LR. Amelioration of type I diabetes-induced osteoporosis by parathyroid hormone is associated with improved osteoblast survival. J Cell Physiol. 2012;227:1326–1334. doi: 10.1002/jcp.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Paula FJ, Horowitz MC, Rosen CJ. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev. 2010;26:622–630. doi: 10.1002/dmrr.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paula FJ, Rosen CJ. Obesity, diabetes mellitus and last but not least, osteoporosis. Arq Bras Endocrinol Metabol. 2010;54:150–157. doi: 10.1590/s0004-27302010000200010. [DOI] [PubMed] [Google Scholar]
- 36.Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–190. 215–357, iii–iv. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 37.The 10-Year Cost-Effectiveness of Lifestyle Intervention or Metformin for Diabetes Prevention: An intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira FA, de Castro JA, dos Santos JE, Foss MC, Paula FJ. Impact of marked weight loss induced by bariatric surgery on bone mineral density and remodeling. Braz J Med Biol Res. 2007;40:509–517. doi: 10.1590/s0100-879x2007000400009. [DOI] [PubMed] [Google Scholar]
- 39.Scibora LM, Ikramuddin S, Buchwald H, Petit MA. Examining the link between bariatric surgery, bone loss, and osteoporosis: a review of bone density studies. Obes Surg. 2012;22:654–667. doi: 10.1007/s11695-012-0596-1. [DOI] [PubMed] [Google Scholar]
- 40.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karczewska-Kupczewska M, Straczkowski M, Adamska A, Nikolajuk A, Otziomek E, Gorska M, Kowalska I. Insulin sensitivity, metabolic flexibility, and serum adiponectin concentration in women with anorexia nervosa. Metabolism. 2010;59:473–477. doi: 10.1016/j.metabol.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 43.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aronis KN, Kilim H, Chamberland JP, Breggia A, Rosen C, Mantzoros CS. Preadipocyte factor-1 levels are higher in women with hypothalamic amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. J Clin Endocrinol Metab. 2011;96:E1634–E1639. doi: 10.1210/jc.2011-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95:407–413. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flemmer M, Scott J. Mechanism of action of thiazolidinediones. Curr Opin Investig Drugs. 2001;2:1564–1567. [PubMed] [Google Scholar]
- 47.Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M, Madiraju M, Jenkinson CP, Cersosimo E, Musi N, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52:723–732. doi: 10.1007/s00125-008-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutchman G, Promrat K, Kleiner DE, Heller T, Ghany MG, Yanovski JA, Liang TJ, Hoofnagle JH. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol. 2006;4:1048–1052. doi: 10.1016/j.cgh.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 50.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 51.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 52.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–976. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 55.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 58.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 59.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106:232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 61.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 62.Ichida F, Nishimura R, Hata K, Matsubara T, Ikeda F, Hisada K, Yatani H, Cao X, Komori T, Yamaguchi A, et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279:34015–34022. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 63.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 64.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep. 2004;5:1007–1012. doi: 10.1038/sj.embor.7400254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M, Pan LC, Simmons HA, Li Y, Healy DR, Robinson BS, Ke HZ, Brown TA. Surface-specific effects of a PPARgamma agonist, darglitazone, on bone in mice. Bone. 2006;39:796–806. doi: 10.1016/j.bone.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone) Calcif Tissue Int. 2004;75:329–337. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- 69.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 70.Wei W, Wang X, Yang M, Smith LC, Dechow PC, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bendixen AC, Shevde NK, Dienger KM, Willson TM, Funk CD, Pike JW. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1. Proc Natl Acad Sci U S A. 2001;98:2443–2448. doi: 10.1073/pnas.041493198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hounoki H, Sugiyama E, Mohamed SG, Shinoda K, Taki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Miyahara T. Activation of peroxisome proliferator-activated receptor gamma inhibits TNF-alpha-mediated osteoclast differentiation in human peripheral monocytes in part via suppression of monocyte chemoattractant protein-1 expression. Bone. 2008;42:765–774. doi: 10.1016/j.bone.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 74.Kawai M, Rosen CJ. Insulin-like growth factor-I and bone: lessons from mice and men. Pediatr Nephrol. 2009;24:1277–1285. doi: 10.1007/s00467-008-1040-6. [DOI] [PubMed] [Google Scholar]
- 75.Di Iorgi N, Mittelman SD, Gilsanz V. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 2008;32:1854–1860. doi: 10.1038/ijo.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gimble JM. The function of adipocytes in the bone marrow stroma. New Biol. 1990;2:304–312. [PubMed] [Google Scholar]
- 78.Tavassoli M. Marrow adipose cells and hemopoiesis: an interpretative review. Exp Hematol. 1984;12:139–146. [PubMed] [Google Scholar]
- 79.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 80.Payne MW, Uhthoff HK, Trudel G. Anemia of immobility: caused by adipocyte accumulation in bone marrow. Med Hypotheses. 2007;69:778–786. doi: 10.1016/j.mehy.2007.01.077. [DOI] [PubMed] [Google Scholar]
- 81.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Cornish J, MacGibbon A, Lin JM, Watson M, Callon KE, Tong PC, Dunford JE, van der Does Y, Williams GA, Grey AB, et al. Modulation of osteoclastogenesis by fatty acids. Endocrinology. 2008;149:5688–5695. doi: 10.1210/en.2008-0111. [DOI] [PubMed] [Google Scholar]
- 83.Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on Denosumab. J Oral Maxillofac Surg. 68:959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 85.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2011 doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huggins C, Blocksom BH. Changes in Outlying Bone Marrow Accompanying a Local Increase of Temperature within Physiological Limits. J Exp Med. 1936;64:253–274. doi: 10.1084/jem.64.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17:34–37. [PubMed] [Google Scholar]
- 89.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 90.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 91.Wagner W, Bork S, Lepperdinger G, Joussen S, Ma N, Strunk D, Koch C. How to track cellular aging of mesenchymal stromal cells? Aging (Albany NY) 2010;2:224–230. doi: 10.18632/aging.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–838. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 93.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- 94.Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–423. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez JP, Rios S, Fernandez M, Santibanez JF. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J Cell Biochem. 2004;92:745–754. doi: 10.1002/jcb.20119. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272:12897–12900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- 97.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 98.Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, Hjertner O, Gordeladze JO, Drevon CA. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. [DOI] [PubMed] [Google Scholar]
- 99.Hess R, Pino AM, Rios S, Fernandez M, Rodriguez JP. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem. 2005;94:50–57. doi: 10.1002/jcb.20330. [DOI] [PubMed] [Google Scholar]
- 100.Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- 101.Pino AM, Rodriguez JM, Rios S, Astudillo P, Leiva L, Seitz G, Fernandez M, Rodriguez JP. Aromatase activity of human mesenchymal stem cells is stimulated by early differentiation, vitamin D and leptin. J Endocrinol. 2006;191:715–725. doi: 10.1677/joe.1.07026. [DOI] [PubMed] [Google Scholar]
- 102.Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, Hunziker W, Weber P, Martin I, Bendik I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology. 2004;145:848–859. doi: 10.1210/en.2003-1014. [DOI] [PubMed] [Google Scholar]
- 103.Janssen JM, Bland R, Hewison M, Coughtrie MW, Sharp S, Arts J, Pols HA, van Leeuwen JP. Estradiol formation by human osteoblasts via multiple pathways: relation with osteoblast function. J Cell Biochem. 1999;75:528–537. doi: 10.1002/(sici)1097-4644(19991201)75:3<528::aid-jcb16>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- 104.Schweikert HU, Wolf L, Romalo G. Oestrogen formation from androstenedione in human bone. Clin Endocrinol (Oxf) 1995;43:37–42. doi: 10.1111/j.1365-2265.1995.tb01890.x. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka S, Haji M, Nishi Y, Yanase T, Takayanagi R, Nawata H. Aromatase activity in human osteoblast-like osteosarcoma cell. Calcif Tissue Int. 1993;52:107–109. doi: 10.1007/BF00308318. [DOI] [PubMed] [Google Scholar]
- 106.Sasano H, Uzuki M, Sawai T, Nagura H, Matsunaga G, Kashimoto O, Harada N. Aromatase in human bone tissue. J Bone Miner Res. 1997;12:1416–1423. doi: 10.1359/jbmr.1997.12.9.1416. [DOI] [PubMed] [Google Scholar]
- 107.Compston J. Local biosynthesis of sex steroids in bone. J Clin Endocrinol Metab. 2002;87:5398–5400. doi: 10.1210/jc.2002-021420. [DOI] [PubMed] [Google Scholar]
- 108.Issa S, Schnabel D, Feix M, Wolf L, Schaefer HE, Russell DW, Schweikert HU. Human osteoblast-like cells express predominantly steroid 5alpha-reductase type 1. J Clin Endocrinol Metab. 2002;87:5401–5407. doi: 10.1210/jc.2001-011902. [DOI] [PubMed] [Google Scholar]
- 109.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology. 2002;143:2349–2356. doi: 10.1210/endo.143.6.8854. [DOI] [PubMed] [Google Scholar]
- 111.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 112.Gennari L, Nuti R, Bilezikian JP. Aromatase activity and bone homeostasis in men. J Clin Endocrinol Metab. 2004;89:5898–5907. doi: 10.1210/jc.2004-1717. [DOI] [PubMed] [Google Scholar]
- 113.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 114.Javaid MK, Prieto-Alhambra D, Lui LY, Cawthon P, Arden NK, Lang T, Lane NE, Orwoll E, Barrett-Conner E, Nevitt MC, et al. Self-reported weight at birth predicts measures of femoral size but not volumetric BMD in eldery men: MrOS. J Bone Miner Res. 2011;26:1802–1807. doi: 10.1002/jbmr.411. [DOI] [PMC free article] [PubMed] [Google Scholar]