Abstract
Stylet sheath formation is a common feature among phytophagous hemipterans. These sheaths are considered essential to promote a successful feeding event. Stylet sheath compositions are largely unknown and their mode of solidification remains to be elucidated. This report demonstrates the formation and solidification of in āere (in air) produced stylet sheaths by six hemipteran families: Diaphorina citri (Psyllidae, Asian citrus psyllid), Aphis nerii (Aphididae, oleander/milkweed aphid), Toxoptera citricida (Aphididae, brown citrus aphid), Aphis gossypii (Aphididae, cotton melon aphid), Bemisia tabaci biotype B (Aleyrodidae, whitefly), Homalodisca vitripennis (Cicadellidae, glassy-winged sharpshooter), Ferrisia virgata (Pseudococcidae, striped mealybug), and Protopulvinaria pyriformis (Coccidae, pyriform scale). Examination of in āere produced stylet sheaths by confocal and scanning electron microscopy shows a common morphology of an initial flange laid down on the surface of the membrane followed by continuous hollow core structures with sequentially stacked hardened bulbous droplets. Single and multi-branched sheaths were common, whereas mealybug and scale insects typically produced multi-branched sheaths. Micrographs of the in āere formed flanges indicate flange sealing upon stylet bundle extraction in D. citri and the aphids, while the B. tabaci whitefly and H. vitripennis glassy-winged sharpshooter flanges remain unsealed. Structural similarity of in āere sheaths are apparent in stylet sheaths formed in planta, in artificial diets, or in water. The use of ‘Solvy’, a dissolvable membrane, for intact stylet sheath isolation is reported. These observations illustrate for the first time this mode of stylet sheath synthesis adding to the understanding of stylet sheath formation in phytophagous hemipterans and providing tools for future use in structural and compositional analysis.
Introduction
Many phytophagous hemipterans (true bugs) are characterized by common structural mouthparts [1] that penetrate host plants inter- or intra-cellularly to feed on contents of vascular tissues or other vegetative cell types. The Order Hemiptera is divided into four clade groups (suborders), the Auchenorrhyncha, Coleorrhyncha, Heteroptera, and Sternorrhyncha (for a systematic review of Hemiptera - see Forero, 2008, [1]). The Sternorrhyncha [2], [3], [4] including Psyllidae (e.g. psyllids), Aleyrodidae (e.g. whiteflies), Aphididae (e.g. aphids), Pseudococcidae (e.g. mealybugs), and Coccidae (e.g. scales) and the Auchenorrhyncha including Cicadoidea (e.g. cicadas), Membracoidea (e.g. leafhoppers and treehoppers), Fulgoroidea (e.g. planthoppers), and Cercopoidea (e.g. spittlebugs) contain many agronomically important plant pests that are vectors of pathogens causing plant diseases resulting in vast crop losses worldwide [5], [6], [7].
Phytophagous hemipterans feed by penetration of a stylet bundle into plant tissues. A common trait of these insects is the concurrent formation of a solidifying sheath structure (termed stylet sheath) that encapsulates the stylet bundle while they penetrate into the plant tissues. As the stylets penetrate various plant tissues, they secrete liquid droplets that solidify to form a solid hollow tube extending from the leaf surface to the point of feeding within the plant tissue, often terminating in the plants vascular tissue [8]. Watery and gelling (sheath) saliva represent two common forms of salivary secretions that are implicated in stylet sheaths composition and hemipteran feeding [8], [9], [10], [11], [12], [13], [14], [15]. The exact function(s) of the stylet sheath in feeding are not known; however, trait conservation across phytophagous hemipterans [5], implies biological importance. Stylet sheaths are thought to provide stability and directional orientation to the stylets during the piercing process [16], to aid in proper feeding [16], to ‘cloak’ the stylets from host (plant) defense responses [9], and to seal up cell damage caused by stylet probing [17], [18].
Commonly, sheath initiation occurs concurrently with labium contact with host plant surfaces [19]. It has been suggested that solidification of sheath material requires interaction with host (plant) components [20]. Additionally, oxygen interactions have been suggested for proper gelling of the flange (portion on the leaf surface) and the sheath (section within the leaf tissue) [11], [21], with a recent report supporting this hypothesis [10]. Numerous reports using artificial diet systems have allowed the visualization and study of stylet sheaths [9], [19], [22], [23], [24], [25], [26]. In plant tissues, a typical stylet sheath structure includes a flange (exterior) on the leaf surface, followed (interior) by a narrowing (‘neck’ region) as the stylet sheath traverses the upper/lower (outer) epidermis of the leaf [22], [27]. This is subsequently followed by a thicker sheath ‘trunk’ having a continuous sequential bulbous structure as it traverses the tissue to the target vascular region (phloem or xylem) where the sheath may remain as a single channel or branch laterally throughout the vascular tissue, also in a continuous sequential bulbous form [22], [27], [28]. Recently, sheath material was induced using a brushing technique in sharpshooter [29]; however, the shape of this induced solidified sheath material differs from those formed naturally [30], [31].
In this report, we demonstrate the non-induced formation of stylet sheaths ‘in air’ (in āere) across a single-layer membrane surface for Diaphorina citri (Psyllidae, Asian citrus psyllid) housed in ‘mock’ feeding chambers (MFC) lacking diet. Membrane probing and stylet sheath formation by the D. citri was virtually instantaneous upon caging in MFCs. The caged D. citri penetrated the single layer membrane with their stylets, depositing fully formed in āere stylet sheaths. To find out if this attribute is common in other phytophagous hemipteran families, we similarly tested: Aphis nerii (Aphididae, oleander aphid), Aphis gossypii (Aphididae, cotton/melon aphid), Toxoptera citricida (Aphididae, brown citrus aphid), Bemisia tabaci biotype B (Aleyrodidae, whitefly), Homalodisca vitripennis (Cicadellidae, glassy-winged sharpshooter), Ferrisia virgata (Pseudococcidae, striped mealybug), and Protopulvinaria pyriformis (Coccidae, pyriform scale) each producing in āere stylet sheaths. Subsequent to this, we developed a technique to isolate intact stylet sheaths utilizing a water-soluble polyvinyl alcohol membrane and differential filtration. We present this method of sheath production as a tool for downstream sheath compositional and structural analyses without complicating interactions of foreign material of either plant or artificial diet origin.
Materials and Methods
Insects
We obtained Diaphorina citri, Homalodisca vitripennis, Bemisia tabaci biotype B, Toxoptera citricida, and Ferrisia virgata from colonies maintained at the USDA-ARS, United States Horticultural Research Laboratory (USHRL) at Fort Pierce, FL. Aphis nerii were isolated from milkweed (Asclepias tuberose) plants (GPS: N 27 25.730, W 80 24.538), Aphis gossypii from hibiscus (Hibiscus rosa-sinensis) plants (GPS: N 27 25.696, W 80 24.503), and Protopulvinaria pyriformis from Confederate Jasmine (Trachelospermum jasminoides) vine (GPS: N 27 25.703, W 80 24.536) located within the USHRL property. Insect identifications were obtained from Dr. Susan Halbert and Dr. Ian Stocks, Florida Department of Agriculture and Consumer Services, Division of Plant Industries, Gainesville, FL.
Insect Chambers
Insect chambers consisted of single species of insects (see section 2.1) caged individually and/or in groups within standard (100 mm×15 mm) or smaller (35 mm×10 mm) plastic Petri dishes. A single Parafilm® or clear plastic ‘kitchen’ wrap (multiple brands tried, each being viable) layer was stretched across the top of the plate to seal it from insect escape and to provide a stylet penetrable surface for insect probing; hereafter called a ‘mock feeding chamber’ (MFC). Parts of the formed stylet sheaths on the membrane interior (directly accessible to the insect) and exterior (insect stylet probe accessible) surfaces are hereafter designated ‘flange’ and ‘sheath face’, respectively.
MFCs were maintained within clear Pyrex® baking dishes with the membrane (film) side up and the baking dish covered with a single layer of clear plastic wrap to prevent external contaminant interactions with the membrane surface during incubation. MFCs were incubated at temperature (25°C), relative humidity (75%), and light:dark cycle of 14∶10 hours during experiments that ranged from 0–10 minutes up to 72 hours in duration.
Whole Stylet Sheath Collection
To collect intact D. citri stylet sheaths (sheath with flange attached), we used Solvy™ Stabilizer (Sulky®, Kennesaw, GA), a water-soluble polyvinyl alcohol membrane, that provided a stylet penetrable probing surface for sheath deposition. After deposition, the membrane was removed from the MFC and washed with 100% ethanol (Aaper, Shelbyville, KY) to remove insect debris. The membrane was then immersed in Nanopure H2O to dissolve and liberate the sheaths into solution. This solution was sequentially filtered using a Nalgene reusable vacuum filtration system (Thermo Scientific, Waltham, MA) with a combination of Spectra/mesh® polyester membranes (Spectrum Labs, Rancho Dominguez, CA) of 50 µm and 10 µm pore sizes. The filter (50 µm) was used to separate large particulates from the sheath/Solvy™ solution and the filtrate was then passed through a 10 µm filter capturing intact stylet sheaths. The filter (10 µm) was then carefully removed from the vacuum filtering system and gently washed with a gentle stream of 100% ethanol to rinse the sheaths from the filter’s surface. The ethanol/stylet sheath rinse was carefully collected into a clean glass container and stored at −4°C for analyses. Spectra/mesh membrane pore sizes were adjusted depending on the size of the expected sheaths for capture/harvest.
Scanning Electron Microscopy (SEM)
SEM micrographs were obtained using two methods. The first method employed a ‘tape lift’ procedure using 6 mm diameter SEM carbon adhesive tabs (Electron Microscope Sciences [EMS], Hatfield, PA) affixed to the center of SEM aluminum specimen mounts (EMS). Utilizing the stub base as a handle, the exposed adhesive surface was gently affixed and subsequently detached (repeatedly) across the sheath face of a MFC directly attaching air formed sheaths to the carbon adhesive tab surface, resulting in partial sheath isolation (minus the flange). These were then directly analyzed by SEM (pre-sputtering) using a Hitachi S-4800 Scanning Electron Microscope, (Hitachi High-Technologies Corporation, Tokyo, Japan) at 5 KV, 7 KV, 10 KV or 25 KV. Subsequently the stubs were gold/palladium sputter coated using a Gold/Palladium Hummer ™ 6.2 sputtering system (Anatech USA, Union City, CA) then reanalyzed by SEM.
The second method employed a novel technique for direct SEM imagery of both membrane surfaces (flange and sheath faces). Briefly, Cellstar® 35 mm×10 mm tissue culture (Greiner Bio-One North America Inc., Monroe, NC) MFCs using clear plastic wrap membrane were made for each of the insects used in this study. Chambers were incubated for 2 to 24 hours depending on the insect used and its propensity to probe the membrane. Following this, the sidewalls of the MFCs were drilled with two opposing holes using a flame heated 20 gauge needle to puncture through the plastic sidewall allowing the MFCs interior to equilibrate in the vacuum of the gold/palladium sputter coating system. Given the stringency involved with direct surface flange/sheath SEM analysis, plastic wrap provided a greater material stability/endurance during the sputter plating and SEM processes as opposed to the more ductile Parafilm®.
To analyze the flange face (interior membrane surface), the membrane was carefully removed from the MFC of interest and replaced inverted (flange face up) and stretched across a like-sized Petri dish. These new chambers were subsequently drilled, sputter coated, and the flange deposits of selected insects were observed by SEM. With SEM micrograph imagery capture was performed using the FE-PC SEM bundled software included in the SEM microscope control system.
Transmission Electron Microscopy (TEM)
D. citri adults feeding on citrus leaves in propylene tubes [32] were immobilized (while feeding) by placing the rearing tube in a −20°C freezer for 10 minutes. These adults were then gently pulled away from the leaf (with their stylets still extended) using fine forceps under a stereomicroscope. D. citri heads with extended stylets were immediately fixed in 2.5% glutaraldehyde in sodium cacodylate buffer (pH 7.4) at 4°C for 3 to 4 days, post fixed in 2% osmium tetroxide in the same buffer for 1 to 2 hours at 4°C, washed in buffer, dehydrated in ethanol and acetonitrile (substitute for acetone), then embedded in EMBed-812 (both from EMS). Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Hitachi S-4800 electron microscope (in transmission mode) at 25 KV.
Confocal Microscopy
For confocal differential interference contrast (DIC) and confocal laser microscopy, a Zeiss LSM 510 AXIO Imager M1 confocal microscope was used (Carl Zeiss MicroImaging GmbH, Jena, Germany) controlled by Zen software version 2009 SP2 (Zeiss). To mount the in āere stylet sheaths on glass slides, 50 µL of mounting medium (70% glycerol in 30% PBS) was applied directly to the sheath surface of the MFC for direct harvest of partial sheaths from the membrane surface using a bent glass rod. Then 10 µL of this medium (with harvested stylet sheaths) was directly pipetted onto pre-cleaned microscope slides. Glass covers (No. 1 thickness, Fisher Scientific, Hampton, NH) were applied and their edges sealed with clear nail polish (EMS). Solvy™ isolated whole D. citri stylet sheaths, were imaged by confocal DIC post stylet sheath collection, mounted on pre-cleaned microscope slides, covered, and sealed with nail polish as described previously. In some cases, D. citri nymphal exuviae were gently pulled away from the leaf (with their stylets still extended) under a stereomicroscope, preserved in 70% ethanol, then mounted and examined by confocal DIC as described. Additionally, hand sections of Duncan grapefruit (Citrus paradisi Macfadyen) leaf midribs fed on by D. citri were stained with propidium iodide following standard protocols and mounted for confocal laser and confocal DIC microscopy as described previously [33].
Stereoscopic Microscopy
Stereoscopic micrographs and video were acquired using a Leica M60 Stereomicroscope and Leica DFC290 HD camera system with image and movie capture by LAS (Leica Application Suite V3.7.0 build 681, Leica Microsystems, Wetzlar, Germany) imagery software.
Light Direct Sheath Face Mock Feeding Chamber Microscopy
Direct MFC sheath face microscopy employed the 35 mm×10 mm chambers or 100 mm×15 mm MFCs. For direct sheath face light microscopy, we used an Olympus IX70 inverted microscope (Olympus, Tokyo, Japan). Micrographs were captured using an Olympus DP73 camera system (Olympus) coupled with cellSens® Dimension version 1.6 (build 9457) imaging software (Olympus). The direct examination of the MFC membrane affixed stylet sheaths was accomplished by inverting the MFC, membrane/sheath face down, directly over an inverted microscope objective lens. This allowed for direct visualization of membrane-affixed stylet sheaths using 4, 10, 20, and 40X objectives. The addition of mounting medium and subsequent overlay with a thin glass cover slip directly to the MFC membrane surface, permitted use of 60 and 100X objectives with oil immersion. Illumination of the stylet sheaths employed either in-line (parallel light to the objective) or 90° lighting (perpendicular to the objective, provided by an external halogen light).
Video capture of live action insect mock feeding attempts employed the Sony DKC-5000 camera system (Sony, Tokyo, Japan) coupled with Image Pro Plus 6.0 software (version 6.0.0.260, Media Cybernetics, Inc., Bethesda, Maryland).
Micrograph and Video Tools
Paint.Net version 3.5.6 (Copyright© 2010 dotPDN LLC, Rick Brewster, and contributors, http://www.getpaint.net/index.html) was used for addition or repositioning of scale bars, size cropping of images, or image rotation as needed. ImageJ version 1.42q (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011) was used to render confocal images into 3D projects and for size measurements. Windows Live Movie Maker version 2011 (Microsoft) was used for video editing, and Any Video Converter version 3.5.2 (AVCLabs, www.avclabs.com) was used for video conversion and compression.
Results
Formation of in āere Stylet Sheaths in Tested Phytophagous Hemipterans
During the D. citri stylet sheath studies using artificial diet sandwiched between two layers of Parafilm® membrane [34], we observed that immediately upon caging in a Petri dish covered with a single layer of Parafilm® prior to diet overlay, the D. citri initiated feeding behavior across the membrane (Video S1). Subsequent to this observation, D. citri were caged in MFCs using different membrane types including a variety of clear plastic wrap(s) and Solvy™ stabilizers (water-soluble poly-vinyl alcohol membranes). On all of these membranes, the D. citri produced stylet sheaths in āere (e.g. Figure 1; Figure 2A–D; and Figure S1G–I).
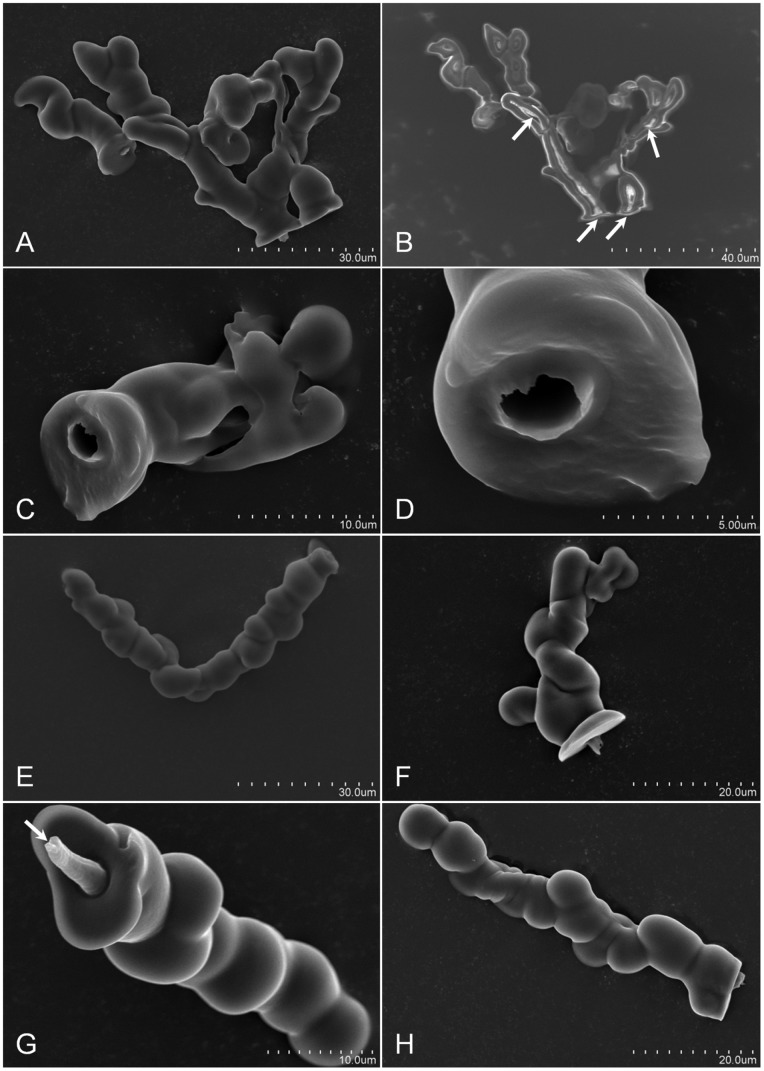
Figure 1. Scanning electron micrographs of D.citri in a¯ere stylet sheaths.
Panel A, is an image of two sheaths, the first (left sheath) is single-canal and the second (right) is a multi-branched sheath. Panel B is a non-gold sputtered image of Panel A with the white arrows indicating internal hollow canal tracks for the D. citri stylets. Panels C and D indicate the hollow canal of the D. citri stylets track with Panel D being an enlargement Panel C stylets canal opening. The opening is ∼3 µm in diameter. Panels E and F, indicate angular stylets probing (in āere) with panel E having a ∼93° bend within a single-canal sheath. Panels G and H are typical of linear sheaths formed in āere with panel G (white arrow indicating a closure of the stylet canal) suggesting secretion of sheath material as the stylet was retracted from the sheath.
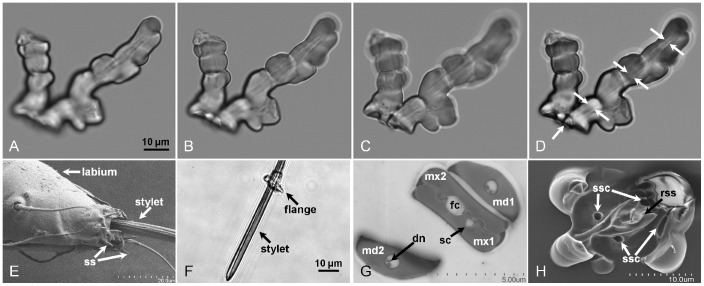
Figure 2. Micrograph images of D.citri in a¯ere formed stylet sheath and flange, and D. citri labrum and stylet bundle.
Panels A – C are individual confocal slices, with Panel D being a merged composite image of Panels A – C confocal slice images. Panel D, white arrows, indicate the visible internal stylet canal traversing the central core of the stylet sheath extending from the base to the terminal end of the sheath. Panel E is a SEM micrograph of a D. citri labium, stylets, and labial tip sensilla (ss). Panel F is a single slice confocal DIC micrograph of a D. citri third instar nymph exuvial stylet bundle with a detached flange circumambient the stylets. Panel G is a TEM micrograph cross-section of an adult D. citri stylet bundle indicating interlocking maxillary (mx) with mandibular (md) stylets, salivary (sc), food (fc), and dendrite (dn) canals. Panel H is a SEM micrograph of an adult D. citri flange formed in a mock feeding chamber. White arrows designate the location of indentations in the flange are sensilla cavities (ssc) with the central protrusion (black arrow) indicating a mound of retraction secreted sheath (rss) material formed upon withdrawal of the stylets from the sheath.
To determine if in āere stylet sheath production was an attribute of other phytophagous hemipterans, similar MFCs were tested with: P. pyriformis (Figure 3A–C), F. virgata (Figure 3D–E), H. vitripennis (Figure 4A and B), B. tabaci (Figure 4E and F), and aphid (Figure 4I and J) insects with each of these produced in āere stylet sheaths (see also Video’s S2– S6). Observations indicated that D. citri, F. virgata, and P. pyriformis in āere sheaths were longer and more complex (including commonly multi-branched stylet sheaths being formed) than those deposited by aphids, B. tabaci, and H. vitripennis (compare Video’s S1, S5, and S6 to Video’s S2, S3, and S4) under this artificial system. Penetration of the stylet bundle and formation of the stylet sheath for each of these insects typically occurred within the first 0–10 minutes post caging in the MFCs, with extended incubation times (beyond 10 minutes and up to 72 hours post caging in the MFC) providing an increase of deposited stylet sheaths for D. citri, aphids, and WFs (data not shown).
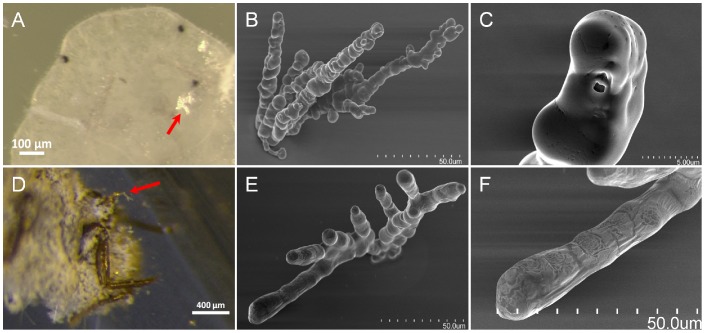
Figure 3. P.pyriformis and F. virgata in āere formed stylet sheaths.
Panels A – C and D – F are P. pyriformis and stripped F. virgata associated micrographs, respectively. Panels A and D are stereoscopic views of a P. pyriformis and F. virgata (respectively) mock feeding across a clear plastic membrane, with red arrows indicating multi-branched air formed sheaths. Panels B and E are SEM micrographs of P. pyriformis and F. virgata multi-branched sheaths, respectively. Panels C and F are magnified SEM micrographs of selected terminal sheath branch tips from Panels B and E, respectively.
Figure 4. H.vitripennis, B. tabaci, and A. nerii in āere formed sheaths and flanges.
Panels A – D, E – H, and I – L are sheath and flanges from H. vitripennis, B. tabaci, and A. nerii, respectively. Panels A, B, E, F, I, and J are sheath and Panels C, D, G, H, K, and L are flange SEMs, respectively. For each, the sheaths are short and lack the branching as seen previously for D. citri, P. pyriformis, and F. virgata (for comparison see Figure 1 - Panels A and B, Figure 2 - Panel D composite, and Figure 4 - Panels B and E). Flanges for both the H. vitripennis (Panel C) and B. tabaci (Panel H) indicate an open access point into the central canal (designated by the black arrows) for the stylets. Panel D is an increased magnification SEM of the H. vitripennis flange opening for the stylets indicating the hollow canal for the stylets. Panels K and L indicate that the A. nerii flange are sealed closed by retraction secreted sheath material (green arrows). White arrows indicate sensilla cavities in the flange surface (Panels C, H and L), yellow arrows indicate the labial groove imprint on the H. vitripennis flange surface (Panel C), the red arrow indicating the coalescence of secreted whitefly ‘waxy’ cuticular lipid hydrocarbons shed and covering the flange (Panel G), and the black arrows (Panels B and H) indicate the open cavity of the stylet canal for both the H. vitripennis and B. tabaci flanges.
To gauge the proclivity of singularly caged D. citri (in a MFC) to form stylet sheaths in āere versus ‘in diet’ over a 24 hours period, we found these averaged ∼16.2±2.1 stylet sheaths in āere relative to 53.0±9.6 (mean±standard error, n = 6) for singularly caged ‘in diet’ fed psyllids.
Contrastingly, it was observed that singularly MFC caged H. vitripennis produced approximately 2–7 sheaths during the first ten minutes after caging; however, subsequently the H. vitripennis ceased probing over the following 24-hour period. For F. virgata and P. pyriformis, these were observed to produce complex multi-branched (Figure 3) in āere stylet sheaths with continued probing attempts over the 24-hour period; however, the total number of sheaths produced by these remained low, perhaps due to the sedentary nature of these or the complexity of their stylet sheaths formed in the MFC environment.
Direct Surface Microscopy (SEM, Stereo, and Light)
The MFCs (see methods) proved to be an adaptable platform to allow for new observational methods by SEM and light microscopy to obtain both high resolution still and video images of form and formation of stylet sheaths for these insects. For direct surface SEM both the sheath face (Figure 3G and E; Figure 4A and B, E and F, I and J; and Video’s S1, S2, S3, and S6) and the flange face (Figure 2H; Figure 4C and D, G and H, K and L; Figure S1A–F; and Video S1) of the MFC membranes were observed. Stereomicroscopy (Figure 3A and D; and Video’s S1, S2, S4, S5 and S6) and inverted light microscopy (Video’s S1, S3, and S5) of MFC chambers provided both still and video imagery of stylet sheath formation without solid or liquid diet obstruction.
Descriptions of in āere Stylet Sheaths of Tested Insects
Diaphorina citri
Characteristic attributes of in āere D. citri stylet sheaths include: a sealed flange (Figure 2H, Figure S1A; and Video S1), labial sensilla impression cavities in the flange surface (Figure 2H and Figure S1A), neck region (Figure 5A–D; Figure S1G–I; and Video S1), and bulbous secretions on the sheath segment of the stylet sheath (Figure 1A, E–H; Figure 2D, Figure 5A – D; Figure S1G–I; and Video S1). D. citri in āere stylet sheaths frequently included multiple branches (Figure 1A; Figure 5C and D; Figure 6C; Figure S1I; and Video S1). These multi-branched stylet sheaths showed that branch forks can occur within the flange (Figure 5D and Figure S1I) or within a sheath segment (Figure 1A; Figure 5C and D; and Video S1). This is consistent with in planta D. citri stylet sheaths, Figure 6, which show similar traits of bulbous secretions (panels A and B) and multiple branching points (panel C). The branching in this instance occurs within the phloem region of the leaf tissue (Figure 6C).
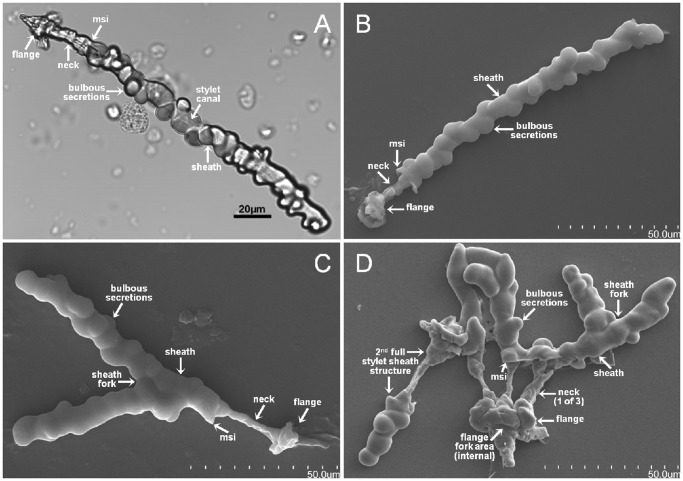
Figure 5. D.citri in a¯ere formed stylet sheaths from water-soluble Solvy™ stabilizer membranes.
Panel A image is DIC and panels B – D are SEM micrographs of D. citri Solvy™ isolated stylet sheaths. Structural features of these intact (attached sheath with flange) stylet sheaths are indicated including: ‘neck’ segments that correspond to the membrane traversing span connecting the flange and sheath segments (Panels A – D), the membrane sheath interface (msi) on the sheath face side (Panels A – D), continuous bulbous secretion formations and fork locations in the sheath segment (Panels C and D), or the flange (Panel D) segment.
Figure 6. Naturally formed D.citri stylet sheaths within leaf midrib of Duncan grapefruit (Citrus paradisi Macfadyen).
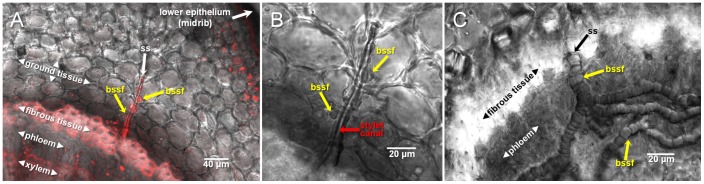
Panel A indicates a stylet sheath segment having bulbous stylet sheath formations (bssf) within the ground tissue region of the midrib that originated from a D. citri probe on the lower surface of the midrib. Propidium iodide fluorescence staining is used to provide contrasting resolution between the tissue types and the stylet sheath structure. Panel B is a magnified view of Panel A stylet sheath (ss) region minus fluorescence, indicating the bssf areas as well as the apparent stylet canal traveling parallel to the stylet sheath core. Panel C illustrates bssf continuing within the phloem tissue and additionally illustrates the multi-branching of the sheath portion within the phloem tissue. Micrographs are DIC.
Additional in āere sheath morphologies are illustrated by the D. citri stylet bundles ability to angularly probe as Figure 1E contains a single track stylet sheath having an ∼93° bend central to the sheath. SEM and light microscopy illustrate that the central canals are generally hollow through the sheath core (Figure 1B–D; Figure 2A–D; Figure 5A; Figure S1G–H; and Video S1), having an average canal diameter of ∼2.5 to 3.5 µm diameter for adult D. citri stylet sheaths; however, Figure S1G–H (with insets) illustrate these canals can be both hollow and filled-in/obstructed at points along the central canal track. Video S1 illustrates that the hardening of the D. citri in āere stylet sheath material appears to occur in as few as 45 seconds upon first detection of the secretion of saliva from the stylet bundle post piercing of the MFC membrane. Individual MFC probing events by D. citri can be highly variable, ranging from a few seconds, to a few minutes, and can extend beyond ten minutes (Video S1 and observations).
Aphis nerii, toxoptera citricida, aphis gossypii
Characteristic attributes of in āere aphid stylet sheaths indicate sealed flanges are common across these aphid genera (Figure 4K and L; Figure S1B). A. nerii (Figure 4I and J) and T. citricida (data not shown) commonly have diminutive sheaths; however, A. gossypii illustrates the potential to make multi-branch stylet sheaths (Video S2) in āere. Bulbous secretions are apparent on the stylet sheath (Figure 4I and J) and the flange displays the presence of labial sensilla cavity impressions (Figure 4L; and Figure S1B).
Bemisia tabaci biotype B
B. tabaci in āere stylet sheaths were commonly short, most often having a single canal (Figure 4E and F; Video S3), but occasionally with multiple canals (Video S3). In general, bulbous secretions constitute the structural morphology of these short sheath structures (Figure 4E and F; and Video S3). B. tabaci in āere formed flanges remain open to the stylet canal (Figure 4H), and labial sensilla impression cavities are evident in the flange (Figure 4H). Secreted whitefly ‘waxy’ cuticular lipid hydrocarbons [35], [36], [37] are prominently shed across the surface of the flange membrane face, coalescing in high numbers on the B. tabaci flange (Figure 4G and H; and Figure S1C), often obstructing the view of the flange.
Homalodisca vitripennis
Attributes of in āere H. vitripennis stylet sheaths appear as semi-bulbous to somewhat vertical amorphous protrusions from the sheath face of the MFC membrane (Figure 4A and B; and Video S4). Relative to the size of this insect and its stylet bundle, their stylet sheaths appear as short, single canal structures, and do not indicate multi-branching in āere (Figure 4A and B; and Video S4). The solidification rate of H. vitripennis in āere stylet sheaths may differ relative to the probing membrane used for the MFC as clear plastic wrap and parafilm surfaces seem to indicate a difference in rigidity upon sheath retraction of the H. vitripennis (Video S4). The H. vitripennis flanges indicate an open central canal of the stylet sheath (Figure 4C and D; and Figure S1D–F) with sensilla impressions in the flange surface and the labial groove imprints apparent (Figure 4C; and Figure S1D).
Ferrisia virgate
Characteristics of in āere F. virgata stylet sheaths appear almost exclusively as multi-branched structures having a continuous bulbous structural morphology from the sheath base. Branching is usually apparent along the main ‘trunk’ portion of the sheath protrusion away from the MFC membrane surface akin to branches from a tree (Figure 3D and E and Video S5). The branches appear to exist on the same plane and do not form a radial pattern from the branching point (Figure 3E and Video S5). F. virgata in āere flanges were not studied for this report. Behavioral movements of the F. virgata during stylet sheath production in āere indicate both branching and radial movements of the sheath encased stylet bundle and a ‘push-up’ like action is employed by the F. virgata that appears to assist in the partial retraction of the stylet bundle to a branching point within the stylet sheath (Video S5). F. virgata in āere complete sheath formation time is a prolonged event that can last up to 17 minutes for a single (multi-branched) stylet sheath (Video S5).
Protopulvinaria pyriformis
Characteristic attributes of in āere P. pyriformis stylet sheaths appear almost exclusively to be multi-branched structures, having a continuous bulbous structural morphology originating from the stylet sheath base through to the tip of the sheath structure (Figure 3B and C; and Video S6). No evidence for single canal P. pyriformis stylet sheaths was observed. Branching appears to occur in a radial pattern from the sheath base near the MFC membrane surface (Figure 3A and B; and Video S6). This P. pyriformis basal branching appears similar to certain D. citri stylet sheaths that contained branching points located within the flange region (Figure 5D; and Figure S1I). P. pyriformis flanges were not studied for this report. Time for sheath production does not appear to be a comparable feature as the P. pyriformis probed in a single location from which it did not appear to move during a 24-hour observational period.
Using Solvy™ Water-soluble Membrane for D. citri Stylet Sheath Collection
To collect full D. citri stylet sheaths for structural analyses two methods were used. Initially, we used a bent glass rod in combination with a small volume of water applied directly to the MFC membrane surface opposite the surface on which the psyllids fed. The bent glass rod was used to scour the MFC surface to dislodge D. citri stylet sheaths, which were then collected in the water/stylet slurry for analyses including microscopic observations (Figure 1 and Figure 2A–D). Less than 5% of the available stylet sheaths were recovered from either clear plastic wrap or parafilm membranes using this method. To increase the efficiency of sheath harvest, Solvy™ stabilizer (a water-soluble polyvinyl alcohol film) was used as the probing membrane for MFCs. This was used in combination with filters (see methods) to harvest (conservatively) an estimated >75% of the available stylet sheaths. This technique also allowed the harvest of intact stylet sheaths including the flange, neck, and sheath portions (Figure 5). In contrast, only the sheath portion was harvested by the bent glass rod/scour method (Figure 1 and Figure 2A–D).
Discussion
The formation of in āere stylet sheaths by D. citri, A. nerii, A. gossypii, T. citricida, B. tabaci, H. vitripennis, F. virgata, and P. pyriformis illustrates formation of sheaths without host tissue (plant) or non-host liquid or agar medium interactions or stimuli. Additional findings from this study include: i) stylet sheath formation does not require a persistent resistance against the stylets (post penetration of the membrane) as a stimulation to elicit sheath secretions as previously suggested [19]; as the stylet bundle penetrates through the membrane material, air resistance is minimal, and sheath formation still occurs (Video’s S1– S6); ii) stylet probing of the membrane was virtually instantaneous upon caging in the MFCs for each of these phytophagous hemipterans; iii) the insects did not require an additional stimuli to induce these probing events, suggesting a proclivity for exploratory probing of their environment; and iv) solidification of stylet sheaths appeared to occur rapidly in āere. These findings expand our knowledge of stylet sheath formation for these tested hemipterans; however, they do not indicate whether molecular compositional differences exist between in āere versus in planta formed stylet sheaths.
Common similarities in physical/morphological form appear between both in āere (Figure 1, 2, 3, 5 and Figure S1) and in planta (Figure 6) D. citri stylet sheaths, including flange, sheath branching, bulbous formations, and neck regions. These similarities between these D. citri in āere and in planta sheath forms may suggest a common chemical/structural composition as the mechanisms for sheath deposition may be similar; however, comparative chemical analyses of these should clarify this. Interestingly, a comparison of the neck regions from Solvy™ isolated D. citri stylet sheaths (Figure 5A–D) appears to correlate with previous reported descriptions of in planta sheaths from other hemipterans: the psyllid Ctenarytaina spatulata (Brennan et al 2000 Figure 2B [27]) and the planthopper Nilaparvata lugens (Wang et al 2008 Figure 6A, B, and D [22]). In these descriptions, the neck regions are indicated in planta as the stylet sheaths pass through the upper epidermal layer of the host plant tissue.
Common morphological features of in āere and in planta stylet sheaths support the use of ‘mock feeding chambers’ as a viable means for behavioral and stylet sheath compositional analyses for these hemipterans. Here we demonstrate the flexibility of MFC systems by altering the probing membrane types for specific purposes. For instance, the use of clear plastic wrap allowed for highly magnified real-time microscopic observations and live action video of hemipteran stylet sheath formation (e.g. Video S3). The use of Solvy™ water soluble membrane provided the means to isolate intact sheaths providing for in-depth compositional analyses (Morgan, J. K., et al, unpublished data) that lack potentially confounding background elements (traces of artificial diet or host plant tissue). Therefore, varying the membrane type for different MFC configurations may be useful for alternate analytical purposes for hemipteran stylet sheath or related studies.
Our results also present a method from which environmental influences can affect behavioral activities. For example, H. vitripennis, B. tabaci, and aphids each produce short sheaths in āere (Figure 4A and B, E and F, I and J); however, previous literature indicates that stylet sheaths for H. vitripennis, B. tabaci, and aphids have long and/or multi-branched sheaths in planta [31], [38], [39]. Therefore, manipulations of chemical applications to either the flange or sheath faces of an MFC membrane could provide a method to monitored for influences on the feeding behavior in relation to sheath structure and length. This common attribute of short sheaths produced on MFCs by H. vitripennis, B. tabaci, and aphids is most likely a manifestation of these insects ability to ‘sense’ the environment on which it is feeding and ‘deciding’ that the environment represents an ‘unsuitable’ feeding site; hence, these spend less energy by reducing their probing efforts. Clearly, the insects have different responses in this process since the MFC naïve H. vitripennis probe initially often in rapid succession ∼2 to 7 times upon caging (typically within the first ten minutes) and then stop while naïve D. citri probe continually throughout a 24-hours (and beyond) averaging approximately 16 (±2.1 St. Error) probes per 24-hour period. The membrane feeding system we present in this paper should be readily adaptable for further studies on this behavior.
Alternate differences are evident when comparing the flange regions of stylet sheaths of D. citri and aphids versus H. vitripennis and B. tabaci (we did not examine the flanges for F. virgata and P. pyriformis). For D. citri (Figure 2H, Figure S1A) and aphids (Figure 4K and L, Figure S1B), micrographs indicate closure of the flange openings. In contrast, those of H. vitripennis (Figure 4C and D, Figure S1 D–F) and B. tabaci (Figure 4H) indicate the flange central canal remains open upon stylet retraction. Present literature also indicates open flanges are evident on host leaf surfaces fed on by glassy-winged sharpshooter (Leopold et al 2003 Figure 4C and D, Figure S1D–F, Figure 28 [31]) and whiteflies (Freeman et al 2001 Figure 4H, Figure 1 [39]), which is consistent with our in āere flange findings for these insects. These data support a hypothesis that D. citri and aphids secrete sheath material during retraction of the stylet bundle resulting in flange closure.
For D. citri, supplemental evidence supporting sheath material secretion upon stylet bundle retraction is indicated by the variable diameters within individual canals for D. citri stylet canal tracks that appear to range from ∼2.5 ∼3.5 µm (Figure 2D, Figure 5A, and Figure S1G–I). Evidence that stylet canals can be completely filled for D. citri (Figure S1G–I, inset images) also strongly indicate this. As the average adult D. citri stylet bundle diameter is ∼5 µm (Figure 2F, note flange circumambient the stylet bundle indicating that the complete stylet bundle enters the canal of the stylet sheath), a D. citri stylet sheath canal having a diameter less than 5 µm may suggest that stylet bundle retraction secretion is involved or that drying effects of the stylet sheath have constricted the canal diameter [10].
The mechanism for the solidification of stylet sheath has been thought to require interaction with host plant tissues [20] or artificial media, yet these in āere stylet sheaths refute this for these tested Hemipterans (Figure 1, 2, 3, 4, 5, and Video’s S1, S2, S3, S4, S5, S6). As referenced previously, prior work has implied in āere solidified sheath material occurring in sharpshooters [29]. In this prior study, the authors indicate specific protein differences between the profiles of brush induced sheath materials and sheath materials harvested by differing methods (parafilm collected over diet, direct gland removal saliva harvest, and filter paper extracts ‘milking’). Their data indicated the necessity of protein factors for full stylet sheath formation to occur [29]. Other hypotheses suggest that oxygen specific interactions are needed for proper sheath gelling [11] and support for this has recently been indicated for aphid stylet sheaths [10]. Experiments using the in āere system shown here can be envisioned where the gas composition of the feeding chamber can be manipulated to block oxygen from below the membrane surface. This would provide a convenient alternate method of testing oxygen requirements.
This study demonstrates that the ability to form stylet sheaths in āere is a common trait for multiple taxonomically diverse hemipterans (D. citri, A. nerii, A. gossypii, T. citricida, B. tabaci, H. vitripennis, F. virgata, and P. pyriformis). Each probed the membrane of their MFCs regardless of membrane type within the first few minutes of caging. Differences in stylet sheath lengths by species on MFCs solicit further inquiry into potential insect specific sensory capacities. We also demonstrated the adaptability of MFC membranes for alternate analyses related to stylet sheath production. The water-soluble Solvy™ stabilizer MFC membranes provides a simple way to rapidly isolate intact stylet sheaths to perform a variety of chemical and other analyses. Further work is ongoing, but these techniques pave the way for additional rapid advancements in the study of hemipteran stylet sheaths biosynthesis and potential ways to combat these pests by molecular interventions that target the stylet sheaths.
Supporting Information
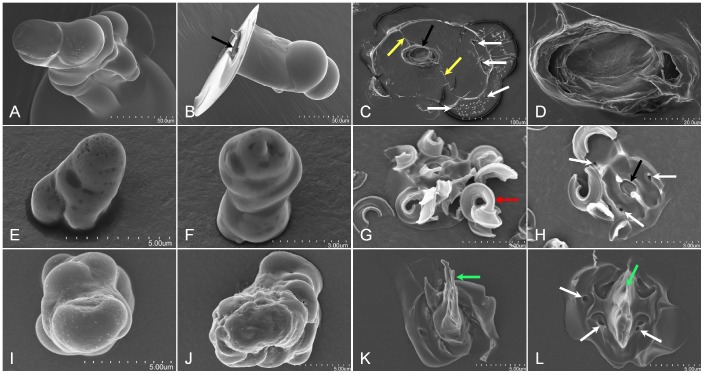
Micrographs of in āere formed flanges and stylet sheaths from: D. citri , T. citricida , B. tabaci , and H. vitripennis . Panels A and B are SEM micrographs of D. citri and T. citricida flanges (respectively), with green arrows indicating retraction secreted sheath (rss) material and white arrows indicating flange sensilla cavities (ssc). Panel C is a SEM micrograph of a typical B. tabaci flange coated with a heavy coalescence of the B. tabaci ‘waxy’ lipid hydrocarbon secretion (red arrow). Panel D is a SEM micrograph overview of a H. vitripennis flange with the black arrow indicating the open cavity entrance for the stylet canal, yellow arrow indicating the labial groove imprint on the flange surface, and the white arrows indicating H. vitripennis ssc imprints. Panel E is a higher magnification SEM micrograph surface focused view of the H. vitripennis flange stylet canal opening from Panel D, with Panel F being a deeper inside view focus SEM micrograph of the Panel D stylet canal opening. Panels G – I (including enlargement inset images), of full D. citri stylet sheaths (from Solvy™) indicating stylet canal closure throughout the central portion of the sheath (black arrows).
(TIF)
Video of a D. citri (Hemiptera: Psyllidae, Asian citrus psyllid) forming an in āere stylet sheath. From approximately 1 minute to 1 minute 45 seconds of the video, stylet sheath material appears to solidify. Additionally, scanning electron micrographs of both single canal and multi-branched sheaths and a flange are indicated, as well as a light microscopy of a fully formed Solvy isolated ss (flange and sheath connected by a neck region).
(MP4)
Video segments of two individual A. gossypii (Hemiptera: Aphididae, cotton/melon aphid) forming in āere stylet sheaths. Additionally, scanning electron micrograph indicates a multi-branch sheath formed in āere.
(MP4)
Video of a B. tabaci biotype B (Hemiptera: Aleyrodidae, whitefly) forming an in āere stylet sheath. Additionally observable are copious cuticular secretions on the flange face of the plastic wrap (appearing as white dust like particles). With scanning electron micrographs that indicate additional B. tabaci short stylet sheaths formed in āere.
(MP4)
Three video segments of Homalodisca vitripennis (Hemiptera: Cicadellidae, glassy-winged sharpshooter) forming in āere stylet sheaths. Video segments 1 and 2 indicate stylet sheath formation in āere across clear plastic wrap. Video segment 3 indicates stylet sheath formation in āere across a Parafilm® membrane.
(MP4)
Video of a single Ferrisia virgata (Hemiptera: Pseudococcidae, striped mealybug) forming a multi-branch stylet sheath in āere . Total elapsed time from start to finish is approximately 17 minutes. Subsequent light micrographs show a detailed view of the sheath.
(MP4)
Video of a Protopulvinaria pyriformis (Hemiptera: Coccidae, pyriform scale) forming a multi-branched in āere stylet sheath. Primary and secondary segments indicate dorsal and ventral stereo micrograph images of a Protopulvinaria pyriformis (Hemiptera: Coccidae, pyriform scale) on a leaf and caged in a mock feeding chamber forming a stylet sheath (respectively). Subsequent to these, a stereoscopic video of a single scale indicates the formation of a multi-branch stylet sheath across the clear plastic wrap of the mock feeding chamber. The final segment indicates a scanning electron micrograph of the multi-branch stylet sheath formed from the pyriform scale of the video.
(MP4)
Acknowledgments
The authors thank the kind access and provisions from: Dr. Cindy McKenzie and John Prokop for Bemisia tobaci (whiteflies), and Dr. Michele Hoffman and Dr. Melissa Doud for Ferrisia virgata (mealybugs) USHRL maintained colonies. We thank EricaRose Egan for her direct involvement and excellent assistance throughout the entirety of this work. Additionally, we thank the Florida Department of Agriculture and Consumer Services Division of Plant Industry for the identification of Aphis nerii, Aphis gossypii, and Protopulvinaria pyriformis used in this study and to Dr. Dov Borovsky and Dr. Aaron Dickey for their critical review of the manuscript.
Mention of trade names or commercial products in this article is solely for providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture.
Funding Statement
Funding for this work was provided by a Citrus Research and Development Foundation (CRDF) grant, CRDF Project # 330. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Forero D (2008) The systematics of the Hemiptera. Rev Colomb Entomol 34: 1–21. [Google Scholar]
- 2.Howard FW, Moore D, Giblin-Davis R, Abad R (2001) Insects on palms; Howard FW, editor. Wallingford, UK: CABI Pub.
- 3. Dejean A, Gibernau M, Bourgoin T (2000) A new case of trophobiosis between ants and Heteroptera. Cr Acad Sci Iii-Vie 323: 447–454. [DOI] [PubMed] [Google Scholar]
- 4.Gullan PJ, Martin JH (2009) Sternorrhyncha (jumping plant-lice, whiteflies, aphids, and scale insects). In: Resh VH, Cardé, R.T., editor. Encyclopedia of Insects 2nd edn. San Diego: Elsevier. 957–967.
- 5. Backus EA, Serrano MS, Ranger CM (2005) Mechanisms of hopperburn: An overview of insect taxonomy, behavior, and physiology. Annu Rev Entomol 50: 125–151. [DOI] [PubMed] [Google Scholar]
- 6. Kempema LA, Cui XP, Holzer FM, Walling LL (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol 143: 849–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goggin FL (2007) Plant-aphid interactions: molecular and ecological perspectives. Curr Opin Plant Biol 10: 399–408. [DOI] [PubMed] [Google Scholar]
- 8. Miles PW (1972) The Saliva of Hemiptera. Advances in Insect Physiology 9: 183–255. [Google Scholar]
- 9. Miles PW (1999) Aphid saliva. Biol Rev 74: 41–85. [Google Scholar]
- 10. Will T, Steckbauer K, Hardt M, van Bel AJE (2012) Aphid Gel Saliva: Sheath Structure, Protein Composition and Secretory Dependence on Stylet-Tip Milieu. PLoS ONE 7(10): e46903 doi:10.1371/journal.pone.0046903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tjallingii WF (2006) Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57: 739–745. [DOI] [PubMed] [Google Scholar]
- 12. Will T, Tjallingii WF, Thonnessen A, van Bel AJE (2007) Molecular sabotage of plant defense by aphid saliva. P Natl Acad Sci USA 104: 10536–10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreno A, Garzo E, Fernandez-Mata G, Kassem M, Aranda MA, et al. (2011) Aphids secrete watery saliva into plant tissues from the onset of stylet penetration. Entomol Exp Appl 139: 145–153. [Google Scholar]
- 14. Carolan JC, Fitzroy CIJ, Ashton PD, Douglas AE, Wilkinson TL (2009) The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics 9: 2457–2467. [DOI] [PubMed] [Google Scholar]
- 15. Backus EA, Andrews KB, Shugart HJ, Greve LC, Labavitch JM, et al. (2012) Salivary enzymes are injected into xylem by the glassy-winged sharpshooter, a vector of Xylella fastidiosa . J Insect Physiol 58: 949–959. [DOI] [PubMed] [Google Scholar]
- 16. Walling LL (2008) Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol 146: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tjallingii WF, Esch TH (1993) Fine-Structure of Aphid Stylet Routes in Plant-Tissues in Correlation with Epg Signals. Physiol Entomol 18: 317–328. [Google Scholar]
- 18. Will T, van Bel AJE (2006) Physical and chemical interactions between aphids and plants. J Exp Bot 57: 729–737. [DOI] [PubMed] [Google Scholar]
- 19. Miles PW (1959) The Salivary Secretions of a Plant-Sucking Bug, Oncopeltus-Fasciatus (Dall) (Heteroptera, Lygaeidae). 1. The Types of Secretion and Their Roles during Feeding. J Insect Physiol 3: 243–255. [Google Scholar]
- 20. Lloyd M, White JA (1987) Xylem Feeding by Periodical Cicada Nymphs on Pine and Grass Roots, with Novel Suggestions for Pest-Control in Conifer Plantations and Orchards. Ohio J Sci 87: 50–54. [Google Scholar]
- 21. Miles PW (1965) Studies on the salivary physiology of plant-bugs: the salivary secretions of aphids. J Insect Physiol 11: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 22. Wang YC, Tang M, Hao PY, Yang ZF, Zhu LL, et al. (2008) Penetration into rice tissues by brown planthopper and fine structure of the salivary sheaths. Entomol Exp Appl 129: 295–307. [Google Scholar]
- 23. Cherqui A, Tjallingii WF (2000) Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J Insect Physiol 46: 1177–1186. [DOI] [PubMed] [Google Scholar]
- 24. Miles PW (1960) The Salivary Secretions of a Plant-Sucking Bug, Oncopeltus-Fasciatus (Dall) (Heteroptera, Lygaeidae).2. Physical and Chemical Properties. J Insect Physiol 4: 209–219. [Google Scholar]
- 25. Miles PW (1960) The Salivary Secretions of a Plant-Sucking Bug, Oncopeltus-Fasciatus (Dall) (Heteroptera, Lygaeidae). 3. Origins in the Salivary Glands. J Insect Physiol 4: 271–282. [Google Scholar]
- 26. Miles PW, Harrewijn P (1991) Discharge by Aphids of Soluble Secretions into Dietary Sources. Entomol Exp Appl 59: 123–134. [Google Scholar]
- 27. Brennan EB, Weinbaum SA, Pinney K (2001) A new technique for studying the stylet tracks of homopteran insects in hand-sectioned plant tissue using light or epifluorescence microscopy. Biotech Histochem 76: 59–66. [DOI] [PubMed] [Google Scholar]
- 28. Lopes JRS, Bonani JP, Fereres A, Garzo E, Miranda MP, et al. (2010) Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol Exp Appl 134: 35–49. [Google Scholar]
- 29. Alhaddad H, Coudron TA, Backus EA, Schreiber F (2011) Comparative Behavioral and Protein Study of Salivary Secretions in Homalodisca spp. Sharpshooters (Hemiptera: Cicadellidae: Cicadellinae). Ann Entomol Soc Am 104: 543–552. [Google Scholar]
- 30. Lopes JRS, Miranda MP, Fereres A, Appezzato-da-Gloria B (2009) Characterization of electrical penetration graphs of Bucephalogonia xanthophis, a vector of Xylella fastidiosa in citrus. Entomol Exp Appl 130: 35–46. [Google Scholar]
- 31. Leopold RA, Freeman TP, Buckner JS, Nelson DR (2003) Mouthpart morphology and stylet penetration of host plants by the glassy-winged sharpshooter, Homalodisca coagulata, (Homoptera : Cicadellidae). Arthropod Struct Dev 32: 189–199. [DOI] [PubMed] [Google Scholar]
- 32. Ammar E, Hall DG (2011) A New Method for Short-Term Rearing of Citrus Psyllids (Hemiptera: Pysllidae) and for Collecting Their Honeydew Excretions. Fla Entomol 94: 340–342. [Google Scholar]
- 33. Ammar E, Hall DG (2012) New and Simple Methods for Studying Hemipteran Stylets, Bacteriomes, and Salivary Sheaths in Host Plants. Ann Entomol Soc Am 105: 731–739. [Google Scholar]
- 34. Hall DG, Shatters RG, Carpenter JE, Shapiro JP (2010) Research Toward an Artificial Diet for Adult Asian Citrus Psyllid. Ann Entomol Soc Am 103: 611–617. [Google Scholar]
- 35. Neal JW, Leonhardt BA, Brown JK, Bentz JA, Devilbiss ED (1994) Cuticular Lipids of Greenhouse-Whitefly and Sweet-Potato Whitefly Type-A and Type-B (Homoptera, Aleyrodidae) Pupal Exuviae on the Same Hosts. Ann Entomol Soc Am 87: 609–618. [Google Scholar]
- 36. Nelson DR, Freeman TP, Buckner JS (2000) Waxes and lipids associated with the external waxy structures of nymphs and pupae of the giant whitefly, Aleurodicus dugesii . Comp Biochem Phys B 125: 265–278. [DOI] [PubMed] [Google Scholar]
- 37. Lapointe SL, Hunter WB, Alessandro RT (2004) Cuticular hydrocarbons on elytra of the Diaprepes root weevil Diaprepes abbreviatus (L.) (Coleoptera : Curculionidae). Agr Forest Entomol 6: 251–257. [Google Scholar]
- 38.Urbańska A (2010) Histochemical analysis of aphid saliva in plant tissue. Electronic Journal of Polish Agricultural Universities 13 (4).
- 39. Freeman TP, Buckner JS, Nelson DR, Chu CC, Henneberry TJ (2001) Stylet penetration by Bemisia argentifolii (Homoptera : Aleyrodidae) into host leaf tissue. Ann Entomol Soc Am 94: 761–768. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Micrographs of in āere formed flanges and stylet sheaths from: D. citri , T. citricida , B. tabaci , and H. vitripennis . Panels A and B are SEM micrographs of D. citri and T. citricida flanges (respectively), with green arrows indicating retraction secreted sheath (rss) material and white arrows indicating flange sensilla cavities (ssc). Panel C is a SEM micrograph of a typical B. tabaci flange coated with a heavy coalescence of the B. tabaci ‘waxy’ lipid hydrocarbon secretion (red arrow). Panel D is a SEM micrograph overview of a H. vitripennis flange with the black arrow indicating the open cavity entrance for the stylet canal, yellow arrow indicating the labial groove imprint on the flange surface, and the white arrows indicating H. vitripennis ssc imprints. Panel E is a higher magnification SEM micrograph surface focused view of the H. vitripennis flange stylet canal opening from Panel D, with Panel F being a deeper inside view focus SEM micrograph of the Panel D stylet canal opening. Panels G – I (including enlargement inset images), of full D. citri stylet sheaths (from Solvy™) indicating stylet canal closure throughout the central portion of the sheath (black arrows).
(TIF)
Video of a D. citri (Hemiptera: Psyllidae, Asian citrus psyllid) forming an in āere stylet sheath. From approximately 1 minute to 1 minute 45 seconds of the video, stylet sheath material appears to solidify. Additionally, scanning electron micrographs of both single canal and multi-branched sheaths and a flange are indicated, as well as a light microscopy of a fully formed Solvy isolated ss (flange and sheath connected by a neck region).
(MP4)
Video segments of two individual A. gossypii (Hemiptera: Aphididae, cotton/melon aphid) forming in āere stylet sheaths. Additionally, scanning electron micrograph indicates a multi-branch sheath formed in āere.
(MP4)
Video of a B. tabaci biotype B (Hemiptera: Aleyrodidae, whitefly) forming an in āere stylet sheath. Additionally observable are copious cuticular secretions on the flange face of the plastic wrap (appearing as white dust like particles). With scanning electron micrographs that indicate additional B. tabaci short stylet sheaths formed in āere.
(MP4)
Three video segments of Homalodisca vitripennis (Hemiptera: Cicadellidae, glassy-winged sharpshooter) forming in āere stylet sheaths. Video segments 1 and 2 indicate stylet sheath formation in āere across clear plastic wrap. Video segment 3 indicates stylet sheath formation in āere across a Parafilm® membrane.
(MP4)
Video of a single Ferrisia virgata (Hemiptera: Pseudococcidae, striped mealybug) forming a multi-branch stylet sheath in āere . Total elapsed time from start to finish is approximately 17 minutes. Subsequent light micrographs show a detailed view of the sheath.
(MP4)
Video of a Protopulvinaria pyriformis (Hemiptera: Coccidae, pyriform scale) forming a multi-branched in āere stylet sheath. Primary and secondary segments indicate dorsal and ventral stereo micrograph images of a Protopulvinaria pyriformis (Hemiptera: Coccidae, pyriform scale) on a leaf and caged in a mock feeding chamber forming a stylet sheath (respectively). Subsequent to these, a stereoscopic video of a single scale indicates the formation of a multi-branch stylet sheath across the clear plastic wrap of the mock feeding chamber. The final segment indicates a scanning electron micrograph of the multi-branch stylet sheath formed from the pyriform scale of the video.
(MP4)