Abstract
Long-term planning to prevent malaria epidemics requires in-depth understanding of immunity to Plasmodium falciparum in areas of unstable transmission. Cytokine responses to immunodominant epitope peptides from liver stage antigen 1 (LSA-1) and thrombospondin-related adhesive protein (TRAP) were evaluated over a nine-month interval in adults and children in Kenya from a malaria epidemic–prone highland area after several years of low transmission. The proportion and magnitude of interferon-gamma ELISPOT responses and the proportion of interleukin-10 responders to LSA-1 and TRAP peptides tended to be higher in adults than children. Frequencies of interferon-gamma responders to these peptides were similar at the two time points, but responses were not consistently generated by the same persons. These results suggest that T cell memory to pre-erythrocytic stage malaria antigens is maintained but may be unavailable for consistent detection in peripheral blood, and that these antigens induce both pro-inflammatory and anti-inflammatory cytokine responses in this population.
INTRODUCTION
Over the past two decades, Plasmodium falciparum malaria epidemics in the highlands of east Africa have occurred with increasing frequency, with 34 million persons estimated to be at risk. 1 Long-term planning to prevent malaria epidemics in areas of unstable transmission must include vaccine development, which requires an in-depth understanding of anti-malarial immunity in these areas.
Pre-erythrocytic malaria vaccines have been tested in non-immune and semi-immune populations living in malaria-holoendemic areas. 2 Malaria vaccine trials typically use clinical and parasitologic endpoints to determine efficacy because these are relatively common events in holoendemic areas and can be easily measured in challenge studies in non-immune persons under controlled exposure conditions. Attempts to establish immunologic correlates of protection that could be used in lieu of clinical endpoints have yielded inconsistent results. 3 The inability to reach a consensus regarding immunologic correlates of protection against malaria may in part be caused by comparing responses generated in areas experiencing vastly different intensity of infections that could influence the development and maintenance of immunologic memory. The impact of concurrent asymptomatic malaria infections, parasite density, or reinfection genotype on malaria vaccine efficacy and the development of protective immunity have yet to be adequately addressed.
Those residing in malaria-holoendemic areas acquire anti-disease and/or anti-parasite immunity with age and cumulative exposure. 4 The development of anti-malarial immunity in holoendemic areas may differ compared with areas with sporadic transmission where periods between malaria epidemics may span years. 5 Examining T cell immunity to pre-erythrocytic antigens during a prolonged period of low or absent transmission is an important step in understanding the development and maintenance of T cell memory against malaria.
The importance of cytokines in mediating resistance to pre-erythrocytic stage malaria infections has been demonstrated in mouse models 6 and in observational studies of humans living in areas with stable malaria transmission 7–11 or those vaccinated with irradiated-sporozoites. 12 To date, only one study has directly compared cytokine responses from a malaria-holoendemic area to those generated in a highland population, 13 and only one study has examined protective cytokine response to malaria pre-erythrocyte stage antigens in epidemic-prone populations. 14 These studies demonstrated that adults had more robust cytokine responses to pre-erythrocytic antigens compared with children and that age and malaria transmission influenced recall responses. To better understand the maintenance of T cell memory to pre-erythrocytic malaria antigens in a highland area of western Kenya during an extended period of extremely low malaria transmission, we conducted a repeat cross-sectional study with a sub-group analysis in a prospective adult cohort. Immunogenic liver stage antigen 1 (LSA-1) and thrombospondin-related adhesive protein (TRAP) peptides were evaluated at two time points over a nine-month interval by interferon-gamma (IFN-γ) ELISPOT and interleukin-10 (IL-10) enzyme-linked immunosorbent assay (ELISA) for proportion, magnitude, and concordance of responders.
MATERIALS AND METHODS
Ethical review and informed consent
Review and approval of this study was obtained from the Ethical Review Committee for the Kenya Medical Research Institute (KEMRI) and the Institutional Review Board for Human Studies at University Hospitals of Cleveland, Case Western Reserve University (CWRU). Written informed consent was obtained from all participants ≥ 18 years of age and from guardians of participants younger than these ages.
Study population and surveillance for clinical malaria
Study participant recruitment was conducted in the sub-location of Kipsamoite, Nandi District, Rift Valley Province, Kenya, which is located in an epidemic-prone rural highland area with unstable malaria transmission. Altitude ranges from 1,910 meters to 2,154 meters. The de facto population in this study site was 3,200 persons. Immunologic measurements were conducted twice at a nine-month interval in asymptomatic persons. The first venous blood sample collection for immunologic studies was conducted in November 2000 and the second was conducted in August 2001. Study participants were enrolled at random with a subset re-captured during the second time point. Those enrolled in the immunologic studies were tested for P. falciparum malaria infection.
Malaria infection was diagnosed by microscopic inspection of thick and thin blood smears. Blood smears were prepared and stained with Giemsa, and slides examined by two experienced microscopists from the Division of Vector Borne Diseases of the Kenya Ministry of Health. A thick blood smear was considered negative when no parasites were observed after counting microscopic fields that included at least 200 leukocytes. The density of parasitemia was expressed as the number of asexual P. falciparum per microliter of blood assuming a leukocyte count of 8,000/μL.
Persons enrolled in the study in whom symptoms of malaria subsequently developed were asked to follow-up for evaluation and treatment of malaria at the local health center. Clinical malaria was defined as a having symptoms of malaria (history of fever, chills, headache, or severe malaise) accompanied by a positive blood stage infection by microscopy. A parasite density threshold was not used in our definition of clinical malaria because asymptomatic parasitemia is uncommon in this highland area. 15 All persons with clinical malaria were treated according to Kenyan Ministry of Health guidelines (sulfadoxine/pyrimethamine at the time of this study). Pregnant women and anyone who had been treated for malaria two weeks prior to venous blood collections were excluded from the analysis. Chart review of all persons attending the Kipsamoite Health Center who were given a clinical diagnosis of malaria by the clinical officer over a 72-month period from 1996 through 2002 was also performed.
Blood sample collection and processing
Adult (≥ 18 years of age) study participants donated 12–16 mL of venous blood and children (2–9 years of age) donated 5 mL of blood collected in sodium-heparin tubes (BD BioScience, San Jose, CA) for immunologic studies. Blood was collected on slides for microscopic determination of P. falciparum and other human Plasmodium species infections. Samples were transported at room temperature and processed the same day at the CWRU-KEMRI laboratory in Kisumu, Kenya. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density centrifugation then resuspended in complete RPMI 1640 medium containing 10% human AB serum, 10 mM L-glutamine, 10 μg/mL of gentamicin. Typical yields were 2 × 106 PBMC/mL of blood from children and 1 × 106 PBMC/mL of blood from adults. Sufficient PBMC for cytokine analyses were obtained from 91 adults and 36 children during the first collection and 114 adults and 85 children during the second collection. Of the 326 samples obtained, 28 had uninterruptible results because of high background and were not included in the analysis. The denominator for each cytokine assay and each stimulation condition is indicated on each table in the Results. Fifty-four adults and 28 children donated blood at both time points. The study population was composed of 48% females.
LSA-1 and TRAP peptides
Immunodominant LSA-1 and TRAP epitope peptides selected for this study had been previously shown to elicit both major histocompatibility complex (MHC) Class I and Class II T cell responses 6,8,16,17 ( Table 1 ), and selection of shorter peptides were further refined based on the MHC Class I molecular types present in our study population. 18 Peptides from LSA-1 and TRAP were synthesized using fluorenylmethyloxycarbonyl chemistry and purified by high-performance liquid chromatography to > 95% purity (Sigma Genosys, Gainesville, TX) for use in stimulation assays.
Table 1.
LSA-1 and TRAP peptides used for this study*
| Malaria antigen (amino acid position†) |
Amino acid sequence | HLA affinity | Abbreviation |
|---|---|---|---|
| LSA-1 (84-92) | LPM SNV KNV | HLA-B7 | ls84 |
| LSA-1 (94-102) | QTN FKS LLR | A3 supertype‡ | ls94 |
| LSA-1 (1881-1889) |
LFH IFD GDN | A2 supertype‡ | ls1881 |
| LSA-1 (1813-1835) |
NEN LDD LDE GIE KSS EEL SEE KI |
HLA Class II | T3 |
| TRAP (51-59) | LLM DCS GSI | HLA-A2.1/2.2 | tr51 |
| TRAP (539-547) | TPY AGE PAP | B7 supertype‡ | tr539 |
| TRAP (51-70) | LLM DCS GSI RRH NWV NHA VP |
Class I and II epitopes |
tp6 |
IFN-γ ELISPOT and IL-10 ELISA
The IFN-γ ELISPOT was performed, as previously described, after PBMCs were cultured at 37°C in an atmosphere of 5% CO2 for 5.5 days (approximately 132 hours). 8 The positive control was mitogen phytohemagglutinin (PHA) used at a concentration of 1 μg/mL. The negative control was phosphate-buffered saline (PBS). Plates were scanned using C. T. L. ImmunoSpot Scanning and Imaging Software Version 5.0 (Cellular Technology Ltd., Shaker Heights, OH) to count the number of spot-forming units (SFU) per well. Results are expressed as SFU per 106 PBMC. Persons without PHA responses were excluded from the analysis (n = 28). An IFN-γ ELISPOT result was considered positive if the proportion of SFU in the stimulated well was significantly greater than that of the unstimulated background well ( P < 0.05). The significance test was a chi-square comparison of two proportions adjusting for small sample size and assuming Poisson distribution.
The IL-10 responses were generated as previously described 8 after stimulating 2 × 105 PBMC/well with malaria peptides, PHA, or PBS. Cultures were incubated at 37°C in an atmosphere of 5% CO2 for 5.5 days (approximately 132 hours). Supernatants were collected and frozen at −20°C until IL-10 ELISAs were performed. Optical densities were measured at 405 nm using an OpsysMR™ Microplate Reader and analyzed using Revelation QuickLink software, version 4.04 (Dynex Technologies, Chantilly, VA). Cytokine values for the unstimulated background wells were subtracted from the stimulated wells to give a net response.
Malaria peptides were tested for IFN-γ and IL-10 responses under the same incubation conditions described above using PBMC obtained from malaria non-exposed control study participants in Cleveland, Ohio. None of the malaria-naive study participants generated an IFN-γ in response to malaria peptides. A pool of EBV peptides was used as positive 9-mer peptide controls and positive responses were observed in the both the malaria-exposed and malaria-naive volunteers. The cut-off value for a positive IL-10 response to a malaria peptide by study participants from Kenya was defined as greater than the mean plus 2 standard deviations of the values of malaria-unexposed controls. If no response above baseline was seen in negative control subjects, a cut-off value of 20 pg/mL was used. For the malaria peptides tested, cut-off values were T3, 20 pg/mL; ls84, 85 pg/mL; ls94, 43 pg/mL; ls1881, 51 pg/mL; and tr539, tr51, and tp6, 20 pg/mL.
Statistical analysis
A chi-square test of homogeneity was used to determine whether the proportion of children with IFN-γ and IL-10 responses were significantly different than that of the adults. A two-sided Wilcoxon (Mann-Whitney) rank sum test was used to determine whether one group had a lower IFN-γ precursor frequency or lower mean IL-10 response than another group. The correlation between responses to both cytokines was assessed by Spearman rank test. A chi-square test of homogeneity was used to determine whether the proportion of persons with cytokine responses was significantly different between time points. All analyses were conducted in SAS version 8.2 (SAS Institute, Carey, NC) or SPSS version 14.0 (SPSS Inc., Chicago, IL).
RESULTS
Cases of malaria and parasitemia prevalence in the highlands
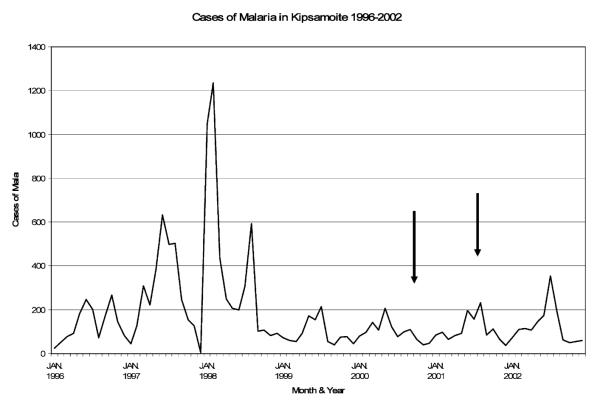
To establish that Kipsamoite experienced unstable malaria transmission, data on all patients presenting to the Kipsamoite Health Center from January 1996 through 2002 who were given a diagnosis of malaria either confirmed by microscopy or when microscopy was unavailable defined by clinical signs and symptoms (i.e., fever, headaches, chills, and severe malaise in the absence of an alternative clinical diagnosis) were collected ( Figure 1 ). These data demonstrate that malaria transmission in this highland area was sporadic with epidemics in February and August 1998, more than two years prior to the first immunologic study conducted in November 2000. There was no indication of an epidemic in the nine-month period that encompassed both collections (November 2000–August 2001), although the monthly number of malaria cases had increased by August 2001 ( Figure 1 ). The prevalence of asymptomatic P. falciparum parasitemia among study participants was 3.1% (5 of 159) in the first collection and 1.3% (3 of 232) in the second collection. Malaria cases were seen in low numbers in the years after the epidemics of 1997 and 1998, but were never completely absent from the area ( Figure 1 ), and asymptomatic parasitemia in this period was also infrequent. 15
Figure 1.
Cases of clinical malaria in the highland site of Kipsamoite, Kenya, 1996–2002 All persons diagnosed with malaria at the local health center in Kipsamoite during 1996–2002 are reported by month. Clinical malaria was defined either by documented fever accompanied by positive blood smear for Plasmodium falciparum parasites or by clinical diagnosis (in the absence of microscopy confirmation). Black arrows indicate when the immunologic measurements were conducted in November 2000 (time point 1) and August 2001 (time point 2) on a subset of residents enrolled in this study.
IFN-γ ELISPOT responses to pre-erythrocytic malaria peptides
To characterize immune responses to pre-erythrocyte stage malaria antigens (i.e., LSA-1 and TRAP) in this study population, we first compared the proportion of children and adults with IFN-γ ELISPOT responses at each time point ( Table 2 ). At first collection, the proportion of adults with IFN-γ responses to LSA-1 peptides tended to be higher than for children. This was not the case for the TRAP peptides tested. Differences that reached significance were IFN-γ responses to LSA-1 peptides ls94 (11% of children compared with 32% of adults; P = 0.017) and T3 (8% of children and 31% of adults; P = 0.008). However, during the second time point, almost three years after the last malaria epidemic, significantly fewer children had IFN-γ responses to all LSA-1 and TRAP peptides tested compared with adults ( Table 2 ).
Table 2.
Proportion of children and adults with IFN-γ ELISPOT responses to LSA-1 and TRAP*
| Age group comparisons |
Age group comparisons |
||||||
|---|---|---|---|---|---|---|---|
| LSA-1 peptides | % Children (n) | % Adults (n) | P | TRAP peptides | % Children (n) | % Adults (n) | P |
| ls84 | tr539 | ||||||
| Time point 1 | 14% (5/36) | 28% (24/88) | 0.110 | Time point 1 | 26% (8/31) | 14% (12/83) | 0.156 |
| Time point 2 | 5% (3/61) | 27% (26/98) | 0.001† | Time point 2 | ND | 28% (23/83) | ND |
| P | 0.121 | 0.909 | P | ND | 0.036* | ||
| ls94 | tr51 | ||||||
| Time point 1 | 11% (4/36) | 32% (28/88) | 0.017† | Time point 1 | 23% (5/22) | 22% (14/83) | 0.961 |
| Time point 2 | 8% (6/74) | 37% (36/98) | 0.001† | Time point 2 | 11% (8/74) | 34% (33/98) | 0.001† |
| P | 0.607 | 0.481 | P | 0.152 | 0.119 | ||
| ls1881 | tp6 | ||||||
| Time point 1 | 20% (7/35) | 21% (28/84) | 0.862 | Time point 1 | ND | ND | ND |
| Time point 2 | ND | 28% (25/90) | ND | Time point 2 | 14% (10/73) | 34% (33/98) | 0.003† |
| P | ND | 0.332 | P | ND | ND | ||
| T3 | |||||||
| Time point 1 | 8% (3/36) | 31% (27/88) | 0.008† | ||||
| Time point 2 | 9% (7/74) | 21% (21/98) | 0.038† | ||||
| P | 0.847 | 0.139 | |||||
IFN-γ = interferon-gamma; LSA-1 = liver stage antigen 1; TRAP = thrombospondin-related adhesive protein; ND = not done. A total of 93 adults and 66 children were enrolled in the first collection, of whom adequate cells were available for IFN-γ ELISPOT testing in 88 adults and 36 children. During the second collection, 114 adults and 119 children were enrolled, of whom of whom adequate cells were available for IFN-γ ELISPOT testing in 98 adults and 74 children. In some persons, there were insufficient peripheral blood mononuclear cells to test all antigen stimulation conditions, which was reflected accordingly in the denominator.
P < 0.05 is significant.
To determine if IFN-γ responses differed over time within each age cohort, we compared the proportion of responders to each peptide ( Table 2 ). The proportion of adults or children with IFN-γ responses did not significantly differ over time to any of the peptides tested with the exception of more adults responding to one TRAP peptide, tr539, at the second time point (14% versus 28%; P = 0.04). The cross-sectional frequency of IFN-γ responses was consistent over time for each age group. However, there was a non-significant trend for fewer children to respond to LSA-1 peptides at the second time point, rendering the difference observed between adults and children over time greater. There was no significant difference between cytokine responses of persons observed once or multiple times.
We then compared the magnitude of IFN-γ response for each peptide to determine if adults had a stronger response compared with children. The ELISPOTs not only determined who was responding but the magnitude of that response. This was ascertained by counting the number of SFU, which indicates how many cells are secreting IFN-γ. For this assessment, we combined positive responders from both time points because there was no significant difference in magnitude of responses between time points. Overall, adults had a significantly greater number of IFN-γ–secreting cells to all peptides compared with children ( P < 0.0001). When examining each peptide ( Figure 2 ), adults had significantly higher median IFN-γ levels compared with children for ls94 ( P = 0.013), tr51 ( P = 0.021), tr539 ( P = 0.026), and tp6 ( P = 0.038). The magnitude of IFN-γ ELISPOT responses did not differ significantly for ls84, ls1881, or T3 when comparing children with adults.
Figure 2.
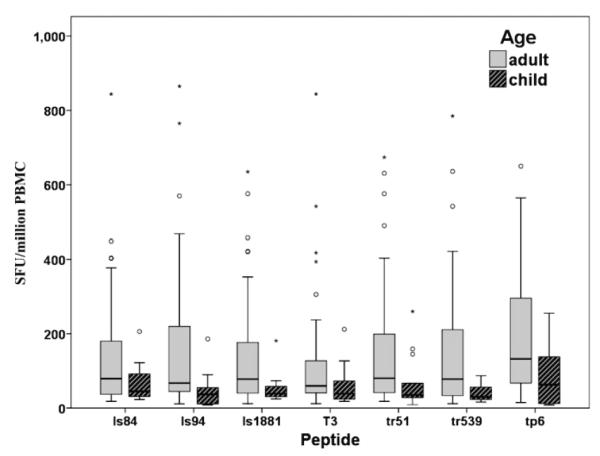
Magnitude of interferon-gamma (IFN-γ) ELISPOT responses to liver stage antigen 1 (LSA-1) and thrombospondin-related adhesive protein (TRAP) peptides for adults and children. Box plot distribution of the number of IFN-γ spot-forming units (SFU) per million peripheral blood mononuclear cells (PBMC) minus the phosphate-buffered saline (PBS) background (range = 0–5 SFU per well) for each peptide tested in adults and children with positive responses at either time point (because there was no significant difference in magnitude of response between time points, results were combined). Adults had significantly higher median IFN-γ levels compared with children for ls94 ( P = 0.013), tr51 ( P = 0.021), tr539 ( P = 0.026), and tp6 ( P = 0.038). The magnitude of IFN-γ ELISPOT responses did not significantly differ for ls84, ls1881 or T3 when comparing children with adults. Circles represent outliers above the 95th percentile and asterisks are extreme points ≥ 99th percentile.
IL-10 ELISA responses to pre-erythrocytic malaria peptides
To further characterize immune responses to pre-erythrocytic stage malaria peptides, we compared IL-10 responses between adults and children. Of those enrolled, after plating PBMC for IFN-γ ELISPOT assays, sufficient PBMC remained for parallel peptide stimulation and to perform IL-10 ELISAs on supernatants from 63 adults and 19 children during the first collection and on supernatants from 72 adults and 47 children during the second collection ( Table 3 ). The IL-10 responses tended to be less frequent for children than adults, but this difference reached significance only at the second time point for ls84 (3% versus 27%; P = 0.008) and tr51 (4% versus 22%; P = 0.008). When IL-10 responses were compared over time within each group, adults had significantly fewer responders at the second collection for the longer LSA-1 peptide, T3 (17–36%, P = 0.012). Otherwise, IL-10 responses did not significantly change over time within each age group, and there was no significant difference between cytokine responses of persons observed once or multiple times. Comparison of the magnitude of IL-10 ELISA responses between adults and children did not show a significant difference. The range of IL-10 responses for any malaria peptide was 90–200 pg/mL.
Table 3.
Proportion of children and adults with IL-10 ELISA responses to LSA-1 and TRAP*
| Age group comparisons |
Age group comparisons |
||||||
|---|---|---|---|---|---|---|---|
| LSA-1 peptides | % Children (n) | % Adults (n) | P | TRAP peptides | % Children (n) | % Adults (n) | P |
| ls84 | tr539 | ||||||
| Time point 1 | 5% (1/19) | 24% (15/63) | 0.074 | Time point 1 | 0% (0/6) | 29% (12/41) | 0.125 |
| Time point 2 | 3% (1/30) | 27% (17/64) | 0.008† | Time point 2 | ND | 37% (12/32) | ND |
| P | 0.739 | 0.721 | P | ND | 0.713 | ||
| ls94 | tr51 | ||||||
| Time point 1 | 15% (2/13) | 23% (11/48) | 0.556 | Time point 1 | ND | ND | ND |
| Time point 2 | 15% (7/47) | 22% (16/72) | 0.322 | Time point 2 | 4% (2/46) | 22% (16/72) | 0.008† |
| P | 0.965 | 0.929 | P | ND | ND | ||
| ls1881 | tp6 | ||||||
| Time point 1 | 0% (0/11) | 21% (9/44) | 0.096 | Time point 1 | ND | ND | ND |
| Time point 2 | ND | 17% (6/35) | ND | Time point 2 | 10% (4/42) | 23% (16/71) | 0.080 |
| P | ND | 0.673 | P | ND | ND | ||
| T3 | |||||||
| Time point 1 | 16% (3/19) | 36% (22/61) | 0.096 | ||||
| Time point 2 | 11% (5/47) | 17% (12/32) | 0.343 | ||||
| P | 0.562 | 0.012* | |||||
IL-10 = interleukin-10; ELISA = enzyme-linked immunosorbent assay; LSA-1 = liver stage antigen 1; TRAP = thrombospondin-related adhesive protein; ND = not done. A total of 93 adults and 66 children were enrolled in the first collection, of whom adequate cells were available after interferon-gamma (IFN-γ) ELISPOT for measuring, in parallel, IL-10 expression by ELISA in 63 adults and 19 children. During the second collection, 114 adults and 119 children were enrolled, of whom of whom adequate cells were available for antigen-specific IL-10 measurements in 72 adults and 47 children. In some persons, there were insufficient peripheral blood mononuclear cells to test all antigen stimulation conditions, which was reflected accordingly in the denominator.
P < 0.05 is significant.
Stability of cytokine responses to pre-erythrocytic stage malaria peptides in adults
The concordance of IFN-γ responses to LSA-1 and TRAP peptides over time was assessed in the subset of adults who were tested at both time points (54 adults). Stability of malaria-peptide specific cytokine response was determined using IFN-γ ELISPOT results for adults only because children had an overall decrease in responses over time, as shown in Table 2 , and the low number of repeat observations among this age group ( N = 28). IL-10 responses were too infrequent to determine stability by this method. Therefore, of the 54 adults tested at both time points, 39 had adequate cells and/or PHA responses to provide interpretable IFN-γ response results. The proportion of responders for each peptide in this subset of adults was similar to that reported for the entire adult group. Among those evaluated at both time points, persons were categorized as responders if they had an IFN-γ response at both time points. If they only responded during the first collection, they were considered to have lost their response if they failed to respond during the second collection. Conversely, those who did not respond during the first collection but did during the second collection were categorized as those who gained a response.
Stability of IFN-γ responses for all pre-erythrocytic peptides tested is summarized in Table 4. As would be expected with an overall increase in the proportion of responders for tr539 as seen in Table 2, 27% gained a response when assessing concordance. However, for those peptides that elicited the same proportion of responders at each time point, most of such responses were not generated by the same persons. For example, 28% of the adults had an IFN-γ response to ls84 during the first time point and 27% had a response to the same peptide nine months later ( Table 2 ). However, these responses were not generated by the same persons because only 8% maintained their response, 15% lost a response, and 23% gained a response. This instability of recall responses was observed for the other LSA-1 peptides and for one of the TRAP peptides (tr51).
Table 4.
Stability of IFN-γ ELISPOT responses in adults over a nine-month interval, Kenya*
| Category | % In each category for each peptide (n/total) |
|||||
|---|---|---|---|---|---|---|
| ls84 | ls94 | ls1881 | T3 | tp539 | tp51 | |
| Stable response | 8% (3/39) | 8% (3/38) | 3% (1/37) | 5% (2/39) | 3% (1/33) | 10% (3/31) |
| Lost response | 15% (6/39) | 18% (7/38) | 19% (7/37) | 23% (9/39) | 6% (2/33) | 10% (3/31) |
| Gained response | 23% (9/39) | 37% (14/38) | 30% (11/37) | 15% (6/39) | 27% (9/33) | 32% (10/31) |
| Non-responders | 54% (21/39) | 37% (14/38) | 49% (18/37) | 56% (22/39) | 64% (21/33) | 48% (15/31) |
IFN-γ = interferon-gamma.
DISCUSSION
Epidemiologic evidence suggests that the development of protective immunity to malaria under natural exposure conditions is dependent on age, intensity and duration of exposure. Studies of mice and humans vaccinated with pre-erythrocytic stage malaria antigens demonstrate that antigen-specific IFN-γ responses are associated with protection against challenge infection. 19–22 However, studies of naturally infected humans show that malaria-specific IFN-γ and IL-10 responses to pre-erythrocyte stage antigens do not consistently correlate with protection 10,14 and may be unstable and short-lived. 8,9 The low IL-10 levels measured in our highland malaria population support conclusions presented by others suggesting that IL-10 plays a role in preventing malaria-associated pathology 23–26 in contrast to preventing infections, which may explain in part why adults in epidemic-prone areas are susceptible to malaria-induced morbidity. Apparent inconsistencies in these studies may be caused by differences in malaria exposure intensities or distinctions between infection status versus severity of disease. In keeping with this conundrum, effector and memory T cell differentiation has been suggested to be dependent on signal strength, 27,28 with a higher dose of antigen being less efficient at generating memory T cells, whereas moderate antigen concentrations succeed.
In this prospective cohort study, we demonstrated that IFN-γ and IL-10 responses to LSA-1 and TRAP peptides can be recalled even after extended periods of low exposure to new malaria infections. These findings are consistent with our previous highland study of LSA-1 reporting similar age-dependent proportions of IFN-γ and IL-10 responders during the dry season. 14 The fact that the prevalence of IFN-γ responses did not wane but that responses were not stable in the same persons further suggests that T cell memory is induced but that the frequency of peptide-specific T cells may be too low to be consistently detectable and/or that memory T cells are sequestered in the lymph nodes awaiting recruitment into the periphery. Although there was no significant decrease in IFN-γ responses within age groups over time to any LSA-1 or TRAP peptide, there was a decrease from adults in IL-10 responses to LSA-1 peptide T3. This finding is consistent with that of our previous highland study, in which IL-10 responses to longer LSA-1 peptides decreased during a period of low transmission, 14 and suggests that IL-10 responses to pre-erythrocytic antigens may be less stable than IFN-γ responses. An alternate explanation could be the type of T cell (CD4 versus CD8) elicited by a longer peptide epitope, which may wane as antibodies develop. Our observation that LSA-1 and TRAP peptides stimulate different cytokine responses has implications for malaria vaccine development and immunogenic epitope selection. Differential cytokine expression upon stimulation with peptides to a malaria blood stage antigen, merozoite surface protein 1, 29 is consistent with our findings and supports the existence of functionally divergent or poly-functional malaria-specific T cell populations, as has been described in other infectious diseases. 30
Our findings that the cross-sectional prevalence of cytokine responses to LSA-1 or TRAP peptides in the study population is not generated by the same persons is consistent with the results of a similar study conducted in a nearby malaria holoendemic area. 8 If the two studies are compared, the proportion of highland adults with IFN-γ and IL-10 responses to LSA-1 and TRAP is similar to adults from the holoendemic area. However, the responses from highland children were significantly less frequent and less robust than responses from the children in the holoendemic area. The consistent finding of lower levels and frequencies of cytokine responses, particularly IFN-γ, to pre-erythrocytic antigens in children from areas of unstable transmission reinforces the premise that a threshold of exposure is required to induce a memory immune response. Curiously, the proportion and magnitude of cytokine responses was not greater for adults living in a malaria-holoendemic area than in a highland area, which suggests an upper threshold for the generation of these responses under natural exposure conditions.
As a repeated cross-sectional design, this study does not have the benefit of individual follow-up. However, this design is likely to be the type of population survey relevant to vaccine studies and trials. The authors acknowledge that statistical assumptions of independence are violated when some persons are observed in both time points. Because of the small sample size of repeat observations, it was decided not to report paired analyses. However, the results in Tables 2 and 3 primarily demonstrated a greater effect among adults relative to children and were consistent across strata of observation frequency and paired samples described in Table 4. Potential confounding factors such as bed net use and travel, which could affect risk of malaria and therefore immune response to malaria, were also not assessed, but in subsequent studies we have documented that these factors are infrequent in this population (John C, unpublished data). Another limitation of this study was insufficient resources to HLA genotype all our study participants and to directly assess HLA restriction of the epitope peptides used in our cytokine recall assays. One might argue that classifying peptides by HLA supertype families 31 might account for observed inconsistency in IFN-γ responses. However, IFN-γ recall instability was observed regardless of HLA affinity or CD4 compared with CD8 T cell response, making this an unlikely explanation for our findings. Given the breadth of Plasmodium epitopes presented to genetically variant host T cells, the challenge of consistently measuring such low frequency events remains.
Most malaria vaccines have been tested in either immunologically naive volunteers or residents of areas with some of the highest malaria transmission intensities in the world. 2 Thus, the findings from these studies may not apply to areas of unstable sporadic or highly seasonal transmission. In either vaccine-induced or naturally developed immunity, protection against malaria does not appear to be long lasting. The findings presented here demonstrate that even after prolonged periods of low malaria transmission, when the prevalence of P. falciparum blood stage infections was extremely low, cytokine responses to LSA-1 and TRAP remain detectable. The clinical significance of this finding is unclear because adults in this area have similar frequencies of IFN-γ responses to adults in areas of high transmission, yet adults in the highland area are far more susceptible to development of clinical malaria, as shown by the large number of these adults who developed clinical malaria during the malaria epidemic in 2002. 15,32 The IFN-γ responses in children in this area were also consistently lower than in adults. Together, the findings suggest that T cell memory to pre-erythrocytic antigens may occur in adults even in areas of low transmission, but that these responses are infrequent in children. This study provides further support for the idea that age and intensity of natural exposure should be taken into account when testing malaria vaccines in malaria-endemic populations, as well as consideration for different mediators of protection against diseases and infection.
Acknowledgments
We thank Dan Rosen (Centers for Disease Control and Prevention) for statistical assistance and Kiprotich Chelimo (Kenya Medical Research Institute) for laboratory assistance. This study was published with the permission of the Director of the Kenya Medical Research Institute.
Financial support: This study was supported by grants U01 AI 43906 (James W. Kazura) and K08 AI-01572 (Chandy C. John) from the National Institutes of Health.
Footnotes
Authors’ addresses: Ann M. Moormann, Paula Embury, Charles H. King, and James W. Kazura, Center for Global Health and Diseases, Case Western Reserve University, 2103 Cornell Road 4-130 Wolstein Research Building, Cleveland, OH 44106-7286, moorms@ case.edu, pbe@case.edu, chk@case.edu, and jxk14@case.edu. Peter Odada Sumba, Center for Global Health Research, Kenya Medical Research Institute, PO Box 1578, Kisumu, Kenya, odadaka sumba@yahoo.com. Daniel J. Tisch, Department of Epidemiology and Biostatistics, School of Medicine WG-37, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106-4945, daniel.tisch@case.edu. Chandy C. John, Global Pediatrics Program, University of Minnesota Medical School, 717 Delaware Street, SE, Room 363, MMC #1932, Minneapolis, MN 55455, ccj@umn.edu.
REFERENCES
- 1.Cox J, Hay SI, Abeku TA, Checchi F, Snow RW. The uncertain burden of Plasmodium falciparum epidemics in Africa. Trends Parasitol. 2007;23:142–148. doi: 10.1016/j.pt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vekemans J, Ballou WR. Plasmodium falciparum malaria vaccines in development. Expert Rev Vaccines. 2008;7:223–240. doi: 10.1586/14760584.7.2.223. [DOI] [PubMed] [Google Scholar]
- 3.Richie T. High road, low road? Choices and challenges on the pathway to a malaria vaccine. Parasitology. 2006;133(Suppl):S113–S144. doi: 10.1017/S0031182006001843. [DOI] [PubMed] [Google Scholar]
- 4.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 5.Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, Snow RW. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002;8:543–548. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doolan DL, Hoffman SL. Pre-erythrocytic-stage immune effector mechanisms in Plasmodium spp. infections. Philos Trans R Soc Lond B Biol Sci. 1997;352:1361–1367. doi: 10.1098/rstb.1997.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connelly M, King CL, Bucci K, Walters S, Genton B, Alpers MP, Hollingdale M, Kazura JW. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect Immun. 1997;65:5082–5087. doi: 10.1128/iai.65.12.5082-5087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moormann AM, John CC, Sumba PO, Tisch D, Embury P, Kazura JW. Stability of interferon-gamma and interleukin-10 responses to Plasmodium falciparum liver stage antigen-1 and thrombospondin-related adhesive protein in residents of a malaria holoendemic area. Am J Trop Med Hyg. 2006;74:585–590. [PubMed] [Google Scholar]
- 9.Flanagan KL, Mwangi T, Plebanski M, Odhiambo K, Ross A, Sheu E, Kortok M, Lowe B, Marsh K, Hill AV. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am J Trop Med Hyg. 2003;68:421–430. [PubMed] [Google Scholar]
- 10.Kurtis JD, Hollingdale MR, Luty AJ, Lanar DE, Krzych U, Duffy PE. Pre-erythrocytic immunity to Plasmodium falciparum : the case for an LSA-1 vaccine. Trends Parasitol. 2001;17:219–223. doi: 10.1016/s0169-4758(00)01862-7. [DOI] [PubMed] [Google Scholar]
- 11.Luty AJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Ulbert S, Migot-Nabias F, Dubois B, Deloron P, Kremsner PG. Parasite antigen-specific interleukin-10 and antibody reponses predict accelerated parasite clearance in Plasmodium falciparum malaria. Eur Cytokine Netw. 1998;9:639–646. [PubMed] [Google Scholar]
- 12.Hoffman SL, Isenbarger D, Long GW, Sedegah M, Szarfman A, Waters L, Hollingdale MR, van der Meide PH, Finbloom DS, Ballou WR. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 1989;244:1078–1081. doi: 10.1126/science.2524877. [DOI] [PubMed] [Google Scholar]
- 13.John CC, Moormann AM, Sumba PO, Ofulla AV, Pregibon DC, Kazura JW. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun. 2004;72:5135–5142. doi: 10.1128/IAI.72.9.5135-5142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John CC, Sumba PO, Ouma JH, Nahlen BL, King CL, Kazura JW. Cytokine responses to Plasmodium falciparum liver-stage antigen 1 vary in rainy and dry seasons in highland Kenya. Infect Immun. 2000;68:5198–5204. doi: 10.1128/iai.68.9.5198-5204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg. 2005;99:780–786. doi: 10.1016/j.trstmh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Aidoo M, Lalvani A, Allsopp CE, Plebanski M, Meisner SJ, Krausa P, Browning M, Morris-Jones S, Gotch F, Fidock DA. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet. 1995;345:1003–1007. doi: 10.1016/s0140-6736(95)90754-8. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan KL, Lee EA, Gravenor MB, Reece WH, Urban BC, Doherty T, Bojang KA, Pinder M, Hill AV, Plebanski M. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J Immunol. 2001;167:4729–4737. doi: 10.4049/jimmunol.167.8.4729. [DOI] [PubMed] [Google Scholar]
- 18.Cao K, Moormann AM, Lyke KE, Masaberg C, Sumba OP, Doumbo OK, Koech D, Lancaster A, Nelson M, Meyer D, Single R, Hartzman RJ, Plowe CV, Kazura J, Mann DL, Sztein MB, Thomson G, Fernandez-Vina MA. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens. 2004;63:293–325. doi: 10.1111/j.0001-2815.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 19.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Charoenvit Y, Corradin G, de la Vega P, Franke ED, Hoffman SL. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 21.Ong’echa JM, Lal AA, Terlouw DJ, Ter Kuile FO, Kariuki SK, Udhayakumar V, Orago AS, Hightower AW, Nahlen BL, Shi YP. Association of interferon-gamma responses to pre-erythrocytic stage vaccine candidate antigens of Plasmodium falciparum in young Kenyan children with improved hemoglobin levels: XV. Asembo Bay Cohort Project. Am J Trop Med Hyg. 2003;68:590–597. doi: 10.4269/ajtmh.2003.68.590. [DOI] [PubMed] [Google Scholar]
- 22.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 23.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 24.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis. 2002;185:971–979. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 25.Kurtzhals JA, Adabayeri V, Goka BQ, Akanmori BD, Oliver-Commey JO, Nkrumah FK, Behr C, Hviid L. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 26.Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 27.Lefrancois L. Dual personality of memory T cells. Trends Immunol. 2002;23:226–228. doi: 10.1016/s1471-4906(02)02190-7. [DOI] [PubMed] [Google Scholar]
- 28.Pearce EL, Shen H. Making sense of inflammation, epigenetics, and memory CD8+ T-cell differentiation in the context of infection. Immunol Rev. 2006;211:197–202. doi: 10.1111/j.0105-2896.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 29.Malhotra I, Wamachi AN, Mungai PL, Mzungu E, Koech D, Muchiri E, Moormann AM, King CL. Fine specificity of neonatal lymphocytes to an abundant malaria blood-stage antigen: epitope mapping of Plasmodium falciparum MSP133. J Immunol. 2008;180:3383–3390. doi: 10.4049/jimmunol.180.5.3383. [DOI] [PubMed] [Google Scholar]
- 30.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 31.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 2008;9:1. doi: 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baird JK. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol. 1998;92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]