Abstract
The unprecedented polymorphism of the non-steroidal anti-inflammatory drug (NSAID) flufenamic acid (FFA) is described here. Nine polymorphs were accessed through the use of polymer-induced heteronucleation (PIHn) and solid-solid transformation at low temperature. Structural elucidation of six of these forms, in addition to the two previously known forms, makes FFA indisputably octamorphic. Although the structure of at least one other form of FFA remains elusive, the occurrence of most of these polymorphs under one crystallization condition through PIHn illustrates that a fine interplay exists among the kinetic factors that lead to phase selection in this NSAID.
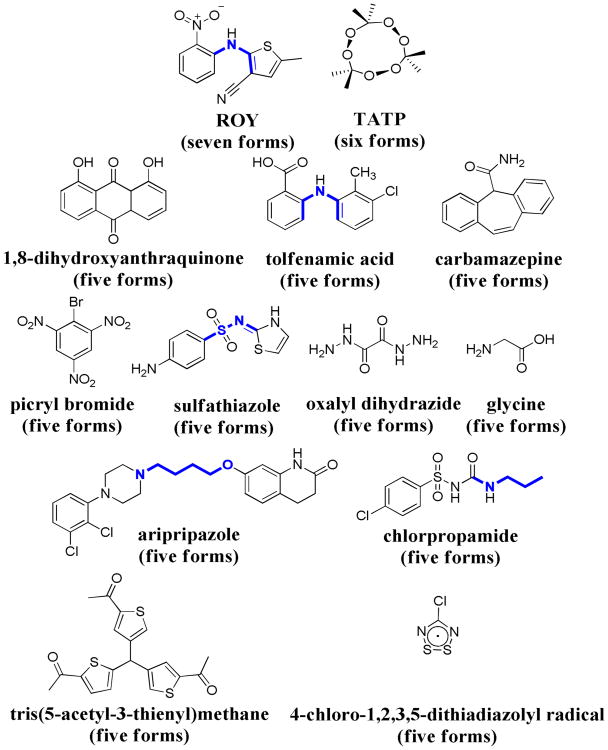
It has been suggested that all compounds can present polymorphism, multiple arrangements of a molecule within a crystal, and that experimental observation of this phenomena in some cases and not in others is mainly a consequence of the time and money invested in their investigation.1 Although this sentiment resonates with the experience of many researchers in the field, there are compounds2 and molecular classes3 more prone to give rise to polymorphism. Moreover, there are legendary compounds purported to give rise to more than 104 or even more than 505 polymorphs at atmospheric pressure. It is therefore sobering that when it comes to the most reliable standard for polymorph characterization, single crystal X-ray diffraction, only one molecule in the Cambridge Structural Database (CSD) is recognized as the most polymorphic compound and has since 2005 been considered the molecule with the most structurally characterized polymorphs; 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY),2 a pharmaceutical intermediate, has seven structurally characterized forms (Figure 1). In 2009 triacetone-triperoxide (TATP), an explosive, was claimed to possess six structurally characterized forms (Figure 1).6 Additionally, eleven different compounds have given rise to five polymorphs7 (Figure 1) according to a recent search of the CSD. Here, we report structural studies on the conformational polymorphism of the non-steroidal anti-inflammatory drug (NSAID) flufenamic acid (FFA), and demonstrate that this pharmaceutical compound possesses at least nine polymorphs. Crystal structure determination of six of these forms, in addition to the two previously known forms, sets a new record for structurally characterized materials presenting polymorphism.
Figure 1.
Molecular structures of highly polymorphic compounds in the CSD, illustrating the torsion angles (τ, bonds in blue) differing among the conformers. In parentheses is the number of structurally characterized polymorphs for each molecule. In some instances the number of polymorphs experimentally observed is greater than the values reported here reflecting additional forms lacking structural characterization.
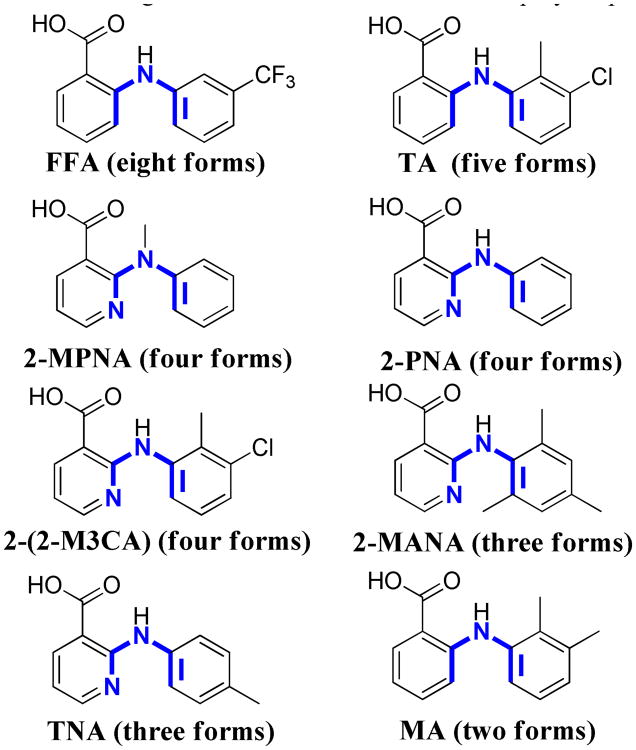
For more than forty years the existence of polymorphism in FFA has been known. During that time numerous investigations concerning the spectroscopic, conformational, and thermodynamic relationships among the proposed FFA poly-morphs have emerged.8 Elucidation of the first crystal structure (form III) occurred in 1973,9 and nearly ten years later the structure of a second form (form I) was reported.10 The existence of polymorphism in this compound has offered a challenge for methodologies developed for polymorph discovery and control from the use of soluble additives11 to epitaxy12 and now polymer-induced heteronucleation (PIHn).13 Furthermore, outside the potential significance of polymorphism for influencing the bioavailability of this drug, its supramolecular chemistry is of considerable interest to test within the fenamic acid series the concept of the polymorphophore: a structural element that when incorporated into a compound favors the formation of polymorphic crystals.14 FFA is a member of a large class of NSAIDs that contain a monocarboxylated diphenyl amine nucleus; several of these analogues display polymorphism (Figure 2) and in particular tolfenamic acid (TA) at five structurally characterized forms is one of the most polymorphic drugs known.15 Here, we report on the polymorphism of FFA by elucidating the crystal structure of six additional forms for the first time. The fact that eight unique crystal structures for polymorphs co-existing at room temperature and atmospheric pressure has only now been achieved for any compound, much less such a well-studied compound, certainly makes a strong case that kinetic access to new polymorphs remains an unmet challenge for experiment.
Figure 2.
Molecular structures of NSAIDs flufenamic acid (FFA), tolfenamic acid (TA), mefenamic acid (MA), and other structural analogues illustrating torsion angles (τ1 and τ2 in blue) differing among the forms. In parentheses is the number of structurally characterized polymorphs for each molecule. In some instances the number of polymorphs experimentally observed is greater than the values reported here reflecting additional forms lacking structural characterization.
Eight crystalline phases of FFA were grown from ethanol solution using polymers as heteronuclei (Supporting Information). Optical examination revealed noticeable differences in the morphology of the crystals. The previously reported colorless and yellow needles (forms I and III) were observed as well as colorless plates (forms II and VIII), colorless prisms (forms IV and V) and colorless needles (forms VII and IX). Furthermore, in the case of form IV, a low temperature transformation (∼—130°C) allowed access to a new polymorph (form VI) which is quite stable at room temperature (Supporting information). Bulk form VI was accessed by submerging a 4 mL vial containing crystals (∼5.0 mg) of form IV into a Dewar flask containing N2(l) for 10-15 minutes. The vial was warmed to room temperature prior to powder X-ray diffraction analysis which confirmed the transformation of form IV to form VI had occurred. Forms I-V and VII could be accessed through conventional solvent based methods while these attempts were unsuccessful in producing forms VIII and IX (Supporting Information). When the samples were examined by Raman vibrational spectroscopy, four main regions (700-810, 1000-1250, 1320-1630, and 3000-3400cm−1) aided in the identification of each polymorph (Supporting Information).
Powder X-ray diffraction (PXRD) established that a unique structural fingerprint exists for all nine polymorphs of FFA (Supporting Information). Comparing the PXRD patterns obtained in this investigation with those found previously for forms 1-III,16 confirms that the undisclosed structure in this set corresponds to FFA form II, whereas diffraction patterns for forms IV-IX are reported here for the first time. Structure determination of forms II, IV, V, VI, VII, and VIII revealed that, as in the case of other highly polymorphic compounds (Figure 1) and each of the structural analogues of FFA presenting multiple packing modes (Figure 2), each FFA polymorph contains different conformers. All structurally characterized forms of FFA present marked conformational changes between the torsion angles (τ1 and τ2) defined by the planes connected by the biphenyl amine functionality, whereas a nearly coplanar arrangement between the carboxyl group and the phenyl ring bearing it (τ3) is observed possibly due to the intramolecular hydrogen bonding occurring between the amino group and the carboxylic acid oxygen in all conformers (Supporting Information).
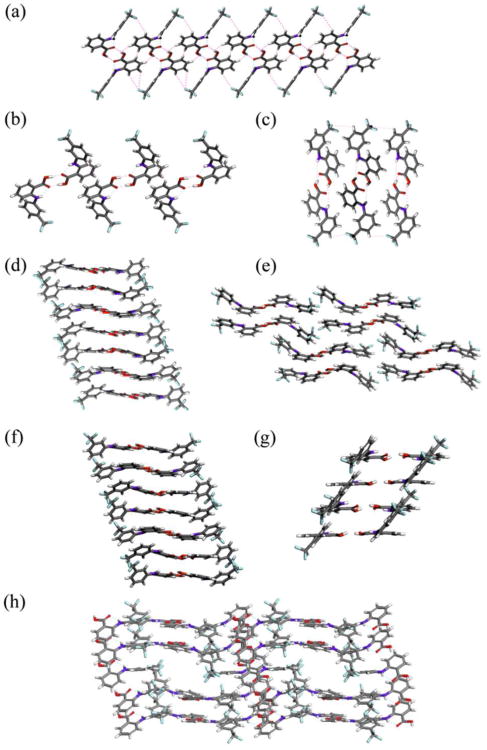
In contrast to ROY, several structures of FFA vary in the number of inequivalent molecules in the asymmetric unit. FFA is known to crystallize in two monoclinic forms, both containing one molecule in the asymmetric unit. The now accessible forms II, V, and VII crystallize in the same space group (P21/c) with Z′= 1, Z′= 4, and Z′= 2, respectively. Form IV crystallizes in the space group P1| with Z′= 3, whereas form VI and VIII crystallize in the same triclinic space group but possess 6 and 9.5 molecules in the asymmetric unit, an exceedingly rare occurrence in the CSD with less than 200 examples for structures with Z′= 6 and no precedent for structures with Z′= 9.5 (Supporting Information). There is an area of severe disorder in the lattice of form VIII at the inversion center which is modeled as a void (Figure 3h). The volume is sufficiently large to accommodate an additional FFA molecule which is required to be disordered about the inversion center; this half molecule yields Z′= 9.5. The calculated contribution of 126 electrons due to diffuse scattering is in good agreement with the 136 electrons expected for the missing molecule. Thermogravimetric analysis (TGA) of FFA form VIII showed no solvent loss upon heating of the sample, confirming that this form is in fact a true polymorph and not a solvated form (Supporting Information); this fact is further confirmed by clean thermal conversion of form VIII to form III upon heating or by mechanical contact. The packing modes present in each of the FFA polymorphs are illustrated in Figure 3. Although the packing modes in forms IV and VI are very similar, both forms present different conformations in each of the molecules of the asymmetric unit and have been demonstrated to coexist at room temperature which supports the notion that form VI is not a superstructure or modulation of form IV occurring at low temperature (Supporting Information) and provides additional evidence of the distinctiveness of each of these forms.
Figure 3.
Molecular packing and hydrogen bonding motifs of FFA polymorphs. (a) form I viewed along the a-axis, (b) form II viewed along the a-axis, (c) form III viewed along the a-axis, (d) form IV viewed along the a-axis, (e) form V viewed along the b-axis, (f) form VI viewed along the a-axis, (g) form VII viewed along the b-axis, and (h) form VIII, ordered molecules only, viewed perpendicular to the bc-plane.
To assess the effect of these conformational and structural differences on the energies of FFA polymorphs, relative stabilities were determined experimentally. The optical absorbance of FFA polymorphs I-IV, VI and VII contacted with water was monitored in situ over time to determine the absorbance at equilibrium at 27°C. Polymorphs I and II slowly transformed during the experimental time frame to form III; therefore the relative free energies presented for these forms are slight underestimations. This study establishes that form III is the thermodynamically most stable form by at least 0.3 kcal/mol among all forms examined (Supporting Information). Enthalpies for FFA polymorphs were determined by experiment and theory. FFA forms I-III melt without transformation prior to melting, whereas for forms IV-VII, additional events were observed in each of the respective thermograms (Supporting Information). According to the lattice energy calculations for forms I-VII, form III has the most negative enthalpy (Supporting Information) which is in accordance with the experimental enthalpies determined among the FFA polymorphs examined. Transformation of forms V, VIII and IX to form III during sample preparation precluded determination of the free energies and/or enthalpies for these forms.
PIHn has allowed access to eight polymorphs of FFA, and an additional form was discovered through solid-solid transformation at low temperature. Six of these forms have been structurally characterized which, in addition to the two previously reported forms, represents a new level of accomplishment in polymorphic compounds. Although the crystal structure of at least one other form remains elusive, the presence of this form has been confirmed through Raman spectroscopy and PXRD. The appearance of a high degree of polymorphism present in FFA and TA, and the occurrence of polymorphism in many of their structurally related analogues supports the notion that certain structural motifs are in fact privileged with regard to polymorphic propensity. Furthermore, this compound is of particular importance to assess the quality of polymorph prediction methods as well as offers a challenge to newly introduced polymorph discovery methods.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Saikat Roy and Kira Landen-berger for useful conversations concerning this project. This work was supported by the National Institutes of Health GM072737.
Footnotes
Supporting Information. See Supporting Information for experimental details, Raman spectroscopy, powder X-ray diffraction, single crystal X-ray diffraction, relative stability studies, conformational energy, lattice energy calculation, thermogravimetric analysis, and Cambridge Structural Database (CSD) search for Z′≥ 6. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.McCrone WC. Physics and Chemistry of the Organic Solid State. Interscience Publishers; London: 1965. [Google Scholar]
- 2.(a) Chen S, Guzei IA, Yu L. J Am Chem Soc. 2005;127:9881. doi: 10.1021/ja052098t. [DOI] [PubMed] [Google Scholar]; (b) Chen SA, Xi HM, Yu L. J Am Chem Soc. 2005;127:17439. doi: 10.1021/ja056072d. [DOI] [PubMed] [Google Scholar]; (c) Yu L, Stephenson GA, Mitchell CA, Bunnell CA, Snorek SV, Bowyer JJ, Borchardt TB, Stowell JG, Byrn SR. J Am Chem Soc. 2000;122:585. [Google Scholar]
- 3.(a) Yang S, Guillory JK. J Pharm Sci. 1972;61:26. doi: 10.1002/jps.2600610104. [DOI] [PubMed] [Google Scholar]; (b) Mesley RJ, Houghton EE. J Pharm Pharmac. 1967;19:295. doi: 10.1111/j.2042-7158.1967.tb08090.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Platteau C, Lefebvre J, Hemon S, Baehtz C, Danede F, Prevost D. Acta Crystallogr. 2005;61:80. doi: 10.1107/S0108768104031143. [DOI] [PubMed] [Google Scholar]; (b) Kato Y, Okamoto Y, Nagasawa S, Ueki T. Chem Pharm Bulletin. 1981;29:3410. [Google Scholar]; (c) Nikolics K, Bidlo G, Nikolics K. Acta Pharm. 1974;44:133. [PubMed] [Google Scholar]; (d) Mesley RJ, Zlements RL. J Pharm Pharmac. 1968;20:341. doi: 10.1111/j.2042-7158.1968.tb09757.x. [DOI] [PubMed] [Google Scholar]
- 5.Cited thereafter; Dixit G, Khile AS, Pradhan NS, Valgeirsson J. Patent 2009/007856. 2009
- 6.Reany O, Kapon M, Botoshansky M, Keinan E. Cryst Growth Des. 2009;9:3661. [Google Scholar]
- 7.(a) Kruger GJ, Gafner G. Acta Crystallogr. 1971;B 27:326. [Google Scholar]; (b) Kruger GJ, Gafner G. Acta Crystallogr. 1972;B 28:272. [Google Scholar]; (c) Blagden N, Davey RJ, Lieberman HF, Williams L, Payne R, Roberts R, Rowe R, Docherty R. J Chem Soc Faraday Trans. 1998;94:1035. [Google Scholar]; (d) Hughes DS, Hursthouse MB, Threlfall T, Tavener S. Acta Crystallogr. 1999;55:1831. [Google Scholar]; (e) Gelbrich T, Hughes DS, Hursthouse MB, Threlfall TL. Cryst Eng Comm. 2008;10:1328. [Google Scholar]; (f) Carper WR, Davis LP, Extine MW. J Phys Chem. 1982;86:459. [Google Scholar]; (g) Bond AD, Haynes DA, Pask CM, Rawson JM. J Chem Soc Dalton Trans. 2002:2522. [Google Scholar]; (h) Clarke CS, Pascu SI, Rawson JM. Cryst Eng Comm. 2004;6:79. [Google Scholar]; (i) Dubnikova F, Kosloff R, Almog R, Zeiri Y, Boese R, Itzhaky H, Alt A, Keinan E. J Am Chem Soc. 2005;127:1146. doi: 10.1021/ja0464903. [DOI] [PubMed] [Google Scholar]; (j) Ahn SY, Guo F, Kariuki BM, Harris KDM. J Am Chem Soc. 2006;128:8441. doi: 10.1021/ja0573155. [DOI] [PubMed] [Google Scholar]; (k) Drebushchak TN, Chukanov NV, Boldyreva EV. Acta Crystallogr. 2006;62:4393. [Google Scholar]; (l) Drebushchak TN, Chukanov NV, Boldyreva EV. Acta Crystal-logr. 2007;63:355. doi: 10.1107/S010827010701952X. [DOI] [PubMed] [Google Scholar]; (m) Drebushchak TN, Chukanov NV, Boldyreva EV. Acta Crystallogr. 2008;64:623. doi: 10.1107/S0108270108034550. [DOI] [PubMed] [Google Scholar]; (n) Parrish DA, Deschamps JR, Gilardi RD, Butcher RJ. Cryst Growth Des. 2008;8:57. [Google Scholar]; (o) Rohl AL, Moret M, Kaminsky W, Claborn K, McKinnon JJ, Kahr B. Cryst Growth Des. 2008;8:4517. [Google Scholar]; (p) Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. Cryst Growth Des. 2009;9:1054. [Google Scholar]; (q) Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. J Pharm Sci. 2009;98:2010. doi: 10.1002/jps.21574. [DOI] [PubMed] [Google Scholar]
- 8.(a) Dhanaraj V, Vijayan M. Acta Crystallogr. 1988;44:406. doi: 10.1107/s0108768188001107. [DOI] [PubMed] [Google Scholar]; (b) Burger A, Ramberger R. Mikrochimica Acta. 1980;1:17. [Google Scholar]; (c) Park K, Evans JMB, Myerson AS. Cryst Growth Des. 2003;3:991. [Google Scholar]; (d) Surov AO, Terekhova IV, Bauer-Brandl A, Perlovich GL. Cryst Growth Des. 2009;9:3265. [Google Scholar]
- 9.McConnell JF. Cryst Struct Comm. 1973;3:439. [Google Scholar]
- 10.Kirshna Murphy HM, Bhat TN, Vijayan M. Acta Crystalogr. 1982;38:315. [Google Scholar]
- 11.Lee EH, Boerrigter SXM, Rumondor ACF, Chamarthy SP, Byrn SR. Cryst Growth Des. 2008;8:91. [Google Scholar]
- 12.Lee EH, Boerrigter SXM, Byrn SR. Cryst Growth Des. 2010;10:518. [Google Scholar]
- 13.(a) Lang MD, Grzesiak AL, Matzger AJ. J Am Chem Soc. 2002;124:14834. doi: 10.1021/ja0286526. [DOI] [PubMed] [Google Scholar]; (b) Price CP, Grzesiak AL, Matzger AJ. J Am Chem Soc. 2005;127:5512. doi: 10.1021/ja042561m. [DOI] [PubMed] [Google Scholar]
- 14.The term draws on analogy with the pharmacophore and is an abstract definition of molecular features which are necessary for high degrees of polymorphism: Lutker KM, Matzger AJ. J Pharm Sci. 2010;99:794. doi: 10.1002/jps.21873.Lutker KM, Tolstyka ZP, Matzger A. J Cryst Growth Des. 2008;8:136. doi: 10.1021/cg700921w.
- 15.(a) López-Mejías V, Kampf JW, Matzger AJ. J Am Chem Soc. 2009;131:4554. doi: 10.1021/ja806289a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Takasuka M, Nakai H, Shiro M. J Am Chem Soc. 1982:1061. [Google Scholar]; (c) Surov AO, Terekhova IV, Bauer-Brandl AB, Prelovich GL. Cryst Growth Des. 2009;9:3265. [Google Scholar]; (d) Nath NK, Kumar SS, Nangia A. Cryst Growth Des. 2011;11:4594. [Google Scholar]; (e) Long S, Siegler MA, Mattei A, Li T. Cryst Growth Des. 2011;11:414. [Google Scholar]
- 16.Krc J. Microscope. 1977;25:31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.