Abstract
Background
There are reports that bone marrow cell (BM) transplants repaired irradiated salivary glands (SGs) and re-established saliva secretion. However, the mechanisms of action behind these reports have not been elucidated.
Methods
To test if a paracrine mechanism was the main effect behind this reported improvement in salivary organ function, whole BM cells were lysed and its soluble intracellular contents (termed as “BM Soup”) injected into mice with irradiation-injured SGs. The hypothesis was that BM Soup would protect salivary cells, increase tissue neovascularization, function, and regeneration. Two minor aims were also tested a) comparing two routes of delivering BM Soup, intravenous (I.V.) versus intra-glandular injections, and b) comparing the age of the BM Soup’s donors. The treatment-comparison group consisted of irradiated mice receiving injections of living whole BM cells. Control mice received irradiation and injections of saline or sham-irradiation. All mice were followed for 8 weeks post-irradiation.
Results
BM Soup restored salivary flow rates to normal levels, protected salivary acinar, ductal, myoepithelial, and progenitor cells, increased cell proliferation and blood vessels, and up-regulated expression of tissue remodeling/repair/regenerative genes (MMP2, CyclinD1, BMP7, EGF, NGF). BM Soup was as an efficient therapeutic agent as injections of live BM cells. Both intra-glandular or I.V. injections of BM Soup, and from both young and older mouse donors were as effective in repairing irradiated SGs. The intra-glandular route reduced injection frequency/dosage by four-fold.
Conclusion
BM Soup, which contains only the cell by-products, can be advantageously used to repair irradiation-damaged SGs rather than transplanting whole live BM cells which carry the risk of differentiating into unwanted/tumorigenic cell types in SGs.
Introduction
There are an estimated 40,000 new cases of oro-pharyngeal cancer and 8,000 estimated deaths due to this disease each year in the United States [1]. Irradiation (IR) is a key component of therapy for these patients, but this causes damage to the salivary glands (SGs), particularly the acinar cells [2]. These cells are the principal site of fluid secretion (saliva) and such patients experience considerable morbidity and discomfort, ranging from symptoms such as xerostomia, dysphagia, dental caries, altered taste, oro-pharyngeal infections and pain [3]. In many patients, all the salivary secretory tissue is lost, which means no suitable treatment is available since existing pharmacologic therapy depends on stimulating residual acinar cells.
Studies of adult stem cell-based therapy have reported an overall improvement of (non-hematologic) organ function. The proposed mechanisms by which bone marrow (BM) cells improve organ functions have been investigated for the past 10–15 years. Initial reports proposed the ability of BM cells to (trans)differentiate into cells of a non-marrow/non-hematopoietic lineage (such as the brain, heart) was the mechanism of action for tissue regeneration [4], [5]. Then reports on fusion of BM cells with parenchymal cells of other tissues were documented [6], [7]. The third mechanism proposed was vasculogenesis from endothelial progenitor cells contained within peripheral blood stem cells or from BM mononuclear cells [8]. Lastly, the most recent mechanism of action is that BM cells provide a local paracrine effect [9], [10], [11]. These four mechanisms of action by BM cells have been thoroughly discussed in recent reviews [12], [13]. In summary, tissue and vascular regeneration have been initially proposed as mechanisms of stem cell action [10], [11], [14]. However at closer investigation, the low frequency of stem cell engraftment or the low number of newly generated parenchymal and vascular cells (either by cell transdifferentiation or fusion) could not fully explain organ improvement [10]. Accordingly, several authors have proposed an alternative paradigm that transplanted stem cells release soluble factors that, acting in a paracrine fashion, contribute to organ repair and regeneration. This hypothesis suggests that cytokines, growth and other factors induce cytoprotection, neovascularization, and mediate endogenous tissue regeneration via activation of resident tissue stem cells. In addition, tissue remodeling and organ function is affected by these paracrine factors [10].
Recent findings from our group demonstrated BM cell transplants repaired SGs and re-established saliva secretion in mice that received IR to their neck area [15]. This current paper aims to test if a paracrine mechanism is the main cause behind the reported improvement of salivary organ function. To test this, whole BM cells will be lysed and its soluble intracellular contents injected into mice with IR-injured SGs. A former group of researchers has coined this product as “bone marrow cell extract” [16], but this paper will rename it as “Bone Marrow Soup” (BM Soup) to first remind the readers that this product is made of a mixture of unknown soluble factors, and second, as a historical analogy to Chicken Soup (reported with medicinal properties, but its specific composition/ingredients are still not known) [17], [18], [19].
The first aim of this study is to test the efficiency of BM Soup and investigate its mechanisms of action in repairing SGs of mice damaged by IR. The hypothesis is that BM Soup protects salivary cells, increases tissue neovascularization, function, and regeneration. In addition, two minor aims are tested: a) comparing two routes of delivering BM Soup to SGs (I.V. versus intra-glandular injection), and b) comparing the age of the BM Soup’s donors.
Results
BM Soup Restored Salivary Organ Function, Protected Cells, Increased Proliferation of Cells and Blood Vessels, and up-regulated Expression of Tissue Repair/regenerative Genes
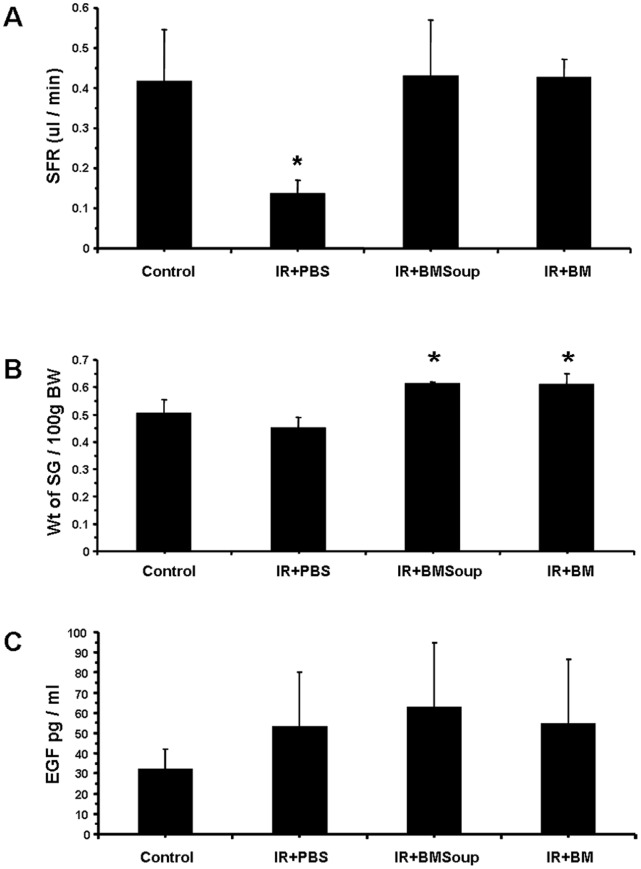
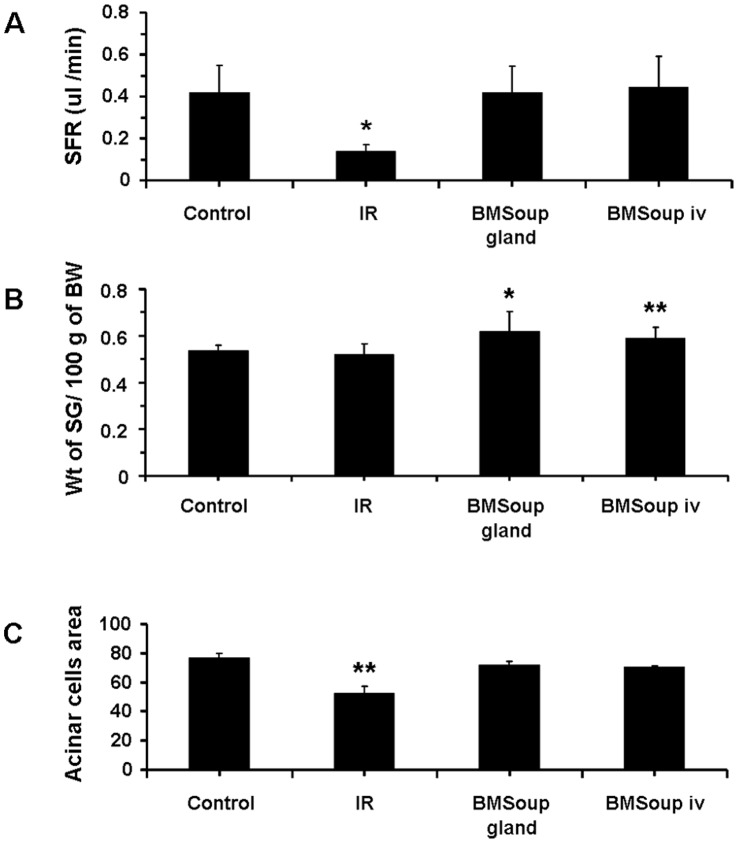
The first part of this study assessed the efficacy of BM Soup in repairing SGs that were damaged by IR injury. At week 8 post-IR, BM Soup-treated mice (IR+BMSoup) had their salivary flow rate (SFR) restored to normal levels (100%) while PBS-treated mice (IR+PBS) had a 60% reduction (Fig. 1A, p<0.05). SFR of BM Soup-treated mice were comparable to that of mice in the Control or BM-treated group (IR+BM). These data indicated that BM Soup treatment was superior to no treatment (IR+PBS injection) in re-establishing saliva secretion. Weights of the SGs (relative to their body weights) were higher in IR+BM Soup mice when compared to IR+PBS mice (Fig. 1B, p<0.05). All mouse groups had comparable concentrations of salivary EGF (Fig 1C).
Figure 1. Bone Marrow Soup re-established saliva secretion following irradiation.
A. At week 8 post-irradiation, BM Soup-treated mice (IR+BMSoup) had their salivary flow rate (SFR; ul/min/per g of body weight) restored to normal levels (100%; 0.4 ul/min) when compared to sham-irradiated mice (Control) and those transplanted with whole live BM cells (IR+BM). Mice receiving an intravenous injection of PBS (IR+PBS; vehicle control group) had 60% reduction in SFR (* P<0.05). B. Weights of the salivary glands relative to 100g of their body weights were higher in IR+BMSoup and IR+BM mice (* P<0.05). C. Saliva levels of epidermal growth factor (EGF) were not statistically different among the four groups of mice (P>0.05). N = 5 to 9 mice per group.
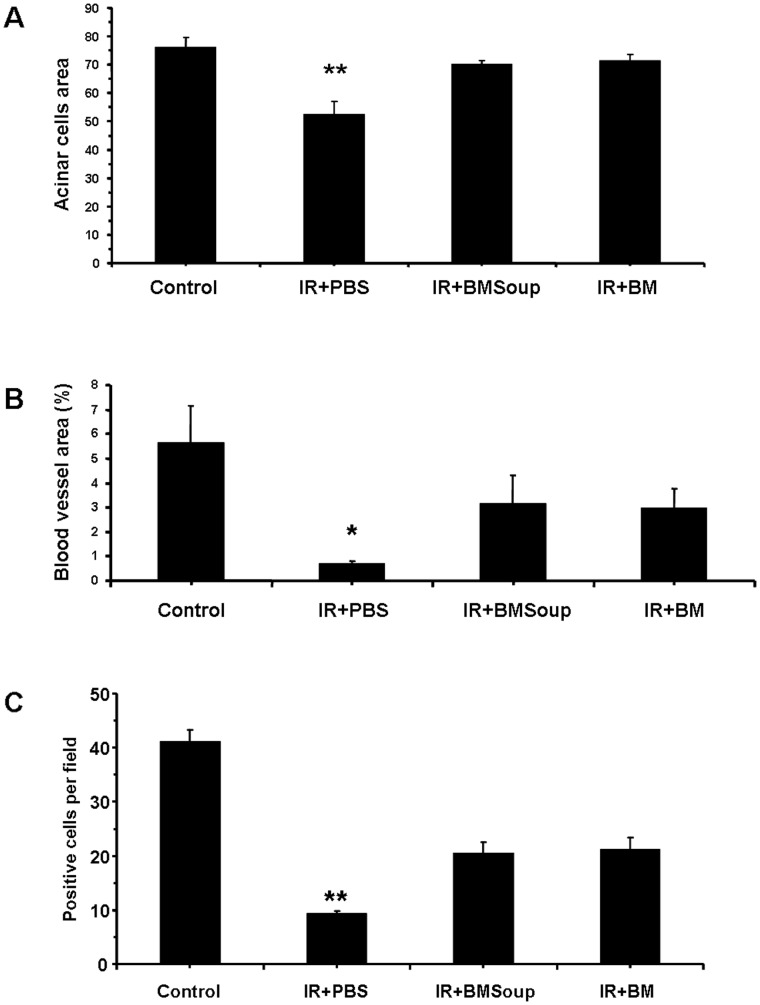
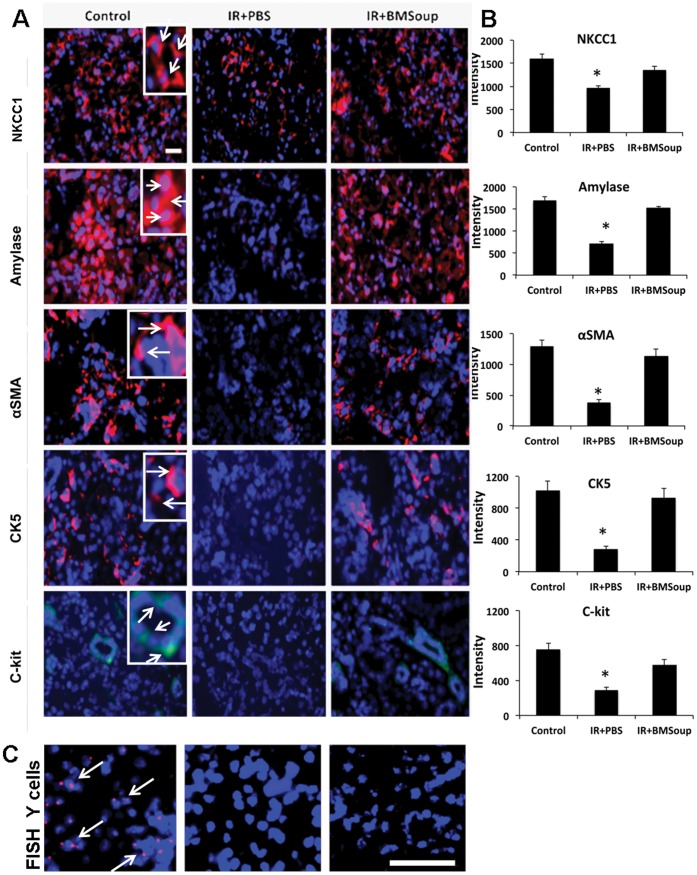
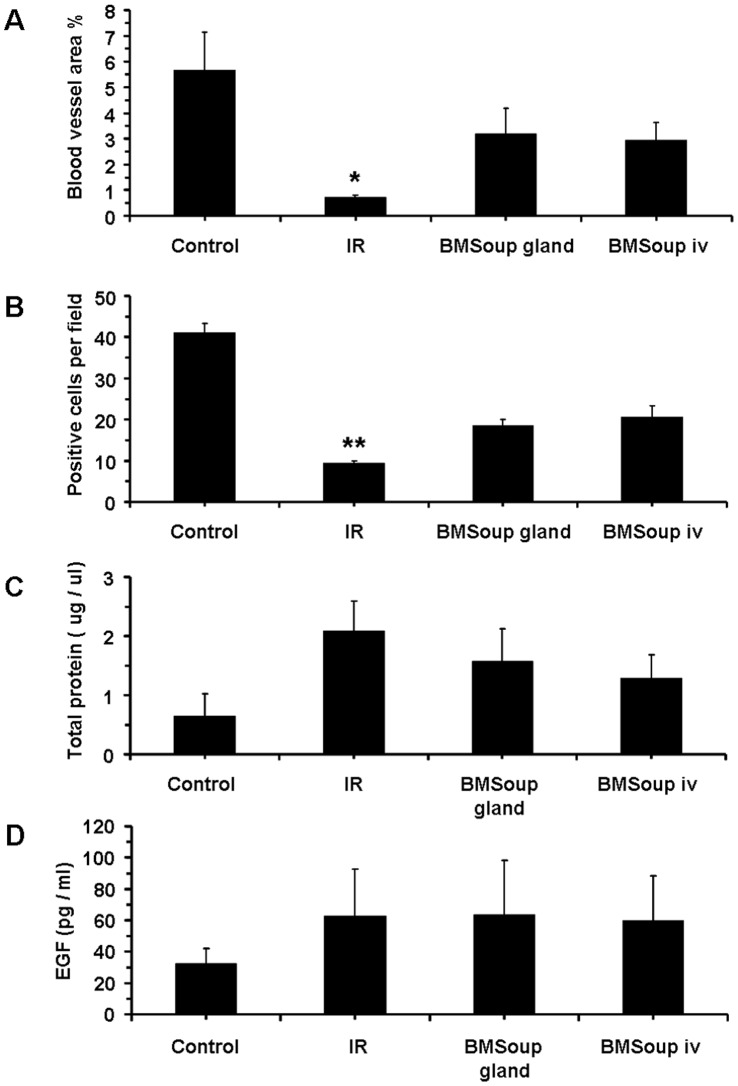
BM Soup was found to protect several cell populations in irradiated-SGs. Alcian blue and periodic acid-Schiff (PAS) staining revealed that the percentage of surface area occupied by acinar cells was comparable in Control, IR+BMSoup and IR+BM mice, while significantly increased when compared to IR+PBS treated mice (Fig. 2A, p<0.05). Von Willebrand factor staining showed that the number of blood vessels was markedly decreased (6-fold less) in IR+PBS mice when compared to non-IR control mice, but that BM Soup or BM cells treatment allowed (4-fold) more blood vessels to proliferate (Fig. 2B, p<0.05). BM Soup was found to favor tissue repair and remodeling. Cell proliferation (PCNA) was 2.5-fold higher in BM Soup-treated mice than IR+PBS mice (Fig. 2C, p<0.05). Immunostaining was used to localize and quantify specific subpopulations of cells. BM Soup-treated mice had a higher number of cells positive for amylase and NKCC1 (markers of acinar cells), smooth muscle actin (marker for myoepithlial cells), CK5 and c-kit (markers for ductal and salivary progenitor cells) than PBS-treated mice (Fig. 3A–B, p<0.05). To guarantee that BM Soup contained no live or whole cells, we examined under the microscope for any remaining cells and nuclei using trypan blue or Hoescht33342 dye (Invitrogen). None was observed (data not shown). Also, since BM Soup was obtained from male mouse donors, we subsequently used a Y-chromosomal probe and fluorescence in situ hybridization (FISH) to detect any male cells in SGs of female C3H mice. No male cells were observed (Fig. 3C). These experiments confirmed that the solution of BM Soup did not contain any cells.
Figure 2. Role of Bone Marrow Soup on acinar cells, blood vessels and cell proliferation rate.
A. Area in salivary gland occupied by acinar cells (quantification of 10 representative fields per gland/mouse with NIH image J software). BMSoup-treated group had a comparable percentage of surface area occupied by acinar cells as Control (sham-irradiated) and IR+BM groups, but a higher surface area with acinar cells than irradiated mice (IR+PBS) (*P<0.05, **P<0.01; N = 5 mice per group). B. BMSoup-treated mice had higher density of blood vessels (by von Willebrand factor) than irradiated mice. C. BMSoup-treated mice had higher cell proliferation (by PCNA) than irradiated mice.
Figure 3. Role of Bone Marrow Soup in cytoprotection.
A. Immunofluorescence staining of specific subpopulations of cells in submandibular glands at 8 wks post-irradiation. Cell markers are shown in red (or green for c-kit); cell nuclei are in blue. BM Soup-treated mice had a higher number of cells positive for NKCC1 and amylase (used here as markers of acinar cells; see white arrows in inset of a magnified region), smooth muscle actin (marker for myoepithlial cells), CK5 and c-kit (markers for ductal and salivary progenitor cells) than PBS-treated mice. All photographs were taken at the same magnification; scale bar (white) is 220 um; inset shows a higher magnification of selected cells. B. Quantification of protein fluorescence (intensity) expressed in 3–5 fields of 400 um2 area/per gland by Volocity software (N = 5 mice per group). BMSoup shows comparable proportions of acinar, ductal, myoepithelial, and stem cells as non-irradiated control/healthy mice. IR+PBS group has less protein intensity when compared to Control or BMSoup-treated mice (*P<0.05). C. Fluorescence in situ hybridization using a Y-chromosomal probe. Left picture with “red dots” localize (expected) male cells in a male mouse salivary gland (positive control). White arrows are pointing to some male cells. Cell nuclei are in blue. Middle picture was taken from a female mouse (negative control; no red cells are seen). Right picture confirmed that BM Soup did not contain any Y-chromosome (male) transplanted cells in female irradiated salivary tissues. White scale bar is 38 um.
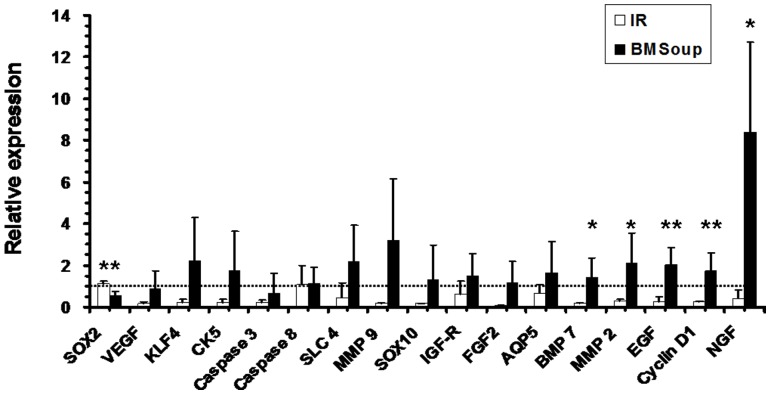
A panel of genes important to SG development, tissue regeneration and repair was examined in BM Soup-treated versus PBS-treated mice. In general, all genes surveyed for this study were up-regulated (Fig 4). Specifically, MMP2 (an enzyme in tissue breakdown), Cyclin D1 (cell cycle G1/Synthesis), and three growth factors involved in salivary gland development/maintenance (BMP7, EGF, NGF) were significantly up-regulated in IR+BM Soup versus IR+PBS mice (Fig 4; p<0.05), while only Sox2 (a transcription factor in stem cell renewal) was down-regulated. Taken together, all these above data (from Figs 1–4) indicated the efficacy of BM Soup in repairing IR-damaged SGs.
Figure 4. Relative gene expression of key factors involved in salivary gland development, repair and regeneration.
qRT-PCR results at 8-week post-irradiation between Control, Irradiated (IR only) and BMSoup-treated mice. mRNAs were significantly up-regulated in BM Soup versus IR mice for MMP2 (an enzyme in tissue breakdown), BMP7, EGF, Cyclin D1 (cell cycle G1/Synthesis) and NGF (*P<0.05, **P<0.01; N = 5 mice per group). There was a downregulation of Sox2 (transcription factor in stem cell renewal). Y-axis shows the relative expression of the gene compared to GAPDH. Horizontal dashed line represents the relative gene expression level of 1 in mice from the Control group (sham-irradiated mice).
Both Intra-glandular and Intravenous Injection Methods were Effective in Delivering BM Soup for the Tissue Repair and Restoration of Salivary Organ Function
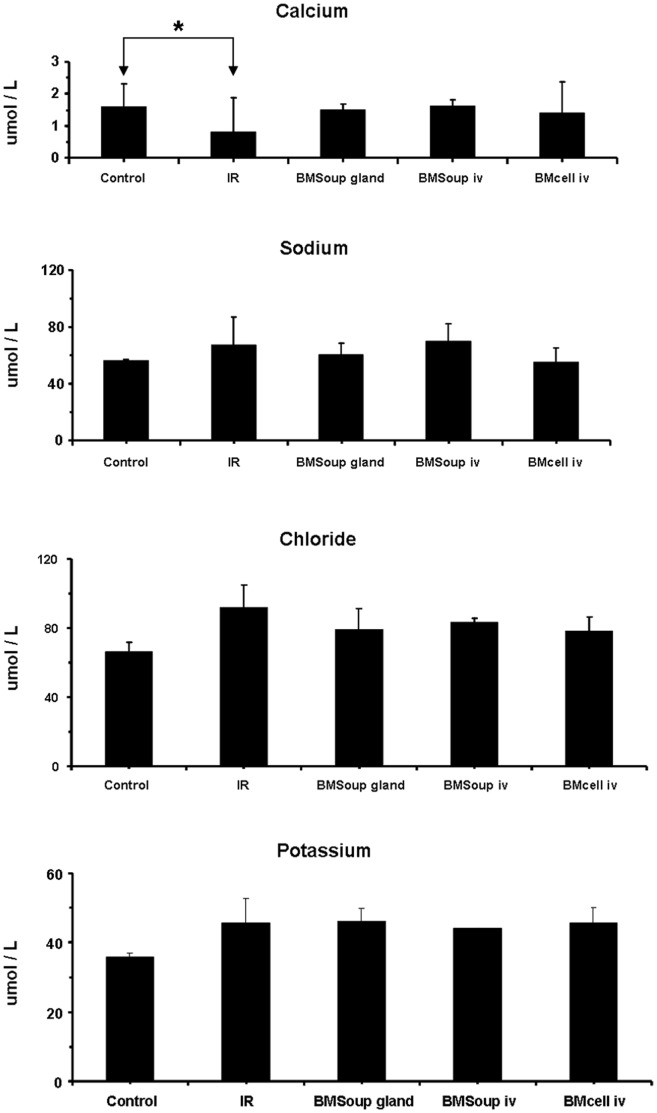
The second part of this study evaluated a systemic (I.V.) versus a local delivery route (intra-glandular injection) for BM Soup to repair SGs that were damaged by IR. Results at week 8 post-IR showed that both groups of mice treated with I.V. and intra-glandular injections of BM Soup had comparable SFRs, gland weights, cell proliferation rate, amount of acinar cells and blood vessels (Figs 5–6). These above mentioned measures were all lowered in IR+PBS mice (Figs 5–6; p<0.05). The composition (quality) of saliva was assessed and all mouse groups had comparable concentrations of salivary EGF, total proteins, and key electrolytes (sodium, potassium, chloride) (Figs 6–7). However, the levels of calcium was lower in IR+PBS mice (Fig 7, p<0.05). Overall, these data indicated that injections of BM Soup directly in the irradiated submandibular glands of C3H mice repaired the glands as efficiently as I.V. injections. One notable advantage for the intra-glandular delivery route was a lower frequency/number of injections. Only one intra-glandular injection was needed for the entire study as compared to four I.V. injections (twice a week for two consecutive weeks).
Figure 5. Intra-glandular and intravenous injections of BM Soup were both effective for restoration of salivary organ function.
A. At week 8 post-irradiation, mice that were injected with BM Soup, either intra- glandularly (BMSoup gland) or intra-venously (BMSoup iv), had their salivary flow rate restored to normal levels (100%; 0.4 ul/min) when compared to sham-irradiated mice (Control). Mice that were irradiated and not treated (IR group) had 60% reduction in SFR (*P<0.05, **P<0.01; N = 5 to 9 mice per group). B. Weights of the salivary glands were higher in BMSoup-treated mice, either by intra-glandular or by IV injection. C. Both intra-glandular and IV delivered BMSoup-treated mice had a comparable percentage of surface area occupied by acinar cells as Control (sham-irradiated) mice. Irradiated mice injected with PBS had significantly a lower percentage of surface area with acinar cells.
Figure 6. Intra-glandular and intravenous injections of BM Soup were both effective for tissue repair.
A and B. Both intra-glandular and IV BMSoup-treated mice had higher density of blood vessels (by von Willebrand factor) and cell proliferation (by PCNA) than irradiated mice. C and D. Saliva levels of total proteins and epidermal growth factor (EGF) were not statistically different among the four groups of mice (P>0.05; N = 5 to 9 mice per group).
Figure 7. Levels of key electrolytes in saliva.
Electrolytes in saliva (such as sodium, potassium, chloride, and calcium concentration levels) were not different among groups, except for calcium levels (P>0.05; N = 5 to 9 mice per group).
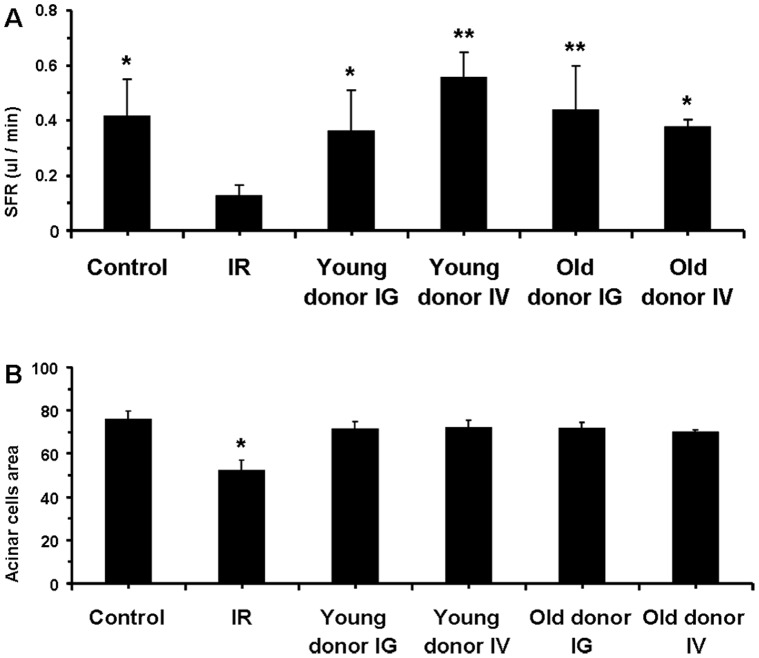
BM Soup of both Young and Older Mouse Donors was Equally Effective in Repairing Irradiated SGs
Reports from adult stem cell-based therapies indicated that more stem/progenitor cells could be isolated from younger donors [20], [21], [22], [23], [24]. Therefore, the third part of our study evaluated if BM Soup therapy success could be affected by the age of the donor mouse. Our data indicated that BM Soup harvested from young (7–8 wks old) and older mice donors (20–22 wks old) were both effective in repairing irradiated SGs. Both SFR and number of acinar cells were comparable between mice receiving BM Soup harvested from young and older donors, and from either an IV or intra-glandular injection delivery route (Fig 8). These findings suggest that BM Soup can be harvested from both young and adult mice, and that the delivery of BM Soup by IV or intra-glandular injection is equally efficient in repairing irradiated SGs.
Figure 8. BM Soup of both young and older mouse donors was as effective in repairing irradiated salivary glands.
A. At week 8 post-irradiation, salivary flow rate (SFR) of groups treated with BM Soup from both young (7–8 wks old) and older mouse donors (20–22 wks old) were comparable to sham-irradiated mice (Control) while the untreated mice (IR group) had 60% reduction in SFR. Both delivery routes for BM Soup (IG, intra-glandular; IV, intra-venous) were equally successful in re-establishing SFR to irradiated mice. B. The percentage of tissue surface area occupied by acinar cells was comparable between mice receiving BM Soup harvested from young and older donors, and from either an IV or IG injection delivery route. All mice treated with BM Soup had significantly a higher percentage of surface area with acinar cells than irradiated untreated mice (IR group). (*P<0.05, **P<0.01; N = 5 mice per group).
Discussion
The findings of this study were that: 1) BM Soup was as effective as whole live BM cells in repairing irradiated-SG, suggesting a paracrine effect of BM cells on SGs; 2) BM Soup restored salivary organ function, protected cells, increased proliferation of cells and blood vessels, and up-regulated expression of tissue repair/regenerative genes; 3) Intra-glandular or intravenous delivery of BM Soup was both effective for tissue repair and restoration of salivary organ function; and 4) BM Soup of both young and older mouse donors was effective in repairing irradiated SGs.
For functional restoration of damaged SGs, three approaches are being tested. The first approach is gene therapy and a recent successful trial restoring function in IR damaged human SGs has been reported [25]. The second approach is building an artificial SG using tissue engineering [26], [27]. The third approach is using (stem) cell-based therapy. Currently, stem/progenitor cells from two different organs are being investigated in mice: a) from the SG itself [28], [29], [30], [31], [32], [33] or b) from the BM [15], [34], [35], [36], [37], [38]. There is a general agreement, although with still a paucity of studies, that transplanted BM cells improved SG function in mouse models; while in humans, organ function has not been examined yet [13], [39], [40]. Both injected and mobilized BM cells (such as with granulocyte-colony stimulating factor) were found effective in partially restoring function in irradiated-SGs. Injections or vector-mediated transfer of growth factors (such as FGF2, IGF, KGF or VEGF) without cell transplants were also reported partially effective in repairing irradiated-SGs [41], [42], [43], [44], [45]. Taken these studies together, the data suggest that BM cells, specific growth factors, or combining both approaches could be used to restore SG organ function. This paper proposes an additional approach by delivering the soluble contents of lysed BM cells to SGs.
This paper highlights the importance of the paracrine effects of BM cell contents on irradiated-SG, and that intact BM cells may not be necessary. Proof-of-concept of paracrine cross-talking between SG cells and other cell populations (such as MSC or amniotic epithelial cells) was demonstrated by using a culture system that physically separated the cell populations [46], [47]. These findings on the paracrine effects of MSC versus SG cells are in agreement with studies from other organs. Particularly to those in cardiology where several studies on the paracrine effects of MSC (isolated from the BM) have shown that organ repair was due to the secretion of cytokines, chemokines, and growth factors [48]. The demonstration that injecting conditioned medium from MSC cultures exerted cardiomyocyte protection and improved cardiac function in mouse infarcted hearts provided an elegant proof of concept [49], [50]. However, delivering conditioned media would be impractical clinically. Therefore a group of cardiologists used a clinically-relevant and simple approach for delivering paracrine secretions of BM cells [16]. They hypothesized that if a paracrine mechanism was at play, use of the cell extract derived from whole BM (we use the term BM Soup) would enable them to detect the effects of any likely protein or factor that would be released. This cell extract was obtained by lysing BM cells, removing the insoluble material, and keeping only the intracellular soluble material. Living whole BM cells versus its cell extract were injected into mice which had surgically-induced myocardial infarction. Results showed similar improvement of cardiac function and a smaller infarct size in both mouse groups. This approach has many advantages (described below), in addition to being simple and likely to be translated to a clinical setting. Using BM Soup does not require the injection of live (stem) cells, which carry the risk of differentiating into unwanted/tumorigenic cell types in SGs. BM Soup includes all cell types of whole BM and consequently numerous proteins, cytokines and paracrine factors [16]. BM Soup is quicker to obtain than the use of a cell-derived conditioned media, which needs to be grown in culture and thus requires more time. BM Soup is not patient-specific. Recently, BM Soup from human origin was reported to improve cardiac function in a mouse model of myocardial infarction, without the need for immuno-compromised animals or antibiotic therapy [51]. Thus, eliminating the issues of cell rejection for allogeneic cells and negating the need for invasive BM harvesting for autologous cell transplantation could be additional advantages for BM Soup [51]. Overall, BM Soup can be obtained from an accessible cell source (i.e. bone marrow). There is potentially less need for histocompatible donors, immune suppression drugs, or immune rejection. Also there is no need to differentiate stem cells, if any, into the correct cell lineage desired and less tumorigenicity risk is associated because there are no live cells.
Salivary flow rate (SFR) directly reflects function of the glands and its decrease is the major clinical finding in patients with dry mouth following head and neck IR. Therefore SFR was the critical quantitative measure used throughout this study and BM Soup was found efficient in restoring salivary function (Figs 1, 5, 8). In addition, we complemented salivary flow data with measurements for proteins (total proteins), salivary electrolytes (Na, Cl, K, Ca), and a growth factor (EGF). These measurements on the composition (quality) of saliva were used as indicators of acinar or ductal cell function [52]. Most proteins secreted into saliva come from acinar cells and measuring the total protein concentration allowed functional assessment of the secretory status of acinar cells [52]. EGF is mainly produced in SGs and this study measured EGF in saliva as a way to assess the functional secretory status of the granular ductal cells. Electrolytes were measured because most NaCl is reabsorbed while potassium is secreted by ductal cells; these levels can be used as an indicator of ductal cell function [52]. Calcium was used as an indicator of acinar cell function (exocrine protein packaging). All measurements on the composition of saliva, used in this study, did not show a statistically significant difference between the control, IR, and treated mice (Figs 6–7). Only the salivary calcium concentration in non-treated irradiated mice was found lower than the non-irradiated control mice. This suggests a reduced function (or number) of acinar cells in irradiated mice.
Testing different routes of delivering therapeutic agents is of critical importance in treating a disease. For SG damaged by IR, an I.V. delivery can provide cytoprotection to endothelial cells, which affect the survival of acinar cells [42], [53]. On the other hand, a local delivery can provide higher levels of BM Soup in the gland, which is the main site of tissue injury (acinar cells), as well as reducing any systemic adverse side-effects of the therapeutic agent, if any. Our study unexpectedly found that, one time, injections of BM Soup in submandibular glands were as effective in restoring SG function as four I.V. injections through the tail vein (see Materials and Methods section). Therefore the intra-glandular delivery route is less time-consuming and only required a quarter of the BM Soup dosage. Clinically, we envision two feasible methods to deliver BM Soup to SGs. First is by intra-glandular injections. Such an approach is currently used to deliver botulinum toxin to reduce drooling [54]. Second is by cannulation of the SG duct and retrograde infusion, such as currently used in sialoscopy/sialography [55]. Retrograde infusion and cannulation is also being used in research to transfer genes to SG of IR patients [25]. The majority of cells in SG exist as a monolayer and cannulation allows the delivered therapeutic agent or viral vector direct access to all SG cells. Our group and collaborators have been cannulating mouse Wharton’s (submandibular) and Stensen’s (parotid) ducts intraorally and infusing products retrogradelly to the SGs [56], [57]. We have begun to test retrograde infusion of BM Soup to SGs of irradiated mice and rats.
Age of the donor reduces the recovery of stem cells in bone marrow and in certain tissues [20], [21], [22], while other studies have reported no significant differences [23], [24], [58]. Our study only compared donor mice aged 8 wks versus 20 wks old while studies such as the one by Wang and colleagues [20] used BM cells from donor mice as old as 2.5 yrs. Our goal, for this study, was to test that the therapeutic effect of BM Soup on irradiated SGs is not limited to young mice only, but was also feasible in more matured/older mice. We continue to house C3H donor mice from the same batch/lot as the ones used in the current study and will sacrifice them approximately at 2 yrs of age to test their BM Soup efficiency.
There are limitations to this study. First our IR model used a single high dose of 15 Gy. While certainly useful experimentally in mice, this is unlike patients receiving fractionated IR, with 1.5–2.5 Gy/day, 5 days/week, and for up to 6 weeks. Dr Bruce Baum’s group reported that a 15 Gy single-dose scheme and a 5-day × 6 Gy fractionated scheme both yielded severe salivary hypofunction (40–60% salivary flow reduction) [59], [60], [61]. This adapted scheme is more convenient than the human regimen, yet one that leads to significant salivary hypofunction for testing in C3H mice. Future experiments testing BM Soup will use this fractionate IR scheme. The second limitation of this study was that BM Soup was injected between day 5–7 post-IR, and not in a situation where there was already an established reduction in salivary flow (e.g., at 8 weeks post-IR in the mouse model or 12 months post-IR in humans). Thus the therapeutic effect of BM Soup in these situations remains to be tested. The third limitation of this study is it was not aimed at characterizing all the factors/ingredients of BM Soup (such as proteins, transcription factors, growth factors, cytokines, etc). The current study was specifically geared at examining a subset of factors known to be involved in SG development/repair/regeneration, in relation to a proposed new treatment, the BM Soup. Our gene expression data showed that several growth factors (FGF2, IGF, VEGF, BMP7, EGF, NGF) were up-regulated in BM Soup-treated mice (Fig. 4). However, we only examined these mRNA levels at one time point (8 wks post-IR) and thus this gene expression profile may change at subsequent time points. It is possible that the therapeutic effect of BM Soup will lessen during a longer follow-up period (such as 16 and 24 week post-IR). Also, we plan to test which “Cell Soup” (from subpopulation of cells in BM or from other tissues) can best restore SG function in irradiated mice. Needless to say that this study reports a proof-of-concept of the successful use of BM Soup into irradiated SG, and that much more work remains to characterize the components and utilization of BM Soup.
Materials and Methods
Animals
All procedures with animals were carried out under protocols approved by the Facility Animal Care Committee of McGill University. Female C3H mice of 8 weeks old (Charles River, Montreal, QC, Canada) were used as recipient mice. Donor mice were 8 wks or 20–22 wks old male C3H mice.
Irradiation (IR)
Mice were anesthetized with 0.3 µl/g body weight of a 60 mg/ml ketamine and 8 mg/ml xylazine (Phoenix Scientific) solution given by intra-muscular injection, and placed in a restraining device. Salivary glands (SGs) of female C3H mice (8-wk old) were damaged by local neck IR of 15 Gy using a Varian Clinac 6EX linear accelerator in a single field anterior-posterior setup (Fig. 9). The radiation field was defined by precise jaw collimation to a slit of 1.2 cm, thereby confining the IR region in the mice to the area of the SGs. The machine output for these specialized narrow fields was measured using a PTW microLion chamber (Type 31018) cross-calibrated against a calibrated secondary standard Farmer-type ionization chamber in a 10×10 cm2 reference field size. Additional buildup material was used to ensure a uniform dose as a function of depth throughout the IR region. Typically 5 mice were IR simultaneously and a typical IR took 4.4 min. The IR caused a 40–60% reduction in saliva output at day 5 post-IR. Mice were randomized into 4 different groups (N = 5 to 9 mice per group) and were followed for 8 weeks post-IR. The treatment group (“IR+BM Soup”) consisted of IR female mice receiving I.V. injections (tail vein) of BM Soup from male donors between day 5–7 post-IR and were compared to IR mice receiving I.V. PBS injections (“IR+PBS”, negative control group, vehicle injection group). The positive control group (“Control”) was C3H mice with normal SG function which were sedated and placed in the restraining device inside the irradiator but the unit was off (i.e. sham irradiation). Therefore these “Control” mice were expected to have normal SG function during the 8 weeks follow-up period. A treatment comparison group (“IR+whole BM”) receiving injections of “live whole BM cells” was included in the present study because we have previously shown that these transplants were relatively efficient in re-establishing normal functions to IR-damaged SGs [15], and wanted to compare its therapeutic efficiency against our newly proposed BM Soup treatment.
Figure 9. Bone Marrow Soup (BM Soup) preparation and study design.
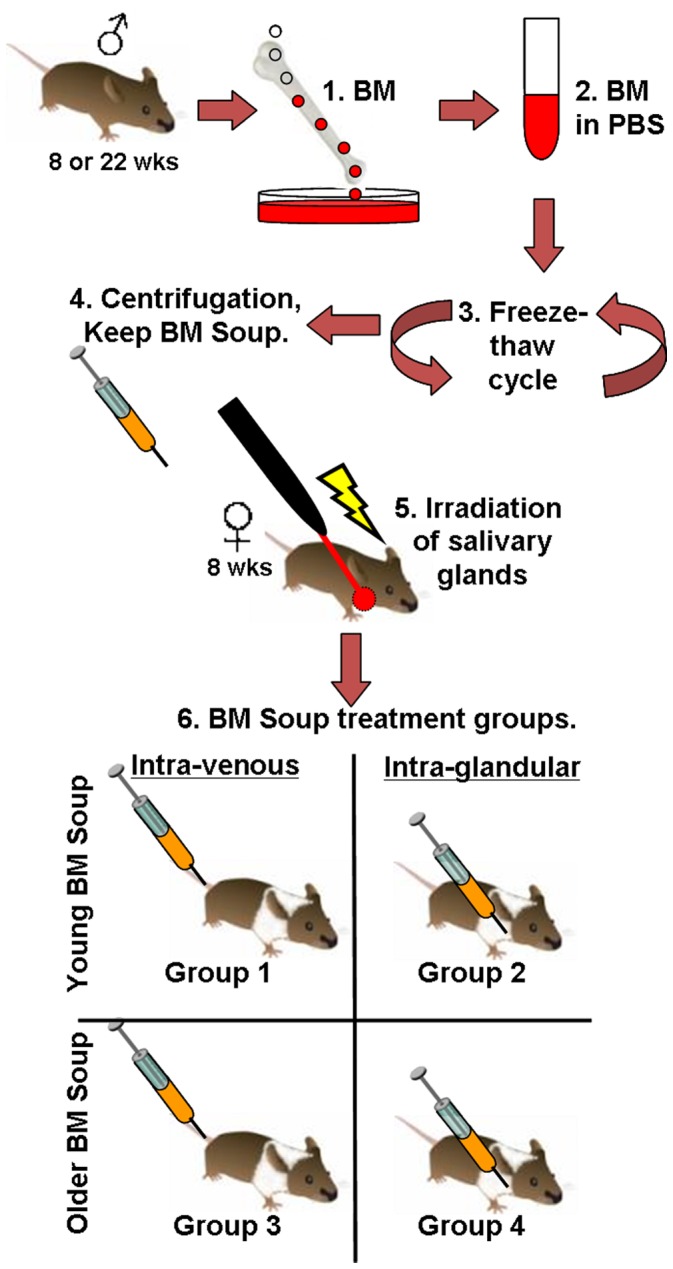
Step 1. Bone marrow (BM) is flushed from femur/tibia bones of young (8 wk-old) or older (22 wk-old) male donor mice. 2. BM cells are suspended in PBS, strained through a filter and washed. 3. BM Soup is prepared by subjecting the BM cells to three freeze-thaw cycles of -80C to +37C degrees. 4. Microcentrifugation at 13,500 g×30 min, 4C degrees to remove insoluble materials. The supernatant (BM Soup) is kept on ice until injections. 5. Salivary glands of recipient female mice (8 wks old) are locally irradiated with 15 Gy. 6. Within 5–7 days post-irradiation, BM Soup (from young or older donor mice) is injected intra-venously (tail vein) or intra-glandularly (submandibular gland). Mice were monitored for 8 wk post-irradiation.
Bone Marrow (BM) Cells or BM Soup Preparation and Transplantation
BM cells were harvested from 8 wk-old male C3H mice. Briefly, BM cells were flushed from tibias and femurs with cold PBS. The suspension was then strained through a 40-µm filter and washed again with PBS (Fig. 9). Cell concentration was adjusted to 107 cells/100 µl PBS. BM Soup was prepared as described by Yeghiazarians et al [16], with modifications, by subjecting BM cells to three cycles of freeze-thaw on a container with dry ice placed in a -80C freezer and thawing in a 37C bath, followed by microcentrifugation at 13,500 g at 4C for 30 min to remove insoluble materials. Protein concentration of BM Soup was ∼1.3 ug/ul (from 107 cells). BM Soup or BM cells were kept on ice until injections in irradiated female recipient C3H mice. The injection of 100 ul containing 1×107 BM cells or BM Soup was done via the tail vein, and these transplantations were repeated twice per week during two consecutive weeks. For experiments where BM Soup was delivered by intra-glandular injections, an incision in the neck area was performed and the mouse submandibular glands localized. BM Soup was injected through the capsule of both submandibular glands. Two injections of 25 ul each/per gland were given. Each delivery route (either IV or intra-glandular) was adjusted to 1×107 cell equivalents of BM Soup/mouse. Also each group of female mice receiving BM Soup I.V. or intra-glandular was further subdivided into BM Soup from a young (8 wks old) versus an older (20–22 wks old) mouse donor. The total number of mice used for this study was 77 mice.
Measurements of Salivary Function
We performed both quantitative (salivary flow) and qualitative (composition) measurements of saliva (described below).
Secretory Function of the Salivary Glands (Salivary Flow Rate; SFR)
SFR of mice was obtained by intra-muscular injection of 0.3 µl/g body weight of a 60 mg/ml ketamine and 8 mg/ml xylazine mixture. Whole saliva was collected after stimulation of secretion using 0.5 mg Pilocarpine (Sigma)/kg body weight administered subcutaneously. Saliva was obtained from the oral cavity by micropipette, placed into pre-weighed 0.5 ml microcentrifuge tubes. SFR was determined by volume-weight of saliva/10 min/body weight. SFR was determined at baseline, day 5, and at week 8 post-IR. At time of sacrifice (week 8 post-IR), the mice submandibular glands were harvested and weighed.
Analysis of Saliva Quality/composition
Concentrations of epidermal growth factor (EGF) in saliva were measured by ELISA method (R&D Systems, Minneapolis, MN, USA) [15]. The total protein concentration of saliva was measured by the bicinchoninic acid assay method (BCA; Thermo Scientific, Pierce, IL, USA). Salivary electrolytes such as sodium, potassium, chloride, and calcium were analyzed on a Beckman Coulter DXc 800 automated chemistry analyzer using the urine chemistry mode (Beckman Coulter Synchron systems, CA) [36].
Salivary Tissue Analysis
1. Blood vessel/capillary density
Five-micrometer thickness tissue sections were immuno-histochemically stained using the Blood Vessel Staining Kit (Chemicon, MA, USA) with an antibody to von Willebrand Factor (vWF). After deparaffinization and rehydration, tissue sections were treated three times with a 10 mM citrate buffer solution (pH 6.1) in a 600 W microwave and then allowed to cool to room temperature for 30 min before blocking overnight at 4°C. Rabbit anti-mouse vWF was used (1∶100) for 2 hours at room temperature. After that, we followed the manufacturer’s instructions. The percentage of surface area occupied by blood vessels was assessed by light microscopy under 400× magnification using 3 sections for each slide. At least 10 fields per section were accounted using NIH Image J software (NIH, Bethesda, USA).
2. Cell proliferation
Tissue sections were blocked for endogenous peroxidase activity with 3% H2O2 in methanol for 10 min. Cell proliferation staining was performed with the Zymed PCNA staining kit (Invitrogen, Carlsbad, CA, USA). Before antibody labeling, the slides were treated three times with a citrate buffer solution (mentioned above) in a 600 W microwave for 5 min. Thereafter, slides were processed with routine indirect immunoperoxidase techniques. Two examiners independently counted the absolute number of PCNA positive cells in a blinded manner in five randomly chosen sections (n = 5 glands/group) at the magnification of 400×.
3. Periodic acid–Schiff stain (PAS)
Acinar cells were detected using PAS method (Sigma-Aldrich, St-Louis, MO, USA). The percentage of surface area occupied by acinar cells (reported as a percentage of the salivary tissue surface examined) was assessed by light microscopy under X 400 magnification of 10 representative fields per gland/mouse with NIH Image J software.
4. Immunostaining
Frozen submandibular gland tissue sections (6–8 µm) were obtained from control (sham-irradiated), irradiated, and BM Soup-treated C3H mice. The following primary antibodies were used: a) rat anti-sodium-potassium-2 chloride co-transporter, (NKCC1, donated by RJ Turner, NIH) to localize salivary acinar and intercalated ductal cells, b) rat anti-c-Kit (CD117, BD Biosciences, Mississauga, ON, Canada) to localize salivary progenitor cells [31], c) rabbit anti-α-amylase (Sigma-Aldrich) for serous acini cells, d) rabbit anti-α-smooth muscle actin (Abcam, Cambridge, MA, USA) for myoepithelial cells, e) rabbit anti-cytokeratin-5 (CK5, Sigma-Aldrich, Oakville, ON, Canada) for salivary progenitor cells found in basal ductal cells. In addition, a non-immune rat or rabbit isotype control antibody (Zymed Labs) was used as a ‘negative control primary antibody’. Tissue sections were incubated with the respective primary antibody (or its isotype control antibody) overnight at 4°C. Tissue sections were then incubated with a secondary antibody for 1 h at room temperature in the dark. Secondary antibodies used were either a donkey anti-rat- fluorescein isothiocyanate-conjugated (FITC), rabbit- Rhodamine Red-X-conjugated (RRX), or rat-RRX (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Then, 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen, San Francisco, CA, USA) was added for 3–5 min. All primary and secondary antibodies were diluted (1∶100) in phosphate-buffered saline (PBS) containing 5% donkey serum (Jackson ImmunoResearch Labs). Fluorescence images were taken using a Leica DM4000 fluorescent microscope equipped with Volocity software (PerkinElmer Inc). Images shown are representative of at least 5 separate experiments with multiple images taken per slide. Quantification of the protein fluorescence (i.e. its intensity) was determined by Volocity software. The average fluorescence of the protein expressed within 3–5 fields of 400 um2 area/per gland was computed.
5. Fluorescence in situ hybridization (FISH)
Serial frozen tissue sections (8 um thickness) were fixed in a 2% paraformaldehyde fixative for 10 minutes at room temperature. Following, serial dehydration, a digoxigenin-labeled riboprobe was used to detect a repeat sequence (pY353B) on the mouse Y-chromosome. The digoxigenin-labeled probe was applied to the tissue section and hybridized at 81°C for 12 min, then followed by 30 minutes incubation at 55°C. Slides were washed in saline-sodium citrate (SSC) followed by a stringency wash at 40°C. Then, a HRP-conjugated antibody to digoxigenin (1/400, Roche, Indianapolis, IN, USA) was added to detect the riboprobe. The positive Y chromosomal signal was observed as a red color once the high affinity substrate of HRP, Alexa-tyramide-594 reagent (TSA System, Invitrogen) was applied. Finally, all sections were stained with DAPI (Invitrogen) to label all nuclei and mounted with 10 mM Tris (hydroxymethyl) - aminomethane, [pH 7.4]) buffer. The slides were visualized using a Leica DM4000 fluorescent microscope equipped with Volocity software.
6. Quantitative Real-Time PCR (qRT-PCR)
Gene expression analyses were performed using an Applied BioSystems qRT-PCR (model 7500). Total RNA was extracted from the submandibular glands with TRIZOL reagent (Invitrogen, Carlsbad, CA). The first-strand cDNA synthesis was performed by using Thermoscript RT-PCR system (Invitrogen). qRT-PCR was done using 1 µg RNA per sample and TaqMan Universal Master Mix (Applied Biosystems). The probes and primers chosen were for angiogenic growth factors such as VEGF and FGF2 (assay IDs were Mm01281449 and Mm00433287, respectively), resident ductal progenitor cells (CK5, assay ID: Mm00503549), acinar cells (AQP5, assay ID: Mm00437578), transcription factors involved in stem cell renewal or differentiation such as Sox2, Klf4, and Sox10 (assay IDs: Mm00488369, Mm00516104, and Mm01300162), growth factors that impact cell fate decision such as BMP7, EGF, NGF, IGF-1R (assay IDs: Mm00432102, Mm00438696, Mm00443039, and Mm00802841), cell cycle or apoptosis such as Cyclin-1, Caspase-3 and Caspase-8 (assay IDs: Mm00432359, Mm01195085, Mm00802247), bicarbonate anion exchanger (SLC4 solute carrier family 4 anion exchanger, assay ID: Mm01347935), and tissue remodeling such as MMP-2 and MMP-9 (assay IDs: Mm00439498 and Mm00442991). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, assay ID: Mm99999915) was used as an endogenous reference running at 50°C for 2 min, 95°C for 10 min, and 40 cycles [95°C for 15 s, 60°C for 1 min]).
7. Statistical analysis
ANOVA analysis with Tukey’s Post-Hoc test were used to determine statistical differences (P<0.05) between the mouse groups. The statistical program was SPSS version 17 (IBM, USA).
Acknowledgments
We thank Drs. Sofiane Azzi and Janet Henderson for advice and discussion during the preliminary steps of this study, to Drs. Mari Kaartinen, Svetlana Komarova and Dieter Reinhardt for access to laboratory equipment. We also thank the McGill Centre for Bone and Periodontal Research.
Funding Statement
This study was supported in part by the Canadian Institutes of Health Research (CIHR) Institute Musculoskeletal Health and Arthritis (IMHA), and the Canada Research Chairs (CRC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, et al. (2010) Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 78: 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhide SA, Miah AB, Harrington KJ, Newbold KL, Nutting CM (2009) Radiation-induced xerostomia: pathophysiology, prevention and treatment. Clin Oncol (R Coll Radiol) 21: 737–744. [DOI] [PubMed] [Google Scholar]
- 4. Anderson DJ, Gage FH, Weissman IL (2001) Can stem cells cross lineage boundaries? Nat Med 7: 393–395. [DOI] [PubMed] [Google Scholar]
- 5. Grove JE, Bruscia E, Krause DS (2004) Plasticity of bone marrow-derived stem cells. Stem Cells 22: 487–500. [DOI] [PubMed] [Google Scholar]
- 6. Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, et al. (2004) Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430: 350–356. [DOI] [PubMed] [Google Scholar]
- 7. Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, et al. (2004) Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 305: 90–93. [DOI] [PubMed] [Google Scholar]
- 8. Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, et al. (2004) Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104: 2084–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tyndall A, Gratwohl A (2009) Adult stem cell transplantation in autoimmune disease. Curr Opin Hematol 16: 285–291. [DOI] [PubMed] [Google Scholar]
- 10. Gnecchi M, Zhang Z, Ni A, Dzau VJ (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prockop DJ (2009) Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, et al. (2008) Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA 299: 925–936. [DOI] [PubMed] [Google Scholar]
- 13. Tran SD, Sumita Y, Khalili S (2011) Bone marrow-derived cells: A potential approach for the treatment of xerostomia. Int J Biochem Cell Biol 43: 5–9. [DOI] [PubMed] [Google Scholar]
- 14. Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 25: 2896–2902. [DOI] [PubMed] [Google Scholar]
- 15. Sumita Y, Liu Y, Khalili S, Maria OM, Xia D, et al. (2011) Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol 43: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, et al. (2009) Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther 17: 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnett ML (1996) Alternative medicine: more than chicken soup? J Dent Res 75: 1721–1722. [DOI] [PubMed] [Google Scholar]
- 18. Bahary N, Zon LI (2001) Development. Endothelium–chicken soup for the endoderm. Science 294: 530–531. [DOI] [PubMed] [Google Scholar]
- 19. Ziment I (2006) Spicy chicken soup. Lancet 368: 646. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Takagawa J, Haddad DJ, Pinnamaneni K, Zhang Y, et al.. (2011) Advanced Donor Age Impairs Bone Marrow Cell Therapeutic Efficacy for Cardiac Disease. J Tissue Sci Eng S3. [DOI] [PMC free article] [PubMed]
- 21. Gago N, Perez-Lopez V, Sanz-Jaka JP, Cormenzana P, Eizaguirre I, et al. (2009) Age-dependent depletion of human skin-derived progenitor cells. Stem Cells 27: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 22. Liang Y, Van Zant G, Szilvassy SJ (2005) Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcus AJ, Woodbury D (2008) Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med 12: 730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Westerman KA, Penvose A, Yang Z, Allen PD, Vacanti CA (2010) Adult muscle ‘stem’ cells can be sustained in culture as free-floating myospheres. Exp Cell Res 316: 1966–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, et al. (2012) Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A 109: 19403–19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aframian DJ, Palmon A (2008) Current status of the development of an artificial salivary gland. Tissue Eng Part B Rev 14: 187–198. [DOI] [PubMed] [Google Scholar]
- 27. Baum BJ, Tran SD (2006) Synergy between genetic and tissue engineering: creating an artificial salivary gland. Periodontol 2000 41: 218–223. [DOI] [PubMed] [Google Scholar]
- 28. Sugito T, Kagami H, Hata K, Nishiguchi H, Ueda M (2004) Transplantation of cultured salivary gland cells into an atrophic salivary gland. Cell Transplant 13: 691–699. [DOI] [PubMed] [Google Scholar]
- 29. Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, et al. (2008) Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3: e2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP (2009) Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 92: 466–471. [DOI] [PubMed] [Google Scholar]
- 31. Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, et al. (2011) Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 99: 367–372. [DOI] [PubMed] [Google Scholar]
- 32. Redman RS, Ball WD, Mezey E, Key S (2009) Dispersed donor salivary gland cells are widely distributed in the recipient gland when infused up the ductal tree. Biotech Histochem 84: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neumann Y, David R, Stiubea-Cohen R, Orbach Y, Aframian DJ, et al. (2012) Long-term cryopreservation model of rat salivary gland stem cells for future therapy in irradiated head and neck cancer patients. Tissue Eng Part C Methods 18: 710–718. [DOI] [PubMed] [Google Scholar]
- 34. Lombaert IM, Wierenga PK, Kok T, Kampinga HH, deHaan G, et al. (2006) Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res 12: 1804–1812. [DOI] [PubMed] [Google Scholar]
- 35. Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, et al. (2008) Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res 14: 7741–7750. [DOI] [PubMed] [Google Scholar]
- 36. Khalili S, Liu Y, Sumita Y, Maria OM, Blank D, et al. (2010) Bone marrow cells are a source of undifferentiated cells to prevent Sjogren’s syndrome and to preserve salivary glands function in the non-obese diabetic mice. Int J Biochem Cell Biol 42: 1893–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin CY, Chang FH, Chen CY, Huang CY, Hu FC, et al. (2011) Cell therapy for salivary gland regeneration. J Dent Res 90: 341–346. [DOI] [PubMed] [Google Scholar]
- 38.Lim JY, Yi T, Choi JS, Jang YH, Lee S, et al.. (2012) Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. [DOI] [PubMed]
- 39. Tran SD, Redman RS, Barrett AJ, Pavletic SZ, Key S, et al. (2011) Microchimerism in salivary glands after blood- and marrow-derived stem cell transplantation. Biol Blood Marrow Transplant 17: 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Souza LN, Faria DR, Dutra WO, Gomes CC, Gomez RS (2011) Microchimerism in labial salivary glands of hematopoietic stem cell transplanted patients. Oral Dis 17: 484–488. [DOI] [PubMed] [Google Scholar]
- 41. Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, et al. (2008) Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 26: 2595–2601. [DOI] [PubMed] [Google Scholar]
- 42. Cotrim AP, Sowers A, Mitchell JB, Baum BJ (2007) Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 15: 2101–2106. [DOI] [PubMed] [Google Scholar]
- 43. Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH (2010) Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer 10: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Limesand KH, Said S, Anderson SM (2009) Suppression of radiation-induced salivary gland dysfunction by IGF-1. PLoS One 4: e4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kojima T, Kanemaru S, Hirano S, Tateya I, Suehiro A, et al. (2011) The protective efficacy of basic fibroblast growth factor in radiation-induced salivary gland dysfunction in mice. Laryngoscope 121: 1870–1875. [DOI] [PubMed] [Google Scholar]
- 46. Maria OM, Tran SD (2011) Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev 20: 959–967. [DOI] [PubMed] [Google Scholar]
- 47. Huang GL, Zhang NN, Wang JS, Yao L, Zhao YJ, et al. (2012) Transdifferentiation of human amniotic epithelial cells into acinar cells using a double-chamber system. Cell Reprogram 14: 377–383. [DOI] [PubMed] [Google Scholar]
- 48. Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 49. Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, et al. (2006) Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 291: H886–893. [DOI] [PubMed] [Google Scholar]
- 50. Gnecchi M, He H, Liang OD, Melo LG, Morello F, et al. (2005) Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368. [DOI] [PubMed] [Google Scholar]
- 51. Angeli FS, Zhang Y, Sievers R, Jun K, Yim S, et al. (2012) Injection of human bone marrow and mononuclear cell extract into infarcted mouse hearts results in functional improvement. Open Cardiovasc Med J 6: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang J, Voutetakis A, Mineshiba F, Illei GG, Dang H, et al. (2006) Effect of serotype 5 adenoviral and serotype 2 adeno- associated viral vector-mediated gene transfer to salivary glands on the composition of saliva. Hum Gene Ther 17: 455–463. [DOI] [PubMed] [Google Scholar]
- 53. Xu J, Yan X, Gao R, Mao L, Cotrim AP, et al. (2010) Effect of irradiation on microvascular endothelial cells of parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys 78: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ellies M, Laskawi R, Rohrbach-Volland S, Arglebe C (2003) Up-to-date report of botulinum toxin therapy in patients with drooling caused by different etiologies. J Oral Maxillofac Surg 61: 454–457. [DOI] [PubMed] [Google Scholar]
- 55. Koch M, Iro H, Zenk J (2008) Role of sialoscopy in the treatment of Stensen’s duct strictures. Ann Otol Rhinol Laryngol 117: 271–278. [DOI] [PubMed] [Google Scholar]
- 56.Kuriki Y, Liu Y, Xia D, Gjerde EM, Khalili S, et al.. (2011) Cannulation of the Mouse Submandibular Salivary Gland via the Wharton’s Duct. J Vis Exp. [DOI] [PMC free article] [PubMed]
- 57. Zheng C, Shinomiya T, Goldsmith CM, Di Pasquale G, Baum BJ (2011) Convenient and reproducible in vivo gene transfer to mouse parotid glands. Oral Dis 17: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Obokata H, Kojima K, Westerman K, Yamato M, Okano T, et al. (2011) The potential of stem cells in adult tissues representative of the three germ layers. Tissue Eng Part A 17: 607–615. [DOI] [PubMed] [Google Scholar]
- 59. Cotrim AP, Hyodo F, Matsumoto K, Sowers AL, Cook JA, et al. (2007) Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res 13: 4928–4933. [DOI] [PubMed] [Google Scholar]
- 60. Zheng C, Cotrim AP, Rowzee A, Swaim W, Sowers A, et al. (2011) Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res 17: 2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baum BJ, Adriaansen J, Cotrim AP, Goldsmith CM, Perez P, et al. (2010) Gene therapy of salivary diseases. Methods Mol Biol 666: 3–20. [DOI] [PubMed] [Google Scholar]