Abstract
Slow delayed rectifier potassium current (IKs) is important in action potential (AP) repolarization and repolarization reserve. We tested the hypothesis that there are sex-specific differences in IKs, AP, and their regulation by β-adrenergic receptors (β-AR’s) using whole cell patch-clamp. AP duration (APD90) was significantly longer in control female (F) than in control male (M) myocytes. Isoproterenol (ISO, 500nM) shortened APD90 comparably in M & F, and was largely reversed by β1-AR blocker CGP 20712A (CGP, 300 nM). Inhibition of IKs with chromanol 293B (10 μM) resulted in less APD prolongation in F at baseline (3.0% vs 8.9%, p<0.05 vs M) and even in the presence of ISO (5.4% vs 20.9%, p<0.05). This suggests that much of the ISO-induced APD abbreviation in F is independent of IKs. In F, baseline IKs was 42% less and was more weakly activated by ISO (19% vs 68% in M, p<0.01). ISO enhancement of IKs was comparably attenuated by CGP in M and F. After ovariectomy, IKs in F had greater enhancement by ISO (72%), now comparable to control M. After orchiectomy, IKs in M was only slightly enhanced by ISO (23%), comparable to control F. Pre-treatment with thapsigargin (to block SR Ca release) had bigger impact on ISO-induced APD shortening in F than that in M (p<0.01). In conclusion, we found that there are sex differences in IKs, AP, and their regulation by β-AR’s that are modulated by sex hormones, suggesting the potential for sex-specific antiarrhythmic therapy.
Keywords: Potassium current, Sex differences, Repolarization, Adrenergic receptor
Introduction
The slow delayed rectifier potassium current (IKs), an important repolarizing current in heart (44, 50) that is encoded by KCNQ1 (KvLQT1) and KCNE1 (minK) genes, is an antiarrhythmic drug target (44). Sympathetic nervous system regulation of cardiac action potential duration (13, 44) is primarily mediated by β-adrenergic receptor (β-AR) activation, which increases IKs (44). In mammalian cardiomyocytes, β1-ARs play a predominant role in regulating myocyte contractility while β2-ARs may play a more modest role in regulating inotropic and lusitropic responses. Both receptors signal through Gs and adenylyl cyclase, although the signals are compartmentalized differently possibly due to localization in distinct membrane microdomains and/or dual coupling of the β2-AR to Gs and Gi (68). IKs is modulated by levels of intracellular Ca ([Ca]i) (52) such as the increase in [Ca]i during contraction due to Ca release from the sarcoplasmic reticulum (SR); so action potential duration (APD) shortening by isoproterenol (ISO) may be influenced by ISO-induced changes in SR Ca release.
Sex hormones may also play an important role in regulating repolarizing K currents, APD, and β-adrenergic responsiveness (2, 10, 14, 18, 22, 63). It has been recognized that the corrected QT interval (QTC) is longer in women than in men (43). In addition, female sex is associated with greater drug-induced QT prolongation and accounts for more torsades des pointes in response to certain drugs that prolong ventricular repolarization (19, 37, 43, 49). Sex differences in potassium currents in females appear to underlie increased action potential (AP) duration, QT interval, and incidence of torsades des pointes. However there is little quantitative and mechanistic characterization of sex-specific differences (and the role of sex hormones) in IKs and action potential characteristics and their responsiveness to β-adrenergic stimulation.
In the present study, we investigated sex differences in repolarization, ISO-induced APD shortening, and β-adrenergic receptor subtype selectivity in left ventricular (LV) myocytes from control male and female rabbits. We used an IKs blocker to evaluate the contribution of IKs to APD and ISO-induced APD shortening. We also assessed sex differences in IKs and its responsiveness to ISO. The contribution of sex hormones to these female vs male differences was assessed with studies in ovariectomized (OVX) and orchiectomized (ORCH) rabbits. Lastly, thapsigargin (SR Ca uptake inhibitor) was used to assess sex differences in the contribution of Ca handling to APD shortening with ISO.
Materials and methods
Animals
All experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). New Zealand rabbits weighing 3.5–4.0 kg of either sex were used. In selected studies, orchiectomized male and ovariectomized female rabbits were used. The protocols were approved by the Animal Studies Committees of the University of Illinois at Chicago and the University of Alabama at Birmingham.
Ovariectomy and Orchiectomy
In brief, female and male rabbits were gonadectomized at ~4 months old and sedated with intramuscular ketamine, intubated, and mechanically ventilated. Anesthesia was maintained with 1–3% isoflurane delivered in 100% oxygen. For ovariectomy, a 3–4 cm midline skin incision was made centered over the area of the cranial pole of the bladder. The linea alba was incised to access the peritoneal cavity. The ovary was separated from the broad ligament and the suspensory ligament. The ovarian vessels were double ligated with synthetic absorbable sutures and transected between the ligatures and the ovary. The procedure was repeated on the contralateral side to remove both ovaries. For orchiectomy, a 1–3 cm incision was made on the ventral surface of the scrotum of each testicle. The testicle was exposed by incising the spermatic fascia and the parietal tunic. The spermatic cord, vas deferens, and associated blood vessels were clamped and double-ligated with synthetic absorbable sutures and the testicles were removed. The waiting period after gonadectomize is ~8 weeks before the gonadectomized rabbits were sacrificed for myocyte isolation at 6 months old.
Cardiac myocyte isolation
LV Cardiac myocytes were isolated as previously described (47, 48). Briefly, 6 months old rabbits (25.9±0.5 weeks in control females, 26.3±0.5 weeks in control males) were anesthetized (pentobarbital sodium; 50 mg/kg) after being heparinized, and the heart was quickly removed, rinsed in cold 0 mM Ca2+ Tyrodes, placed on a Langendorff apparatus, and perfused through the aorta with a 0 Ca2+ solution at 37°C. The heart was then perfused with a MEM solution containing Liberase Blendzyme 4 (Roche Applied Science). Digestion was stopped by adding a bovine serum albumin (BSA)-containing solution (1 mg/ml), and midmyocardial LV tissue was used. Furthermore we have limited the heterogeneity by cutting off both the basal 4–8 mm and the apical 5–10 mm of the rabbit hearts. Tissue was gently minced and filtered. Cells were stored in 50 μM Ca2+ MEM solution at room temperature.
Single cell electrophysiology
Cardiac myocytes adhering to laminin-coated glass coverslips were placed in a small transparent recording chamber mounted on the stage of an inverted microscope (TE200S; Nikon; Tokyo, Japan). The whole-cell patch-clamp technique was used to record the membrane currents in single rabbit LV myocytes. Patch-clamp micropipettes were made from glass capillaries (Harvard Apparatus Limited, Kent, United Kingdom) using a DMZ-Universal puller (Zeitz-Instruments GmbH, Munich, Germany). These electrodes were filled with an intracellular solution containing (mM) KOH 120, KCl 20, MgCl2 2, CaCl2 1.8, Mg-ATP 5, EGTA 5, HEPES 10, aspartic acid 100, adjusted with KOH to pH 7.2. The external solution contained (mM) NaCl 132, KCl 4, CaCl2 1.8, MgCl2 1.2, BaCl2 0.2, HEPES 10, glucose 10, 4-aminopyridine 5, nifedipine 0.01, dofetilide 0.003, pH 7.4. A Multiclamp 700A amplifier (Axon Instruments, Union City, CA, USA) was used to record the membrane current in the whole-cell configuration of the patch-clamp technique. Capacitance was canceled and series resistance was compensated as needed. Membrane currents were recorded from a holding potential of −50 mV to test-pulse potentials ranging in 10-mV steps from −40 mV to +50 mV. Membrane currents were digitized using a Digidata 1322A data-acquisition system (Axon Instruments, Union City, CA, USA). All current measurements were normalized to total cell capacitance (i.e. pA/pF) to allow comparison between cells of various sizes. Slow delayed rectifier potassium (IKs) step current density was measure at the end of a 3s depolarizing voltage step from a holding potential of −50 mV as the dofetilide-resistant current. Slow delayed rectifier potassium tail current was measure as peak density of tail current elicited by repolarizing to −50 mV following 3s depolarizing voltage steps. Chromanol 293B (10 μM; Tocris) was used as a blocker of the slow delayed rectifier K+ current (IKs), and IKs effect was measured as the chromanol 293B-sensitive current in action potential recordings. Action potentials were recorded in current clamp mode with whole cell patch clamp. The pipette solution contained (mM) KCl 30, K-aspartate 110, NaCl 8, Mg-ATP 5, HEPES 5 adjusted with KOH to pH 7.2. Cells were superfused with a solution containing (mM) NaCl 140, KCl 4, MgCl2 1, CaCl2 2, HEPES 5, glucose 10, pH 7.4. Isolated rabbit LV myocytes were stimulated at a frequency of 1 Hz. The APD was measured at 90% repolarization (APD90). Experiments were performed at room temperature. We recorded IKs and APD from different cells. Experiments were performed in the absence or presence (>5 min) of ISO (500 nM; Sigma), CGP 20712A (300 nM; Sigma), ICI-118,551 (100 nM; Tocris), chromanol (10 mM; Tocris) or after pretreatment with thapsigargin (2 μM; Tocris) for 15 minutes. ISO was added for β-adrenergic stimulation. CGP 20712A was used as a selective β 1-AR antagonist for ISO-mediated effects. ICI-118,551 was used as a selective β 2-AR antagonist. In selected studies, myocytes were pretreated with thapsigargin (2 μM; Tocris) to deplete SR Ca2+.
ECG studies
ECG recordings were obtained from conscious control male and female rabbits in lead III at baseline and following a 90 second administration of ISO (1.0 μg/kg/min). QT intervals were measured from QRS onset to the end of the T wave by custom-built analysis software. Corrected QT (QTC) intervals were calculated using Carlsson’s formula (QTC=QT-0.175(RR-300))(24).
Statistics
Data are reported as means ± SEM. Data analyses were performed using Clampfit 9.2 (Axon Instruments, Union City, CA), Origin 7 (Originlab, Northampton, MA), and SAS 9.2 (SAS Institute, Cary, NC). Comparisons between the changes in APD and ISO values obtained on the same group of rabbit myocytes were done using the paired t-test. Comparisons between values on two individual groups of rabbit myocytes were done using the two-group (unpaired) t-test. One-way analysis of variance (ANOVA) was used for measurements obtained from multiple groups. Analysis of covariance (ANCOVA) was used to compare APD at baseline ± ISO for myocytes from males and females treated with thapsigargin) vs untreated controls. Terms included in the ANCOVA model were sex (male or female), thapsigargin (presence or absence of), and the interaction between sex and thapsigargin. The Tukey-Kramer multiple comparisons test was then used to determine specific pairwise differences between the means. All statistical tests were two-sided and were performed using a significance level of 5% (i.e. alpha=0.05).
Results
Sex differences in action potential characteristics
Rabbit LV myocyte AP’s were continuously elicited at a stimulation rate of 1 Hz. Representative AP recordings of control female and male hearts are shown in Figs. 1A & 1B. The AP duration at 90% repolarization (APD90) was 11% longer in female vs male myocytes (672.7±3.7 vs 604.9±3.6 ms, n=26 (24 hearts), 29 (24 hearts), p<0.01 for female vs male). Resting membrane potential (RMP) was not different (−77.0±0.4 mV for female vs −77.3±0.3 mV for male; n=26 (24 hearts), 29 (24 hearts), p=NS).
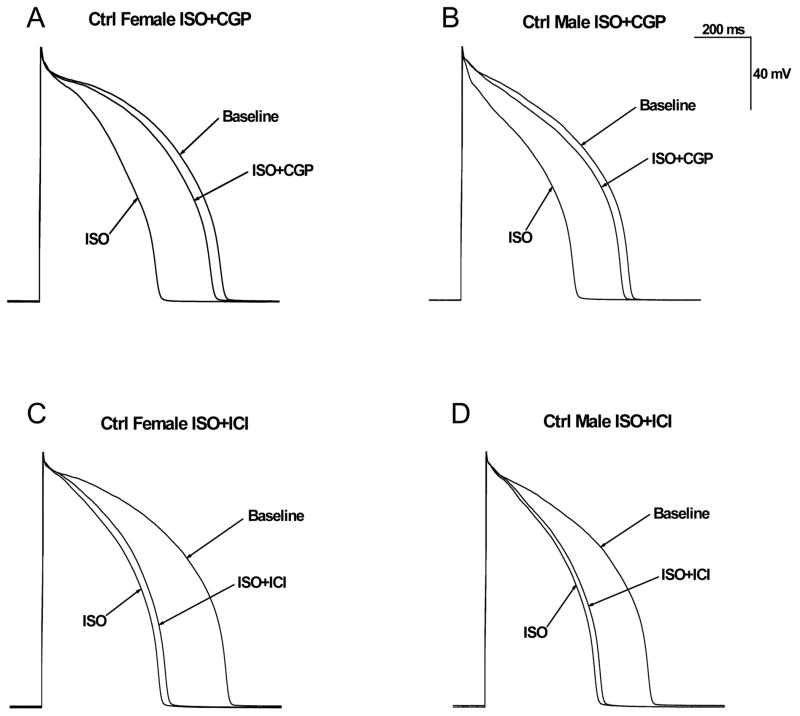
Figure 1. Effect of β1-adrenergic and β2-adrenergic receptor blocker on isoproterenol-induced action potential duration shortening in control male and female rabbits.
A–B, Representative action potential recordings from female (A) and male (B) rabbit LV myocytes at a stimulation rate of 1 Hz at baseline, after the administration of 500nM isoproterenol (ISO) alone, or 500nM ISO plus 300nM β1-adrenergic receptor blocker CGP 20712A (ISO+CGP). C–D, Representative AP recordings from LV myocytes from female (C) and male (D) rabbits at baseline, after 500nM ISO, or 500nM ISO + 100nM ICI-118,551 (β2-adrenergic receptor blocker).
Role of β1- and β2-adrenergic receptor stimulation in isoproterenol-induced APD shortening in control male and female rabbits
Because some cardiac channels are modulated by stimulation of β-adrenergic receptors, we examined ISO-induced APD shortening in control female and male rabbits. ISO (500 nM) shortened APD90 in rabbit myocytes paced at cycle length of 1000 ms comparably (p=NS) in females (by 36%; from 664.4±7.9 to 426.5±10.9 ms; n=6 (5 hearts)) and in males (by 32%; from 609.5±10.0 to 412.9±6.9 ms; n=6 (5 hearts)) comparable (p=NS). We also examined the effect of the selective β1-adrenergic receptor blocker CGP 20712A (CGP) on APD90 in the presence of 500 nM ISO. As shown in Table 1, the ISO-induced APD shortening was comparably (p=NS) prevented by CGP in females (by 84.3±4.8% from 426.5±10.9 to 627.1±6.0 ms) and in males (by 87.9±0.8%, from 412.9±6.9 to 585.7±9.1 ms). Thus β1-adrenergic receptor plays a critical role in ISO-induced APD shortening in rabbit LV myocytes.
Table 1.
Comparison of APD90, isoproterenol-induced APD90 shortening, and attenuation by CGP 20712A and ICI-118,551 in control male and female rabbit LV myocytes
| Baseline | ISO | ISO+CGP | ISO+ICI | Recovery (%) | n | |
|---|---|---|---|---|---|---|
| Male | 609.5±10.0# | 412.9±6.9‡ | 585.7±9.1 | 87.9±0.8 | 6 (5 hearts) | |
| Female | 664.4±7.9#† | 426.5±10.9‡ | 627.1±6.0 | 84.3±4.8 | 6 (5 hearts) | |
| Male | 598.5±6.4#† | 408.3±7.6 | 422.4±5.5 | 7.4±2.0 | 7 (6 hearts) | |
| Female | 679.9±7.3#† | 431.7±6.5‡ | 455.3±5.4 | 9.4±0.7 | 7 (7 hearts) |
P<0.05 for Baseline vs isoproterenol (ISO).
P<0.05 for Baseline vs ISO+CGP
P<0.05 for ISO vs ISO+CGP/ICI
To investigate the role of β2-adrenergic stimulation in ISO-induced APD shortening, we examined the effect of the β2-adrenergic receptor blocker ICI-118,551. Typical recordings are shown in Figs. 1C & 1D. ISO-induced shortening of APD was comparably (p=NS) attenuated by 9.4±0.7% in control female and 7.4±2.0% in control male (Table 1). Thus the effects of ISO-induced APD shortening in LV myocytes from control female and male rabbits were mediated primarily by β1-adrenergic stimulation. Data are summarized in Table 1.
Effects of chromanol 293B on ventricular action potentials in control male and female rabbits
We investigated the effects of the IKs blocker chromanol 293B on ventricular action potentials from female and male rabbit hearts. Consistent with the previous results, baseline APD90 was significantly longer in control females compared with control males. Representative AP’s in the presence and absence of 10 μM chromanol 293B in female and male rabbit LV myocytes at a cycle length of 1000 ms are shown in Figs. 2A & 2B. Inhibition of IKs with 10 μM chromanol 293B resulted in a minimal 3.0% increase in APD90 in females (from 675.5±8.3 to 695.6±10.4 ms, n=6 (6 hearts)), but in a modest (8.9%) APD90 prolongation in males (from 604.7±7.7 to 658.4±6.6 ms, n=8 (6 hearts), p<0.05 vs females). The effects of chromanol 293B on APD90 are summarized in Table 2.
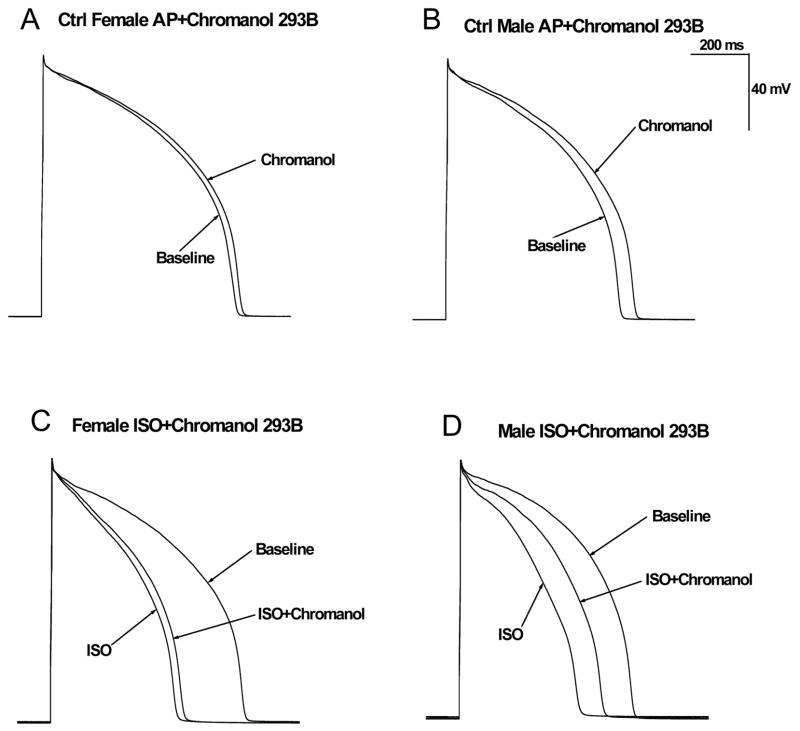
Figure 2. Effects of chromanol 293B on ventricular action potentials in the absence and presence of β-adrenergic stimulation in control male and female rabbits.
A–B, Shown are representative AP’s recorded in LV myocytes from a control female (A) and a control male rabbit (B) with and without 10 μM chromanol 293B (IKs blocker) at a cycle length of 1000 ms. C–D, Representative AP’s recorded in myocytes from a control female (C) and a control male (D) rabbit at baseline, in the presence of 500nM ISO, and after 500nM ISO + 10 μM chromanol 293B (all at a cycle length of 1000 ms).
Table 2.
Effects of Chromanol 293B on APD90 in the absence or presence of ISO in control male and female rabbit LV myocytes
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Chromanol | n | Baseline | Chromanol | n | |||
| −ISO | 604.7±7.7* | 658.4±6.6 | 8 (6 hearts) | 675.5±8.3* | 695.6±10.4 | 6 (6 hearts) | ||
| Baseline | ISO | ISO+Chromanol | n | Baseline | ISO | ISO+Chromanol | n | |
| +ISO | 607.3±5.7#† | 416.5±5.6‡ | 503.4±7.9 | 8 (7 hearts) | 670.1±6.2#† | 435.5±5.6‡ | 459.1±8.2 | 7 (6 hearts) |
P<0.05 for Baseline vs Chromanol
P<0.05 for Baseline vs ISO
P<0.05 for Baseline vs ISO+ Chromanol
P<0.05 for ISO vs ISO+ Chromanol
Electrophysiological effects of IKs blocker in the presence of β-adrenergic stimulation
Because IKs is modulated by sympathetic tone, we further examined the effect of chromanol 293B on ISO-induced APD shortening. Stimulation of β-adrenergic receptors by ISO shortened APD90 in rabbit myocytes paced at cycle length of 1000 ms comparably (p=NS) in females (by 35%; 670.1±6.2 to 435.5±5.6 ms; n=7 (6 hearts)) and in males (by 31% from 607.3±5.7 to 416.5±5.6 ms; n=8 (7 hearts)). Addition of chromanol 293B (10 μM) in the continuous presence of 500 nM ISO produced little (5.4%) APD prolongation (from 435.5±5.6 to 459.1±8.2 ms) in females, but more marked pronounced (20.9%) prolongation of APD in males (from 416.5±5.6 to 503.4±7.9 ms, p<0.05 vs females). Representative AP recordings at baseline, with 500 nM ISO, and with 500 nM ISO in the presence of 10μM chromanol 293B are shown in Figs. 2C & 2D. Summary data for the effects of ISO and chromanol 293B on the change in APD90 are in Table 2.
Effect of SR Ca2+ on ventricular action potentials in the presence of β-adrenergic stimulation
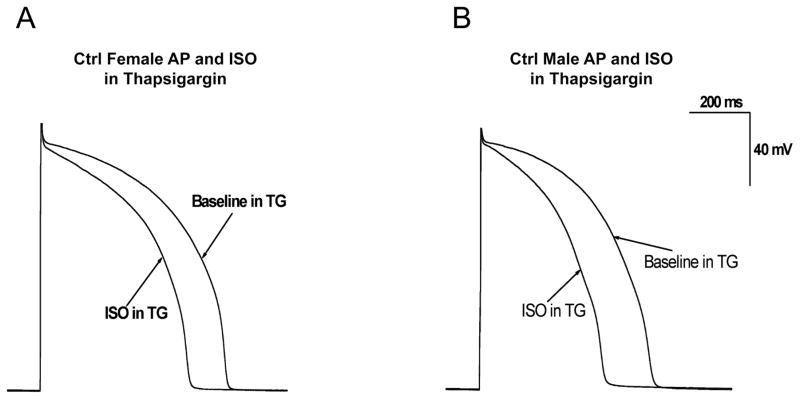
To assess the effects of Ca2+ handling on APD and its modulation by β-adrenergic stimulation, experiments were conducted after pre-incubation with 2 μM thapsigargin to deplete SR stores so that no released SR Ca2+ would be available to influence APD (4). Representative recordings of the effects of ISO in control female and male rabbit myocytes pretreated with thapsigargin are shown in Fig. 3. In thapsigargin-pretreated myocytes paced at cycle length of 1000 ms, ISO shortened APD90 by 20.7±1.8% in females (from 658.1±2.5 to 522.2±13.3 ms; n=7 (7 hearts)) and by 26.4±1.5% in males (from 592.6±3.1 to 435.8±7.8 ms; n=7 (6 hearts)). Overall, thapsigargin block of SR Ca release resulted in greater attenuation of ISO-induced APD shortening in female than in male LV myocytes (p<0.01 by analysis of covariance). Since chromanol 293B had minimal effects on ISO-induced APD shortening in females, these collective results suggest that Ca-sensitive channels other than IKs play a greater role in APD shortening by ISO in myocytes from females.
Figure 3. Effects of isoproterenol in control male and female rabbit myocytes pretreated with thapsigargin.
A–B, Representative AP recordings in absence and presence of 500nM ISO from LV myocytes from control females (A) and males (B) that were pretreated with SR Ca-ATPase inhibitor thapsigargin (2 μM) for 15 min.
β-adrenergic stimulation regulates IKs in control male and female rabbits
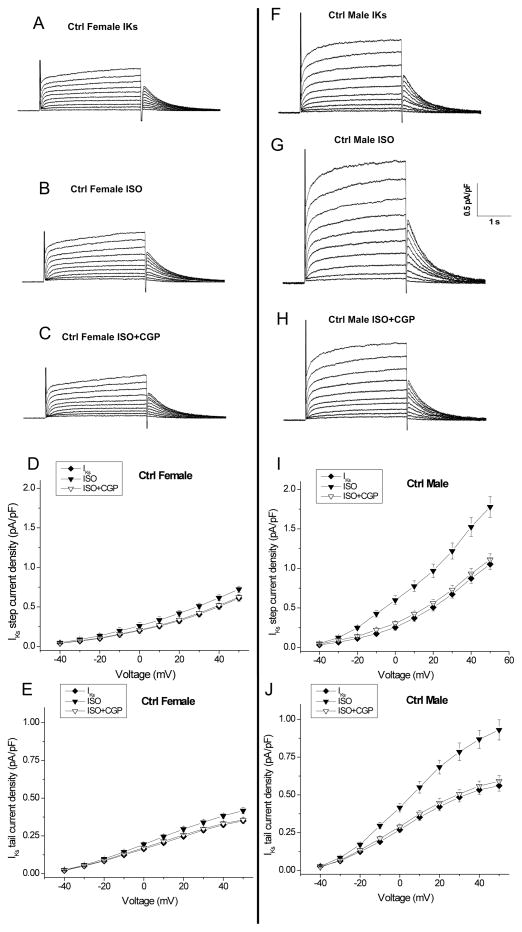
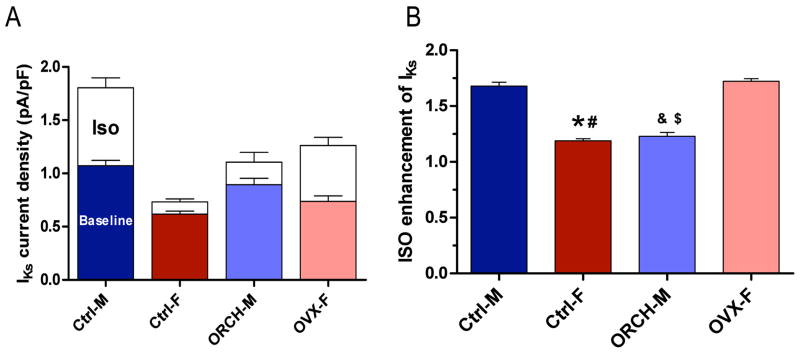
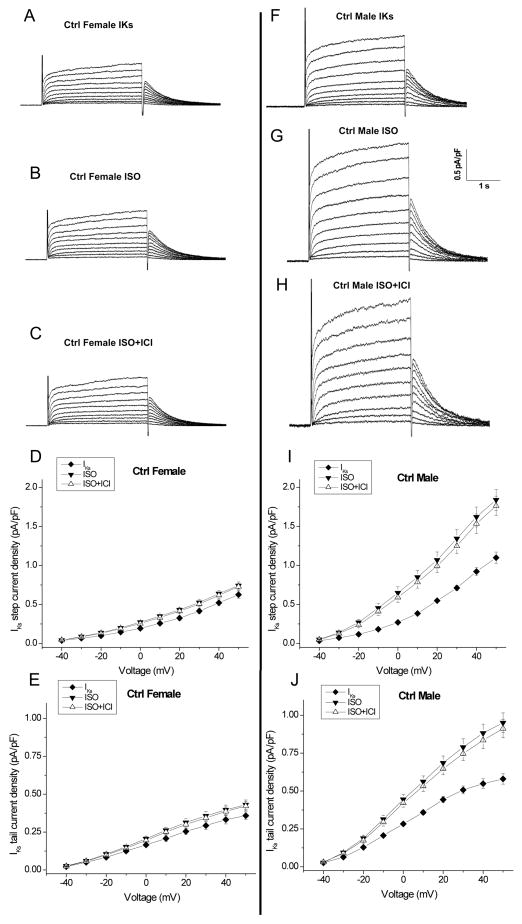
We studied slow delayed rectifier potassium current (IKs) in control female and male rabbits. Fig. 4 shows representative IKs tracings at baseline (A, F), in the presence of ISO (B, G), and after subsequent addition of CGP (C, H). IKs in females was 42% less (p<0.01) than in males (at +50mV, step: 0.61±0.04 vs. 1.05±0.07 pA/pF, p<0.01, n=5 (5 hearts), 9 (9 hearts), p<0.01 for females vs males, Figs. 4A & 4F, and Fig. 5). Because sympathetic tone enhances IKs, we examined IKs in response to β-adrenergic receptor stimulation with ISO (500 nM) in female and male myocytes (Figs. 4 & 5). In females ISO increased IKs by only 19% (at +50mV, Step: 0.61±0.04 to 0.72±0.04 pA/pF) while in males ISO enhanced IKs by 68% (1.05±0.07 to 1.78±0.13 pA/pF, p<0.01 vs females; Fig. 4G & Fig. 5).
Figure 4. β1-adrenergic stimulation regulates slow delayed-rectifier potassium current in control male and female rabbits.
A–C, Representative IKs traces from control female rabbit myocytes are shown at baseline (A), in the presence of 500nM ISO (B), and after subsequent addition of 300nM CGP 20712A (β1-adrenergic receptor blocker) (C) at test potentials ranging from −40 and +50 mV. D & E, Summarized data for step and tail current-voltage (I-V) relationship for IKs in control females (n=5 (5 hearts)). Currents are normalized to cell capacitance. Density of peak currents (mean ± SEM) were plotted vs test potentials. F–H, Representative control male IKs traces are shown at baseline, in the presence of 500nM ISO, and after subsequent addition of 300nM CGP 20712A. I–J, Summarized step and tail current-voltage (I-V) relationship for IKs at baseline and in the presence of 500nM ISO ± 300nM CGP 20712A in control male myocytes (n=9 (9 hearts)).
Figure 5. IKs enhancement with ISO.
A, Summarized data for IKs step current at +50 mV and its response to 500nM ISO in myocytes from control male and female, orchiectomized male (ORCH-M) and ovariectomized female (OVX-F) rabbits. B, Ratio of IKs current (+50 mV test pulse) post- vs. pre- 500nM ISO application in control males, control females, ORCH males and OVX females (, p<0.05 vs Ctrl-M; #, p<0.05 vs OVX-F;&, p<0.05 vs Ctrl-M; $, p<0.05 vs OVX-F).
We evaluated the β-adrenergic receptor subtypes involved in this ISO-induced increase in IKs. The β1-receptor blocker CGP 20712A comparably prevented ISO enhancement of IKs by 90.2±1.3% in females and 91.8±0.8% in males (Fig. 4). We also assessed the contribution of β2-adrenergic receptor stimulation in the IKs response to ISO. Fig. 6 shows representative traces IKs at baseline (A, F), in the presence of ISO (B, G), after subsequent addition of ICI-118,551 (ICI, 100 nM, a selective β2-AR antagonist) (C, H), and the results of IKs current-voltage relationship in females and males. The ISO-induced increase in IKs was comparably prevented by ICI by an average of 11.2±1.2% (n=5 (5 hearts)) in females and 10.0±0.7% (n=7 (7 hearts)) in males (Fig. 6), suggesting that while β1-adrenergic stimulation is the primary modulator of IKs, β2-adrenergic stimulation does contribute, at least in part, to IKs responsiveness to ISO.
Figure 6. β2-adrenergic stimulation enhances slow delayed-rectifier potassium current.
A–C, Representative IKs traces from control females are shown at baseline and in presence of 500nM ISO ± 100nM ICI-118,551 (β2-adrenergic receptor blocker). D–E, Summarized step and tail current-voltage (I-V) relationship for IKs in control females. F–H, Representative IKs traces from control males are shown at baseline and in presence of 500nM ISO ± 100nM ICI-118,551. I–J, Summarized step and tail current-voltage (I-V) relationship for IKs are shown at baseline and in presence of 500nM ISO ± 100nM ICI-118,551 for control males (n=7 (7 hearts)).
Sex differences in IKs in response to isoproterenol
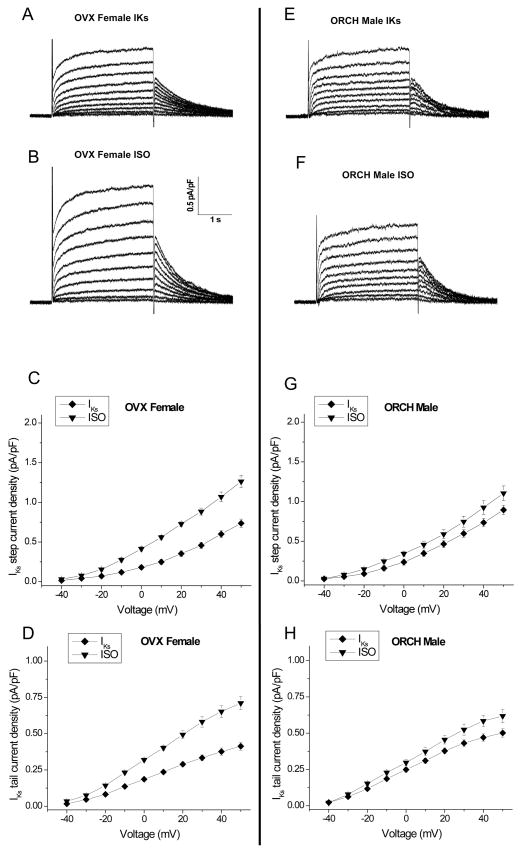
Our results above showed that IKs was enhanced in the presence of the β-adrenergic receptor agonist ISO, most notably in control male rabbits, suggesting a role for sex hormones. We did hormonal manipulation by ovariectomy (OVX) of female rabbits and orchiectomy (ORCH) of male rabbits. Ovariectomy decreased estrogen levels from 27.3±2.2 to 10.9±1.4 pg/ml (n=4,4; p<0.01). Representative current traces and current-voltage relations in OVX females and ORCH males are shown in Fig. 7. OVX females demonstrated a 21% increase in IKs (vs control females) and a much greater enhancement with ISO (at +50mV, Step: 0.74±0.05 to 1.26±0.08 pA/pF, n=7 (5 hearts), p<0.05 vs control females; Figs. 5 & 7). Fig. 5 shows the ratio of IKs in the presence of ISO to the control for a test pulse to +50 mV; OVX increased the ratio from 1.19±0.02 in control non-OVX females to 1.72±0.02 in OVX females (p<0.05). ORCH males demonstrated a 14% decrease in IKs (vs control males) and showed only slight change with ISO (at +50mV, Step: 0.90±0.06 to 1.11±0.09 pA/pF, n=7 (5 hearts), p<0.05; Figs. 5 & 7); ORCH decreased the ISO-enhanced ratio from 1.68±0.03 in control non-ORCH males to 1.23±0.04 in ORCH males, p<0.05. Thus, we found that a 72% increase in IKs step current in OVX females was comparable to the 68% increase that we observed in control males (p=NS). Likewise, the slight 23% increase in IKs step current in ORCH male was comparable to the 19% increase seen in control females (p=NS).
Figure 7. Effects of isoproterenol on slow delayed-rectifier potassium current in orchiectomized (ORCH) male and ovariectomized (OVX) female rabbits.
A–B, Representative IKs current traces in the absence (A) or presence (B) of 500nM ISO in an OVX female rabbit. C–D, Summarized step and tail current-voltage relationships from OVX females (n=7 (5 hearts)). E–F, Representative IKs current traces in absence (E) and presence (F) of 500nM ISO in LV myocytes from an ORCH male rabbit. G–H, Summarized step and tail current-voltage relationships in ORCH males.
Sex differences on QTC interval in response to isoproterenol
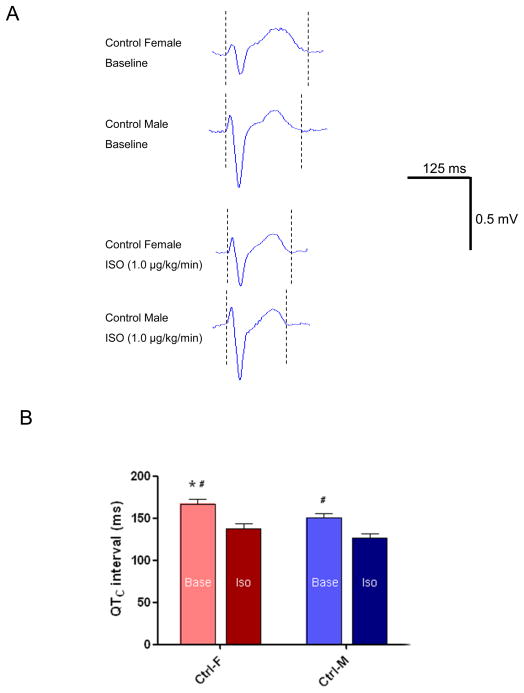
To investigate sex differences in repolarization and the response to β-AR stimulation in vivo, we performed additional in vivo ECG studies in the absence and presence of ISO infusion. Figure 8A shows representative ECG recordings from control female and male rabbits at baseline and during ISO infusion. As shown in Figure 8B, QTC at baseline were significantly longer in female vs male (167.0±5.3 vs 150.4±5.1 ms, n=5, 7, p<0.05 female vs male). ISO shortened QTC interval to a comparable degree in females and males. QTC intervals with ISO infusion (1.0 μg/kg/min) were comparably shortened by 17% to 137.7±6.3 ms (n=5) in females and by 16% to 126.9±4.4 ms (n=7) in males. These findings were similar to sex differences in action potential and ISO-induced APD shortening in control female and male rabbit LV myocytes.
Figure 8. Sex differences in QTC interval in response to isoproterenol.
A, Representative surface ECG recordings at baseline and in response to isoproterenol (ISO, 1 μg/kg/min) in control female and male rabbits. B, Summarized data for QTC intervals at baseline and in response to ISO from control females and males (, p<0.05 vs Ctrl-M; #, p<0.05 vs ISO).
Discussion
The present studies provide novel information about sex differences of IKs in response to β-AR stimulation, ISO-induced APD shortening, chromanol’s effect on APD in the absence or presence of ISO, and responsiveness of IKs to ISO in gonadectomized rabbits.
IKs and its role in repolarization
IKs modulates the repolarization phase of the AP (31, 50), especially in the setting of β-adrenergic stimulation (65). It counterbalances the depolarizing effects of increased L-type Ca current (ICa) associated with β-adrenergic stimulation, and plays an important role in cardiac repolarization reserve, especially in the setting of increased sympathetic tone (65). The important role of IKs is evident from finding that congenital LQTS is associated with mutations in genes encoding KCNQ1 and KCNE1 (32). IKs is composed of KCNQ1 and KCNE1 as part of a macromolecular complex that includes PKA, PP1 and the targeting AKAP protein, yotiao (44). Sex differences in IKs expression and activity may be due to sex differences in IKs channel subunits (KCNQ1 and KCNE1). We find decreased KCNE1 but unchanged KCNQ1 expression in female rabbit mid myocardium LV (unpublished data). Since KCNE1 coassembles with KCNQ1 (3, 35, 51, 54, 57), this could explain the reduced IKs amplitude in females. In our electrophysiological studies, we used mid-myocardium to limit the contribution of endocardium and epicardium. We also limited heterogeneity by excluding base and apex.
We performed our studies in rabbit LV myocytes in which IKs resembles that of humans (38, 50, 62). Chromanol 293B (10 μM) blocks >90% of IKs in rabbit myocytes (46). We found that blockade of IKs with 10 μM chromanol 293B caused a mild increase in APD in males and caused little increase in females. Published results of IKs blocker effects on ventricular AP’s remain controversial. In a number of whole heart and tissue studies, IKs blockers (chromanol 293B, HMR 1556 and L-735,821) did not prolong ventricular repolarization in rabbits (38, 55). On the other hand, in cellular studies chromanol 293B (10 μM) prolonged AP (42), and L-768,673 modestly increased APD of epicardial and endocardial myocytes in rabbits myocytes (69). Likely explanations for these different findings could be different experimental conditions or the low level of baseline IKs.
After β-AR stimulation (which enhances IKs), effects of IKs blockers on APD are more consistent (65). Indeed, in males we found that the IKs blocker chromanol 293B counteracted ~50% of the ISO-induced APD shortening. However, in females chromanol 293B had minimal impact on the ISO-induced APD shortening. This is totally consistent with the weaker ability of ISO to increase IKs in females (as either a percentage change (19 vs. 68%) or an absolute IKs density change (0.11 vs. 0.73 pA/pF)). Our results in males are consistent with studies by So et al, in which IKs block by HMR 1556 reversed the repolarization shortening by ISO without rate-dependence in perfused control male rabbit hearts (55). Reduced response to chromanol 293B in females may relate to lower IKs current and sex differences in β-AR responsiveness (discussed below).
β-adrenergic regulation of IKs and action potential duration
Consistent with previous results (29), we found that APD90 was significantly longer in control females compared to control males. While APD prolongation may enhance SR Ca load and to some degree balance out sex differences in (i.e. reduced) Ca handling and β-AR responsiveness (12, 31, 63), the end result may be detrimental, with an increased propensity in females for EADs and torsades des pointes when challenged with further block of K channels (e.g. drugs that inhibit IKr (including antiarrhythmics) or LQTS).
IKs is enhanced by β-AR stimulation (53). β-adrenergic regulation of IKs is mediated by a macromolecular signaling complex consisting of KCNQ1, KCNE1 and Yotiao (an AKAP) that regulates channel activity by PKA-dependent phosphorylation of Ser27 in the N-terminal KCNQ1 (44), and of Ser43 in the N-terminal of Yotiao (11, 36). KCNE1 is required for this transduction of PKA effects on KCNQ1 (35). ISO’s effects on IKs were primarily mediated by β1-AR’s, but with a small but definite contribution of β2-AR stimulation to ISO’s effect. KCNQ1 and β2-AR are localized to the intercalated discs, surface sarcolemma, and transverse tubules of isolated ventricular myocytes (17). β 2-AR stimulation can modulate IKs under conditions of increased β2-AR expression (17), and β2-AR regulation of IKs might be greater in the setting of HF where there is increased β2-AR arrhythmogenicity mediated in part by β2-AR upregulation (15). β3-AR stimulation has been shown to have effects on IKs and APD, although the results have been variable (7, 23, 33). ISO-induced β3-AR stimulation of IKs in guinea pig ventricular myocytes (in the presence of combined β1-AR and β2-AR blockade) was shown to inhibit IKs (7), although in other studies β3-AR stimulation was shown to shorten repolarization (23). Little role for β3-AR in control rabbit ventricular myocytes is likely due to the low level of β3-AR’s in control rabbit ventricular myocytes, but this might be otherwise in states such as HF where there is β3-AR upregulation (45).
Sex differences in repolarization and the role of sex hormones
Women are known to have longer QTC intervals than men. Moreover, women are at greater risk for arrhythmias associated with long QTC intervals. Repolarization-prolonging drugs induce torsades des pointes (TdP) more frequently in women than men (37, 43), and female sex is the major predictor of d-sotalol-induced mortality (49), and an independent risk factor for the incidence of syncope and sudden death in the inherited long QT syndrome. Similar to humans, female rabbits show a greater cycle length-dependent increase in QT interval than males (18, 40, 41, 60).
With regards to the role of sex hormones on repolarization, ovariectomy shortened the QT interval of isolated hearts (18), and consistent with this, 17-β-estradiol treatment in ovariectomized rabbits caused ventricular APD prolongation. QT intervals and QT prolongation with quinidine was greater in hearts from ovariectomized female rabbits treated with estradiol than those treated with testosterone (18). Protection from excess QT prolongation in males may be attributable to testosterone (46), and testosterone may be responsible for shorter QT intervals in men (46). Overall, these results suggest gonadal steroids can determine sex differences in electrophysiology at the level of control of ion channels.
We found that IKs current was substantially smaller in control female myocytes (vs their male counterparts). A number of studies have examined sex differences in repolarization and its modulation by β-AR, but there are little data on sex differences in IKs other than a study in canine Purkinje fibers that showed limited chromanol-sensitive APD shortening with ISO in females (1). Estrogen inhibits IKs in Xenopus oocytes (9) and testosterone enhances IKs in ventricular myocytes isolated from female guinea pigs (2). Testosterone-induced APD shortening is mainly caused by enhancement of IKs, although suppression of L-type Ca2+ currents (ICa,L) (2) may contribute. These data suggest that estrogen and testosterone may directly modulate IKs and ventricular repolarization.
In our experiments, sex differences in IKs and importantly also its responsiveness to ISO are contributors to sex-based cardiac repolarization differences. IKs has a significant impact on repolarization in the presence of ISO in males, but minimally so in females. Our results suggest that sex hormones (including estrogen and testosterone) play an important role in the regulations of IKs responsiveness to ISO. OVX and its associated estrogen withdrawal enhanced the ISO responsiveness of IKs in females, while ORCH and its associated testosterone withdrawal had the opposite effects in males.
Sex differences in β-AR responsiveness
We found that IKs and APD in females exhibited less β-AR responsiveness than their male counterparts. Two effects may explain the differential β-AR effects in male vs female. First, KCNE1 is required for the β-AR effects on KCNQ1 current (35). Thus, our findings of reduced IKs responsiveness to β-AR stimulation in females may be due to the lower KCNE1 expression in females. That is, KCNQ1 channels without KCNE1 fail to be activated by β-AR stimulation (35) and mutant KCNE1 reduces functional regulation of channel by PKA-dependent phosphorylation (35). Second, reduced IKs responsiveness could also be due to lower β-AR density in female cardiac myocytes, along with reduced levels of cAMP. Early observations suggested β-AR downregulation in estrogen-treated rats (22). Vizgirda et al also characterized sex differences in rat cardiac myocyte response to β-AR stimulation (63), and found lower β-AR density and reduced levels of cAMP in cardiac myocytes from female rats (63). In addition, hearts from ovariectomized rats exhibit upregulation of β1-AR compared with hearts from control female rats (12, 58).
Repolarization reserve and the role of [Ca2+]i
Our findings suggest that decreased IKs in LV myocytes of females may confer less repolarization reserve (vs males) (67). This, along with the prolonged baseline APD in females, may account for the greater propensity for females to develop torsades des pointes in response to drugs that block IKr or in the setting of long QT syndrome (37, 43). However, myocytes from females still demonstrated shortening of APD in response to ISO that was comparable to that in males. The smaller IKs, the reduced β-AR responsiveness of IKs to ISO stimulation, and the lack of chromanol-induced APD prolongation in females (but not males) suggest that factors other than IKs are likely to cause ISO-induced shortening of APD. We pre-incubated myocytes in thapsigargin for 15 min to deplete SR Ca stores and reduce the rise in [Ca2+]i during systole, as has been demonstrated by Bassani et al (4), and we found a greater attenuation of ISO-induced APD shortening vs. males. Our thapsigargin and chromanol studies suggest that, in females, ISO-induced APD shortening is due in large part to currents other than IKs, possibly secondary to ISO-induced changes in Ca or Na handling. The nature of the responsible current other than IKs in females remains to be determined. The smaller ISO-dependent enhancement of inward ICa and INCX (due to less Ca transient enhancement) in females than males (~30% vs ~90%) (14, 56, 63) would limit the APD-prolonging influence of these changes in females and partly explain the difference. However, there should also be a net increase in outward current as well for net APD shortening. IKr has been reported to be smaller in females (40), making it unlikely to contribute to ISO-induced APD shortening (unless IKr in females is more activated by ISO). IK1 is suppressed directly by ISO (34) as well as the rise in [Ca]i (14) induced by ISO, making that an unlikely explanation. ISO also stimulates outward Na/K-ATPase current in myocytes (16) and increased outward pump current is involved in reduced APD in response to increasing heart rate (26), making this a plausible contributor. Other possible explanations include Ca-activated channels such as ICl(Ca) (70) and IK(Ca) (59), but sex-differences in these channels have not been explored.
Limitations
There is heterogeneity of IKs current, not only transmurally (known differences between endocardial, epicardial and M cells) (8), but also between different parts of the heart (RV vs LV, apex vs base) (64) that could be related to differences in sympathetic innervation. We studied myocytes from the midmyocardium of the LV and have excluded the base and apex. We cannot rule out that some of the myocytes that we studied were M cells (that have been reported to reside in midmyocardium (39), but whose presence in all mammalian species is in dispute). Even if M cells exist and have intrinsically long APD’s, their contribution could easily be masked in tissue where these cells are “clamped” by adjacent cells that have shorter APD; Recent studies showing lack of delayed repolarization in the midmyocardium of intact LV (30) supports this.
While we conducted our cellular experiments at room temperature (much like other studies in the literature (21, 25, 27, 61, 66)), similar results of sex differences in APD and IKs were found at physiological temperature. Valverde et al reported that AP duration (APD90) was significantly longer in control female than in control male LV myocytes from adult rabbits at 37°C at cycle length of 5000, 1000 and 500ms (60). Moreover, the 13% difference between females and males they found are very similar to our result at room temperature where we found APD was 11.2% longer in female vs male. Our in vivo ECG studies also show that control females have significant longer QTC interval than control males and that QTC intervals were comparably shortened after ISO infusion, further supporting and validating our results.
We would assert that the mechanistic effects that we analyze at 1 Hz would be mechanistically the same at physiological pacing rate (and at 37°C), even though quantitative differences may occur. Moreover, 1 Hz may be a “physiological frequency” for room temperature in rabbit (e.g. rabbit heart rate drops from around 240 beats/min at 38°C to 64 beats/min (close to 1 Hz) at room temperature (5) and that is similar to the ~70% drop in heart rate in humans going from 37°C to room temperature (20, 28)). Since all processes are temperature-sensitive with Q10 in the 2–3 range this also makes sense. Indeed, for Ca transporters all are slower at room temperature vs. 35°C, but their relative contributions are unaltered (6).
Implications
Our findings of sex differences in IKs activity and response to β-AR stimulation, and the different mechanisms for repolarization reserve, suggest that IKs is more functionally prominent in male vs. female (both at baseline and upon β-AR stimulation). Enhancement of IKs (by direct channel effects or effects on interacting proteins that are part of the IKs macromolecular complex) may be a preferentially effective approach to prevent drug-induced QT prolongation in females. With regard to prevention of ventricular arrhythmias due to reentry, IKs blockade and possibly β-AR blockade may be more effective antiarrhythmic strategies in males than females. Indeed, our findings support the potential and feasibility of sex-specific antiarrhythmic therapy.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute Grants R01HL073966 and R01HL046929 (to SMP) and partially supported by 5UL1 RR025777-03 from the NIH National Center for Research Resources.
Footnotes
Disclosures
None
References
- 1.Abi-Gerges N, Small BG, Lawrence CL, Hammond TG, Valentin J-P, Pollard CE. Gender differences in the slow delayed (IKs) but not in inward (IK1) rectifier K+ currents of canine Purkinje fibre cardiac action potential: key roles for IKs, beta-adrenoceptor stimulation, pacing rate and gender. British journal of pharmacology. 2006;147:653–660. doi: 10.1038/sj.bjp.0706491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai CX, Kurokawa J, Tamagawa M, Nakaya H, Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112:1701–1710. doi: 10.1161/CIRCULATIONAHA.104.523217. [DOI] [PubMed] [Google Scholar]
- 3.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 4.Bassani RA, Bers DM. Rate of diastolic Ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys J. 1995;68:2015–2022. doi: 10.1016/S0006-3495(95)80378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beran AV, Proctor KG, Sperling DR. Hypothermia and rewarming induced by surface and He-O2 inhalate temperature control. J Appl Physiol. 1975;39:337–340. doi: 10.1152/jappl.1975.39.2.337. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers; 2001. [Google Scholar]
- 7.Bosch RF, Schneck AC, Kiehn J, Zhang W, Hambrock A, Eigenberger BW, Rub N, Gogel J, Mewis C, Seipel L, Kuhlkamp V. beta3-Adrenergic regulation of an ion channel in the heart-inhibition of the slow delayed rectifier potassium current I(Ks) in guinea pig ventricular myocytes. Cardiovascular research. 2002;56:393–403. doi: 10.1016/s0008-6363(02)00601-6. [DOI] [PubMed] [Google Scholar]
- 8.Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovascular research. 1998;40:322–331. doi: 10.1016/s0008-6363(98)00133-3. [DOI] [PubMed] [Google Scholar]
- 9.Busch AE, Busch GL, Ford E, Suessbrich H, Lang HJ, Greger R, Kunzelmann K, Attali B, Stuhmer W. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. British journal of pharmacology. 1997;122:187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Petranka J, Yamamura K, London RE, Steenbergen C, Murphy E. Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol. Am J Physiol Heart Circ Physiol. 2003;285:H2657–2662. doi: 10.1152/ajpheart.00557.2003. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem. 2005;280:31347–31352. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 12.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium-handling proteins, beta-adrenergic receptors, and function in rat heart. Life Sci. 2006;79:1257–1267. doi: 10.1016/j.lfs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Conrath CE, Opthof T. Ventricular repolarization: an overview of (patho)physiology, sympathetic effects and genetic aspects. Prog Biophys Mol Biol. 2006;92:269–307. doi: 10.1016/j.pbiomolbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Curl CL, Wendt IR, Kotsanas G. Effects of gender on intracellular. Pflugers Arch. 2001;441:709–716. doi: 10.1007/s004240000473. [DOI] [PubMed] [Google Scholar]
- 15.Desantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM, Pogwizd SM. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circulation research. 2008;102:1389–1397. doi: 10.1161/CIRCRESAHA.107.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia L-G, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circulation research. 2005;97:252–259. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 17.Dilly KW, Kurokawa J, Terrenoire C, Reiken S, Lederer WJ, Marks AR, Kass RS. Overexpression of beta2-adrenergic receptors cAMP-dependent protein kinase phosphorylates and modulates slow delayed rectifier potassium channels expressed in murine heart: evidence for receptor/channel co-localization. J Biol Chem. 2004;279:40778–40787. doi: 10.1074/jbc.M406010200. [DOI] [PubMed] [Google Scholar]
- 18.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 19.Drici MD, Clement N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf. 2001;24:575–585. doi: 10.2165/00002018-200124080-00002. [DOI] [PubMed] [Google Scholar]
- 20.Emslie-Smith D, Sladden GE, Stirling GR. The significance of changes in the electrocardiogram in hypothermia. Br Heart J. 1959;21:343–351. doi: 10.1136/hrt.21.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedida D, Braun AP, Giles WR. Alpha 1-adrenoceptors reduce background K+ current in rabbit ventricular myocytes. J Physiol. 1991;441:673–684. doi: 10.1113/jphysiol.1991.sp018772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fregly MJ, Thrasher TN. Response of heart rate to acute administration of isoproterenol in rats treated chronically with norethynodrel, ethinyl estradiol, and both combined. Endocrinology. 1977;100:148–154. doi: 10.1210/endo-100-1-148. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. Functional beta3-adrenoceptor in the human heart. J Clin Invest. 1996;98:556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gbadebo TD, Trimble RW, Khoo MS, Temple J, Roden DM, Anderson ME. Calmodulin inhibitor W-7 unmasks a novel electrocardiographic parameter that predicts initiation of torsade de pointes. Circulation. 2002;105:770–774. doi: 10.1161/hc0602.103724. [DOI] [PubMed] [Google Scholar]
- 25.Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. Journal of molecular and cellular cardiology. 48:112–121. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J, Massaeli H, Xu J, Jia Z, Wigle JT, Mesaeli N, Zhang S. Extracellular K+ concentration controls cell surface density of IKr in rabbit hearts and of the HERG channel in human cell lines. J Clin Invest. 2009;119:2745–2757. doi: 10.1172/JCI39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicks CE, McCord MC, Blount SG., Jr Electrocardiographic changes during hypothermia and circulatory occlusion. Circulation. 1956;13:21–28. doi: 10.1161/01.cir.13.1.21. [DOI] [PubMed] [Google Scholar]
- 29.James AF, Choisy SCM, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Progress in biophysics and molecular biology. 2007;94:265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Janse MJ, Coronel R, Opthof T. Counterpoint: M cells do not have a functional role in the ventricular myocardium of the intact heart. Heart Rhythm. 8:934–937. doi: 10.1016/j.hrthm.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 31.Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG, Varro A. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 32.Kass RS. Genetically induced reduction in small currents has major impact. Circulation. 1997;96:1720–1721. [PubMed] [Google Scholar]
- 33.Kathofer S, Zhang W, Karle C, Thomas D, Schoels W, Kiehn J. Functional coupling of human beta 3-adrenoreceptors to the KvLQT1/MinK potassium channel. The Journal of biological chemistry. 2000;275:26743–26747. doi: 10.1074/jbc.M003331200. [DOI] [PubMed] [Google Scholar]
- 34.Koumi S, Backer CL, Arentzen CE, Sato R. beta-Adrenergic modulation of the inwardly rectifying potassium channel in isolated human ventricular myocytes. Alteration in channel response to beta-adrenergic stimulation in failing human hearts. J Clin Invest. 1995;96:2870–2881. doi: 10.1172/JCI118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurokawa J, Chen L, Kass RS. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc Natl Acad Sci U S A. 2003;100:2122–2127. doi: 10.1073/pnas.0434935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc Natl Acad Sci U S A. 2004;101:16374–16378. doi: 10.1073/pnas.0405583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann MH, Hardy S, Archibald D, quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d, l-sotalol. Circulation. 1996;94:2535–2541. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 38.Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (I(Ks)) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circulation research. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 40.Liu XK, Katchman A, Drici MD, Ebert SN, Ducic I, Morad M, Woosley RL. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. The Journal of pharmacology and experimental therapeutics. 1998;285:672–679. [PubMed] [Google Scholar]
- 41.Liu XK, Katchman A, Whitfield BH, Wan G, Janowski EM, Woosley RL, Ebert SN. In vivo androgen treatment shortens the QT interval and increases the densities of inward and delayed rectifier potassium currents in orchiectomized male rabbits. Cardiovasc Res. 2003;57:28–36. doi: 10.1016/s0008-6363(02)00673-9. [DOI] [PubMed] [Google Scholar]
- 42.Lu Z, Kamiya K, Opthof T, Yasui K, Kodama I. Density and kinetics of I(Kr) and I(Ks) in guinea pig and rabbit ventricular myocytes explain different efficacy of I(Ks) blockade at high heart rate in guinea pig and rabbit: implications for arrhythmogenesis in humans. Circulation. 2001;104:951–956. doi: 10.1161/hc3401.093151. [DOI] [PubMed] [Google Scholar]
- 43.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 44.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 45.Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 46.Pham TV, Sosunov EA, Gainullin RZ, Danilo P, Jr, Rosen MR. Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by I(K)-blocking drugs. Circulation. 2001;103:2207–2212. doi: 10.1161/01.cir.103.17.2207. [DOI] [PubMed] [Google Scholar]
- 47.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 48.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 49.Pratt CM, Camm AJ, Cooper W, Friedman PL, MacNeil DJ, Moulton KM, Pitt B, Schwartz PJ, Veltri EP, Waldo AL. Mortality in the Survival With ORal D-sotalol (SWORD) trial: why did patients die? Am J Cardiol. 1998;81:869–876. doi: 10.1016/s0002-9149(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 50.Salata JJ, Jurkiewicz NK, Jow B, Folander K, Guinosso PJ, Jr, Raynor B, Swanson R, Fermini B. IK of rabbit ventricle is composed of two currents: evidence for IKs. Am J Physiol. 1996;271:H2477–2489. doi: 10.1152/ajpheart.1996.271.6.H2477. [DOI] [PubMed] [Google Scholar]
- 51.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 52.Sanguinetti MC, Jurkiewicz NK. Role of external Ca2+ and K+ in gating of cardiac delayed rectifier K+ currents. Pflugers Archiv : European journal of physiology. 1992;420:180–186. doi: 10.1007/BF00374988. [DOI] [PubMed] [Google Scholar]
- 53.Sanguinetti MC, Jurkiewicz NK, Scott A, Siegl PK. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circulation research. 1991;68:77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- 54.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.So PP, Backx PH, Hu XD, Dorian P. I(Ks) block by HMR 1556 lowers ventricular defibrillation threshold and reverses the repolarization shortening by isoproterenol without rate-dependence in rabbits. J Cardiovasc Electrophysiol. 2007;18:750–756. doi: 10.1111/j.1540-8167.2007.00812.x. [DOI] [PubMed] [Google Scholar]
- 56.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circulation research. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 57.Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res. 2005;96:e25–34. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- 58.Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of beta 1-adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813–1824. doi: 10.1016/s0024-3205(02)02473-6. [DOI] [PubMed] [Google Scholar]
- 59.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA, Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. American journal of physiology Heart and circulatory physiology. 2005;289:H2714–2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 60.Valverde ER, Biagetti MO, Bertran GR, Arini PD, Bidoggia H, Quinteiro RA. Developmental changes of cardiac repolarization in rabbits: implications for the role of sex hormones. Cardiovasc Res. 2003;57:625–631. doi: 10.1016/s0008-6363(02)00791-5. [DOI] [PubMed] [Google Scholar]
- 61.Varro A, Lathrop DA, Hester SB, Nanasi PP, Papp JG. Ionic currents and action potentials in rabbit, rat, and guinea pig ventricular myocytes. Basic Res Cardiol. 1993;88:93–102. doi: 10.1007/BF00798257. [DOI] [PubMed] [Google Scholar]
- 62.Virag L, Iost N, Opincariu M, Szolnoky J, Szecsi J, Bogats G, Szenohradszky P, Varro A, Papp JG. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovascular research. 2001;49:790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 63.Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW. Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2002;282:H256–263. doi: 10.1152/ajpheart.2002.282.1.H256. [DOI] [PubMed] [Google Scholar]
- 64.Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99:206–210. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- 65.Volders PGA, Stengl M, van Opstal JM, Gerlach U, Spatjens RLHMG, Beekman JDM, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- 66.Whalley DW, Wendt DJ, Starmer CF, Rudy Y, Grant AO. Voltage-independent effects of extracellular K+ on the Na+ current and phase 0 of the action potential in isolated cardiac myocytes. Circ Res. 1994;75:491–502. doi: 10.1161/01.res.75.3.491. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y, Anderson ME. Reduced repolarization reserve in ventricular myocytes from female mice. Cardiovascular research. 2002;53:763–769. doi: 10.1016/s0008-6363(01)00387-x. [DOI] [PubMed] [Google Scholar]
- 68.Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, Cheng H. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci. 2004;25:358–365. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Xu X, Rials SJ, Wu Y, Salata JJ, Liu T, Bharucha DB, Marinchak RA, Kowey PR. Left ventricular hypertrophy decreases slowly but not rapidly activating delayed rectifier potassium currents of epicardial and endocardial myocytes in rabbits. Circulation. 2001;103:1585–1590. doi: 10.1161/01.cir.103.11.1585. [DOI] [PubMed] [Google Scholar]
- 70.Zygmunt AC, Gibbons WR. Calcium-activated chloride current in rabbit ventricular myocytes. Circulation research. 1991;68:424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]