Abstract
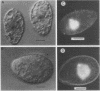
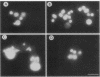
A temperature-sensitive mutation was isolated that blocks cilia regeneration and arrests growth in Tetrahymena thermophila. Protein and RNA synthesis and ATP production appeared to be largely unaffected at the restrictive temperature, suggesting that the mutation is specific for cilia regeneration and growth. At the restrictive temperature, mutant cells arrested at a specific point in the cell cycle, after macronuclear S phase and shortly before micronuclear mitosis. Arrested cells did not undergo nuclear divisions, DNA replication, or cytokinesis, so the mutation appears to cause true cell cycle arrest. Surprisingly, the mutation does not appear to affect micronuclear mitosis directly but rather some event(s) prior to micronuclear mitosis that must be completed before cells can complete the cell cycle. The cell cycle arrest was transiently complemented by wild-type cytoplasm exchanged during conjugation with a wild-type cell. Each starved, wild-type cell apparently contained enough rescuing factor to support an average of six cell divisions. Thus, this mutation affects assembly and/or function of at least one but not all of the microtubule-based structures in T. thermophila.
Full text
PDF


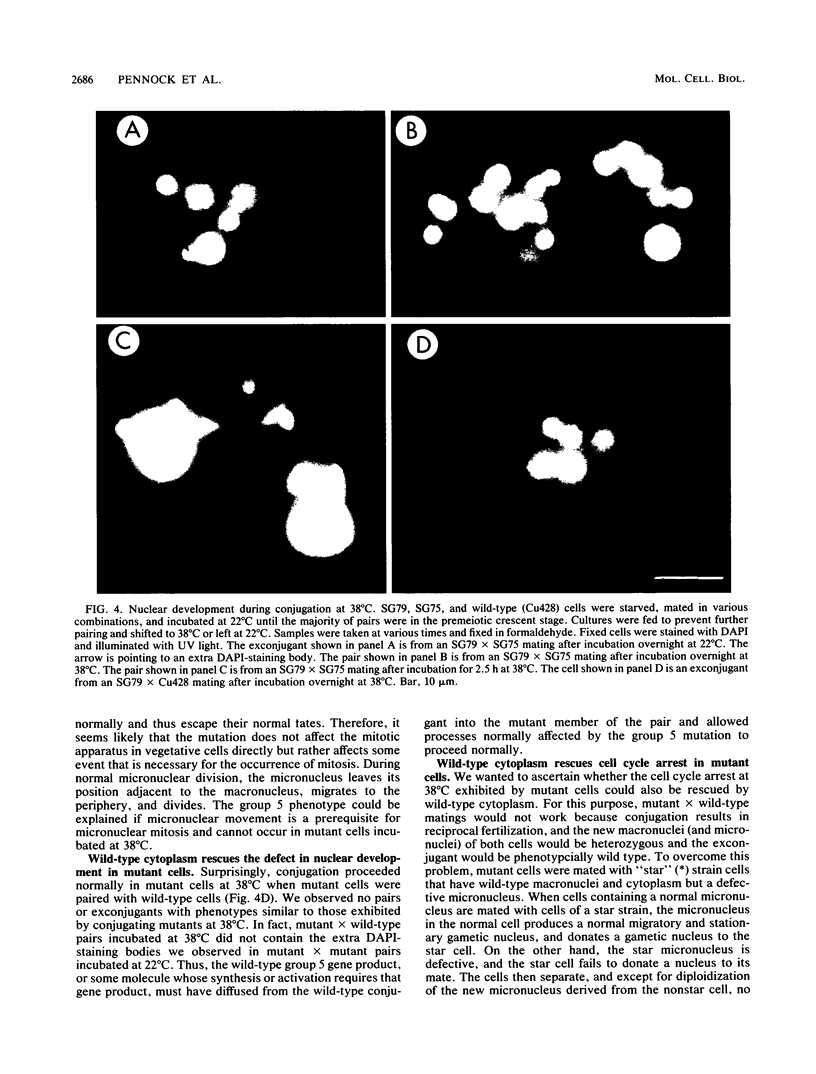
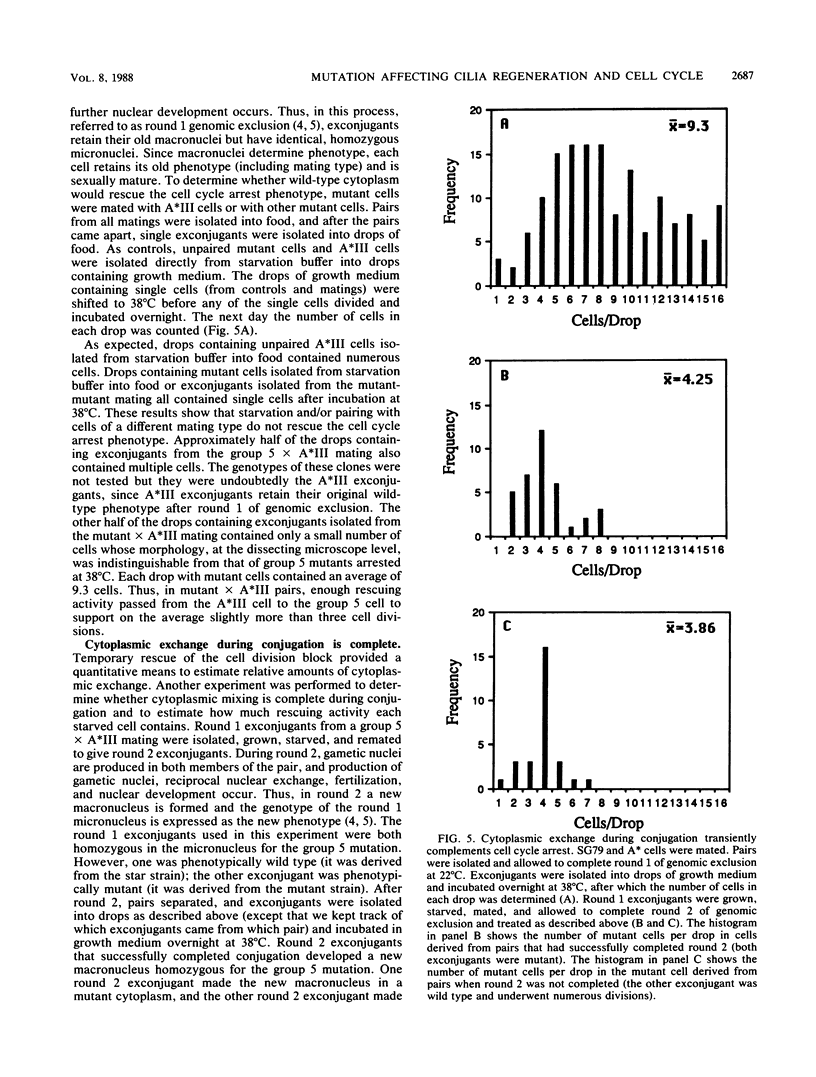


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. L. LINKAGE STUDIES IN VARIETY 1 OF TETRAHYMENA PYRIFORMIS: A FIRST CASE OF LINKAGE IN THE CILIATED PROTOZOA. Genetics. 1964 Apr;49:617–627. doi: 10.1093/genetics/49.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D. Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. J Protozool. 1967 Nov;14(4):553–565. doi: 10.1111/j.1550-7408.1967.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Allen S. L. Cytogenetics of genomic exclusion in Tetrahymena. Genetics. 1967 Apr;55(4):797–822. doi: 10.1093/genetics/55.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. L. Genomic exclusion: a rapid means for inducing homozygous diploid lines in Tetrahymena pyriformis, syngen 1. Science. 1967 Feb 3;155(3762):575–577. doi: 10.1126/science.155.3762.575. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Positive selection for mating with functional heterokaryons in Tetrahymena pyriformis. Genetics. 1974 Nov;78(3):831–841. doi: 10.1093/genetics/78.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Gorovsky M. A. Cilia regeneration in Tetrahymena. A simple reproducible method for producing large numbers of regenerating cells. Exp Cell Res. 1982 Aug;140(2):471–476. doi: 10.1016/0014-4827(82)90144-6. [DOI] [PubMed] [Google Scholar]
- Davidson L., LaFountain J. R., Jr Mitosis and early meiosis in Tetrahymena pyriformis and the evolution of mitosis in the phylum Ciliophora. Biosystems. 1975 Nov;7(3-4):326–336. doi: 10.1016/0303-2647(75)90010-6. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Diblasi S. L. Recombination and assortment in the macronucleus of Tetrahymena thermophila: a theoretical study by computer simulation. Genetics. 1984 Dec;108(4):1035–1045. doi: 10.1093/genetics/108.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder F. P. Regulatory Serotype Mutations in TETRAHYMENA PYRIFORMIS, Syngen 1. Genetics. 1973 May;74(1):81–106. doi: 10.1093/genetics/74.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J., Nelsen E. M., Martel E. Development of the ciliature of Tetrahymena thermophila. II. Spatial subdivision prior to cytokinesis. Dev Biol. 1981 Nov;88(1):39–54. doi: 10.1016/0012-1606(81)90217-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel-Williams R. Nuclear divisions with reduced numbers of microtubules in Tetrahymena. J Cell Sci. 1978 Dec;34:303–319. doi: 10.1242/jcs.34.1.303. [DOI] [PubMed] [Google Scholar]
- LaFountain J. R., Jr, Davidson L. A. An analysis of spindle ultrastructure during prometaphase and metaphase of micronuclear division in Tetrahymena. Chromosoma. 1979;75(3):293–308. doi: 10.1007/BF00293473. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. Conjugation in Tetrahymena thermophila. A temporal analysis of cytological stages. Exp Cell Res. 1982 Jul;140(1):227–236. doi: 10.1016/0014-4827(82)90172-0. [DOI] [PubMed] [Google Scholar]
- McCoy J. W. Linkage and Genetic Map Length in TETRAHYMENA THERMOPHILA. Genetics. 1977 Nov;87(3):421–439. doi: 10.1093/genetics/87.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen E. M., Frankel J., Martel E. Development of the ciliature of Tetrahymena thermophila. I. Temporal coordination with oral development. Dev Biol. 1981 Nov;88(1):27–38. doi: 10.1016/0012-1606(81)90216-5. [DOI] [PubMed] [Google Scholar]
- Nilsson J. R., Williams N. E. An electron microscope study of the oral apparatus of Tetrahymena pyriformis. C R Trav Lab Carlsberg. 1966;35(7):119–141. [PubMed] [Google Scholar]
- Orias E., Bruns P. J. Induction and isolation of mutants in Tetrahymena. Methods Cell Biol. 1976;13:247–282. [PubMed] [Google Scholar]
- Orias J. D., Hamilton E. P., Orias E. A microtubule meshwork associated with gametic pronucleus transfer across a cell-cell junction. Science. 1983 Oct 14;222(4620):181–184. doi: 10.1126/science.6623070. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Carlson K. Cilia regeneration in Tetrahymena and its inhibition by colchicine. J Cell Biol. 1969 Feb;40(2):415–425. doi: 10.1083/jcb.40.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick S. B., Bruns P. J. Conditional lethality associated with macronuclear development in Tetrahymena thermophila. Dev Biol. 1982 Sep;93(1):216–225. doi: 10.1016/0012-1606(82)90253-6. [DOI] [PubMed] [Google Scholar]
- Suprenant K. A., Hays E., LeCluyse E., Dentler W. L. Multiple forms of tubulin in the cilia and cytoplasm of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6908–6912. doi: 10.1073/pnas.82.20.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. E. Regulation of microtubules in Tetrahymena. Int Rev Cytol. 1975;41:59–86. doi: 10.1016/s0074-7696(08)60966-3. [DOI] [PubMed] [Google Scholar]
- Williams N. E., Williams R. J. Macronuclear division with and without microtubules in Tetrahymena. J Cell Sci. 1976 Jan;20(1):61–77. doi: 10.1242/jcs.20.1.61. [DOI] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Gorovsky M. A. Cell-cycle regulation as a mechanism for targeting proteins to specific DNA sequences in Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2205–2209. doi: 10.1073/pnas.85.7.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]