Abstract
In humans, systemic heme homeostasis is achieved via coordinated regulation of heme synthesis, transport and degradation. Although the heme biosynthesis and degradation pathways have been well characterized, the pathways for heme trafficking and incorporation into hemoproteins remains poorly understood. In the past few years, researchers have exploited genetic, cellular and biochemical tools, to identify heme transporters and, in the process, reveal unexpected functions for this elusive group of proteins. However, given the complexity of heme trafficking pathways, current knowledge of heme transporters is fragmented and sometimes contradictory. This review seeks to focus on recent studies on heme transporters with specific emphasis on their functions during erythropoiesis.
Introduction
Heme homeostasis is a highly coordinated process during erythropoiesis, marked by a dramatic increase of heme synthesis which is essential for proper hemoglobinization of red blood cells (RBCs) [1,2]. Heme is also involved in transcriptional and translational regulation of erythroid specific gene expression, which is critical for coupling heme synthesis with protein production for erythroid cell differentiation [3,4]. In addition, a large amount of heme-iron is recycled for re-packing into hemoglobins by erythrophagocytosis (EP) in macrophages of the reticuloendothelial system (RES) [1,5,6]. Although heme biosynthesis and its regulation have been well characterized, the mechanisms for heme transport in eukaryotes remain poorly understood. Comprehensive reviews for generic heme trafficking and interorganellar transfer pathways have been covered elsewhere [5–8]. In this review we will seek to cover the following. How does newly synthesized heme exit the mitochondria for incorporation into hemoglobins and other hemoproteins? How does heme released from lysed RBCs cross the phagolysosomal membrane to be delivered to downstream effectors such as heme oxygenase-1 (HO-1) for degradation? Can heme be redistributed between different tissues through heme transporters and chaperones? Extensive efforts to identify heme trafficking pathways have been underway for over a decade and a number of heme transporters have been identified recently.
Heme import
Heme is a more readily bioavailable iron source and contributes to two-third of body iron, even though heme constitutes only a third of total dietary iron [9,10]. In mammals, dietary heme is apparently taken up intact by enterocytes in the intestine. However, heme is a large amphipathic porphyrin and free heme can be cytotoxic. Thus, specific molecules and pathways are required for heme uptake and trafficking.
HRG-1
Rao et al have demonstrated that the roundworm Caenorhabditis elegans is a unique model for heme trafficking studies because even though it is a heme auxotroph it acquires dietary heme via the intestine and subsequently disseminates heme throughout the organism for viability [11]. Genomic screens in C. elegans identified CeHRG-1 and CeHRG-4 as the first bona fide eukaryotic heme importers [12]. CeHRG-1 has orthologs in vertebrates, while CeHRG-4 is worm-specific. Transient knockdown of hrg-1 in zebrafish resulted in hydrocephalus, yolk tube malformations and severe anemia. Worm HRG-1 fully rescued all phenotypes observed due to knockdown of hrg-1 in zebrafish [12]. The phenotypes resulting from knockdown of zebrafish hrg-1 were restricted specifically to the erythroid lineage and did not impact other hematopoietic cell lineages. Additionally, significant heme-induced inward currents were observed in Xenopus oocytes injected with cRNA for CeHRG-1, CeHRG-4, and the human homolog, hHRG-1, indicating heme-dependent transport across cell membranes [12].
Human HRG-1 (SLC48A1) mRNA was abundant in the brain, kidney, heart and skeletal muscle and in cell lines derived from duodenum, kidney, bone marrow and brain [12]. hHRG-1 localized to acidic endosomal and lysosomal organelles in HEK293 cells, and its affinity for heme decreased with increasing pH. Additionally, tyrosine (YxxxØ) and acidic-dileucine (DxxIL) based sorting motifs were found in the C-terminus of both C. elegans and human HRG-1 [12]. Yanatori and colleagues recently reported hHRG-1 localized to the plasma membrane and lysosomes in non-polarized HEp2 cells. In polarized MDCK cells, hHRG-1 was located to the basolateral membrane and a cytosolic organelle just under the apical membrane [13]. A recent study showed that hHRG-1 interacted with the c subunit of the vacuolar proton ATPase (V-ATPase) pump and enhanced endosomal acidification [14]. Together these studies suggest hHRG-1 plays a role in the transport of heme from the exoplasmic space or lumen of acidic endosome–lysosome compartments into the cytoplasm.
Interestingly, in addition to lysosomal localization in HEK293 cells, hHRG-1 is also recruited and colocalizes with Nramp1 at the erythrophagosomal membrane, surrounding ingested RBCs in bone marrow derived macrophages (BMDMs) [15]. However, the absence of HO-1 at this location indicates that during EP, at least a portion of heme released from degraded hemoglobin is mobilized by hHRG-1 to the cytoplasm [15]. The cytosolic heme can then undergo intracellular redistribution including degradation by HO-1 for iron recycling, or be exported by heme effluxers. Indeed, a recent study shows that HRG1 is essential for macrophage iron homeostasis and transports heme from the phagolysosome to the cytoplasm during EP [16]. HRG1 is strongly expressed in macrophages of the reticuloendothelial system and specifically localizes to the phagolysosomal membranes during EP. Depletion of Hrg1 in mouse macrophages causes attenuation of heme transport from the phagolysosomal compartment suggesting that HRG1 is the heme transporter for heme-iron recycling in macrophages. The study proposes that genetic variation in HRG1 may be an important genetic determinant in inherited iron disorders in humans [16]. HRG-1, as observed for HO-1, was recently identified as a target of the heme-regulated transcription factor BACH1 in microarray expression analysis and ChIP-Seq experiments, further suggesting that HRG-1 may be an important player in erythropoiesis and the phagolysosomal heme transporter [17].
HCP1
Heme carrier protein 1 (HCP1/SLC46A1) is a membrane protein expressed by enterocytes in the duodenum implicated in the absorption of heme in the intestine [18]. Ectopically overexpressing HCP1 in Xenopus oocytes revealed a 2–3 fold increase in heme uptake. Subsequent studies, however, have shown that folate transport by this protein was at least 10-fold higher than that observed with heme, suggesting that folate might be the physiological ligand for HCP1. In addition, a missense mutation in HCP1 in human that leads to the formation of a non-functional protein has been found to be associated with hereditary folate malabsorption. Thus, SLC46A1 is in fact a folate/proton symporter and was renamed as the Proton Coupled Folate Transporter (PCFT) [19]. Interestingly, HCP1 knockout mice displayed severe macrocytic normochromic anemia, which could be a secondary effect of folic acid deficiency. HCP1 deficient erythroblasts failed to differentiate and had a higher apoptosis rate [20]. RNA interference assays of HCP1 in CaCo-2 cells reduced both heme and folate uptake but increase HO-1 expression, suggesting HCP1 could potentially function as a low affinity heme transporter [21].
FLVCR2
Feline leukemia virus subgroup C receptor 2 (FLVCR2), a member of the major facilitator superfamily, was recently reported to import heme in mammalian cells [22]. Knockdown of FLVCR2 in human cells significantly decreased uptake of the fluorescent heme analog, zinc mesoporphyrin (ZnMP). However, unlike HRG-1, ectopically expressing FLVCR2 in yeast did not rescue heme dependent growth or import heme under the assay conditions [23]. Given the high degree of homology between FLVCR2 and the heme effluxer FLVCR1 [24–26], it is possible that FLVCR2 may efflux heme. FLVCR2 is expressed in a broad range of human tissues including the fetal liver, brain and kidney [22]. Recent studies have associated FLVCR2 with Fowler syndrome, a vascular disorder of the brain [27,28]. Currently, a direct physiological role for FLVCR2 in erythropoiesis is unclear.
Heme export
In humans, macrophages phagocytose over 360 billion senescent RBCs and recycles more than 25 mg of iron daily. The heme released from hemoglobin is either degraded by HO-1 to release iron for iron recycling or potentially exported as intact heme [29]. Heme export may serve as a potential detoxification strategy to prevent excess heme from accumulating, a phenomenon that could lead to tissue and cellular damage. Two heme exporters, FLVCR1 and ATP-binding cassette transporter G2 (ABCG2) have been implicated in heme export in humans.
FLVCR1
Quigley et al identified FLVCR1 as a cell surface heme exporter, belonging to the major facilitator family of transmembrane transporters [26]. FLVCR1 is expressed in different hematopoietic cells and showed weak expression in the fetal liver, pancreas and kidney [30]. Ectopically expressing FLVCR1 can reduce intracellular heme levels and mediate efflux of ZnMP in rat renal epithelial and hematopoietic K562 cells [26]. FLVCR1-null mice fail to undergo erythropoiesis and die at midgestation [31]. These mice also exhibit cranio-facial and limb deformities reminiscent of patients with Diamond-Blackfan anemia (DBA), a severe but rare congenital erythroid anemia that presents in infancy. The current model postulates that FLVCR1 exports heme when macrophages phagocytose senescent RBCs. FLVCR1 interacts with the extracellular heme-binding protein hemopexin and export heme at least 100-fold more efficiently in the presence of hemopexin [32]. FLVCR1 has a narrow substrate range including heme, protoporphyrin IX and coproporphyrin, but not bilirubin, the primary breakdown product of heme. Alves et al recently showed that in nucleated RBC precursors (NRBC) from human bone marrow, FLVCR1 expression increased during erythropoiesis and reached maximal level at the intermediate stage of maturation, under condition in which the HO system was disrupted [33]. The authors suggest that FLVCR1 may export excess heme to prevent heme toxicity under conditions in which heme degradation (HO?) is not fully induced.
Interestingly, Tolosano et al recently showed that in addition to full length FLVCR1 (FLVCR1a), there is another isoform, FLVCR1b, which is a smaller protein that localizes to the mitochondria [34]. Overexpression of FLVCR1b increases cytosolic heme, whereas knockdown of FLVCR1b results in mitochondria heme accumulation, indicating that FLVCR1b is a mitochondrial heme exporter. In contrast with previous findings, targeted deletion of FLVCR1a resulted in skeletal defects and vascular abnormalities but did not affect erythropoiesis, whereas knockdown of FLVCR1b impaired erythroid differentiation in vitro. These results suggest that FLVCR1a is dispensable for definitive erythropoiesis, and the failure in erythropoiesis in FLVCR1-null mice may due to cytosolic heme deficiency rather than toxicity associated with excess heme in the cytosol [34].
Recently, four missense mutations in FLVCR1 have been found in patients with the rare autosomal recessive disease posterior column ataxia and retinitis pigmentosa (PCARP) [35,36]. All of the four mutations diminish FLVCR1 heme export activity and mislocalize FLVCR1 to intracellular structures, including lysosomes [37]. Three of the four mutations occur in exon 1 of the gene and are FLVCR1a specific. However, none of the patients carrying these gene variations is anemic. The physiological function of FLVCR1a therefore remains unknown [36].
ABCG2
ABCG2, also known as breast cancer resistance protein (BCRP) has been identified as a second heme exporter in mammals [38]. ABCG2 is expressed in a wide range of tissues including hematopoietic stem cells and erythroid progenitors. Whereas FLVCR1 is highly expressed during erythropoiesis, the expression level of ABCG2 is particularly high in the early stages of hematopoiesis [39,40]. ABCG2 binds to heme directly through the extracellular loop 3 (ECL3) which constitutes a porphyrin-binding domain [39]. Although there is no direct evidence demonstrating that ABCG2 exports heme, ectopically expressed ABCG2 exports ZnMP in K562 cells [39]. As observed in FLVCR1-mediated heme export, ABCG2 possibly exports and transfers heme to extracellular heme-binding proteins, such as albumin [41]. However, unlike FLVCR1, ABCG2 has a broad range of substrates including porphyrin and nonporphyrin substrates, suggesting that ABCG2 may not be a functional backup of FLVCR1. Human patients carrying null mutations in ABCG2 are defined as the Jr(a–) blood group with no apparent defects in erythropoiesis [42,43].
Since both FLVCR1a and ABCG2 appear to be dispensable for erythropoiesis, it raises the question of whether a third heme exporter exist that is essential during erythropoiesis. Severance et al recently performed genome-wide analysis and identified two genes, MRP-5 and F22B5.4, that were involved in heme transport in C. elegans [44]. As opposed to the heme importer HRG-4, knockdown of both MRP-5 and F22B5.4 resulted in ZnMP accumulation in the worm intestine [44]. MRP-5 is the homolog of human ABC transporter ABCC5, and could possibly be a candidate heme exporter in humans.
Intracellular heme trafficking
Membrane proteins
ABCB6 is a mitochondrial ABC transporter that localizes to the mitochondrial outer membrane. ABCB6 was first identified as an iron transporter but later proposed to be a porphyrin/heme importer [45]. Recently, Polireddy et al reported ABCB6 interacts directly with heme in hemin-agarose binding assays, and high-throughput screens identified compounds that competed ABCB6-mediated heme transport in isolated mitochondria [46]. In mice or humans, however, loss of ABCB6 function is not associated with blood defects, even though the expression profile of ABCB6 mimics that of other heme biosynthesis genes [47]. Indeed, recent studies have shown that ABCB6 is dispensable for erythropoiesis and instead of mitochondria, it is localized in the endosomal/lysosomal compartment and in the plasma membrane of mature erythrocytes [48,49]. Two missense mutations in the ABCB6 gene in humans has been associated with Familial Pseudohyperkalemia (FP), which increases leakage of potassium from RBCs [50]. In addition, an L811V mutation of ABCB6 causes ocular coloboma, a developmental defect in the closure of the optic fissure [47].
ABCB10 is another mitochondrial ABC transporter located in the inner mitochondrial membrane. ABCB10 interacts with mitoferrin1 (MFRN1) and ferrochelatase (FECH) and stabilizes the complex [51]. Hyde et al recently reported that ABCB10 is essential for erythropoiesis in vivo. ABCB10 null mice displayed deficiency of primitive erythropoiesis and lack of hemoglobinized cells [52]. However, it is unclear whether heme is the bona fide substrate of ABCB10.
Cytosolic proteins
Free heme is a cytotoxic molecule that generates reactive oxygen species and disrupts lipid bilayers and organelles. As a potent hemolytic agent, free heme can alter the conformation of cytoskeletal proteins in RBCs. Consequently, cytosolic heme carriers or “chaperones” that function to sequester or transport heme are an essential component of heme homeostasis - required for heme detoxification or incorporation into hemoproteins.
Currently, several intracellular heme-binding proteins have been found, including glutathione S-transferases (GSTs), heme-binding proteins (HBPs), and fatty acid binding proteins (FABPs) (Table 1). GSTs are abundant cytosolic proteins that have been shown to bind hemes and porphyrins. Although GSTs are important for heme detoxification in malaria parasites and helminths, little is known regarding their function in mammalian heme trafficking [53,54]. HBPs are proteins that have higher affinity to heme (p22HBP: Kd = 26 nM, HBP23: Kd = 55 nM) and tetrapyrroles than GSTs and FABPs (Kd = 100–200 nM) [55,56]. The expression of p22HBP is induced during erythroid differentiation in mouse erythroleukemia (MEL) cells, whereas the expression of HBP23 is induced by heme, PPIX, and other metalloporphyrins in rat primary hepatocytes or by oxidant stress in peritoneal macrophages [55–57]. Future studies are required to determine if HBPs act as genuine heme carriers/chaperones or they simply scavenge tetrapyrroles. Recently, the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was reported to bind heme and with a preference for Fe(III) and Co(III)-protoporphyrin IX analogs, but do not bind Zn- or the metal-free protoporphyrin IX [58]. It has been shown that GAPDH is required for heme insertion into a soluble hemoprotein - the inducible nitric oxide synthases (iNOS) [59]. Whether GAPDH plays a role as a heme chaperone for other proteins, in addition to its traditional function in glycolysis, needs further investigation. The molecular chaperone HSP90 is also reported to mediate heme insertion into NOS and another hemoprotein, soluble guanylyl cyclase (sGC) [60,61]. However, since HSP90 is a highly conserved molecular chaperone with a wide array of client proteins, its specific role in heme homeostasis is unclear [62].
Table 1.
Putative heme transporters and heme binding proteins.
| Protein | Proposed function | Localization | Heme binding affinity |
Blood disorders | Reference |
|---|---|---|---|---|---|
| HRG-1 (SLC48A1) |
Import heme | PM, Endosome/ Lysosome |
Inherited iron disorders? |
[12] | |
| PCFT/HCP1 | Import heme? | PM | Km = 125 µM (heme) Km = 1.3–56 µM (folate) |
Hereditary familial folate malabsorption, folate-responsive anemia in newborns |
[18,19] |
| FLVCR2 | Import heme? | PM | Fowler syndrome, lethal cerebral vasculopathy |
[22,23] | |
| FLVCRla | Export heme | PM | Posterior column ataxia and retinitis pigmentosa? |
[26] | |
| FLVCRlb | Export heme | IM | Diamond Blackfan anemia? |
[34] | |
| ABCG2/BCRP | Export heme | PM | Increased serum urate level, resulting in gout |
[41] | |
| ABCB6 | Transport coproporphyrinoge n III, heme? |
OM? PM, Endosome/ Lysosome |
Familial Pseudohyperkalemia, Ocular coloboma |
[45,49] | |
| ABCB10 | Stabilize Mfrnl, transport heme? |
IM | KO mice displayed deficiency of primitive erythropoiesis |
[51,52] | |
| p22HBP | Bind heme/tetrapyrroles |
Cytosol | Kd = 26 nM | [56] | |
| HBP23 | Bind heme/tetrapyrroles |
Cytosol | Kd = 55 nM | [55] | |
| GST | Bind heme/tetrapyrroles |
Cytosol | Kd = 100–200 nM |
[66] | |
| FABP | Bind heme/tetrapyrroles |
Cytosol | Kd = 150 nM | [67] | |
| GAPDH | Bind heme, heme insertion into iNOS |
Cytosol | Kd = 24 nM | [58,59] | |
| HSP90 | Heme insertion into NOS, sGC |
Cytosol | [60,61] | ||
| Hemopexin | Bind extracellular heme |
Blood | Kd < 1 pM | [63] | |
| Haptoglobin | Bind extracellular hemoglobin |
Blood | Kd < 1 pM | [63] | |
| Human serum albumin |
Bind extracellular heme |
Blood | Kd = 5 nM | [68] | |
| HRG-3 | Bind heme | Extracellular in C. elegans |
[65] |
PM: plasma membrane, OM: mitochondrial outer membrane, IM: mitochondrial inner membrane, KO: knockout.
Intercellular heme trafficking
To date, several extracellular heme binding proteins have been identified in mammals, including hemopexin, haptoglobin and human serum albumin (HSA). However, none of them has been shown to directly contribute to erythropoiesis [63]. Studies have shown that a portion of heme released from degraded RBCs in macrophage is exported as intact heme during EP, possibly through FLVCR1 or an unknown heme exporter [29,31]. This raises the possibility that heme maybe trafficked between cells and tissues. Mice that are mutant for heme synthesis are viable till embryonic day 8.5, indicating potential intercellular heme transport from a maternal source [64].
In C. elegans, maternal heme levels have a direct impact on embryonic development. Chen et al recently demonstrated HRG-3, a secreted worm protein, is responsible for delivering heme from maternal intestine to the developing embryo [65]. HRG-3 binds heme at a stoichiometry of two protomers to one heme. Although, deletion of HRG-3 had no visible effect on adult worms under low heme conditions, their progeny were either unable to hatch or growth arrested at the first larval stage. This phenotype could be rescued by ectopic expression of HRG-3 in maternal intestine, but not by embryonic specific expression [65]. Even though a functional homolog of HRG-3 in mammals has not been discovered, it is reasonable to speculate that during development and erythropoiesis, a heme chaperone may be required to facilitate the targeted delivery and redistribution of heme between certain tissues and cell types.
Conclusion
In addition to the well characterized heme biosynthetic pathway, intra- and intercellular heme trafficking pathways play an essential role in maintaining systemic heme homeostasis. Emerging studies have discovered several components in heme transport, yet their relationship with human blood disorders remains unclear and sometimes contradictory. Many issues remain unresolved including the function of HRG-1 in intestinal heme absorption and genetic iron disorders, the physiological function of FLVCR paralogs (FLVCR1a, 1b and FLVCR2), and the identity of intra- and intercellular heme chaperones. Tackling these questions will undoubtedly provide a deeper understanding of the chemical role of heme and heme trafficking networks crucial for the genesis of RBCs.
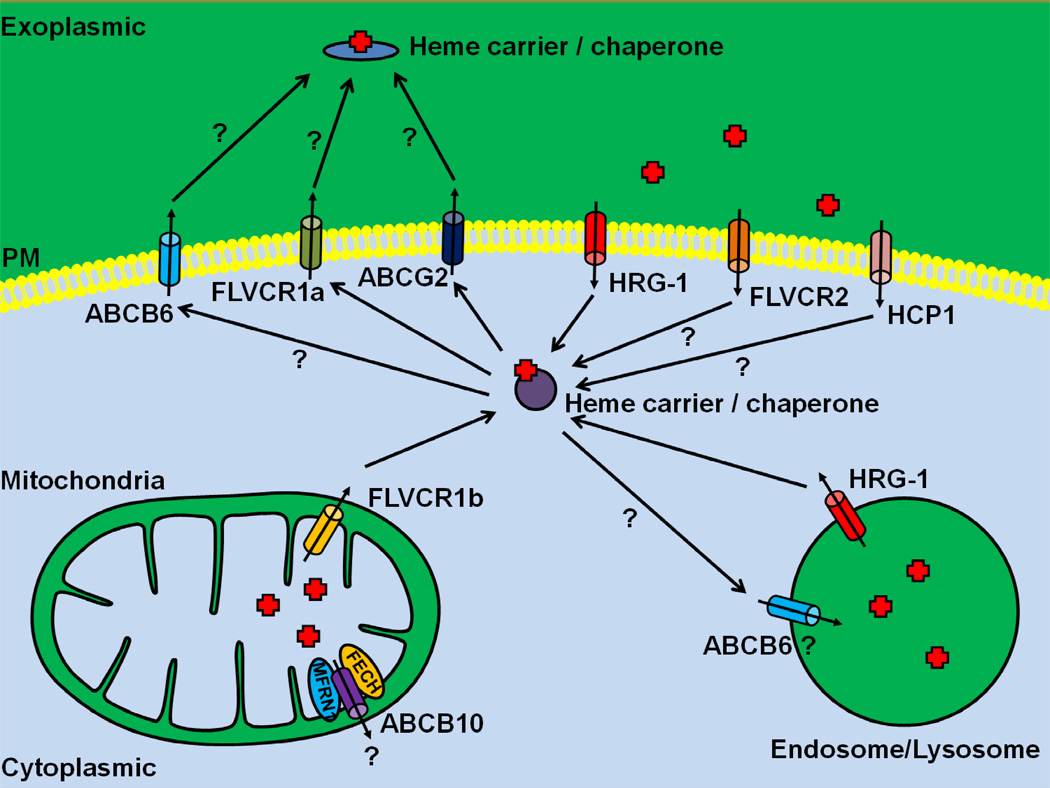
Figure 1. A schematic description of known heme transporters.
HRG-1 is a heme importer that localizes to endosomal/lysosomal compartments, but can traffic to the plasma membrane. HCP1 and FLVCR2 are two putative heme importers. The cell surface FLVCR1a and the ABC transporter ABCG2 have been implicated in heme export in erythroid cells, whereas the mitochondrial isoform FLVCR1b transport heme into the cytosol. ABCB6 was previously proposed to be a mitochondrial porphyrin/heme importer, but has recently been shown to localize to the plasma membrane and endosomal/lysosomal vesicles. ABCB10 forms a complex with MFRN1 and FECH, and stabilizes MFRN1. It is not clear whether ABCB10 transport heme. Heme carrier / chaperone that is responsible for intra- and intercellular heme trafficking remains unknown. Question marks represent the presumptive heme trafficking pathways. PM, plasma membrane.
Highlights.
-
▪
Heme must be transported across membranes for normal erythropoiesis.
-
▪
Recently discovered heme transporters fill a significant knowledge gap.
-
▪
Functional contradictions further complicate the biological role of this enigmatic group of transporters.
Acknowledgements
Our work on heme transport and homeostasis has been generously supported by the National Institutes of Health grants R01DK85035 and R01DK074797 and the Roche Foundation for Anemia Research to I.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1.Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38:61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- 2.Chung J, Chen C, Paw BH. Heme metabolism and erythropoiesis. Curr Opin Hematol. 2012;19:156–162. doi: 10.1097/MOH.0b013e328351c48b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuyama K, Kaneko K, Vargas PD. Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J Exp Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 5. Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. This comprehensive review discusses trafficking and interorganellar transport of heme.
- 6. Hamza I, Dailey HA. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta. 2012;1823:1617–1632. doi: 10.1016/j.bbamcr.2012.04.009. This review discusses the transport of heme and heme synthesis intermediatesheme.
- 7.Hamza I. Intracellular trafficking of porphyrins. ACS Chem Biol. 2006;1:627–629. doi: 10.1021/cb600442b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz IJ, Chen C, Paw BH, Hamza I. Iron and porphyrin trafficking in heme biogenesis. J Biol Chem. 2010;285:26753–26759. doi: 10.1074/jbc.R110.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad ME, Umbreit JN. Iron absorption and transport-an update. Am J Hematol. 2000;64:287–298. doi: 10.1002/1096-8652(200008)64:4<287::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.West AR, Oates PS. Mechanisms of heme iron absorption: Current questions and controversies. World J Gastroenterol. 2008;14:4101–4110. doi: 10.3748/wjg.14.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A. 2005;102:4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. This elegant work describes the identification of HRG-1, the first bona fide heme importer exploiting C. elegans as a model system
- 13.Yanatori I, Tabuchi M, Kawai Y, Yasui Y, Akagi R, Kishi F. Heme and non-heme iron transporters in non-polarized and polarized cells. BMC Cell Biol. 2010;11:39. doi: 10.1186/1471-2121-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Callaghan KM, Ayllon V, O'Keeffe J, Wang Y, Cox OT, Loughran G, Forgac M, O'Connor R. Hemebinding protein HRG-1 is induced by insulin-like growth factor I and associates with the vacuolar H+-ATPase to control endosomal pH and receptor trafficking. J Biol Chem. 2010;285:381–391. doi: 10.1074/jbc.M109.063248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaby C, Rondeau C, Pouzet C, Willemetz A, Pilard N, Desjardins M, Canonne-Hergaux F. Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis. PLoS One. 2012;7:e42199. doi: 10.1371/journal.pone.0042199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White C, Yuan X, Schmidt PJ, Bresciani E, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, Ward DM, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metabolism. 2013 doi: 10.1016/j.cmet.2013.01.005. In press. This study reveals that the human homolog of the C. elegans HRG-1 protein functions in the transport of heme from the phagolysosome in macrophages after erythrophagocytosis.
- 17.Warnatz HJ, Schmidt D, Manke T, Piccini I, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, et al. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J Biol Chem. 2011;286:23521–23532. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 19. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. This study reveals that the previously identified HCP1 is not a heme transporter but actually a high affinity folic acid transporter.
- 20.Salojin KV, Cabrera RM, Sun W, Chang WC, Lin C, Duncan L, Platt KA, Read R, Vogel P, Liu Q, et al. A mouse model of hereditary folate malabsorption: deletion of the PCFT gene leads to systemic folate deficiency. Blood. 2011;117:4895–4904. doi: 10.1182/blood-2010-04-279653. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc S, Garrick MD, Arredondo M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am J Physiol Cell Physiol. 2012;302:C1780–C1785. doi: 10.1152/ajpcell.00080.2012. [DOI] [PubMed] [Google Scholar]
- 22.Duffy SP, Shing J, Saraon P, Berger LC, Eiden MV, Wilde A, Tailor CS. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol Cell Biol. 2010;30:5318–5324. doi: 10.1128/MCB.00690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan X, Protchenko O, Philpott CC, Hamza I. Topologically conserved residues direct heme transport in HRG-1-related proteins. J Biol Chem. 2012;287:4914–4924. doi: 10.1074/jbc.M111.326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JK, Fung C, Tailor CS. Comprehensive mapping of receptor-functioning domains in feline leukemia virus subgroup C receptor FLVCR1. J Virol. 2006;80:1742–1751. doi: 10.1128/JVI.80.4.1742-1751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipovich L, Hughes AL, King MC, Abkowitz JL, Quigley JG. Genomic structure and evolutionary context of the human feline leukemia virus subgroup C receptor (hFLVCR) gene: evidence for block duplications and de novo gene formation within duplicons of the hFLVCR locus. Gene. 2002;286:203–213. doi: 10.1016/s0378-1119(02)00457-2. [DOI] [PubMed] [Google Scholar]
- 26. Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. This important work describes the identification of FLVCR1 as the potential heme exporter.
- 27.Lalonde E, Albrecht S, Ha KC, Jacob K, Bolduc N, Polychronakos C, Dechelotte P, Majewski J, Jabado N. Unexpected allelic heterogeneity and spectrum of mutations in Fowler syndrome revealed by next-generation exome sequencing. Hum Mutat. 2010;31:918–923. doi: 10.1002/humu.21293. [DOI] [PubMed] [Google Scholar]
- 28.Meyer E, Ricketts C, Morgan NV, Morris MR, Pasha S, Tee LJ, Rahman F, Bazin A, Bessieres B, Dechelotte P, et al. Mutations in FLVCR2 are associated with proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome (Fowler syndrome) Am J Hum Genet. 2010;86:471–478. doi: 10.1016/j.ajhg.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and downregulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quigley JG, Burns CC, Anderson MM, Lynch ED, Sabo KM, Overbaugh J, Abkowitz JL. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95:1093–1099. [PubMed] [Google Scholar]
- 31.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Philips JD, Doty RT, Giraudi P, Ostrow JD, Tiribelli C, Smith A, Abkowitz JL. Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin. J Biol Chem. 2010;285:28874–28882. doi: 10.1074/jbc.M110.119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alves LR, Costa ES, Sorgine MH, Nascimento-Silva MC, Teodosio C, Barcena P, Castro-Faria-Neto HC, Bozza PT, Orfao A, Oliveira PL, et al. Heme-oxygenases during erythropoiesis in K562 and human bone marrow cells. PLoS One. 2011;6:e21358. doi: 10.1371/journal.pone.0021358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiabrando D, Marro S, Mercurio S, Giorgi C, Petrillo S, Vinchi F, Fiorito V, Fagoonee S, Camporeale A, Turco E, et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J Clin Invest. 2012;122:4569–4579. doi: 10.1172/JCI62422. This study shows that FLVCR1 is alternatively spliced to yield a mitochondrial isoform that localizes to the mitochondria instead of the expected plasma membrane.
- 35.Rajadhyaksha AM, Elemento O, Puffenberger EG, Schierberl KC, Xiang JZ, Putorti ML, Berciano J, Poulin C, Brais B, Michaelides M, et al. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa. Am J Hum Genet. 2010;87:643–654. doi: 10.1016/j.ajhg.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishiura H, Fukuda Y, Mitsui J, Nakahara Y, Ahsan B, Takahashi Y, Ichikawa Y, Goto J, Sakai T, Tsuji S. Posterior column ataxia with retinitis pigmentosa in a Japanese family with a novel mutation in FLVCR1. Neurogenetics. 2011;12:117–121. doi: 10.1007/s10048-010-0271-4. [DOI] [PubMed] [Google Scholar]
- 37.Yanatori I, Yasui Y, Miura K, Kishi F. Mutations of FLVCR1 in posterior column ataxia and retinitis pigmentosa result in the loss of heme export activity. Blood Cells Mol Dis. 2012;49:60–66. doi: 10.1016/j.bcmd.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Krishnamurthy P, Schuetz JD. The ABC transporter Abcg2/Bcrp: role in hypoxia mediated survival. Biometals. 2005;18:349–358. doi: 10.1007/s10534-005-3709-7. [DOI] [PubMed] [Google Scholar]
- 39.Desuzinges-Mandon E, Arnaud O, Martinez L, Huche F, Di Pietro A, Falson P. ABCG2 transports and transfers heme to albumin through its large extracellular loop. J Biol Chem. 2010;285:33123–33133. doi: 10.1074/jbc.M110.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu XG, Peng SB, Huang Q. [Transcriptional regulation of breast cancer resistance protein] Yi Chuan. 2012;34:1529–1536. doi: 10.3724/sp.j.1005.2012.01529. [DOI] [PubMed] [Google Scholar]
- 41.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 42.Zelinski T, Coghlan G, Liu XQ, Reid ME. ABCG2 null alleles define the Jr(a-) blood group phenotype. Nat Genet. 2012;44:131–132. doi: 10.1038/ng.1075. [DOI] [PubMed] [Google Scholar]
- 43.Saison C, Helias V, Ballif BA, Peyrard T, Puy H, Miyazaki T, Perrot S, Vayssier-Taussat M, Waldner M, Le Pennec PY, et al. Null alleles of ABCG2 encoding the breast cancer resistance protein define the new blood group system Junior. Nat Genet. 2012;44:174–177. doi: 10.1038/ng.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Severance S, Rajagopal A, Rao AU, Cerqueira GC, Mitreva M, El-Sayed NM, Krause M, Hamza I. Genome-wide analysis reveals novel genes essential for heme homeostasis in Caenorhabditis elegans. PLoS Genet. 2010;6:e1001044. doi: 10.1371/journal.pgen.1001044. This study provides a glimpse into the potential complexity of the heme homeostasis network in C. elegans.
- 45.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 46.Polireddy K, Khan MM, Chavan H, Young S, Ma X, Waller A, Garcia M, Perez D, Chavez S, Strouse JJ, et al. A novel flow cytometric HTS assay reveals functional modulators of ATP binding cassette transporter ABCB6. PLoS One. 2012;7:e40005. doi: 10.1371/journal.pone.0040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, He F, Bu J, Zhen Y, Liu X, Du W, Dong J, Cooney JD, Dubey SK, Shi Y, et al. ABCB6 mutations cause ocular coloboma. Am J Hum Genet. 2012;90:40–48. doi: 10.1016/j.ajhg.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, Tanaka M, Deybach JC, Puy H, Le Gall M, et al. ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat Genet. 2012;44:170–173. doi: 10.1038/ng.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, Vallentin A, Vial H, Vidal M, Szakacs G. Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS One. 2012;7:e37378. doi: 10.1371/journal.pone.0037378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andolfo I, Alper SL, Delaunay J, Auriemma C, Russo R, Asci R, Esposito MR, Sharma AK, Shmukler BE, Brugnara C, et al. Missense mutations in the ABCB6 transporter cause dominant familialpseudohyperkalemia. Am J Hematol. 2013;88:66–72. doi: 10.1002/ajh.23357. [DOI] [PubMed] [Google Scholar]
- 51.Chen W, Dailey HA, Paw BH. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood. 2010;116:628–630. doi: 10.1182/blood-2009-12-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyde BB, Liesa M, Elorza AA, Qiu W, Haigh SE, Richey L, Mikkola HK, Schlaeger TM, Shirihai OS. The mitochondrial transporter ABC-me (ABCB10), a downstream target of GATA-1, is essential for erythropoiesis in vivo. Cell Death Differ. 2012;19:1117–1126. doi: 10.1038/cdd.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhan B, Perally S, Brophy PM, Xue J, Goud G, Liu S, Deumic V, de Oliveira LM, Bethony J, Bottazzi ME, et al. Molecular cloning, biochemical characterization, and partial protective immunity of the heme-binding glutathione S-transferases from the human hookworm Necator americanus. Infect Immun. 2010;78:1552–1563. doi: 10.1128/IAI.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiller N, Fritz-Wolf K, Deponte M, Wende W, Zimmermann H, Becker K. Plasmodium falciparum glutathione S-transferase--structural and mechanistic studies on ligand binding and enzyme inhibition. Protein Sci. 2006;15:281–289. doi: 10.1110/ps.051891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwahara S, Satoh H, Song DX, Webb J, Burlingame AL, Nagae Y, Muller-Eberhard U. Purification, characterization, and cloning of a heme-binding protein (23 kDa) in rat liver cytosol. Biochemistry. 1995;34:13398–13406. doi: 10.1021/bi00041a017. [DOI] [PubMed] [Google Scholar]
- 56.Taketani S, Adachi Y, Kohno H, Ikehara S, Tokunaga R, Ishii T. Molecular characterization of a newly identified heme-binding protein induced during differentiation of urine erythroleukemia cells. J Biol Chem. 1998;273:31388–31394. doi: 10.1074/jbc.273.47.31388. [DOI] [PubMed] [Google Scholar]
- 57.Immenschuh S, Nell C, Iwahara S, Katz N, Muller-Eberhard U. Gene regulation of HBP 23 by metalloporphyrins and protoporphyrin IX in liver and hepatocyte cultures. Biochem Biophys Res Commun. 1997;231:667–670. doi: 10.1006/bbrc.1997.6166. [DOI] [PubMed] [Google Scholar]
- 58.Hannibal L, Collins D, Brassard J, Chakravarti R, Vempati R, Dorlet P, Santolini J, Dawson JH, Stuehr DJ. Heme Binding Properties of Glyceraldehyde-3-phosphate Dehydrogenase. Biochemistry. 2012 doi: 10.1021/bi300863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakravarti R, Aulak KS, Fox PL, Stuehr DJ. GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 2010;107:18004–18009. doi: 10.1073/pnas.1008133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh A, Stuehr DJ. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc Natl Acad Sci U S A. 2012;109:12998–13003. doi: 10.1073/pnas.1205854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghosh A, Chawla-Sarkar M, Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011;25:2049–2060. doi: 10.1096/fj.10-180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 63.Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, Altruda F. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- 64.Okano S, Zhou L, Kusaka T, Shibata K, Shimizu K, Gao X, Kikuchi Y, Togashi Y, Hosoya T, Takahashi S, et al. Indispensable function for embryogenesis, expression and regulation of the nonspecific form of the 5-aminolevulinate synthase gene in mouse. Genes Cells. 2010;15:77–89. doi: 10.1111/j.1365-2443.2009.01366.x. [DOI] [PubMed] [Google Scholar]
- 65. Chen C, Samuel TK, Sinclair J, Dailey HA, Hamza I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011;145:720–731. doi: 10.1016/j.cell.2011.04.025. This study describes the identification of small protein termed HRG-3 as an intercellular heme trafficking protein.
- 66.Harvey JW, Beutler E. Binding of heme by glutathione S-transferase: a possible role of the erythrocyte enzyme. Blood. 1982;60:1227–1230. [PubMed] [Google Scholar]
- 67.Vincent SH, Muller-Eberhard U. A protein of the Z class of liver cytosolic proteins in the rat that preferentially binds heme. J Biol Chem. 1985;260:14521–14528. [PubMed] [Google Scholar]
- 68.Adams PA, Berman MC. Kinetics and mechanism of the interaction between human serum albumin and monomeric haemin. Biochem J. 1980;191:95–102. doi: 10.1042/bj1910095. [DOI] [PMC free article] [PubMed] [Google Scholar]