Abstract
Background
β-glucans are fungal cell wall components that bind to the C-type lectin-like receptor dectin-1. Polymorphisms of dectin-1 gene are associated with susceptibility to invasive fungal infection and medically refractory ulcerative colitis. The purpose of this study has been addressing the response of human macrophages to β-glucans under different conditions mimicking the composition of the inflammatory milieu in view of the wide plasticity and large range of phenotypical changes showed by these cells, and the relevant role of dectin-1 in several pathophysiological conditions.
Principal Findings
Serum-differentiated macrophages stimulated with β-glucans showed a low production of TNFα and IL-1β, a high production of IL-6 and IL-23, and a delayed induction of cyclooxygenase-2 and PGE2 biosynthesis that resembled the responses elicited by crystals and those produced when phagosomal degradation of the phagocytic cargo increases ligand access to intracellular pattern recognition receptors. Priming with a low concentration of LPS produced a rapid induction of cyclooxygenase-2 and a synergistic release of PGE2. When the differentiation of the macrophages was carried out in the presence of M-CSF, an increased expression of dectin-1 B isoform was observed. In addition, this treatment made the cells capable to release arachidonic acid in response to β-glucan.
Conclusions
These results indicate that the macrophage response to fungal β-glucans is strongly influenced by cytokines and microbial-derived factors that are usual components of the inflammatory milieu. These responses can be sorted into three main patterns i) an elementary response dependent on phagosomal processing of pathogen-associated molecular patterns and/or receptor-independent, direct membrane binding linked to the immunoreceptor tyrosine-based activation motif-bearing transmembrane adaptor DNAX-activating protein 12, ii) a response primed by TLR4-dependent signals, and iii) a response dependent on M-CSF and dectin-1 B isoform expression that mainly signals through the dectin-1 B/spleen tyrosine kinase/cytosolic phospholipase A2 route.
Introduction
Classical distinction between macrophage types includes the type M1 inflammatory macrophage and the M2 regulatory macrophage. In cultures starting from peripheral blood monocytes, supplementation with human serum, a source of M-CSF but not GM-CSF [1], produces differentiated but non-polarized macrophages (M0 type), and addition of different cytokine cocktails elicit polarization versus the M1 or M2 type. One of the most used macrophage stimuli are zymosan particles. These contain β-glucans and α-mannans, and activate macrophages through different receptors, among which dectin-1 (encoded by the clec7a gene) is the most important receptor for β-glucans [2]. The response of macrophages to β-glucans has mainly been studied in rodents and few reports have been conducted in human macrophages, even though dectin-1 plays a relevant role in human disease since polymorphisms of clec7a are associated with an increased risk of fungal infection [3] and medically refractory ulcerative colitis [4]. The expression of dectin-1 is widely distributed in myeloid cells and is modulated by cytokines and microbial products [5], [6]. Transcription from human clec7a gives rise to the expression of several isoforms of the receptor [5], but a survey of dectin-1 expression along the differentiation of human macrophages has not been conducted yet. This is of pathophysiological relevance since monocytes can display different responses to β-glucans [7], [8]. Systematic studies in mouse macrophages have disclosed that the response to β-glucans depends on the activation state of the cell and, in general, BMDM are poor responders in spite of the expression of dectin-1, thus making the response dependent on myeloid cell programming [9]. Whereas GM-CSF and IFNγ allow for the release of TNFα in response to β-glucans, mouse macrophages differentiated with M-CSF did not produce cytokines [10]. Although a complete mechanistic explanation for these findings is not available, this points to a cell-type specific variability of CARD9-mediated NF-κB activation downstream of dectin-1 ligation, although other dectin-1 associated signals are preserved. Dectin-1 cooperates with other receptors, for instance, CR3 [11], TLR2 [12], [13], DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN) [14], and galectin-3 [15]. Cooperation of dectin-1 and TLR2 is of particular relevance since the sole stimulation of mouse thioglycollate-elicited macrophages with purified β-glucans fails to induce cytokine production even though spleen tyrosine kinase (Syk) activation occurs, whereas combined stimulation of dectin-1 and TLR2 elicits a robust response in a MyD88 and Syk-dependent manner [16]. The case of CR3 is also relevant since it may engage β-glucans through its C-lectin-like domain and it also binds β-glucan particles that have been opsonized with CR3 through the I domain. In fact, opsonisation of zymosan is widely used to induce productive binding in polymorphonuclears and monocytes to study the release of lipid mediators [8], [17] and complement is of central importance for neutrophil response to Candida infection [18]. CR3 signaling involves the recruitment of adaptors containing immunoreceptor tyrosine-based activation motifs (ITAM) such as DNAX-activating protein (DAP)12 and Fc receptor γ-chain, and the tyrosine kinase Syk [19]. Notably, this mechanism mimics dectin-1 signaling that depends on its own ITAM.
A new scenario has emerged after disclosing the involvement of PGE2 in the induction of Th2 type immune responses by particulate crystal-like materials [20]. In fact, alum and silica induce the production of PGE2 through a receptor-independent mechanism triggered from phagolysosomes that leads to the production of antigen-specific serum IgE. This mechanism is also triggered by sodium monourate particles [21] and hemozoin crystals [22], and mimics the reported activation of Syk by receptor-independent, direct membrane binding of monosodium urate crystals in dendritic cells (DC) [23]. In addition to the significance of these findings to vaccine technology, these results are relevant to understand the pathogenic mechanism underlying the inflammation induced by microcrystals in gouty arthritis and silicosis [24] and highlight the complexity of the process of recognition of particles bearing pathogen-associated molecular patterns (PAMP), which may include receptor-dependent and independent mechanisms, and is influenced by receptor expression, the state of differentiation of the cell, and the presence of molecules that affect receptor function.
In this study, we have observed that stimulation of putative M0 macrophages with zymosan induces COX-2 expression, release of PGE2, IL-6, and IL-23, and activation of the NF-κB system in a way that suggests the involvement of phagosomal processing and may be primed by low concentrations of LPS and interferons. Differentiation of monocytes with M-CSF produced an increase of dectin-1 B isoform expression, an increased capacity to release arachidonic acid (AA), and a parallel decrease of the production of IL-6 and IL-23. These results disclose three different patterns of response to zymosan, the features of which are: i) a response consistent with phagosomal processing, ii) a response primed by LPS and cytokines, and iii) a response associated with an enhancement of dectin-1 B isoform expression by M-CSF that mainly conveys signals through the dectin-1/Syk/cPLA2 route.
Materials and Methods
Ethic Statement
The study was approved by the Bioethical Committee of the Spanish Council of Research (CSIC) and the written informed consent of all healthy donor subjects was obtained at Centro de Hemoterapia y Hemodonación de Castilla y León Biobank. The participants received written consent according to the regulations of the Biobank. The researchers received the samples in an anonimous way. The process is documented by the Biobank authority according to the specific Spanish regulations. The ethics committee approved this procedure before starting the study.
Cells and Reagents
Monocytes were isolated from pooled buffy coats of healthy volunteer donors. Differentiation of monocytes into macrophages was carried out by culture in the presence of 5% human serum for seven days or in 10% fetal bovine serum, supplemented with 10 ng/ml M-CSF. Human monocyte-derived dendritic cells (DC) were obtained by culture in the presence of GM-CSF (800 U/ml) and IL-4 (500 U/ml) for 5 days and differentiation assessed by immunofluorescence flow cytometry of CD40, CD80, CD83 and CD86 as reported [14]. Mouse macrophages were obtained from the bone-marrow of dectin-1−/− and wild type (WT) animals in a CB57/BL6 background [25] by differentiation in the presence of murine M-CSF. Zymosan from Saccharomyces cerevisiae, mannan, laminarin, latex beads, and LPS were from Sigma Chemical Co. (St. Louis, MO). Depleted zymosan was from InvivoGen (San Diego, CA). Pure β-glucan was obtained from Dr. David L. Williams from East Tennessee State University. Endotoxin levels in the reagents were below 1 ng/ml as determined by the Limulus Amebocyte Lisate assay (Cambrex Bio Sciences, Walkersville, MD). Moreover, addition of 200 µg/ml polymyxin B did not modify the effect of stimuli other than LPS, what negates the possible involvement of LPS in the responses to β-glucans.
Coating of C3bi to zymosan was conducted by incubation with human serum. TNFα, IL-1β, IL-23, and IL-6 were assayed by ELISA. The expression of the different isoforms of dectin-1 was carried out by RT-PCR and the PCR products identified by DNA sequencing on both strands. The sequences of the primers are shown in Table 1.
Table 1. Oligonucleotide primers used for real time RT-PCR and ChIP.
| Gene | Sequence (5′-3′) | GeneBank AC |
| clec7a (dectin-1) exon 1 forward | GGGCTCTCAAGAACAATGGA | AF400596 |
| exon 6 reverse | TTGGAGATGGGTTTTCTTGG | |
| exon 5 reverse | CCCAGAGCCATGGTACCT | |
| CD209 (DC-SIGN) forward | AGGTCCCCAGCTCCATAAGT | NM_021155 |
| reverse | TCTCTGGAAGCTCACCCACT | |
| tlr2 (TLR2) forward | GCCAAAGTCTTGATTGATTGG | NM_003264 |
| reverse | TTGAAGTTCTCCAGCTCCTG | |
| mrc1 forward | GCTGAACCTGGAAAAAGCTG | NM_002438 |
| reverse | ACGAAGCCATTTGGTAAACG | |
| gapdh forward | GTCAGTGGTGGACCTGACCT | NM_002046.3 |
| reverse | AGGGGAGATTCAGTGTGGTG | |
| ptgs2 promoter forward | AGGAGAGGGAGGGATCAGAC | D28235 |
| reverse | TTTACCCACGGAAATGAGAAA |
mrc1 stands for the mannose receptor gene.
AA Release Assay and Prostaglandin E2 (PGE2) Production
Radioactive labeling with [3H]AA was performed by overnight incubation in the presence of 0.25 µCi/ml [3H]AA in 0.25% essentially fatty acid-free BSA. After labeling, cells were washed and equilibrated at 37°C in medium containing 1% BSA, before the addition of agonists or vehicle. The release of [3H]AA into the culture medium after 60 min stimulation was measured by scintillation counting. PGE2 was assayed with Biotrack ELISA system. The characterization of PGE2 was carried out by reversed phase ultra performance liquid chromatography and electrospray-quadrupole-time-of-flight-mass spectrometry (Materials S1).
Assays for Phagocytosis and Flow Cytometry
For the assay of phagocytosis, cells were incubated with zymosan particles conjugated with Alexa Fluor® 488 at the concentration of five particles per cell at 37°C for the times indicated. Subsequently, cells were washed, treated with 100 units/ml lyticase in phosphate-buffered saline (PBS) for 10 min at room temperature in order to dissolve extracellular zymosan, and then resuspended in 500 µl of PBS supplemented with 0.05% sodium azide and 1 mM EDTA for analysis by flow cytometry [7]. For the assay of the expression of receptors on the cell membranes, adherent cells were scraped and centrifuged for 5 min at 350×g and resuspended in PBS. Ab was added at the concentration of 0.5 µg for 5.105 cells and incubated for 45 min at 4°C. When the Ab were labelled with FITC, cells were washed and fixed in 1% formaldehyde. In the case of non labelled Ab, indirect immunofluorescence was carried out using a FITC-labeled secondary Ab, before washing and formaldehyde fixation steps. Isotype-matched irrelevant Ab were used as control. The analysis was performed in a Gallios Flow Cytometer (Becton Dickinson). At least 10,000 cells were analyzed per sample. Kaluza software version 1.1 and FlowJo software were used for quantitative data analysis and preparation of overlay histograms.
Immunoblots and Immunoprecipitations
Samples were loaded on a 10% SDS-PAGE system. Proteins were transferred to nitrocellulose membranes and the membranes used for immunodetection of cyclooxygenase-2 (COX-2) (Santa Cruz sc-1745, Ab), cytosolic phospholipase A2 (cPLA2), (Cell Signaling # 2832, Ab) and phospho-Syk (Santa Cruz sc-2711, Ab) using the Amersham Biosciences ECL system. β-actin and TATA-box binding protein (TBP) were used as a load control. For the assay of nuclear proteins, the nuclear extracts were obtained with an extraction kit (Active Motif). The membranes were used for the detection of RelA/p65, c-Rel, and p50. Immunoprecipitations were carried out as described [14] using anti-DAP12 Ab (Santa Cruz sc-20783), anti-CD32A Ab (abcam ab41899), and anti-Fc receptor γ-chain Ab (Millipore # 06-727) Ab. Briefly, cells were lysed in a medium containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 1 mM Na3VO4, 10 µg/ml aprotinin and leupeptin, 100 µg/ml soybean trypsin inhibitor, and 1 mM PMSF and clarified by centrifugation at 15,000 rpm for 20 min. The clarified lysates were preabsorbed on protein G-Sepharose and then incubated with precipitating mAb for 4 hours, followed by overnight incubation with protein G-Sepharose beads. Immune complexes were extensively washed, suspended in Laemmli sample buffer and subjected to SDS/PAGE. Blots were stained to assess the tyrosine phosphorylation of FcR-γ-chain, DAP12, and CD32A with anti-phosphotyrosine, clone 4G10 (Millipore # 05-321) mAb.
Cromatin Immunoprecipitation (ChIP) Assay
ChIP assays were conducted with reagents and Ab against c-Rel (Santa Cruz sc-70), RelA/p65 (# 06-418), and Ac-14K-histone H3 (# 07-353) from Upstate Biotechnology as previously reported [26]. Briefly, cells were stimulated and then washed twice with PBS and fixed with 1% formaldehyde. Cross-linking was terminated by 0.125 M glycine. Crude nuclear extracts were collected by microcentrifugation and resuspended in a lysis buffer containing a high salt concentration. Chromatin sonication was carried out using a Bioruptor device from Diagenode (Diagenode, Liege, Belgium). The chromatin solution was precleared by adding Protein A/G PLUS-Agarose for 30 min at 4°C under continuous rotation. After elimination of the beads, Ab was added for overnight incubation al 4°C, and then Protein A/G PLUS-Agarose was added and incubated for an additional period of 2 hours at 4°C. Beads were harvested by centrifugation at 12,000 rpm and sequentially washed with lysis buffer high salt, wash buffer, and elution buffer. Cross-links were reversed by heating at 67°C in a water bath, and the DNA bound to the beads isolated by extraction with phenol/chloroform/isoamylalcohol. PCR reactions were carried out with primers designed from the pgts2 promoter (Table 1).
Real-time RT-PCR
Purified RNA was used for RT reactions. The resulting cDNA was amplified in a PTC-200 apparatus equipped with a Chromo4 detector (BioRad) using SYBR Green I mix containing HotStart polymerase (ABgene). Cycling conditions were adapted to each set of primers. gadph was used as a housekeeping gene to assess the relative abundance of the different mRNA, using the comparative CT (circle threshold) method.
Statistical Analysis
Data are represented as the mean ± S.D. and were analyzed with the Prism 4.0 statistical program (GraphPad Software). Comparison between two experimental groups was carried out using the Student t test. Differences were considered significant for p<0.05.
Results
Particle Uptake and AA Release
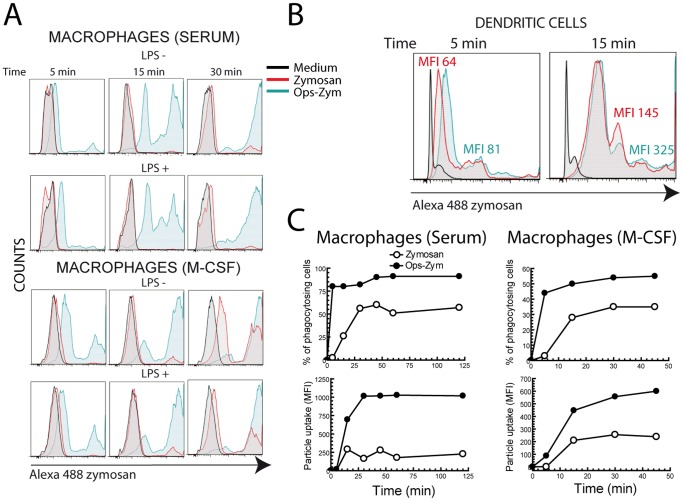
Monocyte-derived macrophages differentiated with serum showed a limited uptake of zymosan particles (Figure 1A). Opsonisation of the particles with fresh serum increased the uptake to the levels observed in DC, which phagocytose both zymosan and opsonized zymosan efficiently (Figure 1B and 1C). The uptake of opsonized zymosan reached maximal levels at ∼30 min as assessed by both the percentage of cells showing phagocytosis and the increase of mean fluorescence intensity (MFI), which is an indicator of the number of particles taken up by each cell. Particle uptake was not affected by priming with 10 ng/ml LPS (Figure 1A) and only slightly by differentiation with M-CSF (Figure 1B, lower panels, and 1C, right panels). Stimulation of monocytes and serum-differentiated macrophages with zymosan, depleted zymosan, and pure β-glucan particles induced a negligible release of [3H]AA (Figure 2A and B). In contrast, zymosan produced a significant release of [3H]AA in both macrophages differentiated with M-CSF and in DC (Figure 2C and D). Notably, whereas zymosan opsonisation did not significantly increase the ability of zymosan to release [3H]AA in DC, opsonisation did increase [3H]AA release in macrophages (Figure 2A–D).
Figure 1. Zymosan uptake by macrophages and DC.
(A, B and C) Macrophages and DC were incubated with Alexa Fluor® 488-labeled zymosan at the concentration of 5 particles per cell at 37°C for the times indicated and the uptake of particles assayed by flow cytometry. Results in (A) show representative experiments in macrophages differentiated in the presence of human serum or M-CSF for seven days and then treated for 3 hours with 10 ng/ml LPS or left untreated prior to the addition of zymosan particles. (B) Zymosan uptake by DC. Experiments in (C) were conducted to compare percentage of phagocytosing cells and MFI in serum and M-CSF differentiated macrophages. Ops-Zym indicates serum opsonised zymosan. Results show a representative experiment of three independent ones with a similar trend.
Figure 2. Release of [3H]AA.
Cells were differentiated and stimulated as indicated. Anti-CD32A antibody was used at the concentration of 10 µg/ml 30 min prior to the addition of the stimuli. Zymosan (Zym), Ops-Zym, depleted zymosan (Dep-Zym), and β-glucan (β-glu) particles were used at the concentration of 1 mg/ml. Immune complexes (IC) were used at the concentration of 100 µg/ml. Results represent mean ± S.D. of 5 to 6 independent experiments. *Indicates p<0.05.
PGE2 Production and COX-2 Induction
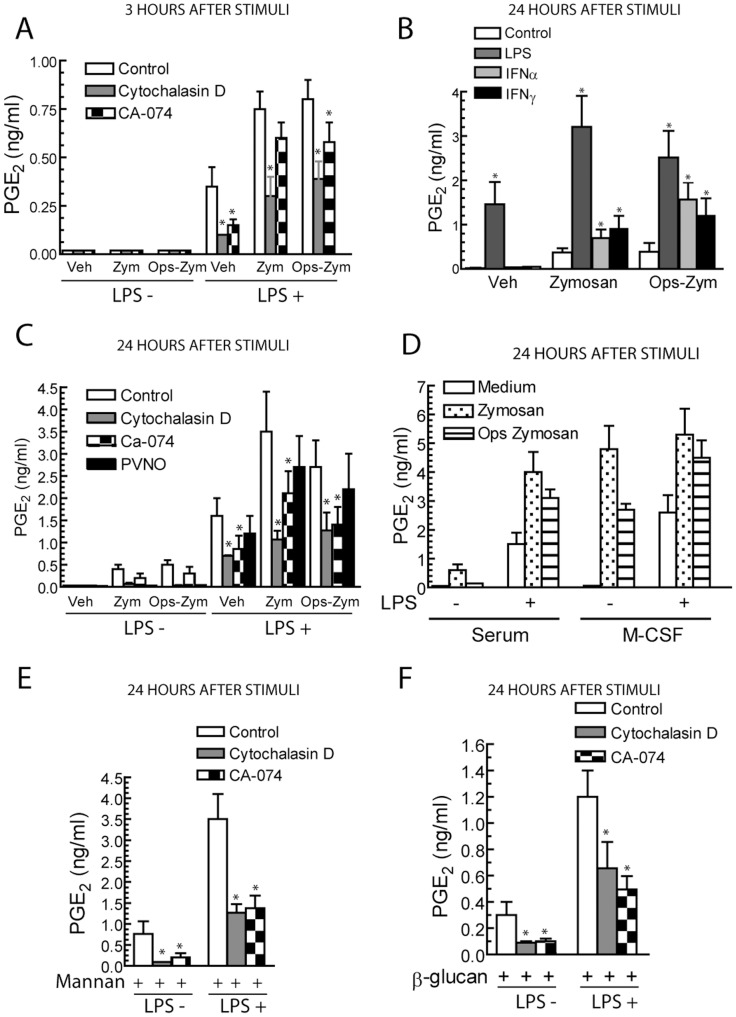
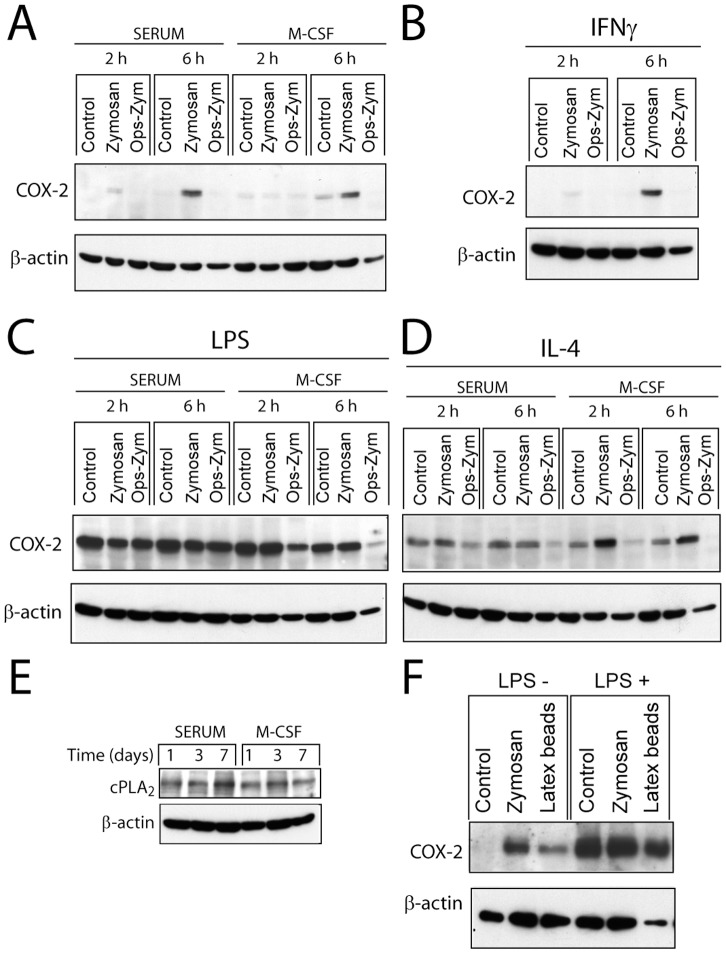
Stimulation of serum-differentiated macrophages with zymosan for 3 hours did not induce the production of PGE2, but concentrations of ∼0.5 ng/ml were detected at 24 hours. Priming with LPS increased the production of PGE2 (Figure 3A–C), thus suggesting that low concentrations of LPS elicit a synergistic effect. Priming with IFNα and IFNγ also increased the production of PGE2 (Figure 3B). In the case of macrophages differentiated with M-CSF, the production of PGE2 was enhanced (Figure 3D), but it did not show further significant increase by LPS priming. Since zymosan contains both α-mannan and β-glucan polymers, additional experiments were carried out with mannan and β-glucan. LPS showed an enhancing effect similar to that observed with zymosan particles (Figure 3E and F). Altogether, these results indicate that the response to zymosan is synergistically enhanced by stimuli used to generate type M1 polarized macrophages and by M-CSF. Since the production of PGE2 depends on the activity of two enzymes: the constitutively expressed COX-1 and the inducible COX-2, the expression of COX-2 was addressed. As shown in Figure 4A and B, zymosan induced the expression of COX-2 at 6 hours. Unlike M-CSF and IFNγ, priming with LPS induced a strong induction of COX-2 protein (Figure 4C). IL-4, which is used to induce macrophage M2 type differentiation elicited a slight induction of COX-2 at 2 hours (Figure 4D). Differentiation with M-CSF did not influence the expression of cPLA2 (Figure 4E). To confirm that products of the COX routes were the predominant eicosanoids released, mass spectrometric analysis was conducted. Stimulation with zymosan of LPS-primed macrophages induced a predominant production of PGE2, a lower production of PGD2, and a minimal amount of leukotriene (LT) B4 (Table 2 and Figure S1). Notably, in cells differentiated with M-CSF, there was a detectable production of LTB4 that agrees with the increased release of AA upon zymosan challenge and processing via the 5-lipoxygenase route. These data indicate that priming with LPS, IFN, and M-CSF increases the production of PGE2.
Figure 3. Production of PGE2.
(A, C, E and F) Macrophages were primed with 10 ng/ml LPS or (B) 100 U/ml IFNα and IFNγ for 3 hours or left untreated, and then stimulated with different additions at a concentration of 1 mg/ml. 10 µM CA-074, 2 µM cytochalasin D, and 20 µg/ml PVNO were added before the stimuli. The supernatants were collected for the assay of PGE2. Results represent mean ± S.D. of 6 to 7 independent experiments. Veh, indicates vehicle, *indicates p<0.05.
Figure 4. Expression of COX-2 protein.
(A–F) Macrophages were differentiated in the presence and absence of M-CSF and then primed with (B) 100 U/ml IFNγ, (C and F) 10 ng/ml LPS, and (D) 500 U/ml IL-4 for 3 hours. At the times indicated after stimulation, cell lysates were collected for the immunodetection of COX-2 and cPLA2 proteins. These are representative of experiments conducted at least in duplicate. (E) The expression of cPLA2 was assayed in macrophages differentiated in the presence of serum and M-CSF. (F) Macrophages were stimulated with latex beads at a concentration of 60 particles per cell.
Table 2. Eicosanoid release by macrophages.
| Differentiation | Treatment | PGE2 | PGD2 | LTB4 |
| Human serum | None | 0 ng/ml | 0.01 ng/ml | 0 ng/ml |
| Human serum | LPS+Zymosan | 7 ng/ml | 1 ng/ml | 0.02 ng/ml |
| M-CSF | None | 0 ng/ml | 0 ng/ml | 0 ng/ml |
| M-CSF | LPS+Zymosan | 6.9 ng/ml | 1.2 ng/ml | 0.2 ng/ml |
Human macrophages were primed for 3 hours with LPS and stimulated for 24 hours with zymosan. Results represent mean values of one experiment with duplicate samples.
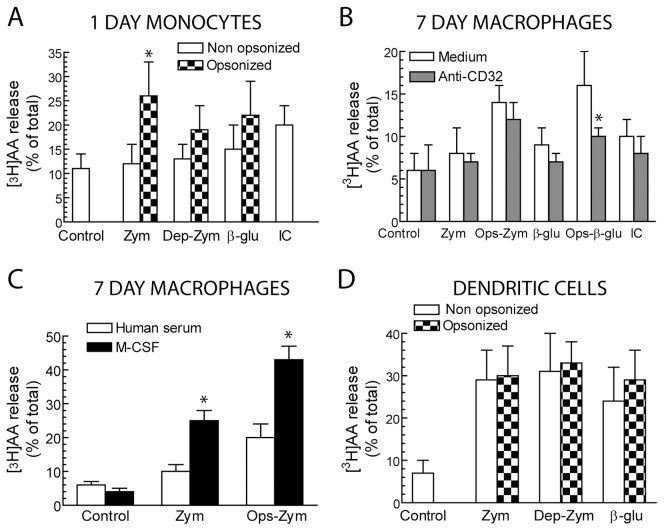
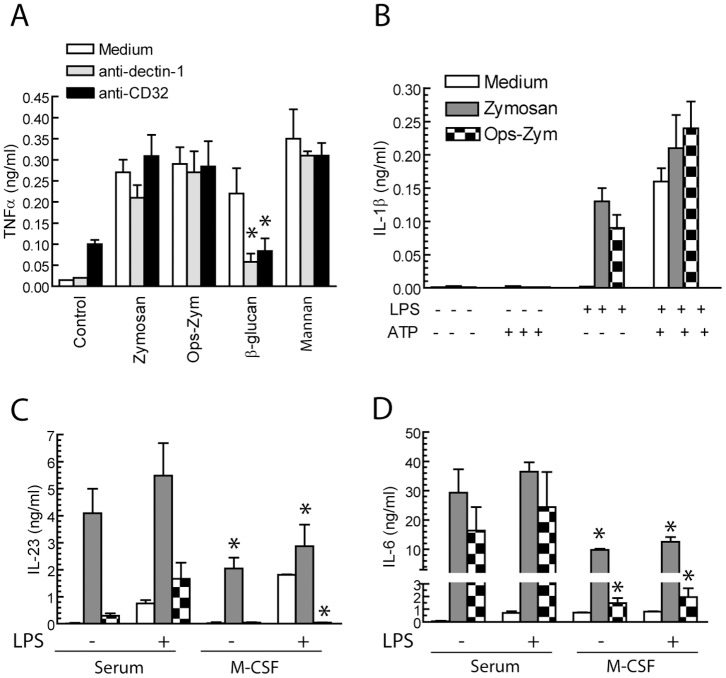
Cytokine Production
Zymosan, which is a potent stimulus for the release of cytokines in many cell systems [27]–[29], induced a low amount of TNFα (Figure 5A), what differs from findings in DC where it behaves as a strong stimulus [28]. In the case of β-glucan particles, the production of TNFα was inhibited by blocking Ab against dectin-1 and CD32A. Zymosan released low amounts of IL-1β, even in the presence of 10 ng/ml LPS, and this effect was increased in the presence of 10 mM ATP (Figure 5B). In contrast, zymosan was a potent inducer of IL-6 and IL-23 production (Figure 5C and D). Unlike what was observed on AA release and PGE2 production, differentiation with M-CSF blunted the release of the cytokines (Figure 5D). These data indicate that zymosan is a robust stimulus for IL-6 and IL-23 production and that this production is modulated by the inflammatory environment.
Figure 5. Zymosan uptake by macrophages and DC.Assay of cytokine production.
Macrophages were stimulated as indicated and after 24 hours the supernatants were collected for the assay of cytokines. (A) Inhibitory anti-CD32 and anti-dectin-1 Ab were used at a concentration of 10 µg/ml, 30 min prior to the addition of the stimuli. (B) The combination of a priming concentration of LPS and 10 mM ATP was used as a control of inflammasome activation. Results represent mean ± S.D. of 4 independent experiments in the case of TNFα (A) and IL-1β (B), and three experiments in the case of IL-23 (C) and IL-6 (D). *Indicates p<0.05. In (C) and (D) comparison has been conducted between serum and M-CSF differentiated macrophages.
Expression of the Receptors Involved in Zymosan Recognition
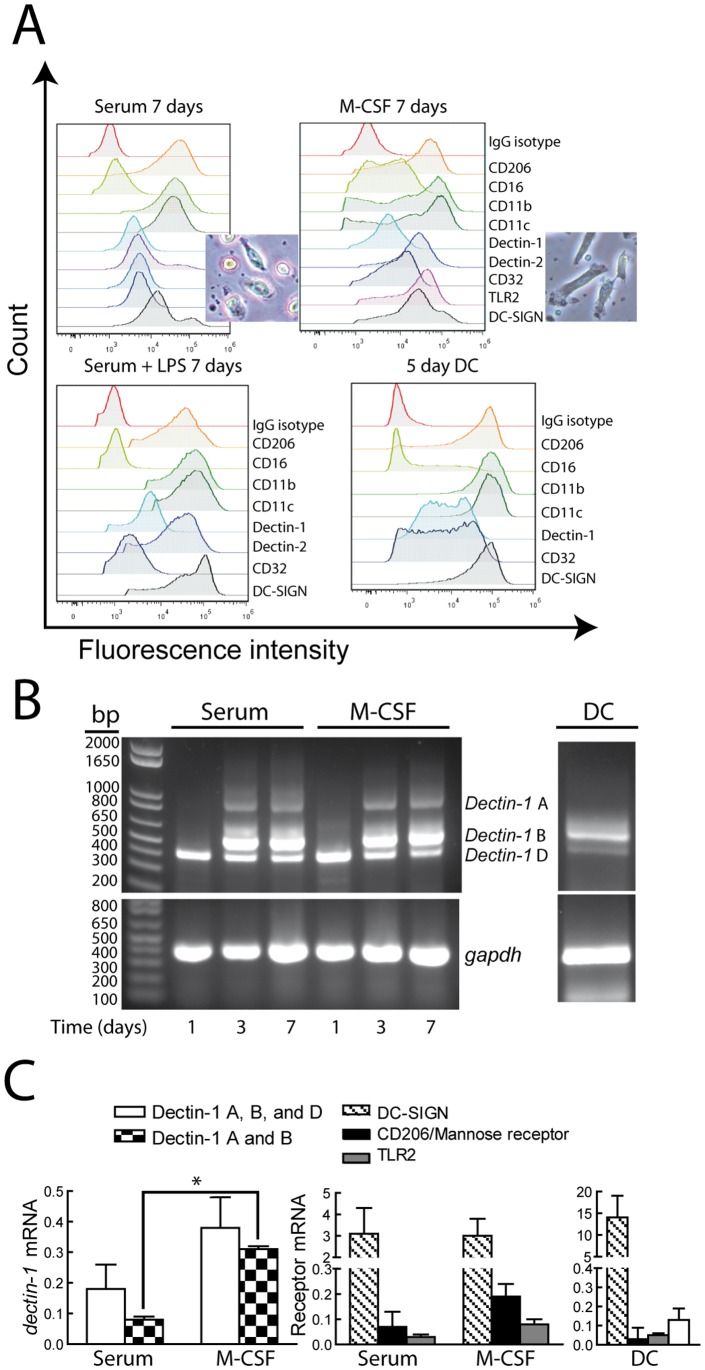
A plausible explanation for the different responses of DC and macrophages to β-glucan-bearing particles pointed to changes in the pattern of expression of receptors and/or in the mechanisms of signal transduction. No major differences were observed in the expression of receptors in serum-differentiated macrophages in the presence and absence of LPS priming (Figure 6A). In contrast, DC showed a higher expression of dectin-1 and DC-SIGN. Serum-differentiated macrophages differed from M-CSF differentiated macrophages by the presence of a lower number of fusiform cells (Fig. 6A). Since dectin-1 shows several isoforms, some of them with deletions in the CRD encoding region and in the transmembrane and stalk regions [5], RT-PCR assays were conducted to address the expression of these isoforms. Day-one monocytes only expressed the mRNA encoding dectin-1 D isoform, which is characterized by the deletion of both the stalk region and a portion of the carbohydrate recognition domain (CRD) (Figure 6B). Incubation with serum and M-CSF induced dectin-1 B and to a lower extent dectin-1 A, whereas dectin-1 B was the predominant isoform in DC. To quantify these changes, real-time RT-PCR was carried with primers spanning the extracellular and intracellular portions of dectin-1 and with a reverse primer designed from the exon skipped in dectin-1 D to assay dectin-1 A and B isoforms. Differentiation with M-CSF enhanced the expression of dectin-1 A and B isoforms as compared to the levels observed in serum-differentiated macrophages (Figure 6C). Analysis of the mRNA encoding other receptors showed that DC-SIGN is the receptor displaying the highest expression increase (Figure 6C, right panels), thus agreeing with the reported effect of M-CSF on regulatory macrophages [30]. These results indicate that differentiation of monocytes into macrophages has a prominent effect on the expression of dectin-1 and DC-SIGN, and that these changes can explain the mechanism whereby the response to β-glucans and α-mannans vary along the differentiation process.
Figure 6. Expression of receptors.
(A) The expression of different receptors was assayed by flow cytometry. The panel represents a typical experiment of two. (B) Expression of dectin-1 mRNA. The image shows a 40 cycles PCR carried out to show the minor band corresponding to dectin-1 A isoform that was not observed with a lower number of cycles. The high number of PCR cycles explains the difficulty to assess in the image the increased expression of dectin-1 B isoform in M-CSF differentiated macrophages over serum-differentiated macrophages that could be detected in real-time RT-PCR. The identification of the different isoforms was carried out by DNA sequencing on both strands of the RT-PCR product. This is a representative experiment of two. (C) Real-time RT-PCR was carried out with reverse primers in exon 5 and exon 6 to assay dectin-1 A and B isoforms, as well as with primers to assay DC-SIGN, the mannose receptor, and TLR2 in 7 day differentiated macrophages and DC. gadph was assayed as a load control and as a reference in real-time RT-PCR. Results represent mean ± S.D. of 5 experiments. *Indicates p<0.05.
Effect of the Deletion of dectin-1
Experiments in mouse bone marrow-derived macrophages (BMDM) were carried out to address the receptors involved in the response to β-glucans. dectin-1−/− mice did not release [3H]AA in response to a set of different β-glucan-bearing stimuli (Figure 7A). However, zymosan was a potent stimulus for the release of PGE2, whereas pure β-glucan was ineffective (Figure 7B). Notably, the production of PGE2 elicited by zymosan was enhanced in dectin-1−/− mice as compared to the WT. A similar pattern of response was observed for IL-6 (Figure 7C). Altogether, these results indicate the central role of dectin-1 in the induction of [3H]AA release by β-glucans.
Figure 7. Effect of clec7a deletion on the response to β-glucans.
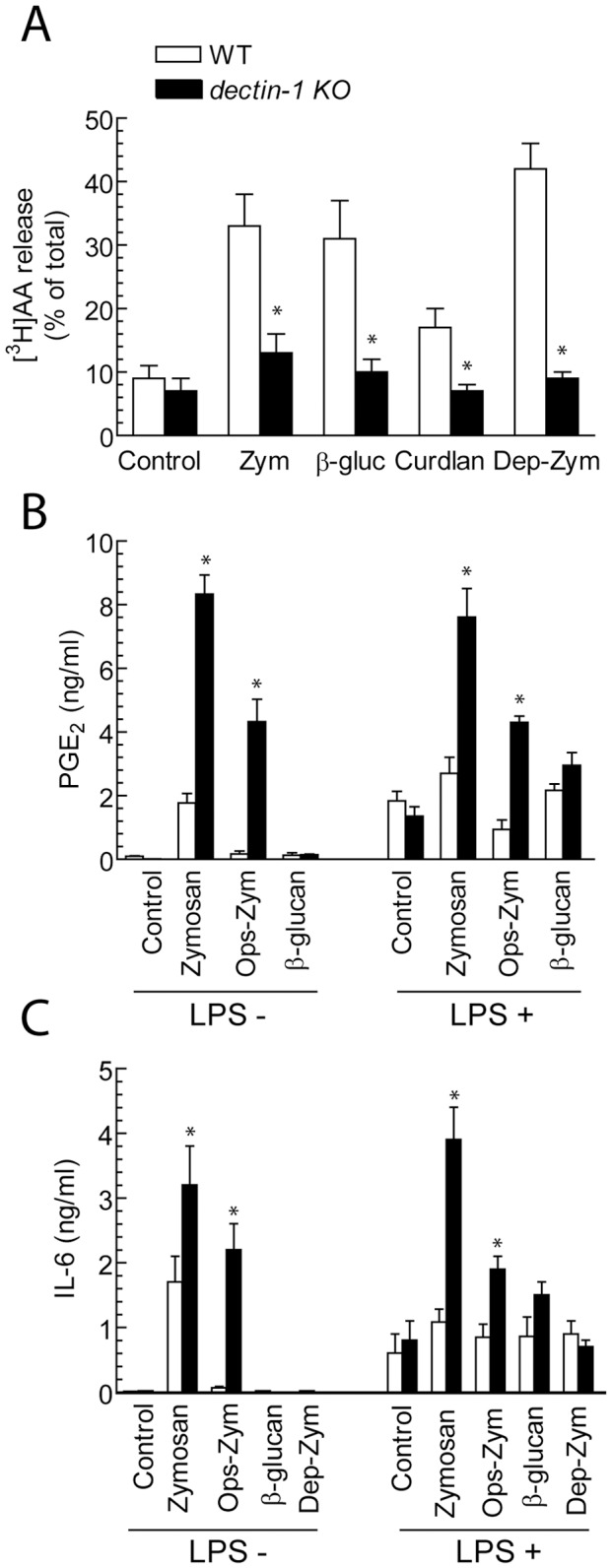
(A) BMDM from WT and dectin-1−/− mice were used for the release of [3H]AA after incubation for 1 hour with the indicated additions at the concentration of 1 mg/ml. (B) The production of PGE2 was assayed after 24 hour in BMDM preincubated for 3 hours with 10 ng/ml LPS or left untreated. (C) Production of IL-6 assayed 24 hours after the addition of the stimuli. Results indicate mean ± S.D. of 4 to 6 independent experiments. *Indicates p<0.05 as compared to WT mice.
Adaptor Proteins and Tyrosine Phosphorylation Reactions
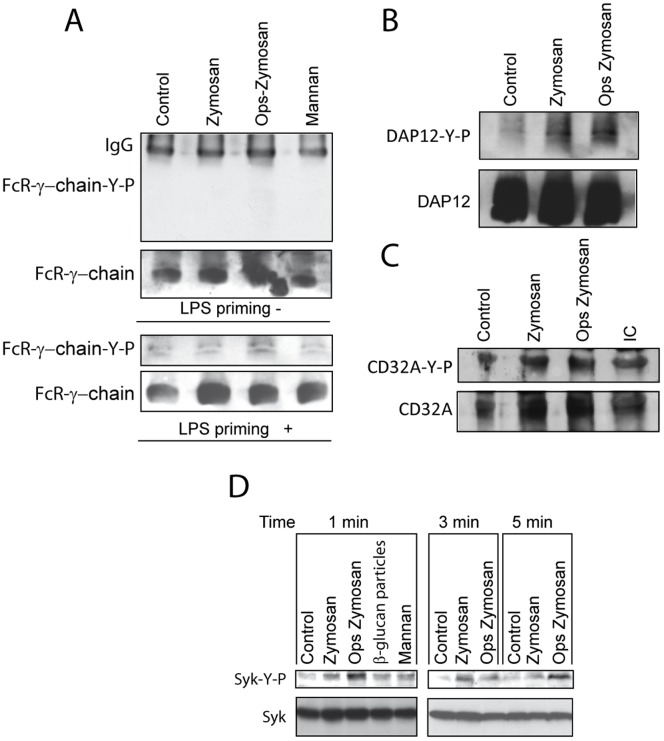
Since opsonisation of zymosan enhances productive binding to the β2-integrin CR3 and tyrosine phosphorylation of ITAM-containing adaptors leads to Syk activation following integrin engagement [19], we addressed whether zymosan induced the phosphorylation of DAP12and FcR γ-chain. Neither zymosan nor opsonized zymosan induced tyrosine phosphorylation of FcR γ-chain (Figure 8A). In contrast, they increased tyrosine phosphorylation of DAP12 and to a lower extent of CD32 (Figure 8B and C). Consistent with the downstream activation of Syk following phosphorylation of adaptor proteins, phosphorylation of Syk Y525/526 was detected as early as 1 min after stimulation (Figure 8D). Taken collectively, these data indicate that in addition to the canonical route involving dectin-1, zymosan particles may activate Syk by a mechanism involving the recruitment of DAP12. In keeping with the involvement of CD32A in CR3 signaling [31], a CD32A blocking Ab significantly inhibited [3H]AA release in response to opsonized β-glucan particles (Figure 2B).
Figure 8. Immunoprecipitation and assay of tyrosine phosphorylation of FcR γ-chain, DAP12, and CD32A.
Macrophages were differentiated with serum and incubated overnight with medium containing BSA. At the end of this period, they were treated for 1 min with the stimuli. Cell lysates were used for the immunoprecipitation of (A) FcR γ-chain, (B) DAP12, and (C) CD32A. After separation of the proteins in SDS/PAGE and transference to nitrocellulose membranes, the different proteins were immunodetected. (D) The tyrosine phosphorylation of Syk was assayed with phosphospecific Ab. These are representative blots of two experiments with similar results.
Activation of the NF-κB Route
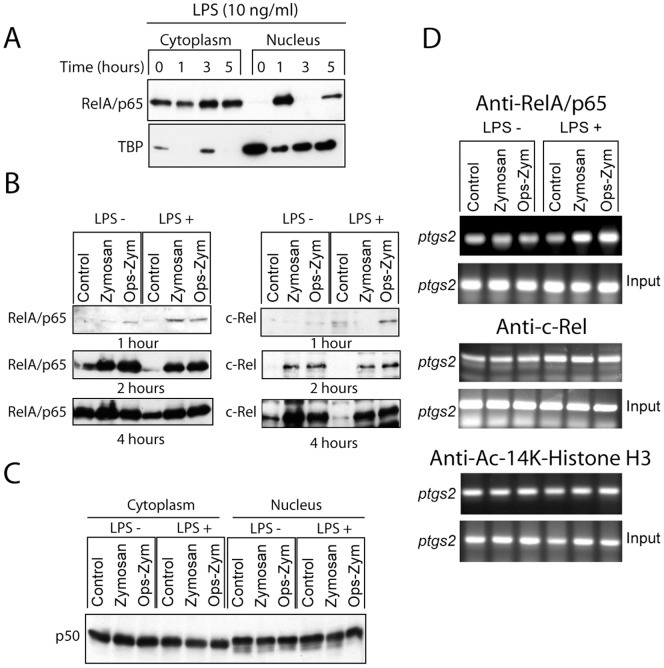
Since the NF-κB family of transcription factors plays a major role in the transcriptional regulation of COX-2, we addressed the involvement of this route. Priming with LPS induced a rapid activation of NF-κB as judged from the nuclear translocation of RelA/p65. This was followed by their disappearance from nuclear fractions and the reappearance of the protein at 5 hours, which can be explained most likely through an indirect mechanism involving the formation of secondary mediators (Figure 9A). When zymosan was used as the stimulus, nuclear translocation of RelA/p65 and c-Rel was observed 2 and 4 hours after the addition of the stimuli, thus suggesting a more delayed time-pattern of response than that elicited by LPS (Figure 9B). In contrast, p50 was detected in the nuclear fractions both upon stimulation and under resting conditions (Figure 9C). Notably, zymosan induced the binding of RelA/p65 to the ptgs2 promoter in LPS-primed cells two hours after addition of the stimuli, but it did not increase c-Rel binding over the level detected in LPS primed cells (Figure 9D). We did not observe any significant change of the level of histone H3 acetylation in the ptgs2 promoter following LPS priming (Figure 9D lower panel). Since NF-κB activation and COX-2 induction have been reported to occur after crystal particle uptake [25], [32] with a time-course similar to that observed with zymosan particles by a mechanism involving the release of cathepsin B from the phagolysosome [20], we addressed the effect of cytochalasin D, an alkaloid that inhibits phagocytosis, the cathepsin B inhibitor CA-074, and the lysosomal stabilizing agent poly-2-vinylpyridine N-oxide (PVNO) on PGE2 production. As shown in Figure 3, cytochalasin D and CA-074 inhibited the response to both zymosan and opsonized zymosan, whereas the inhibition by PVNO did not reach statistical significance. Taken together, these results suggest that particle uptake and cathepsin B can be involved in the delayed production of PGE2 elicited by zymosan. Further evidence of the parallelism between the effect of zymosan and crystal particles was the observation that latex particles also induced COX-2 expression in serum differentiated macrophages (Figure 4F), even taking into account the differences in the load of protein in the different lanes.
Figure 9. Activation of NF-κB proteins by LPS and zymosan.
(A) Macrophages were treated for up to 5 h with 10 ng/ml LPS and RelA/p65 was assayed in the cytoplasm and nucleus. (B) Macrophages were primed for 3 h with LPS and then stimulated for different times. (B–C) The nuclear fractions were used for the assay of RelA/p65, c-Rel, and p50. (D) Binding of RelA/p65 and c-Rel, and histone H3 acetylation of the ptgs2 promoter were assayed in the presence and absence of LPS priming 2 h after stimulation. These are representative experiments of at least two showing identical results.
Discussion
These data show that the response of macrophages to β-glucans depends on their state of differentiation and the presence of LPS and/or cytokines in the microenvironment. Whereas several studies have addressed this issue in mouse macrophages, to the best of our knowledge this is the first systematic study on human macrophages. Our findings can be summarized in three distinct patterns: i) a pattern consistent with phagosomal processing, ii) a pattern induced by priming with LPS, and iii) a pattern elicited by M-CSF, the hallmark of which is an increased expression of dectin-1 B isoform.
The first pattern is reminiscent of both the response to crystals and the increased access to intracellular pattern receptors elicited by phagosomal processing [33]. Notably, a recent study has disclosed that dectin-1 dependent response to Aspergillus conidia occurs when the particles are located in acidified phagolysosomes, thereby stressing the importance of β-glucan signalling from the phagolysosome [34]. Our findings show an early activation of Syk and a more delayed activation of the NF-κB route that leads to the induction of COX-2, IL-6, and IL-23. The production of PGE2 is sensitive to cathepsin B inhibition and to the blockade of phagocytosis. These findings agree with the activation of cathepsin B by β-glucans [35] and with the inhibitory effect of cytochalasin D on cytokine production in DC stimulated with particulate β-glucans reported by Rosas et al. [9], who concluded that inhibition of cytoskeletal assembly by cytochalasins impides the contact with particles. A study addressing caspase recruitment domain-containing protein (CARD)9 by dectin-1 signaling showed that it is possible to distinguish signals downstream Syk such as activation of p38 MAPK, which can occur in the absence of cytokine production, and signals dependent on Syk/CARD9/NF-κB associated with cytokine production [10]. In other words, the dectin-1 route can promote CARD9 phagosomal translocation and enhance TLR-induced cytokine production even though dectin-1 signaling is insufficient to drive cytokine production. As it has been reported that phagosomal degradation increases TLR access to ligands [33], it seems likely that a similar mechanism may be operative following zymosan phagocytosis, thus agreeing with the recognition of Aspergillus conidia by dectin-1 in acidified phagolysosomes [34].
By using BMDM from dectin-1 −/− mice, we observed that dectin-1 is necessary for AA release by β-glucans and dispensable for the cytokine response. The absence of a delayed production of PGE2 by pure β-glucan in WT mice in spite of the occurrence of AA release, can be explained by the absence of COX-2 induction. Unexpectedly, PGE2 and IL-6 production were enhanced in the dectin-1−/− mice, thus suggesting that in the absence of dectin-1, zymosan binding to receptors that activate κB-dependent transcription such as TLR2 might be enhanced. A similar mechanism could explain the lower production of IL-6 and IL-23 in human macrophages differentiated with M-CSF A plausible explanation for the prominent IL-6 response to zymosan particles is that the transcriptional regulation of il6 involves cooperation of NF-κB and cyclic AMP response element binding protein (CREB) to recruit CREB-binding protein (CBP) at the il6 promoter, thus enabling synergistic gene activation [36]. In keeping with these data, the time course of the activation of these factors seems optimal given that NF-κB activation by zymosan shows a delayed time-course that seems coincidental with the generation of PGE2 and the ensuing activation of the E prostanoid receptor/protein kinase A/cyclic AMP/CREB system. This pattern of cooperation differs from other systems where CREB and RelA/p65 bind to different promoters and may compete for the coactivator CBP [37]. The predominant production of IL-23 could be explained by the ability of zymosan to blunt il12a transcription [38].
Macrophages exposed to a low concentration of LPS show an early induction of COX-2 that may be the consequence of the first wave of the activation of NF-κB by the TLR4 route. This agrees with current views on ptgs2 regulation, in that it is a primary response gene with CpG islands and constitutive histone acetylation that allows a transcriptionally permissive state [39]. Although zymosan clearly enhanced the production of PGE2 and the activation of NF-κB over that observed upon LPS exposure, the immunodetection of COX-2 protein did not show a parallel increase, which can be explained by the instability of COX-2 protein [40]. In this connection, COX-2 induction has always been assayed in the presence of load controls. Although the expression of the putative housekeeping gene might show variations among different lanes due to either technical mistakes or inconsistent behaviour of the housekeeping gene. For instance, the β-actin load in the rightmost lane in Figure 4F. It seems likely that the induction of COX-2 has been underestimated rather than overestimated, therefore not affecting the overall significance of the results. The enhancement by zymosan of the effect of a priming dose of LPS was also shown at the ptgs2 promoter as judged from an increased binding of RelA/p65. An effect related to an increased acetylation of the ptgs2 promoter seems unlikely, in view of the steady levels of histone H3 acetylation. As to the effect of IFNγ, we did not observe an enhanced expression of COX-2 protein. This agrees with the reported effect of IFNγ on microsomal PGE synthase expression, since co-stimulation of TNFα with IFNγ reduces COX-2 protein expression, but enhances PGE2 biosynthesis through an increased expression of PGE synthase [41].
M-CSF enhanced the expression of the isoforms of dectin-1 most usually associated with productive binding, specially dectin-1 B, and increased AA release and PGE2 production in the absence of an increased expression of cPLA2 and COX-2. The enhanced production of LTB4 observed upon M-CSF treatment agrees with a rapid formation by 5-lipoxygenase, the activity of which is regulated by AA disposal. Most studies so far conducted have been carried out in GM-CSF treated cells [14], [42] and have disclosed an increased expression of receptors involved in the recognition of α-mannan and β-glucan moieties rather than an increased expression of cPLA2. In contrast, M-CSF has been reported to down-regulate the production of cytokines by mouse macrophages [9], [10]. Given that M-CSF increases the expression of the dectin-1 B isoform as well as the expression of the α-mannan binding receptor DC-SIGN, our findings suggest that similar to the effect of GM-CSF, the main effect of M-CSF on AA metabolism in human macrophages might be exerted on the expression of receptors rather than on the induction of enzymes.
The role of adaptor proteins should be analyzed in the context of their role in integrin signaling [19] and fungal uptake [31]. We have detected phosphorylation of DAP12 and CD32A, but we did not observe phosphorylation of Fc receptor γ-chain. Unlike DAP12 tyrosine phosphorylation, CD32A phosphorylation did not increase upon zymosan challenge, what agrees with the notion that basal ITAM phosphorylation is commonly observed in macrophages in the absence of ligand binding and that low-level ITAM-mediated signaling might provide functionally relevant signals [23], [43]. Since tyrosine phosphorylation of DAP12 followed zymosan stimulation, it seems likely that receptor-independent, direct membrane binding signaling might utilize DAP12 to recruit Syk [23]. A schematic diagram of our findings is shown (Figure 10). Taken collectively, our data show the existence of distinct patterns of response of human macrophages to β-glucan containing particles, the scope of which varies from a limited and delayed response mimicking signaling from the phagosomes to a synergistic response triggered by priming with LPS and cytokines.
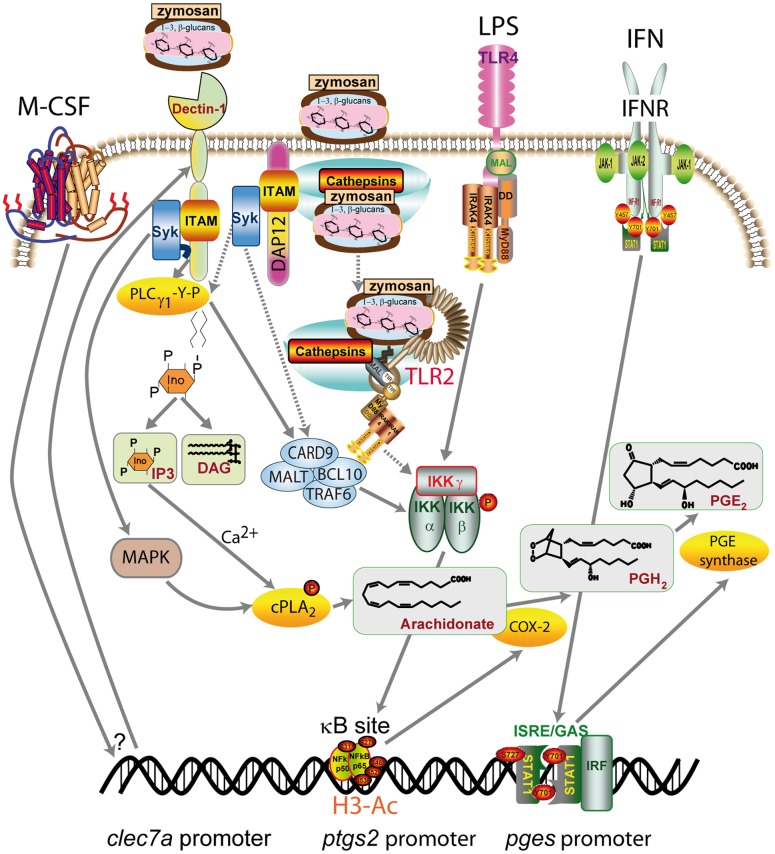
Figure 10. Proposed mechanisms involved in zymosan uptake and signaling in human macrophages.
Zymosan particles can activate Syk via dectin-1 engagement and through the adaptor protein DAP12. After internalization into phagosomes, TLR2 recognition and cathepsin B leakage may occur. In the presence of M-CSF, the expression of dectin-1 B isoform is increased and allows for an enhanced receptor-dependent binding. If the LPS/TLR4 cascade is activated, an additional, concomitant mechanism of NF-κB activation will be triggered. cPLA2 is activated by dectin-1/Syk-dependent mechanisms involving MAPK-dependent Ser-505 phosphorylation and Ca2+-dependent membrane translocation, whereas the induction of COX-2 depends mainly on κB-dependent transcriptional regulation. IFNγ signaling leads to the activation of the promoter of the inducible microsomal isoform of prostaglandin E synthase (pges) by an IFN-stimulated response element (ISRE)-dependent or GAS (interferon-γ activated sequence)-dependent mechanism. The dotted lines indicate the steps associated with phagocytic cargo processing. Ac-H3 indicates acetylated histone H3.
Supporting Information
Mass spectrometric characterization of the eicosanoids released by macrophages. The supernatants of 4 ml of culture medium corresponding to ∼2.106 macrophages differentiated in the absence (A) and presence of M-CSF (B), primed with 10 ng/ml LPS and stimulated with zymosan were extracted and analyzed as explained in Materials S1. The upper panels in (A) and (B) show a display arranged to show maximal intensity of the recording as a function of free AA (m/z 303.233). Middle and lower panels have been adapted to show maximal intensity for LTB4 (m/z 335.222) and PGE2/PGD2 (m/z 351.217), respectively.
(TIF)
Eicosanoid measurements by reversed phase ultra performance liquid chromatography (UPLC) and electrospray-quadrupole-time-of-flight-mass spectrometry.
(DOC)
Acknowledgments
Dr. Gordon D. Brown from Institute of Medical Sciences, University of Aberdeen, is thanked for the gift of clec7a −/− mouse bone marrow and critical review of the manuscript. Dr. David L. Williams from East Tennessee State University is thanked for the gift of pure β-glucan.
Funding Statement
This work was supported by Plan Nacional de Salud y Farmacia (grants SAF2007-60446 and SAF2010-15070), Fundación Ramón Areces, Red Temática de Investigación Cardiovascular, and Junta de Castilla y León (grant CSI003A11-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cebon J, Layton JE, Maher D, Morstyn G (1994) Endogenous haemopoietic growth factors in neutropenia and infection. Br J Haematol 86: 265–274. [DOI] [PubMed] [Google Scholar]
- 2. Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, et al. (2002) Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med 196: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, et al. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN (2012) Interactions between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science 336: 1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willment JA, Gordon S, Brown GD (2001) Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem 276: 43818–43823. [DOI] [PubMed] [Google Scholar]
- 6. Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, et al. (2003) Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol 171: 4569–4573. [DOI] [PubMed] [Google Scholar]
- 7. Fernández N, Renedo M, Alonso S, Sánchez Crespo M (2003) Release of arachidonic acid by stimulation of opsonic receptors in human monocytes: the FcγR and the complement receptor 3 pathways. J Biol Chem 278: 52179–52187. [DOI] [PubMed] [Google Scholar]
- 8. van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, et al. (2009) Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for β-glucan-bearing particles. Mol Immunol 47: 575–581. [DOI] [PubMed] [Google Scholar]
- 9. Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, et al. (2008) The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol 181: 3549–3557. [DOI] [PubMed] [Google Scholar]
- 10. Goodridge HS, Shimada T, Wolf AJ, Hsu YM, Becker CA, et al. (2009) Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol 182: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xia Y, Vetvicka V, Yan J, Hanikýrová M, Mayadas T, et al. (2009) The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol 162: 2281–2290. [PubMed] [Google Scholar]
- 12. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown GD, Jurgen H, Williams DL, Willment JA, Marshall ASJ, et al. (2003) Dectin-1 mediates the biological effects of β-glucans. J Exp Med 197: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valera I, Fernández N, Trinidad AG, Alonso S, Brown GD, et al. (2008) Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocyte-derived dendritic cells. J Immunol 180: 5727–5736. [DOI] [PubMed] [Google Scholar]
- 15. Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, et al. (2011) Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci USA 108: 14270–14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, et al. (2008) Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol 38: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suram S, Brown GD, Ghosh M, Gordon S, Loper R, et al. (2006) Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the β-glucan receptor. J Biol Chem 281: 5506–5514. [DOI] [PubMed] [Google Scholar]
- 18. McDonald JU, Rosas M, Brown GD, Jones SA, Taylor PR (2012) Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLoS One 7(9): e45781 doi:10.1371/journal.pone.0045781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mócsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, et al. (2006) Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol 7: 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, et al. (2011) Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity 34: 514–526. [DOI] [PubMed] [Google Scholar]
- 21. Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, et al. (2011) An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34: 527–540. [DOI] [PubMed] [Google Scholar]
- 22. Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, et al. (2009) Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog 5(8): e1000559 doi:10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, et al. (2008) Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29: 807–818.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Reilly KM, Phipps RP, Thatcher TH, Graf BA, Van Kirk J, et al. (2005) Crystalline and amorphous silica differentially regulate the cyclooxygenase-prostaglandin pathway in pulmonary fibroblasts: implications for pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, et al. (2007) Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez Y, Municio C, Alonso S, Sánchez Crespo M, Fernández N (2009) Induction of IL-10 by fungi in dendritic cells depends on CREB activation by the coactivators CBP and TORC2 and autocrine PGE2 . J Immunol 183: 1471–1479. [DOI] [PubMed] [Google Scholar]
- 27. Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, et al. (2006) Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 116: 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leibundgut-Landmann S, Gross O, Robinson MJ, Osorio S, Slack EC, et al. (2007) Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8: 6630–6638. [DOI] [PubMed] [Google Scholar]
- 29. Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, et al. (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol 13: 246–254. [DOI] [PubMed] [Google Scholar]
- 30. Domínguez-Soto A, Sierra-Filardi E, Puig-Kröger A, Pérez-Maceda B, Gómez-Aguado F, et al. (2011) Dendritic cell-specific ICAM-3-grabbing nonintegrin expression on M2-polarized and tumor-associated macrophages is macrophage-CSF dependent and enhanced by tumor-derived IL-6 and IL-10. J Immunol 186: 2192–2200. [DOI] [PubMed] [Google Scholar]
- 31. Moalli F, Doni A, Deban L, Zelante T, Zagarella S, et al. (2010) Role of complement and Fcγ receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus . Blood 116: 5170–5180. [DOI] [PubMed] [Google Scholar]
- 32.Leea S, Yunb H-S, Kima SH (2011) The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials doi:10.1016/j.biomaterials.2011.08.042. [DOI] [PubMed]
- 33. Wolf AJ, Arruda A, Reyes CN, Kaplan AT, Shimada T, et al. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J Immunol 187: 6002–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faro-Trindade I, Willment JA, Kerrigan AM, Redelinghuys P, Hadebe S, et al. (2012) Characterisation of innate fungal recognition in the lung. PLoS One 7(4): e35675 doi:10.1371/journal.pone.0035675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenzweig HL, Clowers JS, Nunez G, Rosenbaum JT, Daveym MP (2011) Dectin-1 and NOD2 mediate cathepsin activation in zymosan-induced arthritis in mice. Inflamm Res 60: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dendorfer U, Oettgen P, Libermann TA (1984) Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol 14: 4443–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parry GCN, Mackman N (1997) Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J Immunol 159: 5450–5456. [PubMed] [Google Scholar]
- 38. Alvarez Y, Municio C, Hugo E, Zhu J, Alonso S, et al. (2011) Notch- and transducin-like enhancer of split (TLE)-dependent histone deacetylation explain interleukin 12 (IL-12) p70 inhibition by zymosan. J Biol Chem 286: 16583–16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramirez-Carrozzi VR, Braas D, Bhatt DM, Cheng CS, Hong C, et al. (2009) A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell 138: 114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mbonye UR, Yuan C, Harris CE, Sidhu RS, Song I, et al. (2008) Two distinct pathways for cyclooxygenase-2 protein degradation. J Biol Chem 283: 8611–8623. [DOI] [PubMed] [Google Scholar]
- 41. Wright KL, Weaver SA, Patel K, Coopman K, Feeney M, et al. (2004) Differential regulation of prostaglandin E biosynthesis by interferon-γ in colonic epithelial cells. Br J Pharmacol 141: 1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parti RP, Loper R, Brown GD, Gordon S, Taylor PR, et al. (2010) Cytosolic phospholipase A2 activation by Candida albicans in alveolar macrophages: role of dectin-1. Am J Respir Cell Mol Biol 42: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ivashkiv LB (2009) Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol 10: 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mass spectrometric characterization of the eicosanoids released by macrophages. The supernatants of 4 ml of culture medium corresponding to ∼2.106 macrophages differentiated in the absence (A) and presence of M-CSF (B), primed with 10 ng/ml LPS and stimulated with zymosan were extracted and analyzed as explained in Materials S1. The upper panels in (A) and (B) show a display arranged to show maximal intensity of the recording as a function of free AA (m/z 303.233). Middle and lower panels have been adapted to show maximal intensity for LTB4 (m/z 335.222) and PGE2/PGD2 (m/z 351.217), respectively.
(TIF)
Eicosanoid measurements by reversed phase ultra performance liquid chromatography (UPLC) and electrospray-quadrupole-time-of-flight-mass spectrometry.
(DOC)