Abstract
Objective
Germline mutations in BRCA1/2 are related to increased lifetime risk of breast and ovarian cancer. While risk-reducing salpingo-oophorectomy reduces the risk for both cancers, loss of fertility is a major concern. A recent study suggested an association of BRCA1 mutation with occult primary ovarian insufficiency. The aim of this study was to determine whether BRCA1/2 mutation carriers have earlier onset of natural menopause than unaffected women.
Materials and Methods
Caucasian BRCA1/2 carriers (n=382) were identified within the UCSF Breast Cancer Risk Program Registry and compared to non-clinic-based Caucasian women in Northern California (n=765). We compared the two groups regarding median age at natural menopause before and after adjustment for known risk factors, and examined the role of smoking within each group, using the Kaplan-Meier approach for unadjusted analyses and Cox proportional hazards regression analyses for adjusted analyses.
Results
The median age at natural menopause in BRCA1/2 carriers was significantly earlier than the unaffected sample (50 vs 53years, p-value<0.001). The unadjusted hazard ratio for natural menopause comparing BRCA1/2 carriers to unaffected women was 4.06(95% confidence interval 3.03-5.45), 3.98(2.87-5.53) after adjusting for smoking, parity, and oral contraceptive use. For BRCA1/2 carriers who were current heavy smokers (≥20cigarettes/day), the median age at natural menopause was 46 vs.49years for non-smokers (p-value=0.027).
Conclusions
BRCA1/2 mutation was associated with significantly earlier age at natural menopause, and heavy smoking compounded this risk. As the relationship between menopause and end of natural fertility is considered fixed, these findings suggest a risk of earlier infertility among BRCA1/2 carriers.
Keywords: menopause, BRCA1 gene, BRCA2 gene, primary ovarian insufficiency, smoking, carcinogenesis
INTRODUCTION
In women, germ cells encounter one of several fates: they may remain quiescent, be recruited for further development and ovulation, or undergo apoptosis. Although somewhat controversial, over time and without regeneration, the population of oocytes (follicles) are depleted until <1000 remain, and menopause ensues [1] [2]. Natural menopause (the final menstrual period [FMP] followed by 12 months of amenorrhea [3]) occurs at a median age of 51.4 years with a distribution ranging between 40 and 60 years [4]. Before menopause, as the follicle count diminishes with age, the quality of oocytes also declines, resulting in infertility and increased miscarriage rates [5]. It has been proposed that the intervals between the decline in fertility, the age at last birth, and menopause are fixed at 10 years, with varying age for the onset of menopause [6].
Age at menopause is a complex outcome related to a number of factors [7] but is at least partially heritable, suggesting a genetic contribution. The heritability of menopausal age has been estimated to be between 30 and 85% [8] [9] [10], and a notable proportion (15–30%) of primary ovarian insufficiency cases is familial [11] [12] [13] [14]. Although multiple genes control some of the timing of natural menopause, very few genes have been identified that contain common genetic variants that are associated with age of menopause[15] [16].
Recent evidence has suggested that women with mutations in the DNA repair genes, BRCA1 and BRCA2 (BRCA1/2), have an increased risk of primary ovarian insufficiency [17]. The hypothesis asserts that because DNA repair mechanisms are impaired in patients with BRCA1/2 mutations, oocytes are more prone to DNA damage and thereby experience accelerated follicular depletion [17]. If BRCA1/2 carriers are more likely to experience occult ovarian insufficiency, this may lead to a higher incidence of infertility and early menopause. This circumstance may add to the significant psychosocial implications of being a BRCA1/2 carrier and likely have an impact on reproductive decision-making. To test this hypothesis, we sought to determine if BRCA1/2 carriers had an earlier age at menopause than non-carriers.
METHODS
Study population
The data for this study were collected from two populations: 1)The Cancer Risk Program (CRP) at the University of California, San Francisco (UCSF) and 2) the northern California (UC Davis/Kaiser) site of the Study of Women's Health Across the Nation (SWAN) [18]. Institutional review boards at all involved sites approved the study protocol, and all participants provided informed consent.
For all BRCA1/2 carriers identified in the CRP registry, demographic, health behavior and clinical background information was obtained from a self-administered questionnaire completed at enrollment. This information included medical history, surgical history, detailed history and timing of cancer diagnosis and treatment, detailed menstrual history, parity, history of oral contraceptive (OCP) use, recent weight change, age and smoking history. It was then further cross-checked with the hospital charts and operations reports in electronic medical records to confirm its accuracy. Similar data were collected in SWAN, a multi-site, multi-race/ethnic study of health and menopause in midlife women. SWAN's first phase was a cross-sectional telephone survey of women aged 40-55 years, conducted at seven sites across the US.
Specifically, natural menopause was defined as at least 12 consecutive months of amenorrhea[3]. Similar questions were used in both CRP and SWAN cohorts to obtain menopause information. Women were asked if they had had a menstrual period in the past 12 months; if not, they were asked whether their periods stopped due to surgical intervention, medical treatment, pregnancy/breastfeeding, or severe weight loss or other reasons other than menopause. Women who answered no to all of these were asked what year their periods stopped. FMP age was calculated by subtracting the year of last menstruation from birth year. Analyses of the terminal digit for self-reported year in SWAN study had suggested self-reporting was accurate, as there was no digit preference [19].
Almost all BRCA1/2 carriers within the CRP registry are Caucasian. Thus, to increase comparability of the BRCA1/2 carriers and SWAN participants, we included only the Caucasians in the SWAN Northern California site to obtain an estimate of the age at the natural FMP for comparison. The present analyses included all Caucasian research participants with a positive BRCA1/2 test between 1998 and 2006 in CRP program in UCSF. The participants included from the northern California SWAN sample were Caucasians aged 40-55 years who were selected at random from the northern California Kaiser Permanente membership in 1995 and interviewed for eligibility for the longitudinal SWAN cohort in 1996-7.
Statistical Analysis
Statistical analyses were performed with SAS version 9.2. Descriptive analyses were performed with independent t-tests to compare means for continuous data and with chi-square tests to compare frequencies for categorical data. Statistical significance was defined with p-value<0.05.
To compare age at final menstrual period (FMP) in the two study samples (primary analysis), survival analyses were performed to allow for the inclusion of incomplete or censored observations. Age at FMP was censored for women who were still menstruating or whose FMP was not observable due to medical intervention, including exogenous hormone therapy, or to pregnancy/breastfeeding.
Unadjusted differences between BRCA1/2 carriers and SWAN participants were estimated using Kaplan-Meier analyses with log-rank testing and Cox proportional hazards regression, and adjusted differences were estimated using Cox proportional hazards regression to control for potential confounding factors (parity, smoking, and oral contraceptive pill [OCP] use). These predictors were selected based on prior literature indicating their significant association with age at natural menopause [7]. We verified the validity of the proportional hazards assumption for each predictor by testing the significance of its interaction with age [20] and determining whether its inclusion improved model fit, as indicated by the AIC statistic [21].
Sensitivity analyses were run excluding BRCA1/2 participants outside the 40-55 year age range of SWAN participants (172 BRCA1/2 participants, 765 SWAN participants). Primary analyses included women whose FMP was not observable due to medical intervention (chemotherapy, radiation, and bilateral oophorectomy), consistent with previous studies [4]. Their inclusion reduces bias, as these women are not a random subsample of all participants, nor are they a small subset; moreover, they contribute information regarding age at FMP up to the age at which they are censored. To confirm that results were similar with and without these women, secondary analyses were run on 166 BRCA1/2 carriers and 639 SWAN participants with a natural menopause or who were still menstruating, excluding patients who underwent hysterectomy or bilateral oophorectomy, or received chemotherapy or radiotherapy.
RESULTS
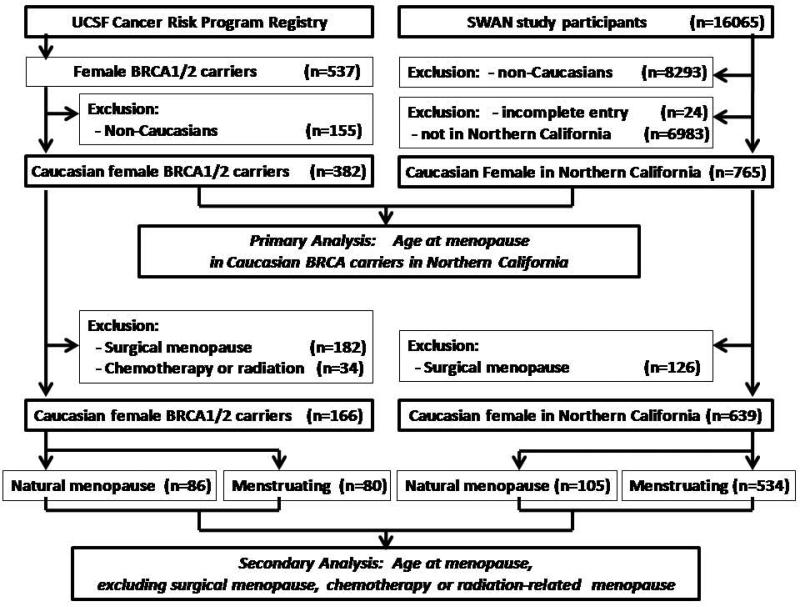
Within the UCSF CRP Registry, a total of 537 female BRCA1/2 carriers were identified, and 382 (238 BRCA1, 144 BRCA2) were Caucasian. Among the SWAN participants, 765 were identified as living in northern California and Caucasian (figure 1). Comparison of health and behavior characteristics of BRCA1/2 carriers to the northern California SWAN sample showed that BRCA1/2 carriers had a higher rate of being nulliparous, a lower mean parity, a lower rate of OCP use, a higher rate of never smokers, but a lower rate of current smokers (table1).
Figure 1.
Study flow- Inclusion and exclusion criteria of the primary analysis and secondary analysis of BRCA1/2 carriers from UCSF cancer risk program registry and SWAN study participants.
Table 1.
Characteristics of general population & BRCA carriers
| % or Mean (SD) | |||
|---|---|---|---|
| Characteristic | SWAN* (N=765) | BRCA carriers** (N=382) | p-value for difference |
| Parity: | |||
| 0 | 25.5 | 39.4 | <0.0001 |
| 1 + | 74.5 | 60.6 | |
| # children | 1.5 (1.2) | 1.3 (1.2) | 0.0017 |
| History of OCP use | 88.2 | 48.0 | <0.0001 |
| History of smoking: | <0.0001 | ||
| Never | 49.3 | 83.7 | |
| Past | 33.6 | 6.4 | |
| Current | 17.1 | 9.9 | |
The samples of general population were Caucasians extracted from the Study of Women's Health Across the Nation (SWAN) populations in Northern California.
The BRCA carriers were obtained through The Cancer Risk Program (CRP) at the University of California, San Francisco (UCSF)
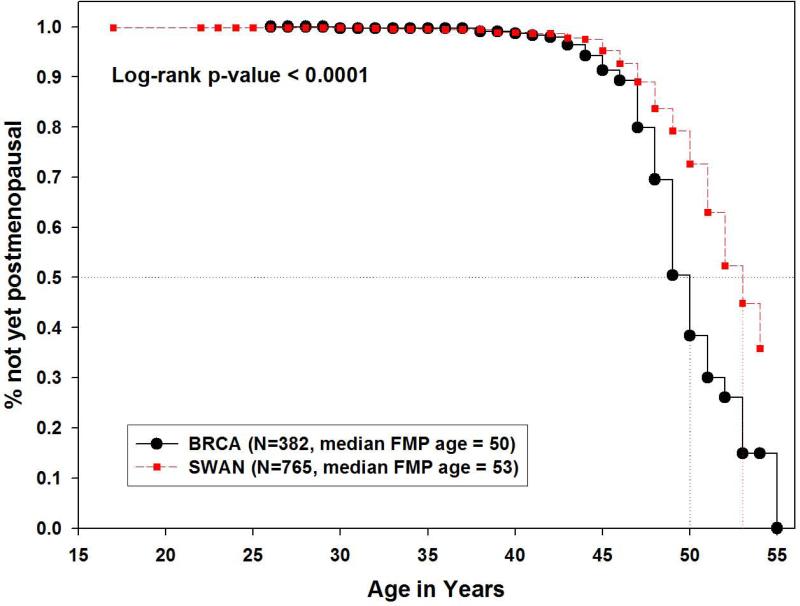
Overall, the median age at menopause for the BRCA1/2 carriers in Northern California was 50 years, which was significantly earlier than the Caucasian population in Northern California at 53 years (p<0.001) (Figure 2), while the median age at natural menopause for the entire SWAN sample was 51.4 years [4], consistent with prior studies [7].
Figure 2.
Kaplan-Meier estimates of age at natural menopause in Northern California Caucasians, comparing BRCA1/2 carriers to SWAN cross-sectional screened sample. (Primary Analysis)
Table 2 presents hazard ratios from the multivariate Cox proportional hazards model of the primary analysis, sensitivity analysis, and secondary analysis. Adjustment for parity, smoking, and OCP use had little impact on the difference in age at menopause between the BRCA1/2 carriers and the northern California SWAN sample. In the subset of Caucasian women with complete covariate information (n=228 in BRCA carriers, n=759 in northern California SWAN), the unadjusted hazard ratio for time to menopause from Cox proportional hazards modeling was 4.06 (95% confidence interval 3.03 – 5.45, p<0.001), and the corresponding adjusted hazard ratio was 3.98 (95% confidence interval 2.87 – 5.53, p<0.001), both indicating a significantly earlier menopause in BRCA1/2 carriers than in northern California Caucasians in SWAN. When restricting the age range for the BRCA1/2 carriers to 40-55 years to be the same as the SWAN participants, the results were similar and remained statistically significant. The median age at menopause was 50 in the BRCA1/2 carriers vs. 53 years in the SWAN participants (p=0.004). In the subset aged 40-55 years with complete covariate information (n=103 in BRCA1/2 carriers, n=759 in northern California SWAN), the unadjusted hazard ratio was 2.69 (95% confidence interval 1.57 – 4.62, p=0.0003), and the corresponding adjusted hazard ratio was 2.82 (95% confidence interval 1.56 – 5.09, p=0.0006).
Table 2.
Results of Cox proportional hazards modeling for age at final menstrual period
| Primary Analyses | Sensitivity Analyses (Ages 40 – 55 Only) | Secondary Analyses (Omit surgery, chemotherapy, radiotherapy) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model-BRCA vs. SWAN | HR (95% CI) | p-value | N | HR (95% CI) | p-value | N | HR (95% CI) | p-value | N |
| Unadjusted (all) | 1147 | 937 | 805 | ||||||
| BRCA | 2.32 (1.74, 3.10) | <0.0001* | 2.21 (1.30, 3.76) | 0.0034* | 4.38 (3.28, 5.85) | <0.0001* | |||
| SWAN | Reference | Reference | Reference | ||||||
| Unadjusted (all with complete data) | 987 | 862 | 780 | ||||||
| BRCA | 4.06 (3.03, 5.45) | <0.0001* | 2.69 (1.57, 4.62) | 0.0003* | 4.70 (3.50, 6.30) | <0.0001* | |||
| SWAN | Reference | Reference | Reference | ||||||
| Adjusted:(c) | 987 | 862 | 780 | ||||||
| BRCA | 3.98 (2.87, 5.53) | <0.0001* | 2.82 (1.56, 5.09) | 0.0006* | 4.33 (3.13, 6.01) | <0.0001* | |||
| SWAN | Reference | Reference | Reference | ||||||
| Parity: | 0.4958 | 0.8565 | 0.4746 | ||||||
| 0 | Reference | Reference | Reference | ||||||
| 1 | 1.25 (0.83, 1.89) | 1.03 (0.59, 1.79) | 0.95 (0.62, 1.47) | ||||||
| 2 | 1.21 (0.76, 1.93) | 0.89 (0.56, 1.43) | 0.80 (0.55, 1.17) | ||||||
| 3+ | 0.97 (0.65, 1.47) | 1.11 (0.66, 1.85) | 0.75 (0.49, 1.14) | ||||||
| History of OCP use: | 0.0659 | 0.0639 | 0.0631 | ||||||
| No | Reference | Reference | Reference | ||||||
| Yes | 0.72 (0.51, 1.02) | 0.65 (0.41, 1.03) | 0.72 (0.51, 1.02) | ||||||
| History of smoking: | 0.0013* | 0.0275* | 0.0085* | ||||||
| Never | Reference | Reference | Reference | ||||||
| Past | 1.27 (0.88, 1.85) | 1.14 (0.74, 1.76) | 1.12 (0.77, 1.62) | ||||||
| Current | 2.01 (1.38, 2.93) | 1.94 (1.19, 3.16) | 1.79 1.23, 2.61) | ||||||
statistical significance; (a) HR=hazard ratio, CI=confidence interval; (b) Multivariate model including as predictors for all variables in the table
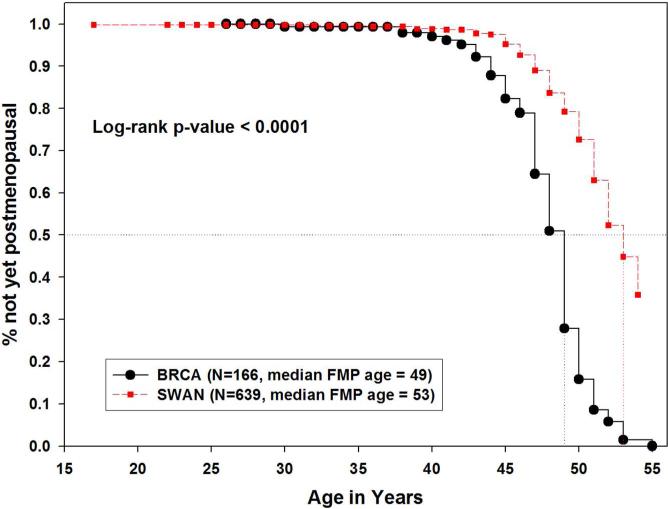
Our secondary analysis excluded those who underwent surgical menopause or chemotherapy- or radiotherapy-related menopause. In this subset, the health and behavior characteristics of BRCA1/2 carriers and the northern California Caucasian SWAN sample were similar to the primary analysis (Table 2). The median age at natural menopause among the BRCA1/2 carriers (n=166) remained significantly earlier than the SWAN sample (n=639), specifically 49 vs, 53 years (p<0.001) (Figure 3). In the subset with complete covariate information (n=146 BRCA1/2, n=634 SWAN), the unadjusted hazard ratio was 4.70 (95% confidence interval 3.50 – 6.30; p<0.001). After adjusting for parity, smoking and OCP use, the difference remained statistically significant; BRCA1/2 carriers had a significantly earlier age onset of menopause with a hazard ratio of 4.33 (95% confidence interval 3.13 – 6.01; p<0.001).
Figure 3.
Kaplan-Meier estimates of age at natural menopause in Northern California Caucasians, comparing BRCA1/2 carriers to SWAN cross-sectional screened sample, excluding medical and surgical menopause. (Secondary Analysis)
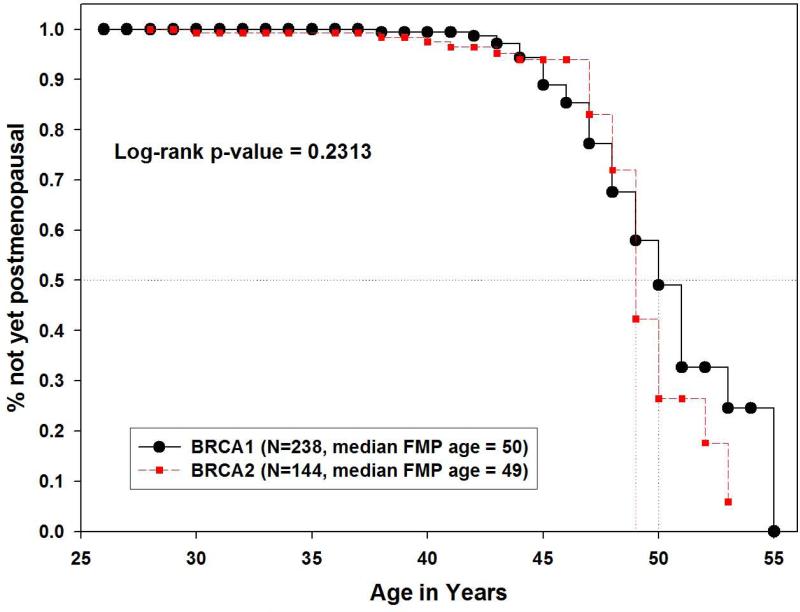
Interestingly, among BRCA1/2 carriers, the Kaplan-Meier survival curves were very similar across the range of possible FMP ages, and no significant difference was observed in the median age at natural menopause between the 238 BRCA1 carriers and the 144 BRCA2 carriers in the primary analysis (50 vs. 49 years, p=0.231) (Figure 4) or in the secondary analysis, excluding those who underwent surgical menopause or chemotherapy- or radiotherapy-related menopause (N= 94 vs. 72, 48 vs 49 years, p=0.759).
Figure 4.
Kaplan-Meier estimates of age at natural menopause in Northern California Caucasians, comparing BRCA1 to BRCA2 carrier.
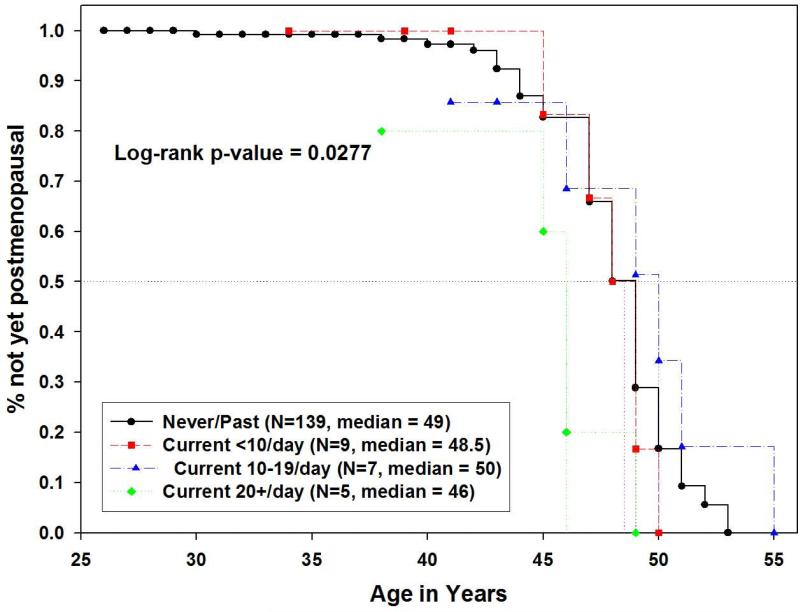
BRCA1/2 carriers who were current smokers (n=25) underwent earlier natural menopause than current smokers in SWAN (n=130) (median age at natural menopause 48 vs. 51 years, p=0.0006). Among BRCA1/2 carriers, the median age at natural menopause was 49 years for both never- and former smokers (hazard ratio=1.35, 95% CI 0.72 – 2.55, p=0.350). Thus, subsequent analyses combined both subgroups as current non-smokers. Current heavy smokers (≥20 cigarettes/day) underwent even earlier menopause than non-smokers or current light or moderate smokers among BRCA1/2 carriers (<10, 10-19 cigarettes/day) (median age was 46 years for heavy smokers, versus 49 years for non-smokers, 48.5 years for light smokers, and 50 years for moderate smokers; p=0.027) (Figure 5). The patterns of smoking-related differences among northern California Caucasian SWAN participants were consistent. In SWAN samples, former and never smokers did not differ (hazard ratio=1.08, 95% CI 0.69 – 1.68, p=0.733), and the median age at natural menopause for never/past smokers (n=631) was 54 years, vs. 52 years for current light smokers (n=31), 49 years for current moderate smokers (n=46), and 51 years for current heavy smokers (n=51) (log-rank p-value=0.0005).
Figure 5.
Kaplan-Meier estimates of age at natural menopause in Northern California Caucasian BRCA1/2 carriers, comparing never/past smokers, current light, moderate, and heavy smokers.
Discussion
The novel results of this study showed that being a Caucasian BRCA1/2 mutation carrier was associated with undergoing natural menopause a median of 3-4 years earlier than a community sample of midlife Caucasian women. In addition, when adjusted for variables known to affect age at menopause, BRCA1/2 carriers had a significant four-fold increased hazard ratio, indicating a significantly earlier menopause than the community sample even after adjustment for such variables. Moreover, those carriers who were current heavy smokers underwent an even earlier age at menopause (median 46 years), an age at which only 7% of northern California Caucasian women had undergone natural menopause.
Data on the age at natural menopause among BRCA1/2 carriers are limited as most women are recommended to undergo risk-reducing salpingo-oophorectomy on completion of childbearing, before menopause. One study reported BRCA1 breast cancer patients to be at risk of premature ovarian failure compared to other breast cancer patients [22]. Although it is a study designed to investigate the association of BRCA1 mutation and estrogenization of ovarian tissues with limited control groups, its menopause findings were consistent with our observation. Another study reported the median age at natural menopause among BRCA1/2 carriers to be 49.1~49.8 years [23]. However, this study was designed to evaluate the associations between menopausal hormone therapy and the risk of ovarian cancer in BRCA1/2 mutation carriers. Although no comparisons were made regarding the age at natural menopause in BRCA1/2 carriers to the general population, the age at natural menopause for BRCA1/2 carriers was consistent with our observations.
Our finding that BRCA1/2 carriers who were current heavy smokers had an even earlier onset of menopause is consistent with the literature on the relation of smoking to earlier age at menopause [24]. Cigarette smoking has also been demonstrated to alter menstrual cycles, estrogen status, spontaneous pregnancy rate, as well as assisted reproduction outcomes [25, 26] [27] [28] [29] [30]. The mechanism is thought to be due to, in part, an accumulation of toxic metabolites [31] [32], such as cadmium, nicotine, cotinine, and benzo[a]pyrene within the follicular fluid and ovarian stroma, which can have detrimental effects on ovarian function, potentially via oxidative damage [33, 34]. Some of these effects would seem to be reversible, as the literature and the present data largely indicates that women who stop smoking have a similar age at menopause to non-smokers [35]. This conclusion is not surprising given many other clinical observations, in addition to age onset of menopause, suggest smoking cessation resolves the additional risks present in current smokers (i.e. stroke) [36] [37].
The implications of BRCA1/2 carriers having an earlier age at menopause may be a narrower reproductive window and possibly an increased risk of infertility. However, few studies have reported on fertility parameters for BRCA1/2 carriers. Our results show that parity was different among carriers and non-carriers, which is in contrast to the previous published studies [38] [39]. However, parity in itself is an inadequate method for determining if the reproductive capacity is decreased, as it does not identify how many tried to conceive but were unable and does not take into account female age at the time of attempted conception. Interestingly, a recent study showed BRCA1 carriers have poor ovarian stimulation responses, implying lower ovarian reserve for a given age than the general population [17]. These findings are of interest, given that lower ovarian reserve has been associated with infertility and earlier onset of menopause [40] [41]. Future studies are needed to clarify if fertility is compromised among BRCA1/2 carriers.
Mechanistically, because DNA repair is deficient in patients with BRCA1/2 mutations, oocytes may be more prone to DNA damage [42]. Prior basic research in non-reproductive cell types demonstrated that when DNA damage is severe and cannot be repaired, apoptotic pathways are activated, though there is no current evidence that this is in fact the case for the oocytes of women with BRCA1/2-mutations[43] [44] [45] [46]. It is possible based on the foregoing that oocytes with deficient BRCA1/2 function may be prematurely eliminated by a similar mechanism, resulting in early depletion of egg reserve [17].
Nevertheless, BRCA1/2 mutations are associated with a marked depletion of germ cells in adult female mice [39]. Mutations in BRCA1/2 also have phenotypic presentations that range from female-to-male sex reversal at gametogenesis stage in zebrafish to impaired spermatogenesis in male mice[47] [48] [49]. In the extreme, in specific double mutants models, sterile males or females were observed, followed by neoplastic proliferation and elevated risk of testicular and ovarian tumors [47]. These observations, as well as the results of the present study, support the idea that the BRCA1/2 genes may be important in reproductive function [17]. However, further basic research will be needed to understand the molecular mechanisms that lead to lower germ population both in animal models and women. This includes why in haplo-insufficient women oocyte yield in response to ovarian stimulation is low and menopause tends to occur earlier as reported here. Only studies that complement animal models with large prospective clinical studies will be able to answer these questions.
While this study has important practical implications it also has several limitations. First, the design was cross-sectional so that we cannot be positively certain of some temporal relations. Potential recall bias and not accounting for all possible confounders may have affected the effect estimates. However, women tend to have good recall about their age at natural FMP [50]. Additionally, while we may not have accounted for all possible confounders, we controlled for the factors that are known to be strongly associated with age at natural FMP, including smoking and OCP use [7]. Further, we limited the analyses to Caucasians who lived within the same geographical area, which likely reduced some of the variability in estimating age at the natural FMP, but also have limited generalizability to other racial/ethnic groups. Another limitation of the present study was that the comparison SWAN sample of women was not tested for BRCA1/2 and was not recruited in a similar fashion to the BRCA1/2 carriers. The carrier rate in general population is 1-2%, so that we may have misclassified a very small proportion of the comparison SWAN sample in considering them to be non BRCA1/2 carriers. However, this misclassification would potentially tend to lead to an earlier observed menopause in the controls and thus reduce the effect estimate of BRCA1/2 carrier status on age at natural FMP such that our difference is a conservative estimate.
In conclusion, our study showed that both BRCA1 and BRCA2 carriers had an earlier age at natural menopause than a similar community-based sample of Caucasian women. Those carriers who were current heavy smokers had an even earlier menopause. These findings suggest that BRCA1/2 carriers may have a narrower reproductive window than non-carriers. Therefore, in addition to discussing risk-reducing salpingo-oophorectomy, we would encourage the early initiation of fertility counseling for BRCA1/2 carriers and consideration of earlier childbearing.
Financial Support and Acknowledgements
We recognize the support of the UCSF Cancer Risk Program Patient Registry supported by the Helen Diller Family Comprehensive Cancer Center. The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Kutluk Oktay is supported by NIH R01HD053112. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
BRCA1/2 carriers do undergo earlier natural menopause compared to the general population, and that current heavy smoking is associated with an even earlier age onset of menopause. This is the first study to explore the association between BRCA1/2 and age at onset of menopause by comparing BRCA1/2 carriers with women in the general population.
No financial disclosure from any authors.
References
- 1.Faddy MJ, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 2.Vaskivuo TE, et al. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab. 2001;86(7):3421–9. doi: 10.1210/jcem.86.7.7679. [DOI] [PubMed] [Google Scholar]
- 3.Research on the menopause in the 1990s. World Health Organization; 1996. [Google Scholar]
- 4.Gold EB, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 5.Rowe T. Fertility and a woman's age. J Reprod Med. 2006;51(3):157–63. [PubMed] [Google Scholar]
- 6.Evers JL. Female subfertility. Lancet. 2002;360(9327):151–9. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38(3):425–40. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kok HS, et al. Genetic studies to identify genes underlying menopausal age. Hum Reprod Update. 2005;11(5):483–93. doi: 10.1093/humupd/dmi024. [DOI] [PubMed] [Google Scholar]
- 9.van Asselt KM, et al. Heritability of menopausal age in mothers and daughters. Fertil Steril. 2004;82(5):1348–51. doi: 10.1016/j.fertnstert.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83(6):1875–80. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 11.Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet. 1998;352(9134):1084–5. doi: 10.1016/S0140-6736(05)79753-1. [DOI] [PubMed] [Google Scholar]
- 12.de Bruin JP, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16(9):2014–8. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- 13.Murabito JM, et al. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90(6):3427–30. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- 14.Kok HS, et al. Age at natural menopause is not linked with the follicle-stimulating hormone receptor region: a sib-pair study. Fertil Steril. 2004;81(3):611–6. doi: 10.1016/j.fertnstert.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Stolk L, et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet. 2009;41(6):645–7. doi: 10.1038/ng.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voorhuis M, et al. Human studies on genetics of the age at natural menopause: a systematic review. Hum Reprod Update. 2010;16(4):364–77. doi: 10.1093/humupd/dmp055. [DOI] [PubMed] [Google Scholar]
- 17.Oktay K, et al. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–4. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowers MF, C.S., Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Loba RA KJ, Marcus R, editors. Menopause: biology and pathology. Academic Press; San Diego: 2000. pp. 175–88. [Google Scholar]
- 19.Crawford SL, Johannes CB, Stellato RK. Assessment of digit preference in self-reported year at menopause: choice of an appropriate reference distribution. Am J Epidemiol. 2002;156(7):676–83. doi: 10.1093/aje/kwf059. [DOI] [PubMed] [Google Scholar]
- 20.PD A. Survival analysis using SAS: A practical guide. SAS Institute, Inc.; Cary, NC: 1995. [Google Scholar]
- 21.Agresti A, C.B. Measures of relative model fit. Computational Statistics & Data Analysis. 2002;39(2):127–136. [Google Scholar]
- 22.Rzepka-Gorska I, et al. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100(1):59–63. doi: 10.1007/s10549-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 23.Kotsopoulos J, et al. Hormone replacement therapy and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Gynecol Oncol. 2006;100(1):83–8. doi: 10.1016/j.ygyno.2005.07.110. [DOI] [PubMed] [Google Scholar]
- 24.Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–30. doi: 10.1080/03014468200005591. [DOI] [PubMed] [Google Scholar]
- 25.Willett W, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–8. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 26.Stanford JL, et al. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40(11):995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 27.Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update. 2000;6(2):122–31. doi: 10.1093/humupd/6.2.122. [DOI] [PubMed] [Google Scholar]
- 28.Murin S, Rafii R, Bilello K. Smoking and smoking cessation in pregnancy. Clin Chest Med. 2011;32(1):75–91. viii. doi: 10.1016/j.ccm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Smoking and infertility. Fertil Steril. 2008;90(5 Suppl):S254–9. doi: 10.1016/j.fertnstert.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Freour T, et al. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed Online. 2008;16(1):96–102. doi: 10.1016/s1472-6483(10)60561-5. [DOI] [PubMed] [Google Scholar]
- 31.Kinney A, et al. Smoking, alcohol and caffeine in relation to ovarian age during the reproductive years. Hum Reprod. 2007;22(4):1175–85. doi: 10.1093/humrep/del496. [DOI] [PubMed] [Google Scholar]
- 32.Kinney A, Kline J, Levin B. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas. 2006;54(1):27–38. doi: 10.1016/j.maturitas.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Zenzes MT, Puy LA, Bielecki R. Immunodetection of benzo[a]pyrene adducts in ovarian cells of women exposed to cigarette smoke. Mol Hum Reprod. 1998;4(2):159–65. doi: 10.1093/molehr/4.2.159. [DOI] [PubMed] [Google Scholar]
- 34.Van Voorhis BJ, et al. The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol. 1996;88(5):785–91. doi: 10.1016/0029-7844(96)00286-4. [DOI] [PubMed] [Google Scholar]
- 35.Midgette AS, Baron JA. Cigarette smoking and the risk of natural menopause. Epidemiology. 1990;1(6):474–80. doi: 10.1097/00001648-199011000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kawachi I, et al. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269(2):232–6. [PubMed] [Google Scholar]
- 37.Wannamethee SG, et al. Smoking cessation and the risk of stroke in middle-aged men. JAMA. 1995;274(2):155–60. [PubMed] [Google Scholar]
- 38.Pal T, et al. Fertility in women with BRCA mutations: a case-control study. Fertil Steril. 2010;93(6):1805–8. doi: 10.1016/j.fertnstert.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 39.Moslehi R, et al. Impact of BRCA mutations on female fertility and offspring sex ratio. Am J Hum Biol. 2010;22(2):201–5. doi: 10.1002/ajhb.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen MP, et al. A lower antral follicle count is associated with infertility. Fertil Steril. 2011;95(6):1950–4. 1954, e1. doi: 10.1016/j.fertnstert.2011.01.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broekmans FJ, et al. Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause. 2004;11(6 Pt 1):607–14. doi: 10.1097/01.gme.0000123643.76105.27. [DOI] [PubMed] [Google Scholar]
- 42.Keefe DL, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women--toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192(4):1256–60. doi: 10.1016/j.ajog.2005.01.036. discussion 1260-1. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Higuera I, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7(2):249–62. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann ER, et al. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr Biol. 2002;12(22):1908–18. doi: 10.1016/s0960-9822(02)01262-9. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107(11):4223–33. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 46.Skalka AM, Katz RA. Retroviral DNA integration and the DNA damage response. Cell Death Differ. 2005;12(Suppl 1):971–8. doi: 10.1038/sj.cdd.4401573. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Mari A, et al. Roles of brca2 (fancd1) in oocyte nuclear architecture, gametogenesis, gonad tumors, and genome stability in zebrafish. PLoS Genet. 2011;7(3):e1001357. doi: 10.1371/journal.pgen.1001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Mari A, et al. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010;6(7):e1001034. doi: 10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Mari A, Postlethwait JH. The Role of Fanconi Anemia/BRCA Genes in Zebrafish Sex Determination. Methods Cell Biol. 2011;105:461–90. doi: 10.1016/B978-0-12-381320-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 50.Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol. 1997;146(9):771–5. doi: 10.1093/oxfordjournals.aje.a009353. [DOI] [PubMed] [Google Scholar]