Abstract
The traditional notion of chelation therapy is the administration of a chemical agent to remove metals from the body. But formation of a metal-chelate can have biological ramifications that are much broader than metal elimination. Exploring these other possibilities could lead to pharmacological interventions that alter the concentration, distribution, or reactivity of metals in targeted ways for therapeutic benefit. This review highlights recent examples that showcase four general strategies of using principles of metal chelation in medicinal contexts beyond the traditional notion of chelation therapy. These strategies include altering metal biodistribution, inhibiting specific metalloenzymes associated with disease, enhancing the reactivity of a metal complex to promote cytotoxicity, and conversely, passivating the reactivity of metals by site-activated chelation to prevent cytotoxicity.
Introduction
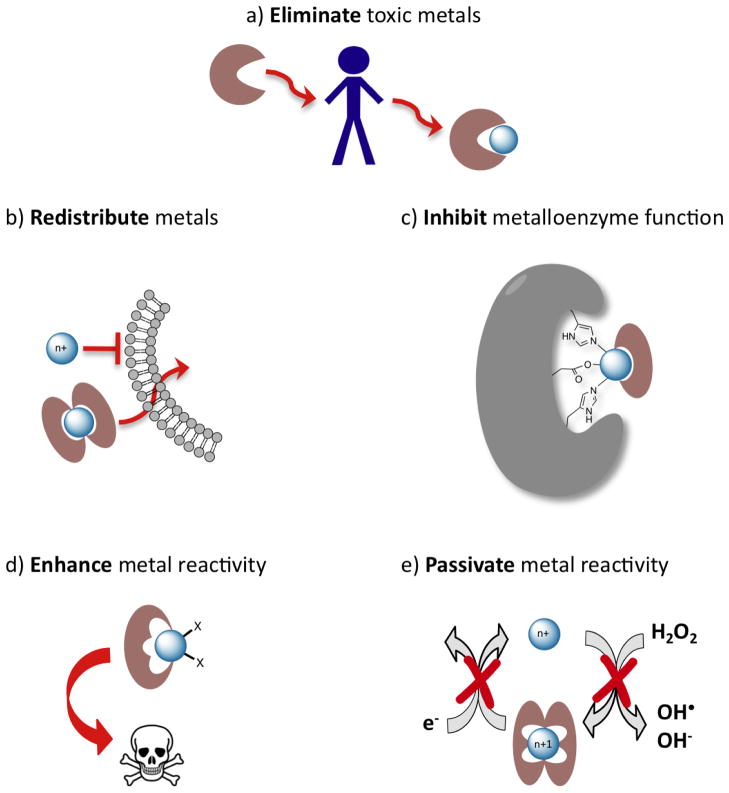
The US Food and Drug Administration has approved the use of chelating agents for conditions of heavy metal overload that are clearly pathogenic and clinically verified. Whether the species to be removed is a native metal like iron or copper, or a contaminant that arrived by nefarious or accidental means, like arsenic, mercury or plutonium, the goal of chelation therapy as defined by its approved medical uses is to inhibit the action of transgressing metals by sequestering them in high-affinity complexes that are excreted through the liver or kidneys. But not all chelating agents are the same. Their chemistry is not the same, and their biological response will certainly not be the same. If a metal ion has a biological role, either beneficial or pathological, there is a common misconception that selectively chelating it will inhibit that biological activity. There are two problems with that misconception. The first is about selectivity, since it is very difficult to have exquisite selectivity for one metal ion over all others, especially in a complex biological environment. The second is about the fate of the resulting metal complex, which may itself have a biological effect. Pharmacological interventions that alter the concentration, distribution, and reactivity of endogenous metals can have profound biological repercussions–for good or bad. The following vignettes showcase the range and scope of chelating agents that may have clinical utility beyond the traditional notion of metal removal. The chosen examples are by no means exhaustive, but represent recent advances in four general categories of the way chelating agents can influence the biological activity of metal species (Figure 1).
Figure 1.
Not all chelating agents are the same. (a) The traditional notion of chelation therapy as reducing the total body burden of a heavy metal. Other approaches that use principles of metal chelation include (b) redistributing a metal across biological membranes via formation of neutral, lipophilic complexes; (c) inactivating an enzyme active site by taking advantage of metal chelation to form protein-metal-chelator ternary complexes; (d) enhancing the reactivity of a metal by forming a chelate complex, for example that promotes redox cycling and generation of reactive oxygen species or other cytotoxic products; (e) inactivating the reactivity of a metal, for example by forming a chelate complex that prevents Fenton chemistry where redox cycling catalyzes formation of reactive oxygen species. Each example shows a generic metal, as these approaches can in principle apply across the periodic table.
Chelating Agents: A Medical Perspective
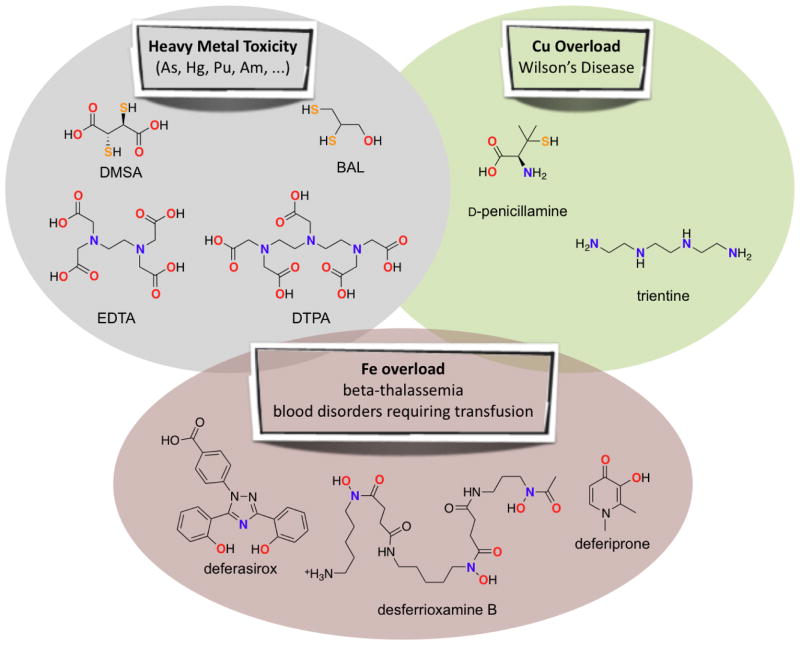
From a clinical viewpoint, chelation therapy refers to the administration of a chemical agent to remove heavy metals from the body. It originated in the mid 20th century when compounds were developed to mitigate arsenic toxicity associated with chemical warfare agents like dichlorovinylarsine, known as Lewisite, as well as arsenic-containing syphilis treatments.[1,2] British Anti-Lewisite (BAL), along with the more hydrophilic and less toxic DMSA (Figure 2), are still used to treat cases of acute metal poisoning by arsenic, mercury, lead and gold. Subsequently, the polyamino carboxylic acid chelators EDTA and DTPA (Figure 2) were adopted for decorporation of contaminating heavy metals, particularly lead and radionuclides like plutonium, americium and curium.
Figure 2.
FDA-approved chelating agents for indications related to heavy metal toxicity, copper overload, and iron overload. Atoms involved in binding to metal ions are highlighted in bold and color-coded. Full names and other abbreviations or common names: DMSA (meso-2,3-dimercaptosuccinic acid, succimer); BAL (dimercaprol, meso-2,3-dimercaptopropanol); EDTA (ethylenediaminetetraacetic acid); DTPA (diethylene triamine pentaacetic acid); D-penicillamine (Cuprimine, Depen, dPen); trientine (triethylenetetramine, trien); desferrioxamine B (DFO, deferoxamine, desferal), deferasirox (Exjade), and deferiprone (Ferriprox).
In addition to removing toxic foreign metals and metalloids that have no native biological function, chelation therapy is also used to reduce levels of essential metals in cases of copper or iron overload disorders. In Wilson’s Disease, mutation in the ATP7B gene results in blockage of normal copper trafficking, which leads to dangerous accumulation of copper in the liver that ultimately spreads to other organs, including the brain.[3] Painful treatments using BAL lead to the development of D-penicillamine and triethylenetetramine (Figure 2).[4]
Iron overload can result from hemochromatosis, a family of genetic diseases resulting in unregulated iron absorption, or from repetitive blood transfusions that are necessary for treating blood diseases like β-thalassemia and sickle cell disease.[5] Three chelators are now approved for iron overload: desferrioxamine B, deferasirox, and deferiprone (Figure 2).
Chelating Agents: An Internet Perspective
With easy access to information on the internet, patients who suffer from incurable diseases, who are not responding to established medical practices, or who are wary of the medical establishment, can readily find marketers of “chelation therapy” who promise cure-alls for all kinds of diseases, including cardiovascular disease, cancer, autism, and dementia. The premise for chelation therapy in these cases is similar to that of its approved uses: remove heavy metals from the body. However, this premise is controversial for most of these indications, and unjustified and harmful in others. For example, EDTA and DMSA have both been erroneously promoted as a treatment for autism based on the now debunked hypothesis that vaccines containing ethylmercury (thimerasol) trigger the disease.[6] Ill-advised chelation therapy can be tragic, as documented for a boy who died of a heart attack after chelation therapy with EDTA.[7] A 2006 clinical trial of Mercury Chelation to Treat Autism, sponsored by the US National Institute of Mental Health, was cancelled in 2008 because of the disproportionate risk of the treatment and marginal confidence in its efficacy.[8] The trial’s cancellation was in part influenced by a study in rats showing that DMSA treatment could indeed improve cognitive outcomes in rats with lead poisoning, but it also caused lasting and pervasive cognitive impairment in normal animals.[9]
Soon after the autism chelation trial was halted, the Trial to Assess Chelation Therapy (TACT) for coronary artery disease was suspended.[8,10] Chelation therapy for cardiovascular disease was described in the 1950s based on the hypothesis that calcium chelation would disperse arterial plaques. Subsequent theories promoted by chelation practitioners posit that EDTA removes heavy metals and reduces oxidative stress, regardless of the well-known catalytic activity of Fe-EDTA to generate reactive oxygen species.[10] Research has found no evidence for efficacy of EDTA chelation therapy for cardiovascular disease.[11]
The over-hyped promises, lack of reputable proof of efficacy, and scientifically unsound justifications for these non-approved uses of chelating agents have contributed to a negative view of chelation. But, is there something there? Over one-third of our proteins are metalloproteins. Metals play integral roles in our biochemistry, acting as cofactors that are required for enzymatic function. Intervening in the bioinorganic processes of a cell or organism by using principles of metal chelation can be powerful. Do the failures and bad reputations of a few chelators mean we should dismiss the whole notion? The answer of course is NO! The reasoning for this answer is embedded in a much broader definition of “chelation.”
Chelating Agents: A Chemist’s Perspective
The word “chelator” derives from the Greek “chele” for “claw”, which provides a good visual cue to the function of these molecules to clamp down on a metal ion the way a lobster claw grasps its prey. A chelator, or chelating agent, refers to a ligand that coordinates to a metal center by more than one point of attachment, thereby forming a ring with the metal atom.[12] The resulting metal complex is referred to as a chelate. The term chelator itself does not distinguish the number of points of attachment to a metal, so a chelator can be bidentate or multidentate, and more than one chelator may be bound to a metal. Due to the chelate effect, these multiple points of attachment provide more stability to the complex compared to one-site ligands of the same atom type. Aside from this thermodynamic advantage, the term does not infer other information about the properties of the chelate, which are very different from the metal ion and ligand individually, and from chelates of the same metal but different ligands. Chemical properties of charge, solubility, lipophilicity, redox potential, shape, pH dependence, and kinetics and thermodynamics of both ligand exchange and metal exchange can all influence the biological outcome of a chelator and its chelates.[13]
Metal Redistribution
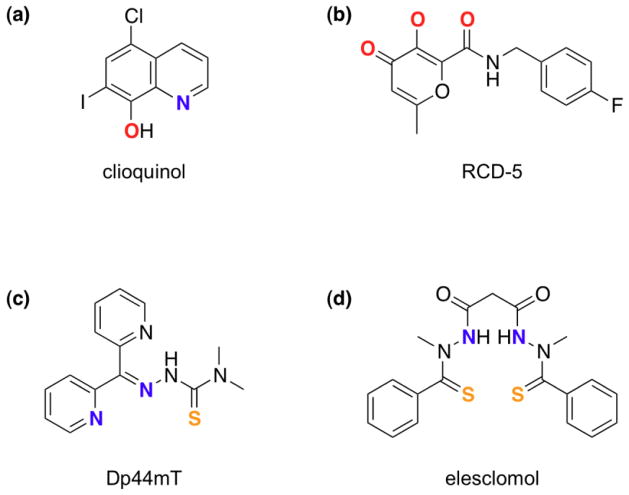
The last 2 decades have seen a growing interest in the hypothesis that chelating agents (chemically defined) may have potential to treat neurodegenerative diseases.[14] Several recent reviews cover the creative attempts of chemists to design molecules for such challenging applications.[2,15–17] One of the drivers behind this surge is the moderate success of clioquinol (Figure 3), a bidentate chelating agent with a history of use as an antimicrobial.[18]
Figure 3.
Specialized chelating agents discussed in the text; atoms proposed to bind to metals are in bold and color-coded. (a) clioquinol acts as an ionophore that can shuttle metal ions across biological membranes; (b) RCD-5 inhibits HIV integrase by binding to the di-magnesium active site of the enzyme via the 3-oxygen chelating motif;[23] (c) Dp44mT is a cytotoxic agent that can bind both iron and copper as reactive complexes;[28] (d) elesclomol’s cancer cell toxicity is thought to be mediated via its redox-active copper complex.[30]
The story of clioquinol in the Alzheimer’s Disease arena highlights the basic but important message of this article: not all chelators are the same. While the premise for its use in Alzheimer’s was rooted in the classic idea of chelator as metal-sequestering agent, its biological mechanism of action is far more complex. It had been observed in vitro that Cu2+ and Zn2+ accelerate the aggregation of the amyloid beta (Aβ) peptide and that compounds like clioquinol prevent that process, even in vivo.[19] Since these initial experiments in the 1990s, significant advances have been made in the biochemical underpinnings of Alzheimer’s and the biological activity of clioquinol and PBT2, a related proprietary compound of Prana Biotechnology. An historical account of the evolving understanding of the mechanism of action of these agents has recently appeared.[20] A key piece to the puzzle was the recognition of the chaperone-like activity of 8-hydroxyquinolines to redistribute metal ions into cells.[21] Upon deprotonation, these ligands bind divalent metals like Zn2+ or Cu2+ in a 2:1 ligand:metal ratio to form neutral, lipophilic species that facilitate diffusion across cellular membranes. This activity is in essence the opposite of the clinical definition of chelation therapy described above, but is completely consistent with the chemical definition of chelating agent.
The current model for the biological mechanism of action of PBT2 involves dislodging extracellular Cu2+ and Zn2+ from amyloid plaques and redistributing them back into the cell, where the metal cargo activates neuroprotective cell-signaling pathways.[20] A key feature of this class of compounds is their moderate affinity for metal ions. While this appears to contradict the prevailing medical definition of chelators as high-affinity sequestering agents, it serves well in this capacity, where they are strong enough to pull Cu2+ and Zn2+ out of Aβ plaques but not strong enough to inhibit or de-metallate metalloenzymes.
Passivating Metalloenzymes with Chelating Inhibitors
Metalloproteins comprise at least one-third of the human proteome, and many critical catalytic processes are carried out by metal centers within enzyme active sites. While high-affinity, non-selective chelating agents that strip metals from these sites are unlikely drug candidates, well-designed inhibitors that use chelating motifs to fill open coordination sites on the metal can block substrate access or otherwise disrupt catalytic function. Metal-binding pharmacophores are not often included in molecular libraries used to screen for drug targets. Cohen and coworkers, however, have recently shown that a chelator fragment library can produce lead compounds that can be further elaborated to generate selective metalloenzyme inhibitors.[22] In a separate study, the same group methodically investigated the structure-reactivity relationship of a series of HIV integrase inhibitors that differed only in the metal-binding headgroup. Their study identified new chelating pharmacophores (one example shown in Figure 3) as promising scaffolds for next-generation HIV integrase inhibitors, and in the process demonstrated the power of using principles of metal coordination chemistry to optimize metalloenzyme inhibition.[23]
Chelators to Generate Cytotoxic Chelates
Continuing on the message that not all chelators are the same, this vignette showcases just how significant the chemical properties of a chelated complex are on the biological activity of a chelator. Because rapidly proliferating cancer cells need iron, iron chelation may be an effective anticancer strategy.[24] While iron sequestration from cancer cells would seem like a plausible strategy, iron chelators with potent anticancer activity have a more complex biological effect that is not associated with iron depletion, but rather with the redox activity of the iron complex itself.[25,26] Furthermore, referring to these agents as “iron chelators” invokes an unintentional misconception that binding iron is their sole mode of action. Recall that most chelators that bind iron will also bind other metal ions. An interesting example is Dp44mT, a chelating agent with potent antitumor activity (Figure 3).[26] While its coordination of iron is likely relevant to tumor toxicity,[27] Richardson and coworkers recently showed that its redox-active copper complexes activate the lysosomal apoptotic pathway.[28] Treatment with Dp44mT causes significant efflux of iron from cells in culture, but has the opposite effect on copper, which accumulates particularly within lysosomal compartments. This latter activity is directly related to its chemical properties: the acidic pH within the lysosome favors protonation to give a charged Dp44mT+ that becomes trapped inside the lysosome. Binding of endogenous copper forms a redox-active complex that promotes formation of reactive oxygen species, which degrade the lysosomal membrane and subsequently lead to apoptosis.[28]
The oncology drug candidate elesclomol (Figure 3), which has been evaluated in clinical trials,[29] appears to operate via a similar copper-associated mechanism.[30] Copper is essential for its selective cancer cell toxicity. Experiments in cell culture showed that elesclomol shuttles copper from the extracellular environment to intracellular compartments, with notable accumulation in mitochondria.[30] Not only do the chemical properties of the Cu-elesclomol chelate direct its subcellular localization, they also provide a redox potential that is key to its activity. The redox-active complex appears to associate and disrupt the mitochondrial electron transport chain, ultimately leading to oxidative stress and cell death.[31]
Passivating Metal Reactivity by Triggering Prochelators
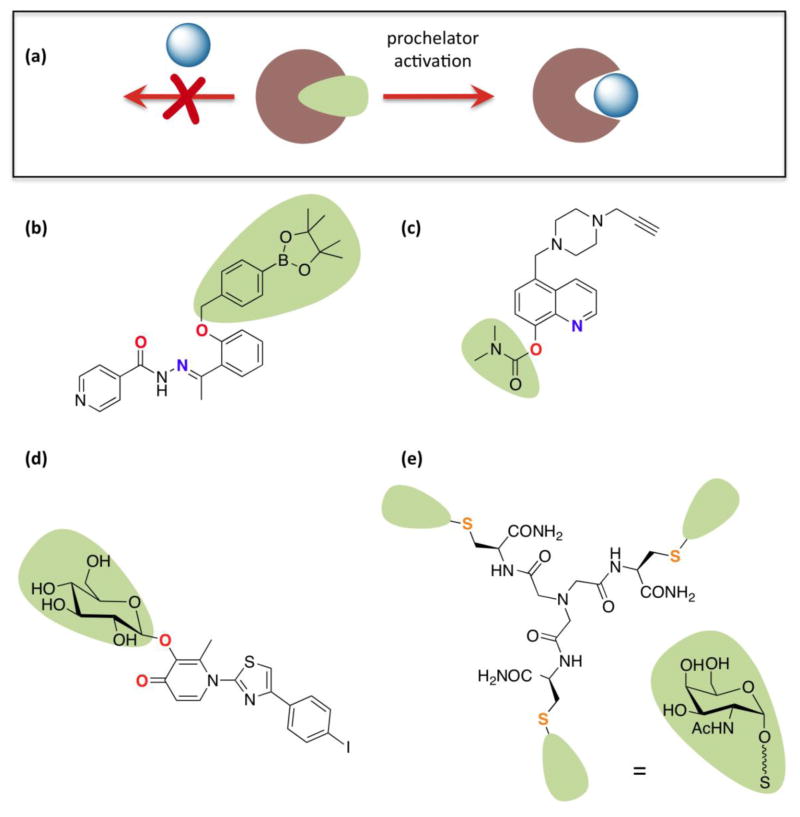
On the flip side of using chelators to generate cytotoxic metal complexes is to use them to passivate redox activity for cytoprotection. Finding the balance between high-affinity metal chelators that passivate the harmful reactions of metals without disturbing normal metal status is the impetus for designing agents that alter their metal-binding capacity in response to a disease-related trigger (Figure 4a). Recognizing that oxidative stress is associated with many diseases, and that metal-catalyzed reactions like the Fenton reaction contribute to oxidative stress (Figure 1e), we introduced boronate-based prochelators in 2006 as agents that do not bind metals until unmasking by hydrogen peroxide releases a chelator that passivates iron and copper against Fenton reactivity.[32] A later-generation prochelator called BHAPI (Figure 4b) has recently been shown to protect cells from damage by paraquat, an herbicide that promotes cellular oxidative stress but is not itself an oxidant.[33] Importantly, BHAPI does not perturb the iron status of non-stressed cells, unlike standard iron chelators.[33]
Figure 4.
(a) General prochelator strategy, where a masking group blocks metal binding until activation releases the chelating unit. For specific prochelator examples, the atoms ultimately involved in metal chelation are in bold and color-coded; (b) BHAPI, which can be activated by H2O2;[33] (c) HLA20A, which is activated by acetylcholinesterase and has multifunctional neurorescueing properties;[35] (d) a multifunctional, amyloid targeting prochelator activated by glucosidase enzymes;[37] (e) a tripodal glycoconjugate that targets hepatocytes and is reductively activated intracellularly to release a Cu(I) chelator (squiggly line represents ethylene glycol linker).[38]
Enzyme activation provides another strategy for site-directed chelator release.[34] Youdim and Fridkin have generated prochelators that are activated by acetylcholinesterase and have multifunctional properties of monoamine oxidase inhibition and metal passivation that make them attractive compounds for applications in neurodegenerative diseases (Figure 4c).[35,36] By using a glycosyl as the chelator-masking group, Orvig and coworkers have created enzyme-activatable prochelators that have the added benefit of hijacking glucose uptake pathways for improving brain uptake of their multifunctional agents (Figure 4d).[37] In a different twist on this theme, Mintz, Delangle and coworkers incorporated N-acetyl-galactosamine units into prochelator masking groups as a way to direct the agent to its most needed location, in this case hepatocytes, which are most affected by copper overload disorders. The tripodal, disulfide-protected prochelator is reduced intracellularly to generate a high-affinity Cu(I) chelator (Figure 4e).[38,39]
Conclusion and outlook
In conclusion, I reiterate a now recognizable theme: not all chelators are the same. The concept that metal–ligand binding produces complexes with unique chemical properties has been a foundational principle of coordination chemistry since Alfred Werner. Yet the subtleties of these interactions continue to amaze, especially in the context of how these complexes and their individual components interface with complex biological environments. We have much still to learn and explore. It is clear from this small sampling of vignettes that the principles of metal chelation provide significant opportunities for drug design that go beyond traditional notions of chelation as metal sequestration and elimination. Establishing which chemical properties are desirable design features for a particular application is an active area of research.[17] Properties like charge, lipophilicity, lability, shape, redox potential, etc. can all be tweaked by synthetic coordination chemists to create custom chelators that can be tested relatively easily in cellular assays. Figuring out if and how those design elements translate from the cellular context to a clinical setting remains an open frontier.
Supplementary Material
Highlights.
Not all chelators are the same: more than metal sequestration and elimination
Metal chelates can redistribute metals across biological membranes
Metal-chelating fragments provide leads for metalloenzyme inhibition
Metal chelation can enhance metal reactivity and thus cytotoxicity
Prochelators provide site-activated metal chelation and passivation
Acknowledgments
I would like to thank my current and former group members for their many contributions. Our work in this area has been funded by the National Institutes of Health (GM084176), the Sloan Foundation, the Camille and Henry Dreyfus Foundation, and the National Science Foundation (CHE-1152054).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Jones CJ, Thornback J. Medicinal applications of coordination chemistry. Cambridge: Royal Society of Chemistry; 2007. [Google Scholar]
- 2.Scott LE, Orvig C. Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chem Rev. 2009 doi: 10.1021/cr9000176. [DOI] [PubMed] [Google Scholar]
- 3.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. The Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 4.Walshe JM. The story of penicillamine: A difficult birth. Move Disord. 2003;18:853–859. doi: 10.1002/mds.10458. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt PV. Coordination chemistry and biology of chelators for the treatment of iron overload disorders. Dalton Trans. 2007:3214–3220. doi: 10.1039/b708133b. [DOI] [PubMed] [Google Scholar]
- 6.Baker JP. Mercury, vaccines, and autism. Am J Pub Health. 2008;98:244–253. doi: 10.2105/AJPH.2007.113159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter AJ, Krenzelok EP. Pediatric fatality secondary to edta chelation. Clin Toxicol. 2008;46:1083–1084. doi: 10.1080/15563650701261488. [DOI] [PubMed] [Google Scholar]
- 8.Mitka M. Chelation therapy trials halted. J Am Med Assoc. 2008;300:2236–2236. doi: 10.1001/jama.2008.607. [DOI] [PubMed] [Google Scholar]
- 9.Stangle DE, Smith DR, Beaudin SA, Strawderman MS, Levitsky DA, Strupp BJ. Succimer chelation improves learning, attention, and arousal regulation in lead-exposed rats but produces lasting cognitive impairment in the absence of lead exposure. Environ Health Perspect. 2007;115:201–209. doi: 10.1289/ehp.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atwood KC, Woeckner E, Baratz RS, Sampson WI. Why the NIH trial to assess chelation therapy (TACT) should be abandoned. Medscape J Med. 2008;10:1–115. [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst E. Chelation therapy for coronary heart disease: An overview of all clinical investigations. Am Heart J. 2000;140:139–141. doi: 10.1067/mhj.2000.107548. [DOI] [PubMed] [Google Scholar]
- 12.Shriver Atkins. Inorganic chemistry. 5. New York: W. H. Freeman; 2010. [Google Scholar]
- 13.Haas KL, Franz KJ. Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev. 2009;109:4921–4960. doi: 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duce JA, Bush AI. Biological metals and Alzheimer’s disease: Implications for therapeutics and diagnostics. Prog Neurobiol. 2010;92:1–18. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Perez LR, Franz KJ. Minding metals: Tailoring multifunctional chelating agents for neurodegenerative disease. Dalton Trans. 2010;39:2177–2187. doi: 10.1039/b919237a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braymer JJ, Choi J-S, DeToma AS, Ko KS, Lim MH. Recent development of bifunctional small molecules to study metal-amyloid-β species in Alzheimer’s disease. Int J Alz Dis. 2011 doi: 10.4061/2011/623051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Rodríguez C, Telpoukhovskaia M, Orvig C. The art of building multifunctional metal-binding agents from basic molecular scaffolds for the potential application in neurodegenerative diseases. Coord Chem Rev. 2012;256:2308–2332. [Google Scholar]
- 18.Bareggi SR, Cornelli U. Clioquinol: Review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther. 2012;18:41–46. doi: 10.1111/j.1755-5949.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y-S, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- •20.Crouch PJ, Barnham KJ. Therapeutic redistribution of metal ions to treat Alzheimer’s disease. Acc Chem Res. 2012;45:1604–1611. doi: 10.1021/ar300074t. Provides a detailed account of how the mechanism of action of clioquinol and other metal ion redistributing agents has been elucidated for potential application in neurodegenerative disease. [DOI] [PubMed] [Google Scholar]
- 21.Treiber C, Simons A, Strauss M, Hafner M, Cappai R, Bayer TA, Multhaup G. Clioquinol mediates copper uptake and counteracts copper efflux activities of the amyloid precursor protein of alzheimer’s disease. J Biol Chem. 2004;279:51958–51964. doi: 10.1074/jbc.M407410200. [DOI] [PubMed] [Google Scholar]
- ••22.Jacobsen JA, Fullagar JL, Miller MT, Cohen SM. Identifying chelators for metalloprotein inhibitors using a fragment-based approach. J Med Chem. 2010;54:591–602. doi: 10.1021/jm101266s. Demonstrates how basic principles of metal chelation can be used to design better inhibitors for metalloenzymes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal A, DeSoto J, Fullagar JL, Maddali K, Rostami S, Richman DD, Pommier Y, Cohen SM. Probing chelation motifs in HIV integrase inhibitors. Proc Natl Acad Sci USA. 2012;109:2251–2256. doi: 10.1073/pnas.1112389109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Wong J, Lovejoy DB, Kalinowski DS, Richardson DR. Chelators at the cancer coalface: Desferrioxamine to triapine and beyond. Clin Can Res. 2006;12:6876–6883. doi: 10.1158/1078-0432.CCR-06-1954. [DOI] [PubMed] [Google Scholar]
- •25.Aye Y, Long MJC, Stubbe J. Mechanistic studies of semicarbazone triapine targeting human ribonucleotide reductase in vitro and in mammalian cells: Tyrosyl radical quenching not involving reactive oxygen species. J Biol Chem. 2012 doi: 10.1074/jbc.M112.396911. This paper details an alternative mechanism for how metal complexes can be cytotoxic that is more specific than general induction of oxidative stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson DR, Sharpe PC, Lovejoy DB, Senaratne D, Kalinowski DS, Islam M, Bernhardt PV. Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J Med Chem. 2006;49:6510–6521. doi: 10.1021/jm0606342. [DOI] [PubMed] [Google Scholar]
- ••28.Lovejoy DB, Jansson PJ, Brunk UT, Wong J, Ponka P, Richardson DR. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011;71:5871–5880. doi: 10.1158/0008-5472.CAN-11-1218. This paper shows that the copper complex of an “iron chelator” plays an important role in the cytotoxic mechanism of action of this interesting class of compounds. [DOI] [PubMed] [Google Scholar]
- 29.O’Day S, Gonzalez R, Lawson D, Weber R, Hutchins L, Anderson C, Haddad J, Kong S, Williams A, Jacobson E. Phase ii, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J Clin Oncol. 2009;27:5452–5458. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- ••30.Nagai M, Vo NH, Shin Ogawa L, Chimmanamada D, Inoue T, Chu J, Beaudette-Zlatanova BC, Lu R, Blackman RK, Barsoum J, et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radical Biol Med. 2012;52:2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. Shows that even drugs that were not designed to interact with metals can harness endogenous metals to effect significant cytotoxicity. [DOI] [PubMed] [Google Scholar]
- 31.Blackman RK, Cheung-Ong K, Gebbia M, Proia DA, He S, Kepros J, Jonneaux A, Marchetti P, Kluza J, Rao PE, et al. Mitochondrial electron transport is the cellular target of the oncology drug elesclomol. PLoS ONE. 2012;7:e29798. doi: 10.1371/journal.pone.0029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charkoudian LK, Pham DM, Franz KJ. A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. J Am Chem Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- •33.Kielar F, Helsel ME, Wang Q, Franz KJ. Prochelator bhapi protects cells against paraquat-induced damage by ROS-triggered iron chelation. Metallomics. 2012;4:899–909. doi: 10.1039/c2mt20069d. This paper shows that a peroxide-activated prochelator can be induced by compounds that generate oxidative stress intracellularly, but are not themselves oxidants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folk DS, Franz KJ. A prochelator activated by beta-secretase inhibits Aβ aggregation and suppresses copper-induced reactive oxygen species formation. J Am Chem Soc. 2010;132:4994–4995. doi: 10.1021/ja100943r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •35.Zheng H, Youdim MBH, Fridkin M. Selective acetylcholinesterase inhibitor activated by acetylcholinesterase releases an active chelator with neurorescuing and anti-amyloid activities. ACS Chem Neurosci. 2010;1:737–746. doi: 10.1021/cn100069c. This paper is a very nice example of incorporating multifunctionality into agents targeting neurodegenerative disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng H, Youdim MBH, Fridkin M. Site-activated chelators targeting acetylcholinesterase and monoamine oxidase for alzheimer’s therapy. ACS Chem Biol. 2010;5:603–610. doi: 10.1021/cb900264w. [DOI] [PubMed] [Google Scholar]
- 37.Scott LE, Telpoukhovskaia M, Rodriguez-Rodriguez C, Merkel M, Bowen ML, Page BDG, Green DE, Storr T, Thomas F, Allen DD, et al. N-aryl-substituted 3-(β-d-glucopyranosyloxy)-2-methyl-4(1H)-pyridinones as agents for Alzheimer’s therapy. Chem Sci. 2011;2:642–648. [Google Scholar]
- •38.Pujol AM, Cuillel M, Jullien A-S, Lebrun C, Cassio D, Mintz E, Gateau C, Delangle P. A sulfur tripod glycoconjugate that releases a high-affinity copper chelator in hepatocytes. Angew Chem Int Ed. 2012;51:7445–7448. doi: 10.1002/anie.201203255. Combines multiple principles in one compound: targets the organ most likely associated with copper overload, and contains a prochelator trigger to prevent adventitious metal binding. [DOI] [PubMed] [Google Scholar]
- 39.Delangle P, Mintz E. Chelation therapy in Wilson’s disease: From d-penicillamine to the design of selective bioinspired intracellular cu(I) chelators. Dalton Trans. 2012 doi: 10.1039/c2dt12188c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.