Abstract
Vascular endothelial growth factors (VEGFs) have been shown to participate in atherosclerosis, arteriogenesis, cerebral edema, neuroprotection, neurogenesis, angiogenesis, postischemic brain and vessel repair, and the effects of transplanted stem cells in experimental stroke. Most of these actions involve VEGF-A and the VEGFR-2 receptor, but VEGF-B, placental growth factor, and VEGFR-1 have been implicated in some cases as well. VEGF signaling pathways represent important potential targets for the acute and chronic treatment of stroke.
Keywords: Vascular endothelial growth factor, Stroke, Ischemia, Neuroprotection, Neurogenesis, Angiogenesis
Introduction
Vascular endothelial growth factors (VEGFs, including VEGF-A, VEGF-B, and placental growth factor, or PlGF) have important roles in the development and function of the circulatory and nervous systems, so it should not be surprising to find them involved in stroke, which occurs at the interface of these systems. VEGFs have been implicated in all phases of vascular, including neurovascular, development: vasculogenesis, or the de novo production of blood vessels from mesenchymal precursor cells [1]; angiogenesis, or the hypoxia-driven sprouting of new capillaries from existing vessels [2]; and arteriogenesis, or the enlargement of anastomotic arteriolar channels in response to blood-pressure gradients [3]. In addition, VEGFs exert direct trophic and protective effects on neurons [4], so that both their vascular and neuronal actions are relevant to stroke.
This review will consider the induction of VEGFs by cerebral ischemia and their role in atherosclerosis, collateral cerebral circulation, cerebral edema, neuroprotection, neurogenesis, cerebral angiogenesis, postischemic brain repair, postischemic vascular repair, and stroke therapeutics.
Atherosclerosis
Stroke results from focal cerebral ischemia or, less commonly, hemorrhage. The causes of focal cerebral ischemia include thrombosis of large or small blood vessels, usually arteries, and artery-to-artery or cardiogenic embolus. Among these processes, VEGF-A has been implicated most clearly in arterial thrombosis due to atherosclerosis (Fig. 1).
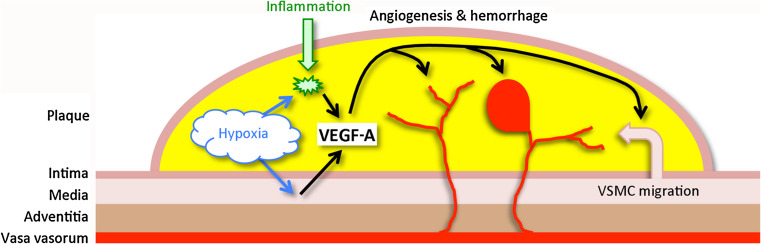
Fig. 1.
VEGF-A and atherosclerosis. Hypoxia and inflammation in the atherosclerotic plaque trigger VEGF-A expression in vascular smooth muscle cells (VSMC) and macrophages. VEGF-A, in turn, acts on vasa vasorum to promote angiogenesis, which may be associated with hemorrhage, and promotes migration of VSMC from the tunica media of the vessel wall into the plaque
Atherosclerosis is a complex inflammatory and degenerative disorder that affects primarily large and medium-sized arteries, especially at branch points. Atherosclerotic plaques cause clinical ischemic syndromes such a stroke when they rupture, releasing thrombogenic and embolic material, or when they directly occlude an artery. Plaques are subject to at least two processes associated with enhanced expression of VEGF-A—hypoxia and inflammation [5]. These increase levels of hypoxia-inducible factor-1 and other transcription factors associated with VEGF-A expression in plaque macrophages and vascular smooth muscle cells. VEGF-A acts on vasa vasorum (“vessels of the vessels”) of atherosclerotic arteries to promote angiogenesis, which in some cases leads to intraplaque hemorrhage and plaque rupture, although whether hemorrhage causes rupture is unresolved. However, increased VEGF-A expression, angiogenesis, and intraplaque hemorrhage have been observed in carotid endarterectomy specimens from symptomatic compared to asymptomatic patients [6].
VEGF-A may also contribute to atherogenesis by stimulating the migration of vascular smooth muscle cells, which has been attributed to its activation of phosphatidylinositol 3-kinase (PI3 K) and extracellular signal-related kinase (ERK) 1/2 [7]. VEGF-B has been implicated in the regulation of fatty acid uptake into endothelial cells [8], which could also influence atherogenesis.
The role of VEGFs in atherosclerosis has been tested in a variety of animal models. In low-density lipoprotein receptor-knockout mice fed an atherogenic diet, who develop hyperlipidemia and atherosclerosis, vaccination against VEGF receptor 2 (VEGFR-2/Flk-1) reduced the size and microvessel density of aortic atherosclerotic lesions [9]. In another study, administration of a plasmid vector coding for the decoy receptor, soluble VEGF receptor 1 (VEGFR-1/Flt-1), to rabbits given a high-lipid diet and subjected to balloon-catheter injury of the iliac artery decreased plaque size and neovascularity [10].
Collateral circulation
Collateral vessels constitute the first line of defense against tissue ischemia, by providing alternative pathways for arterial blood flow. Flow through preexisting collaterals is activated by blood pressure gradients between patent and occluded arteries, and is established almost instantaneously upon occlusion. Sources of collateral blood supply in the cerebral circulation include both extracranial and intracranial vessels, and the adequacy of collateral circulation helps to determine the severity of stroke and response to treatment [11].
The channels through which blood flow is redirected in response to focal hypoperfusion develop through a process termed arteriogenesis, in which arterioles are remodeled and enlarged to accommodate increased flow [3]. In rats, this occurs over days to weeks following middle cerebral artery occlusion leading to infarction [12, 13]. The molecular mechanisms underlying cerebral arteriogenesis are poorly understood, but in a study in rats, granulocyte-macrophage colony-stimulating factor potentiated enlargement of the ipsilateral posterior cerebral artery following occlusion of the middle cerebral artery (MCA) and both vertebral arteries [14].
A study of myocardial ischemia in rats showed that administration of an anti-VEGF neutralizing antibody, which had no acute effect on coronary blood flow, inhibited the growth of collateral coronary vessels [15]. This observation was extended to the cerebral circulation in a study involving VEGF-A hypermorphic, hypomorphic, and wild-type mice. Counting of pial collaterals showed a correlation with VEGF-A expression, and VEGF-A hypermorphs had smaller cerebral infarcts after MCA occlusion [16]. It seems likely, therefore, that VEGF-A helps to mediate the developmental and postischemic growth of collateral vessels in the brain, but not the acute increase in collateral blood flow triggered by cerebral ischemia.
Ischemic induction
Several studies have documented effects of cerebral ischemia on the expression of VEGFs and VEGF receptors, primarily in the ischemic border zone, or penumbra. This region, which surrounds the infarct core, remains salvageable pending reperfusion, and clinical outcome from stroke is strongly influenced by the fate of the penumbra.
VEGF-A protein expression increased in neurons, astrocytes and macrophages, and VEGFR-1 protein expression increased in endothelial cells, over days to weeks following MCA occlusion in rats [17]. VEGF-A mRNA and protein also increased within hours of MCA occlusion in rats, with subsequent rapid decline in neurons and more sustained expression in pial cells [18]. A comparison of transient and permanent MCA occlusion in rats showed elevations of VEGF-A (in neurons and endothelial cells), VEGFR-1 (in neurons, endothelial cells, and astrocytes), and VEGFR-2 (in endothelial cells and astrocytes), which were detectable at 1–3 days, and were generally more prominent in the ipsilateral hemisphere and after permanent MCA occlusion [19]. VEGF-A mRNA and protein were increased in another rat MCA occlusion study, with predominant expression in astrocytes [20], whereas others reported that microglia/macrophages were the main site of VEGF-A mRNA and protein expression [21]. VEGF-A protein levels and expression in neurons also rose within the first 24 h after MCA occlusion in a neonatal rat model of perinatal hypoxic-ischemic injury [22]. A photothrombotic ring model of stroke in rats revealed upregulation of not only VEGF-A, VEGFR-1, and VEGFR-2, but also of VEGF-C and VEGFR-3/Flt-4 proteins [23].
A different rat model—transient forebrain or “global” cerebral ischemia—which is often likened to hypoxic-ischemic encephalopathy following cardiac arrest, has also been used to assess the effects of ischemia on VEGF signaling. In this model, which affects the hippocampus most strikingly, VEGF-A mRNA was induced within hours in neurons and within days in astrocytes [24]. Other studies showed increased expression of VEGF-A mRNA and protein [25] and VEGFR-1 and VEGFR-2 protein [26] in hippocampal and cortical neurons over hours to days post-ischemia.
Cerebral edema
VEGF-A was identified originally based on two biological effects—angiogenesis [27] and vascular permeability [28]. The latter is associated with tissue edema which, in a closed compartment like the skull, can be lethal. For this reason, and because brain edema is a well-documented and often fatal complication of stroke, the ability of VEGF-A to cause leakage from cerebral blood vessels has received considerable attention. The mechanisms involved are thought to include transendothelial transport of small solutes via cytoplasmic fenestrations and plasmalemmal caveolae, as well as leakage of fluid and plasma proteins and extravasation of blood cells through interendothelial tight junctions [29].
A role for VEGFs in brain edema related to stroke was first shown in mice subjected to transient MCA occlusion and treated with a VEGFR-1 fusion protein that served as a VEGF-sequestering decoy receptor [30]. Compared to untreated mice, these animals showed reduced edema volume and infarct size. In a complementary study, intravenous administration of VEGF-A to rats 1 h (but not 48 h) after MCA occlusion increased extravasation of an intravenously administered contrast agent, as measured by magnetic resonance imaging, and caused an increase in infarct size [31]. Subsequent reports documented the ability to counteract the vascular permeability effect of VEGF-A in rodent models of stroke with an anti-VEGF-A neutralizing antibody [32] or the growth factor angiopoietin-1 [33].
Neuroprotection
Although VEGF-A was identified originally based on its effects on endothelium, it has since been recognized to act on several other cell types, including neurons [34]. Neurotrophic effects of VEGF-A have been described in a variety of peripheral [35, 36] and central [37–42] neuronal preparations. VEGF-A promotes neuronal survival in cell culture models of stroke involving oxygen and glucose deprivation [43] or excitotoxicity [44, 45], and has been implicated in some forms of hypoxic preconditioning in vitro [46]. Most of these direct neuronal effects of VEGF-A have been ascribed to activation of VEGFR-2, PI3 K, and ERK1/2. Another VEGF family member, PlGF, which interacts with VEGFR-1 but not VEGFR-2, was reported to be neuroprotective in a cell culture model of oxygen and glucose deprivation in one [47] but not another [43] study; however, in the former case, neuronal survival increased by only about 10 %.
Protection by VEGF-A has also been demonstrated in MCA occlusion models of stroke in vivo (Fig. 2). Topical application of VEGF-A to the cortical surface reduced [48], whereas intraventricular infusion of an anti-VEGF antibody increased [49] infarct volume in rats, suggesting a beneficial effect. In contrast, as noted above in connection with cerebral edema, effectively reducing VEGF levels by administration of a VEGF-sequestering VEGFR-1 fusion protein reduced infarct size in mice, although this seemed to be due to increased edema rather than a direct adverse effect on ischemic brain tissue [30]. As also noted above, one study described a temporally biphasic effect of intravenous VEGF-A on infarct volume in rats, with anatomic worsening when administered 1 h post-ischemia, but improved neurobehavioral function when given at 48 h [31]. Intraventricular administration of VEGF-A to rats beginning 24 h after MCA occlusion and continuing for 3 days, reduced infarct volume by approximately one-third at 1-month post-stroke, and also rescued sensorimotor and cognitive deficits, with behavioral improvement persisting for at least 2 months [50, 51]. Infarct volume was reduced to a similar extent and neurological deficits improved after MCA occlusion in VEGF-A-overexpressing transgenic mice compared to controls [52]. One important lesson from these studies is that the timing and route of VEGF-A administration are critical for achieving a desirable result [53].
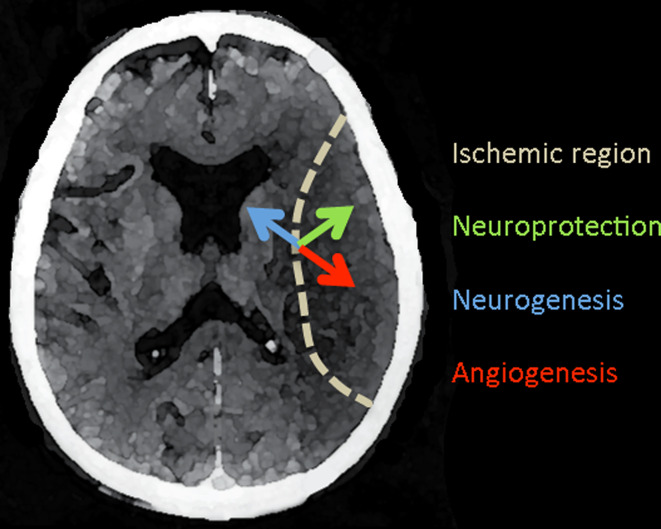
Fig. 2.
VEGF effects in acute ischemic stroke. A horizontal section of the human brain shows an acute infarct within the territory of the middle cerebral artery (dotted line). VEGF-A is induced in the ischemic border zone and acts on local neurons and endothelial cells to promote neuroprotection (green arrow) and angiogenesis (red arrow). VEGF-A also stimulates neurogenesis (blue arrow) in the subventricular zone, from which new neurons migrate to the site of ischemia. Other VEGF family members, including VEGF-B and PlGF, share some of these effects
Although cerebral ischemia is most common in adults, especially the elderly, it is also an important cause of neurological disability and death in the neonatal period. Accordingly, several studies have addressed the potential for protection by VEGF-A in neonatal rat models of hypoxic-ischemic injury. Intraventricular VEGF-A, given to 7-day-old rats after unilateral common carotid artery occlusion coupled with hypoxia, reduced macroscopic and microscopic brain injury at 24 h [54]. Intracerebral administration of a VEGF-A-expressing adenoviral vector 3 days after a similar injury produced like benefit as well as neurobehavioral improvement [55]. Conversely, histological brain damage was exacerbated in 10-day-old rats subjected to transient MCA occlusion and treated for 3 days, beginning 2 days post-ischemia, with the VEGR2 tyrosine kinase inhibitor semaxanib (SU5416) [56].
The possibility that other VEGF family members have protective effects in stroke has received limited attention. However, MCA occlusion in VEGF-B-knockout mice was associated with an increase of about 40 % in infarct size and more severe neurological dysfunction compared to findings in wild-type mice [57]. Like PlGF, VEGF-B activates VEGFR-1 but not VEGFR-2 receptors. Thus, agents acting at either (or both) of these receptor subtypes on neurons might have therapeutic value in stroke. Moreover, VEGF-B [58] and PlGF [59] appear to be much less potent inducers of vascular permeability than VEGF-A, which could be advantageous in limiting stroke-related brain edema.
Neurogenesis
Neurogenesis occurs in the adult as well as the developing mammalian brain, especially in selected regions, among which the best documented are the hippocampal dentate gyrus (DG) and the zone surrounding the lateral ventricles (subventricular zone, or SVZ). Adult neurogenesis is regulated by chemical effectors such as neurotransmitters, hormones, and growth factors; by behavior; and by pathological processes. Of interest in the present context, the former include VEGFs and the latter stroke.
Stroke-induced neurogenesis (Fig. 2) has been demonstrated in a variety of rodent models [60–64] and in humans [65, 66]. An increase in neuroproliferation above physiological baseline levels is observed in both DG and SVZ, and is accompanied by migration of newborn neurons from the latter site toward the ischemic lesion [63–68]. Stroke-induced neurogenesis appears to contribute to improved anatomic and functional outcome from stroke, because postischemic outcome is worse when neurogenesis is ablated by radiation [69], cytotoxic drugs [63, 68], or genetic manipulation [70].
VEGF-A stimulated neurogenesis in embryonic rat brain cultures, as demonstrated by an increase in the number of cells co-expressing markers of cell proliferation (bromodeoxyuridine, or BrdU, labeling) and neuronal lineage (embryonic nerve cell adhesion molecule, or ENCAM) [71]. This was blocked by the VEGR-2 tyrosine kinase inhibitor SU1498, consistent with a VEGFR-2-mediated effect. Intraventricular administration of VEGF-A to rats also enhanced incorporation of BrdU into cells expressing VEGFR-2 and the neuronal lineage marker doublecortin (Dcx) in DG and SVZ in vivo. In a subsequent study [50], intraventricular administration of VEGF-A for 3 days, starting 24 h after MCA occlusion in rats, also increased postischemic BrdU labeling in neuronal (Dcx- or NeuroD-expressing) cells in DG and SVZ. Moreover, stimulation of VEGF expression has been implicated in the neurogenesis-promoting effects of therapeutic agents, such as statins [72] and antidepressants [73]. Thus, VEGF-A enhances neurogenesis not only in normal, but also in ischemic, brain, representing another way in which VEGF-A may contribute to cerebral adaptation to stroke.
VEGF-B also appears to have a role in neurogenesis [74]. Like VEGF-A, VEGF-B also increased BrdU labeling in embryonic rat brain cortical cultures, involving cells expressing the neuroepithelial cell marker nestin. Intraventricular VEGF-B also stimulated BrdU incorporation in DG and SVZ, where BrdU co-localized with the neuronal lineage marker Dcx. In addition, BrdU labeling and Dcx expression were reduced in DG and SVZ from VEGF-B-knockout mice.
Angiogenesis
VEGF-A is a principal mediator of cerebral angiogenesis [75], which is increased following stroke (Fig. 2) in rodents [76] and humans [77, 78]. Several studies have demonstrated a temporal or spatial correlation between upregulation of VEGF-A or VEGF receptors and angiogenesis following MCA occlusion in rats [17, 79, 80]. In addition, administration of VEGF-A has been shown to enhance postischemic angiogenesis. For example, intravenous VEGF-A increased microvessel density in the cortical ischemia penumbra [31] and intraventricular VEGF increased the number of von Willebrand factor-immunoreactive endothelial cells in the ischemic caudate-putamen after MCA occlusion in rats [50]. VEGF-A also appears to mediate NO-induced angiogenesis in the postischemic brain, because the NO donor, DETANONate, increased new vessel formation in the ischemic penumbra when given 24 h after MCA occlusion in rats, and an anti-VEGFR-2 neutralizing antibody inhibited capillary-like tube formation stimulated by DETANONOate in vitro [81].
Indirect evidence suggests that PlGF might also affect stroke-related angiogenesis, because PlGF-knockout mice showed a deficit in hypoxia-induced cerebral angiogenesis compared to wild-type mice [82]. However, VEGF-A levels were also lower in PlGF-knockout mice, so VEGF-A deficiency, rather than PlGF deficiency, might explain this finding.
Postischemic brain repair
Brain repair and functional recovery following stroke depend partly on brain plasticity in non-ischemic regions, such as the peri-infarct cortex and contralateral (non-ischemic) cerebral hemisphere [83, 84]. Manifestations of plasticity-related repair include changes in gene expression [85], neuronal excitability [86], axon sprouting [87], synapse formation [87], somatotopic organization [88], and intra-cortical neuronal connectivity [89, 90]. Evidence for a role of VEGF signaling in remote plasticity (Fig. 3) includes the observations that occlusion of cortical vessels supplying the primary motor (M1 hand) cortex in squirrel monkeys increased neuronal VEGF-A [91] and VEGFR-1 [92] expression in both peri-infarct cortex and remote brain regions (PMv hand and M1 hindlimb) thought to be involved in stroke recovery.
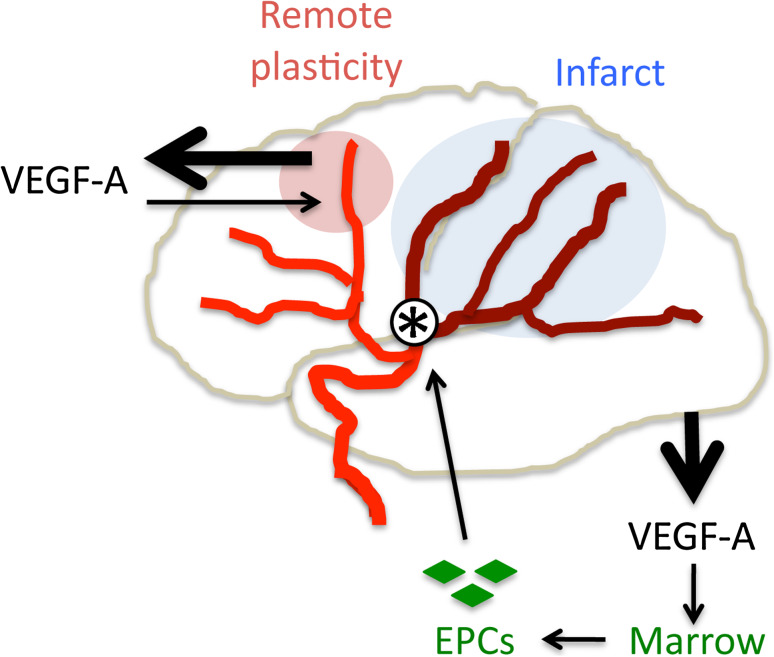
Fig. 3.
VEGF-A-mediated postischemic repair. VEGF-A appears to be a mediator of postischemic brain and vessel repair. Following cerebral infarction (blue) due to arterial occlusion (asterisk), VEGF-A expression is increased (thick arrow, top left) in nonischemic brain regions that exhibit remote plasticity (pink); VEGF-A acting here (thin arrow, top left) may help to restore brain function. Increased serum VEGF-A levels after stroke (thick arrow, bottom right) also mobilize endothelial progenitor cells (EPCs, green) from the bone marrow, enhancing repair of ischemia-damaged vessels (thin arrows, bottom right)
Postischemic vascular repair
Circulating endothelial progenitor cells (EPCs) appear to have an important role in repairing systemic blood vessels after ischemic injury [93] and VEGF-A has been implicated in mobilizing circulating EPCs in response to ischemia [94, 95] (Fig. 3). This effect is likely mediated through both VEGFR-1 and VEGFR-2 receptors, because both receptors are expressed on EPCs, antibodies against either receptor inhibit EPC mobilization or assembly into new vessels, and both VEGF-A and the VEGFR-1-selective agonist PlGF are effective [96]. Regarding the cerebral circulation, low levels of circulating EPCs are reported to correlate with increased stroke risk [97], whereas higher levels of circulating EPCs within the first week after stroke were associated with better functional outcome at 3 months [98]. In addition, serum VEGF-A levels 24 h post-stroke correlated with the growth of EPC colonies from venous blood mononuclear cells in vitro [99].
Therapeutics
In addition to the studies cited above documenting acute neuroprotective effects of VEGFs in experimental stroke [31, 48, 50, 51, 54, 55, 57], other reports have described longer-term, VEGF-mediated beneficial effects of stem cell treatment. Intravenous administration of human neural stem cells (NSCs) together with VEGF-A, beginning 24–48 h after MCA occlusion in rats, was more effective than NSCs or VEGF-A alone in reducing brain atrophy and improving behavioral recovery at 7–28 days [100]. VEGF-A-overexpressing transfected NSCs derived from fetal rat brain, transplanted into the ischemic penumbra 3 days after MCAO in rats, decreased the severity of neurological deficits to a greater extent than control NSCs at 8–12 weeks [101]. A VEGF-A-transfected human NSC clone, transplanted into ipsilesional cerebral cortex 7 days after intracerebral hemorrhage in mice, also produced better behavioral outcome at 1–9 weeks than an untransfected clone [102]. In another study, human mesenchymal stem cells transfected with a PlGF-expressing adenoviral vector, administered intravenously 3 days after MCAO in rats, were superior to cells transfected with a control vector in reducing infarct volume and behavioral impairment at 3–7 days [103].
Perhaps the most interesting studies of the role of VEGFs in neurotransplantation for experimental stroke have involved cells that have not been modified to overexpress VEGFs, but which nevertheless elicit VEGF-A-mediated improvement. In one instance, intrastriatal delivery of mouse embryonic NSCs 3 days prior to MCA occlusion in mice decreased the number of TUNEL-positive nuclei and the loss of NeuN-immunopositive neurons in the peri-infarct region; in vitro studies suggested that the neuroprotective effect of transplanted NSCs might be mediated through VEGFR-2, because it was inhibited by the VEGFR-2 tyrosine kinase receptor inhibitor SU1498 [104]. In another study, this possibility was tested directly in vivo. Human NSCs transplanted into the ipsilesional cerebral cortex 7 days after MCA occlusion in rats reduced infarct size and enhanced behavioral recovery at 2–5 weeks, and these effects were blocked by the humanized monoclonal anti-VEGF-A antibody bevacizumab [105]. Thus, at least some of the beneficial effects of neurotransplantation for stroke may be due to release of VEGF-A from transplanted cells.
Conclusions
VEGFs have a broad array of effects related to stroke. They have been implicated in the pathogenesis of atherosclerosis, a common cause of stroke, and also participate in the process of arteriogenesis underlying the formation of collateral vessels, which can protect ischemic brain from infarction and promote recanalization of occluded cerebral arteries. VEGF-A expression is induced in the ischemic penumbra during stroke and at remote sites involved in post-ischemic brain repair, and VEGFs expressed in this setting have acute neuroprotective, neurogenic, and angiogenic actions. Administration of VEGF-A or VEGF-B, or of VEGF-A together with stem cells, has therapeutic effects in animal models of stroke, and the release of VEGF-A from transplanted stem cells may be responsible for their therapeutic actions. Notwithstanding potential adverse effects of some VEGFs, including their propensity to induce vascular permeability and associated brain edema, these factors may prove to have applications in the acute or chronic treatment of stroke.
Acknowledgments
This study was supported by National Institutes of Health grant R01 NS44921.
References
- 1.Hirashima M. Regulation of endothelial cell differentiation and arterial specification by VEGF and notch signaling. Anat Sci Int. 2009;84:95–101. doi: 10.1007/s12565-009-0026-1. [DOI] [PubMed] [Google Scholar]
- 2.Shibuya M. Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. FEBS J. 2009;276:4636–4643. doi: 10.1111/j.1742-4658.2009.07175.x. [DOI] [PubMed] [Google Scholar]
- 3.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139:1371–1380. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 5.Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- 6.Yang GY, Yao JS, Huey M, Hashimoto T, Young WL. Participation of PI3 K and ERK1/2 pathways are required for human brain vascular smooth muscle cell migration. Neurochem Int. 2004;44:441–446. doi: 10.1016/j.neuint.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 8.Hiyama T, Tanaka T, Endo S, Komine K, Kudo T, Kobayashi H, Shiokawa Y. Angiogenesis in atherosclerotic plaque obtained from carotid endarterectomy: association between symptomatology and plaque morphology. Neurol Med Chir (Tokyo) 2010;50:1056–1061. doi: 10.2176/nmc.50.1056. [DOI] [PubMed] [Google Scholar]
- 9.Petrovan RJ, Kaplan CD, Reisfeld RA, Curtiss LK. DNA vaccination against VEGF receptor 2 reduces atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1095–1100. doi: 10.1161/ATVBAHA.106.139246. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou Y, He L, Hong K, Su H, Wu Y, Wu Q, Han M, Cheng X. Gene delivery of soluble vascular endothelial growth factor receptor-1 (sFlt-1) inhibits intra-plaque angiogenesis and suppresses development of atherosclerotic plaque. Clin Exp Med. 2011;11:113–121. doi: 10.1007/s10238-010-0112-7. [DOI] [PubMed] [Google Scholar]
- 11.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels. 1991;28:183–189. doi: 10.1159/000158860. [DOI] [PubMed] [Google Scholar]
- 13.Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab. 2003;23:621–628. doi: 10.1097/01.WCB.0000057741.00152.E4. [DOI] [PubMed] [Google Scholar]
- 14.Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- 15.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O’Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–2113. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 16.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt: expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–1873. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Abe K, Suzuki H, Itomaya Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 19.Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol. 1998;57:874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in rat focal cerebral ischemia. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- 21.Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Disease. 2003;14:524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Gu W, Brannstrom T, Jiang W, Bergh A, Wester P. Vascular endothelial growth factor-A and -C protein up-regulation and early angiogenesis in a rat photothrombotic ring stroke model with spontaneous reperfusion. Acta Neuropathol (Berl) 2001;102:216–226. doi: 10.1007/s004010100370. [DOI] [PubMed] [Google Scholar]
- 24.Lee MY, Ju WK, Cha JH, Son BC, Chun MH, Kang JK, Park CK. Expression of vascular endothelial growth factor mRNA following transient forebrain ischemia in rats. Neurosci Lett. 1999;265:107–110. doi: 10.1016/s0304-3940(99)00219-0. [DOI] [PubMed] [Google Scholar]
- 25.Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA. Induction of vascular endothelial growth factor and hypoxia-inducible factor-1alpha by global ischemia in rat brain. Neuroscience. 2000;99:577–585. doi: 10.1016/s0306-4522(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 26.Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA. Induction of vascular endothelial growth factor receptors and phosphatidylinositol 3′-kinase/Akt signaling by global cerebral ischemia in the rat. Neuroscience. 2000;100:713–717. doi: 10.1016/s0306-4522(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 27.Leung DW, Cachianes G, Kuang W-J, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 28.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 29.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 30.van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura R, Nakase H, Tamaki R, Sakaki T. Vascular endothelial growth factor antagonist reduces brain edema formation and venous infarction. Stroke. 2005;36:1259–1263. doi: 10.1161/01.STR.0000165925.20413.14. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 35.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 36.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman WF, Krum JM, Mani N, Rosenstein JM. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90:1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 38.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor rescues HN33 neural cells from death induced by serum withdrawal. J Molec Neurosci. 2000;14:197–203. doi: 10.1385/JMN:14:3:197. [DOI] [PubMed] [Google Scholar]
- 39.Ogunshola OO, Antic A, Donoghue MJ, Fan S-Y, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 40.Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Dev Brain Res. 2004;148:59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- 43.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- 45.Svensson B, Peters M, Konig HG, Poppe M, Levkau B, Rothermundt M, Arolt V, Kogel D, Prehn JH. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- 46.Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du H, Li P, Pan Y, Li W, Hou J, Chen H, Wang J, Tang H. Vascular endothelial growth factor signaling implicated in neuroprotective effects of placental growth factor in an in vitro ischemic model. Brain Res. 2010;1357:1–8. doi: 10.1016/j.brainres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Bao WL, Lu SD, Wang H, Sun FY. Intraventricular vascular endothelial growth factor antibody increases infarct volume following transient cerebral ischemia. Chung Kuo Yao Li Hsueh Pao. 1999;20:313–318. [PubMed] [Google Scholar]
- 50.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Galvan V, Gorostiza O, Ataie M, Jin K, Greenberg DA. Vascular endothelial growth factor improves recovery of sensorimotor and cognitive deficits after focal cerebral ischemia in the rat. Brain Res. 2006;1115:186–193. doi: 10.1016/j.brainres.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- 53.Kaya D, Gursoy-Ozdemir Y, Yemisci M, Tuncer N, Aktan S, Dalkara T. VEGF protects brain against focal ischemia without increasing blood–brain permeability when administered intracerebroventricularly. J Cereb Blood Flow Metab. 2005;25:1111–1118. doi: 10.1038/sj.jcbfm.9600109. [DOI] [PubMed] [Google Scholar]
- 54.Feng Y, Rhodes PG, Bhatt AJ. Neuroprotective effects of vascular endothelial growth factor following hypoxic ischemic brain injury in neonatal rats. Pediatr Res. 2008;64:370–374. doi: 10.1203/PDR.0b013e318180ebe6. [DOI] [PubMed] [Google Scholar]
- 55.Zheng XR, Zhang SS, Yang YJ, Yin F, Wang X, Zhong L, Yu XH. Adenoviral vector-mediated transduction of VEGF improves neural functional recovery after hypoxia-ischemic brain damage in neonatal rats. Brain Res Bull. 2010;81:372–377. doi: 10.1016/j.brainresbull.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Shimotake J, Derugin N, Wendland M, Vexler ZS, Ferriero DM. Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke. 2010;41:343–349. doi: 10.1161/STROKEAHA.109.564229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J Cereb Blood Flow Metab. 2004;24:1146–1152. doi: 10.1097/01.WCB.0000134477.38980.38. [DOI] [PubMed] [Google Scholar]
- 58.Mould AW, Greco SA, Cahill MM, Tonks ID, Bellomo D, Patterson C, Zournazi A, Nash A, Scotney P, Hayward NK, Kay GF. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res. 2005;97:e60–e70. doi: 10.1161/01.RES.0000182631.33638.77. [DOI] [PubMed] [Google Scholar]
- 59.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Diff. 1996;7:213–221. [PubMed] [Google Scholar]
- 60.Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 63.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 64.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 65.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- 67.Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 68.Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 70.Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Zacharek A, Li A, Zhang C, Ding J, Roberts C, Lu M, Kapke A, Chopp M. Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaete-scute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience. 2006;141:737–744. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 75.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Chen HH, Chien CH, Liu HM. Correlation between angiogenesis and basic fibroblast growth factor expression in experimental brain infarct. Stroke. 1994;25:1651–1657. doi: 10.1161/01.str.25.8.1651. [DOI] [PubMed] [Google Scholar]
- 77.Liu HM. Neovasculature and blood-brain barrier in ischemic brain infarct. Acta Neuropathol. 1988;75:422–426. doi: 10.1007/BF00687796. [DOI] [PubMed] [Google Scholar]
- 78.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 79.Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 80.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 82.Freitas-Andrade M, Carmeliet P, Charlebois C, Stanimirovic DB, Moreno MJ. PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge. J Cereb Blood Flow Metab. 2012;32:663–675. doi: 10.1038/jcbfm.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 84.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 85.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Reinecke S, Lutzenburg M, Hagemann G, Bruehl C, Neumann-Haefelin T, Witte OW. Electrophysiological transcortical diaschisis after middle cerebral artery occlusion (MCAO) in rats. Neurosci Lett. 1999;261:85–88. doi: 10.1016/s0304-3940(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 87.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 88.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 89.Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Disease. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- 90.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stowe AM, Plautz EJ, Eisner-Janowicz I, Frost SB, Barbay S, Zoubina EV, Dancause N, Taylor MD, Nudo RJ. VEGF protein associates to neurons in remote regions following cortical infarct. J Cereb Blood Flow Metab. 2007;27:76–85. doi: 10.1038/sj.jcbfm.9600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stowe AMPE, Nguyen P, Frost SB, Eisner-Janowicz I, Barbay S, Dancause N, Sensarma A, Taylor MD, Zoubina EV, Nudo RJ. Neuronal HIF-1α protein and VEGFR-2 immunoreactivity in functionally related motor areas following a focal M1 infarct. J Cereb Blood Flow Metab. 2008;28:612–620. doi: 10.1038/sj.jcbfm.9600560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watt SM, Athanassopoulos A, Harris AL, Tsaknakis G. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface. 2010;7(Suppl 6):S731–S751. doi: 10.1098/rsif.2010.0377.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 95.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70:829–834. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 96.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 97.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 98.Sobrino T, Hurtado O, Moro MA, Rodriguez-Yanez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Davalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 99.Sobrino T, Perez-Mato M, Brea D, Rodriguez-Yanez M, Blanco M, Castillo J. Temporal profile of molecular signatures associated with circulating endothelial progenitor cells in human ischemic stroke. J Neurosci Res. 2012;90:1788–1793. doi: 10.1002/jnr.23068. [DOI] [PubMed] [Google Scholar]
- 100.Chu K, Park KI, Lee ST, Jung KH, Ko SY, Kang L, Sinn DI, Lee YS, Kim SU, Kim M, Roh JK. Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia. Neurosci Res. 2005;53:384–390. doi: 10.1016/j.neures.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 101.Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu JH, Zhu Y, Yang GY. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery. 2005;57:325–333. doi: 10.1227/01.neu.0000166682.50272.bc. [DOI] [PubMed] [Google Scholar]
- 102.Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS ONE. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]