Abstract
Infection with Kaposi’s Sarcoma-Associated Herpesvirus (KSHV/HHV-8) is common among men who have sex with men (MSM). Here quantitative anti-KSHV antibody levels were measured using Luciferase Immunoprecipitation Systems (LIPS) in a MSM cohort with and without HIV from the NIH Clinical Center. Antibodies were detected using a mixture of four KSHV antigens in the MSM cohort and in Kaposi Sarcoma (KS) patients. Along with HIV status, these results were compared with K8.1 and ORF73 ELISA, PCR virus detection, and additional LIPS testing. LIPS revealed that 25% (76/307) of the MSM cohort were KSHV seropositive, including 59 HIV+ and 17 HIV− subjects. The anti-KSHV antibody levels detected by LIPS were not statistically different between the KSHV+/HIV+ and KSHV+/HIV− subgroups, but were lower than the KS patients (P<0.0001). ELISA analysis of the MSM cohort detected a 35.5% frequency of KSHV infection and showed agreement with 81% of the samples evaluated by LIPS. Further LIPS testing with v-cyclin, a second ORF73 fragment and ORF38 reconciled some of the differences observed between LIPS and the ELISA immunoassays and the revised LIPS seroprevalence in the MSM cohort was increased to 31%. Additional quantitative antibody analysis demonstrated statistically lower KSHV antibody levels in MSM compared to KS patients, but no difference was found between KSHV infected with and without HIV coinfection. These findings also suggest that antibodies against v-cyclin and ORF38 are useful for identifying patients with asymptomatic KSHV infection.
Keywords: antibodies, Kaposi’s Sarcoma-Associated Herpesvirus (KSHV), MSM
1. Background
Kaposi sarcoma–associated herpes virus (KSHV), also called human herpesvirus-8 (HHV-8), is one of eight known cancer-causing viruses and is the agent responsible for Kaposi sarcoma (KS) (Mesri, Cesarman, and Boshoff, 2010). KSHV infection can also cause rare B-cell lymphoproliferative disorders in HIV-infected individuals including primary effusion lymphoma (PEL), multicentric Castleman’s disease (MCD), and a newly described interleukin 6-related inflammatory syndrome (Gantt and Casper, 2011; Uldrick et al., 2010). Epidemiological surveys based on serological assays demonstrate that KSHV infection occurs more commonly in men who have sex with men (MSM) compared to the general population. Previous studies have detected KSHV seropositivity in the range of 24%-45% in several MSM cohorts, likely reflecting different KSHV infectivity in different populations and possibly the performance of the immunoassays used (Batista et al., 2009; Casper et al., 2006; Casper et al., 2002; Giuliani et al., 2007; Guanira et al., 2008; Martin et al., 1998; Mbulaiteye et al., 2006). Studies have also shown that KSHV infection is associated with a number of factors including coinfection with HIV, the number of sexual partners, and co-infections with several other agents including herpes simplex virus-2 (HSV-2) and syphilis (Casper et al., 2006; Casper et al., 2002; Engels et al., 2007; Mbulaiteye et al., 2006).
Despite the many studies describing the seroprevalence of KSHV in different populations of patients with KS, the diagnostic performance of the immunoassays used to detect KSHV infection, such as immunofluorescence assays and ELISAs, have not been fully validated. This is in part because there is no gold standard technique for evaluating the diagnostic performance of other assays in asymptomatic KSHV-infected individuals without KS. Instead, the performance of most immunoassays is based on studies with KS samples, which may lead to over-estimation of assay sensitivity. Immunofluorescence-based immunoassays for KSHV diagnosis are not highly quantitative and require subjective interpretation. With ELISA, at least two major antigens (K8.1 and latency-associated nuclear antigen (LANA/ORF73) are usually evaluated with diagnostic algorithms to achieve high sensitivity and specificity (Laney et al., 2006; Mbisa et al., 2010). Many of the ELISA used to detect KSHV infection in KS patients show diagnostic performance of 94-99% sensitivity and 98-100% specificity (Laney et al., 2006; Mbisa et al., 2010).
Recently, luciferase immunoprecipitation systems (LIPS), a liquid-phase immunoassay employing light emitting protein, was used to quantitatively measure KSHV antibody levels and diagnose infection (Burbelo et al., 2010; Burbelo et al., 2009b). LIPS testing of K8.1, ORF73 fragment (LANA-Δ2 fragment) and ORF65, along with a new latent KSHV antigen v-cyclin, showed 99% sensitivity and 100% specificity for the diagnosis of patients with KS and showed similar diagnostic performance to performing ELISAs with K8.1 and ORF73 (Burbelo et al., 2009b). In this same study, a mixture of these four antigens in the LIPS format also simplified testing and provided an accurate method to diagnose KS. In another LIPS study, evaluation of antibody levels against latent and lytic KSHV antigens detected statistically significant differences in patients with KS, and MCD (Burbelo et al., 2010). The goal of the current study was to employ LIPS to evaluate anti-KSHV antibody levels in an MSM cohort with and without HIV infection, but without KS and compare the diagnostic results with ELISA to determine the usefulness of LIPS in detecting asymptomatic KSHV infection.
2. Material and methods
2.1 Clinical specimens
Serum samples were obtained between 1988 and 1999 from MSM subjects who were participating in IRB-approved protocols at the NIH Clinical Center. All participants provided signed informed consent for their original protocols, and the current study was approved by the NIAID, IRB. The studies were conducted according to the principles expressed in the Declaration of Helsinki. All of the HIV-1 positive samples were taken before 1996, prior to the availability of HAART (nucleoside analog reverse transcriptase inhibitors were available). For testing by the LIPS mixture and ELISA assays, the MSM samples (n=307) were coded, and the code was broken only after antibody levels and KSHV infection status was determined. This MSM cohort was previously characterized for HIV infection and contained 144 HIV-1 positive and 163 HIV negative samples.
2.2 LIPS analysis
For LIPS analysis, a four KSHV antigen mixture (K8.1, ORF73-Δ2, ORF65 and v-cyclin) was used that has been previously described (Burbelo et al., 2009b). Extracts of Renilla luciferase (Ruc) antigen fusions of KSHV proteins were prepared from Cos1 cells using a transfection protocol essentially as described (Burbelo et al., 2009a). These Ruc-antigen extracts were then used in LIPS with serum samples in a 96-well format at room temperature. Serum samples were first diluted 1:10 in assay buffer A (50 mM Tris, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100) using a 96-well polypropylene microtiter plate. Antibody titers were measured by adding 40 μl of buffer A, 10 μl of diluted human sera (1 μl equivalent), and 1 × 107 light units (LU) of each of the Ruc-KSHV antigens as a mixture or as single antigen tests containing crude Cos1 cell extract to wells of a polypropylene plate and incubated for 60 minutes at room temperature on a rotary shaker. Next, 5 μl of a 30% suspension of Ultralink protein A/G beads (Pierce Biotechnology, Rockford, IL) in PBS were added to the bottom of each well of a 96-well filter HTS plate (Millipore, Bedford, MA). To this filter plate, the 100 μl antigen-antibody reaction mixture was transferred and incubated for 60 minutes at room temperature on a rotary shaker. The washing steps of the retained protein A/G beads were performed on a Biomek Workstation with a vacuum manifold. After the final wash, LU were measured in a Berthold LB 960 Centro microplate luminometer (Berthold Technologies, Bad Wilbad, Germany) using coelenterazine substrate mix (Promega, Madison, WI). All LU data was obtained from the average of at least two independent experiments and have been corrected for background LU values of Ruc Cos-1 cell mixture extract added to protein A/G beads, but not incubated with plasma.
For testing with the mixture of four KSHV antigens called the “LIPS Aggregate Ag”, a defined cutoff (17,000 LU) was used to determine seropositive status. For comparison, an additional set of normal blood donors previously shown to be seronegative for KSHV infection and 34 random KS samples were also studied by LIPS (Burbelo et al., 2009b). Follow-up LIPS tests included evaluation of antibody levels against single KSHV antigens including v-cyclin, ORF-Δ3 (Burbelo et al., 2009b) and ORF38.
For developing a LIPS test against ORF38 (Zheng et al., 2011), the complete coding sequence was amplified by PCR from KSHV genomic DNA and subcloned downstream of Ruc essentially as previously described for other antigens and used to produce recombinant Ruc-ORF38 lysate from transfected Cos1 cell (Burbelo et al., 2009b). The utility of ORF38 for LIPS serological screening was then validated by examining antibody levels in 20 non-KSHV infected blood donors and in 35 KS patients. The cut-off value (6,022 LU) for determining ORF38 seropositive status in the KS patients and selected MSM samples was based on the value of the mean plus 5 standard deviations of the 20 KSHV uninfected blood donors.
2.3 KSHV ELISA and PCR
KSHV ELISA testing employed baculovirus-produced LANA and bacterially-produced K8.1 antigen to measure anti-KSHV antibodies (Mbisa et al., 2010). A diagnostic algorithm assessing the optical density values from ELISA testing was then used to assess KSHV infection status.
PCR detection of KSHV was performed using DNA was extracted from PBMC from the MSM cohort using the QiaAmp DNA mini kit (Qiagen) and viral load was measured as previously described (Uldrick et al., 2011; Whitby et al., 2007). KSHV DNA was detected using primers for the K6 gene. Cellular equivalents were measured using a qPCR assay for the endogenous retrovirus 3 (Yuan, Miley, and Waters, 2001).
2.4 Statistical analyses
The GraphPad Prism software (San Diego, CA) was used for statistical analysis. The antibody levels for LIPS are based on the geometric mean plus 95% confidence interval (CI). Antibody levels among the groups were statistically analyzed using the Mann-Whitney U test. Conditional odds ratios (OR) and 95% confidence intervals (CI) are also reported.
3. Results
3.1 LIPS detection of KSHV antibodies in MSM
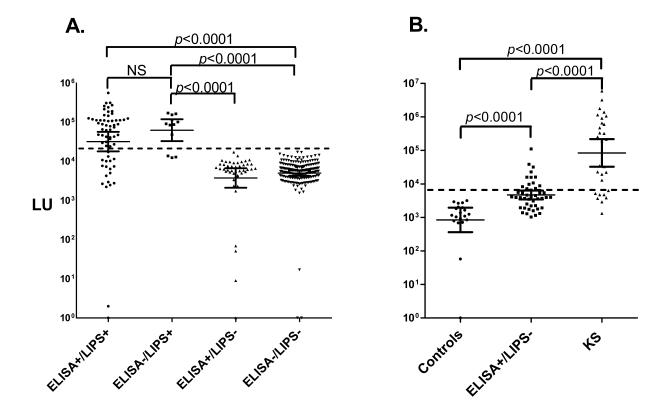
A MSM cohort collected from NIH Clinical Center, NIH was used for assessing the serological detection of asymptomatic KSHV-infected individuals without KS. Testing with the LIPS Aggregate Ag containing K8.1, ORF73 fragment, ORF65 and v-cyclin antigens with 307 serum samples from these men showed highly reproducible antibody values ranging from 0 to 705,414 LU (Fig. 1A). Based on a defined cut-off value corresponding to 17,000 LU, 25% (76 of the 307) of the MSM samples were seropositive for KSHV antibodies (Fig. 1A). Further analysis based on HIV infection status revealed that 48% (146/307) of the MSM samples were KSHV−/HIV−, 28% (85/307) were KSHV−/HIV+, 6% (17/307) were KSHV+/HIV−, and 19% (59/307) were KSHV+/HIV+. In this MSM cohort, KSHV infection was strongly associated with HIV infection (OR, 4.0; 95% CI 2.3-7.0). To confirm immunoreactivity and examine the relative antibody levels in the HIV negative and HIV positive MSM subjects, the KSHV seropositive MSM samples were retested by LIPS alongside serum samples from uninfected blood donors and KS patients. As shown in Fig. 1B, the 17 KSHV+/HIV− samples with a GML of 59,566 LU (95% CI, 41,114 to 86,298 LU) and the 59 KSHV+/HIV+ samples with a GML of 85,310 LU (95% CI, 69,502 to 104,954 LU) showed significantly higher KSHV antibody levels than the KSHV−/HIV− individuals with a geometric mean of 1,871 LU (95% CI, 1,469 to 2,382 LU). In comparison, the KS patients from LIPS testing showed a geometric level of 411,150 LU (95% CI, 294,442 to 574,116 LU) (Fig. 1B). While there was no statistical differences in antibody levels between the KSHV+/HIV+ and KSHV+/HIV− samples (Mann Whitney U test, P=0.10), the KS patients demonstrated statistically higher levels of antibodies than either the KSHV+/HIV+ and KSHV+/HIV− subgroups (Mann Whitney U test, P<0.0001).
Fig 1.
Detection of anti-KSHV antibodies in the MSM cohort by a LIPS Aggregate Ag. (A) Antibodies were evaluated by LIPS with 307 serums samples from the MSM cohort using a 4 antigen mixture. The antibody levels were plotted on the Y-axis using a log10 scale and each symbol represents the values in an individual subject. The geometric mean antibody levels with 95% CI error bars is shown by the short horizontal line and the dashed line represents the cut-off level for determining seropositivity. Based on the cut-off value, 231 samples were KSHV seronegative and 76 were KSHV seropositive. P values for the different groups were calculated using the Mann Whitney U test. (B) All the MSM samples initially detected as KSHV seropositive were retested with the LIPS Aggregate Ag and then segregated based on HIV status. For comparison, the antibody levels are shown for known uninfected controls (n=10) and KS patients (n=34) that were tested side-by side the MSM samples.
3.2 Comparison of LIPS with ELISA for detection of KSHV infection
To gain insight into the diagnostic performance of KSHV LIPS for detecting KSHV seropositivity, serum samples from the MSM cohort were also tested by an ELISA that employed K8.1 and ORF73 KSHV antigens. Analysis of the MSM cohort by ELISA detected a 35.5% frequency of KSHV infection (Table I). Comparison of the ELISA results with LIPS revealed that 21% (64/307) of the MSM samples were positive in both assays, 15% (45/307) were LIPS−/ELISA+, 4% (12/307) were LIPS+/ELISA−, and 60% (186/307) were negative in both immunoassays (Table I). Overall, 81% (250/307) of the serum samples yielded the same serological status between the LIPS and ELISA tests, leaving 19% (57/307) of the serum samples as discrepant. From the 12 samples that were LIPS+/ELISA−, 10 were from HIV+ and 2 from HIV− individuals. Conversely, from the 35 samples that were LIPS−/ELISA+, 34 were HIV+ and 2 were from HIV− individuals (Table I). Altering the cut-off value did not improve concordance with ELISA because ROC analysis showed that lowering the LIPS cutoff from 17,000 LU to 8,169 LU would result in increased seropositivity with 8 of the 35 previous ELISA+/LIPS− samples, but would decrease the specificity because 6 of the previous 186 samples that were LIPS−/ELISA− would now be seropositive.
Table I.
Comparison of MSM Samples 438 for KSHV by ELISA and LIPS Testing
| Assay | Number (%) | HIV+ | HIV− |
|---|---|---|---|
| ELISA+/LIPS Aggregate Ag+ | 64 (21%) | 56 | 8 |
| ELISA−/ LIPS Aggregate Ag− | 186 (60%) | 50 | 136 |
| ELISA−/ LIPS Aggregate Ag+ | 12 (4% ) | 10 | 2 |
| ELISA+/ LIPS Aggregate Ag− | 45 (15% ) | 34 | 11 |
| ELISA+/ LIPS Aggregate Ag−/ORF73−Δ3+ | 13 (4% ) | 6 | 7 |
| ELISA+/ LIPS Aggregate Ag−/ORF38+ | 14 (4%) | 8 | 6 |
| ELISA+/ LIPS Aggregate Ag-/ORF73−Δ3+/ORF38 | 19 (6%) | 14 | 13 |
3.3 q-PCR testing of MSM for KSHV infection
In an effort to reconcile the seropositivity differences between the ELISA and LIPS mixture, q-PCR with KSHV-specific primers were performed on lymphocytes obtained from all the MSM subjects. q-PCR testing identified 31 KSHV positive samples. While 10 of these samples were positive in all three assays, 17 were negative in both LIPS and ELISA. There were two samples positive by the LIPS and q-PCR but negative in ELISA, and two separate samples positive in ELISA and q-PCR but negative in LIPS. These PCR results highlight the difficulty in using nucleic acid testing in determining KSHV infection and motivated us to pursue additional LIPS testing.
3.4 Analysis of v-cyclin antibodies in the MSM cohort
Since the antigen mixture used for LIPS assay, but not the ELISA, employed v-cyclin, the MSM cohort along with the uninfected controls and KS samples was tested for anti-v-cyclin antibodies to determine the individual contribution of this antigen to LIPS positivity. As shown in Figure 2A, 45 of the 64 LIPS+/ELISA+ samples (70%) were positive for v-cyclin antibodies, but none of the LIPS−/ELISA+ or LIPS−/ELISA− were positive for v-cyclin antibodies. Of the 12 samples that were LIPS+/ELISA−, 9 were positive for v-cyclin antibodies using the LIPS assay (Fig. 2A). Additional analysis of the LIPS data revealed that the v-cyclin antibody levels were statistically higher in KS samples compared to either the KSHV+/HIV+ or KSHV+/HIV+ (data not shown).
Fig. 2.
v-Cyclin and ORF38 LIPS testing. (A) Antibodies against v-cyclin were evaluated by LIPS with the entire MSM cohort. The antibody levels were plotted on the Y-axis using a log10 scale and each symbol represents the antibody value for each individual subject. The results were compared to the ELISA and MSM samples were binned into 4 groups according to the previous LIPS mixture and ELISA serological status. The geometric mean antibody levels with 95% CI error bars is shown by the short horizontal line and the dashed line represents the cut-off level for determining seropositivity. P values for the different groups were calculated using the Mann Whitney U test. (B) All the MSM samples that were ELISA+/LIPS− (n=45) along with KSHV uninfected controls (n=20) and KS samples (n=29) were tested with ORF38. The geometric mean antibody level with 95% CI error bars is shown by the short horizontal line and the dashed line represents the cut-off level for determining seropositivity. P values for the different groups were calculated using the Mann Whitney U test.
3.5 Additional antibody testing by LIPS
In light of the inability to resolve the discrepancy between testing with the LIPS mixture compared to the ELISA, additional LIPS serological tests employing other single KSHV antigens were used. Since one potential explanation for the discordant is that the LIPS Aggregate Ag test uses a central ORF73 protein fragment (ORF73-Δ2) rather than the full-length protein that is used in the ELISA (Burbelo et al., 2009b), a second, non-overlapping C-terminal fragment of ORF73 (ORF73-Δ3) that previously showed utility for detecting KSHV infection (Burbelo et al., 2009b) was tested. From LIPS testing the 45 samples that were LIPS−/ELISA+ with ORF73-Δ3, thirteen additional MSM samples were found to be seropositive that were not previously detected by the LIPS Aggregate Ag containing ORF73–Δ2 fragment (Table I). Of these 13 seropositive samples, 6 were HIV+ and 7 were HIV−.
Recently ORF38 was identified as a potential useful serologic lytic antigen (Zheng et al., 2011). Based on these findings, a new LIPS test for ORF38 was generated and used to evaluate antibody immunoreactivity in known uninfected KSHV blood donors (n=20), KS patients (n=29) and in the 45 discrepant serum samples that were LIPS−/ELISA+. Using the ORF38 LIPS test, the geometric mean antibody levels in control uninfected blood donors was 845 LU (95% CI, 363 to 1967 LU), while the antibody levels in the KS samples was 84,240 LU (95% CI, 32,570 to 217,800 LU) (Fig. 2B). Using a cut-off value of 6022 LU that was based on the mean plus 5 SD of the controls, 22/29 (76%) of the KS patients were seropositive. More importantly, applying the cut-off, 14 MSM samples from the LIPS−/ELISA+ were seropositive for anti-ORF38 antibodies (Table I). Thus additional ORF73-Δ3 and ORF38 testing resulted in an additional 6% of the MSM samples as seropositive. Moreover, by combining these results from two additional tests with the LIPS Aggregate Ag revealed an overall KSHV seroprevalence of 31%.
4. Discussion
Here a MSM cohort containing asymptomatically-infected KSHV individuals was studied using LIPS, ELISA and PCR. While in a previous study LIPS and ELISA immunoassays had almost identical diagnostic performance in distinguishing KS patients from control uninfected individuals (Burbelo et al., 2009b), the two immunoassays showed markedly different results with an MSM cohort, in which LIPS initially showed 25% seropositivity and ELISA testing showed 35.5% seropositivity for KSHV infection. Overall, 15% of the samples were initially discrepant, with 4% being LIPS+/ELISA−, and 11% being LIPS−/ELISA+. One explanation for the discrepancies is likely due to the different KSHV antigens used in the assays. For example it is highly likely that many of the LIPS−/ELISA+ samples were missed by LIPS because full length ORF73 was not employed. Nevertheless, additional LIPS testing with two independent antigens, a C-terminal fragment of LANA and the ORF38 antigen, detected an additional 6% of the MSM samples that were potentially seropositive by LIPS and thereby showing a cumulative prevalence of 31% LIPS seropositivity in the MSM cohort. The results regarding testing for antibodies against the new ORF38 target showed that ~70% of the KS patient samples were seropositive and the ORF38 antigen was also useful in detecting KSHV-infected individuals that were positive by ELISA, but negative by the LIPS Aggregate Ag. The diagnostic performance of the ORF38 antigen by LIPS is also consistent with the recent protein array study (Zheng et al., 2011). Together these results suggest that a more detailed examination of the separate immunoreactivity against the 6 different single antigens employed in LIPS assay might be informative for identifying asymptomatic KSHV-infected individuals.
Because one limitation of our study is the unambiguous identification of asymptomatic KSHV infection in this MSM cohort, additional DNA testing was performed. Consistent with other reports, the detection of KSHV DNA in lymphocyte samples was not useful in detecting KSHV infection in the seropositive KSHV-infected subjects. However, several seronegative subjects were detected as KSHV DNA positive. Since negative antibody-based tests are common in the early weeks after infection for many different infectious agents, the inability to detect seropositivity with this panel of antigens supports the idea that these 17 PCR positive samples are likely from early in infection where there has not been enough time for significant antibody production.
Previously, v-cyclin antibodies were commonly detected in many of the KS patient but none of the control uninfected individuals (Burbelo et al., 2009b). Here we found that approximately 70% of the LIPS+/ELISA+ were also positive for v-cyclin antibodies suggesting that antibodies to this latent antigen are not restricted to patients with KSHV-associated malignancies and can be found in asymptomatic individuals. Moreover, 10 of the 12 LIPS+/ELISA− samples were positive for v-cyclin suggesting it likely represent a useful serological antigen for identifying asymptomatic KSHV-infected individuals. Interestingly, a recent protein array failed to detect significant immunoreactivity against v-cyclin (Zheng et al., 2011). We speculate that the likely reason is that the solid phase protein array is unable to detect the important conformational epitopes associated with v-cyclin and is consistent with the known higher performance of liquid phase assays compared to solid phase ELISAs for detecting conformational epitopes (Liu and Eisenbarth, 2007).
In comparing the diagnostic performance of the two assays, the greatest numbers of discrepant MSM samples were positive by ELISA but negative by the LIPS mixture. Based on our previous KS study (Burbelo et al., 2009b), this finding showing a large pool of MSM samples that were ELISA+/LIPS− was unexpected. It is possible that the LIPS antigen mixture approach may have missed many of the borderline samples or that additional epitopes present in the ELISA assay were better at detecting antibodies in the asymptomatic KSHV samples.
As found in previous MSM studies (Casper et al., 2006; Casper et al., 2002; Engels et al., 2007; Guanira et al., 2008; Mbulaiteye et al., 2006), both the ELISA (OR, 10.9; 95% CI, 6.0-19.5) and LIPS (OR, 11.5; 95% CI, 5.6-23.5) showed that KSHV infection was strongly associated with HIV infection. It has been proposed that the increased frequency of KSHV infection in HIV individuals may be due to increased KSHV viral shedding in HIV individuals (Pauk et al., 2000). Despite the prominent hypergammaglobulinemia associated with HIV infection, the KSHV+/HIV+ MSM samples had only a slightly higher anti-KSHV antibody level than the KSHV+/HIV− MSM samples, which was not statistically significant. Our analysis also demonstrated that KS patients had significantly (p=0.001) higher anti-KSHV antibody levels than asymptomatically KSHV-infected individuals. Based on our current and past findings (Burbelo et al., 2010), the overall trend in anti-KSHV antibody levels in different conditions is as follows: PEL> KS-MCD> MCD> KS> KSHV+/HIV+>KSHV+/ HIV−. Given that anti-KSHV antibodies increase with time of infection (Biggar et al., 2003), the higher antibody levels seen in KS, MCD and PEL patients compared to the asymptomatic KSHV-infected individuals may simply correlate with length of infection. As such, it may be that increasing antibody levels could be used to identify patients at risk for developing KSHV-related malignancies. For such clinical testing, further standardize of the LIPS assay are needed including the establishment of the exact cut-off for detecting borderline positive samples.
Acknowledgements
Funding: This work was supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research and the Clinical Center and, in part, by a Bench to Bedside award from the NIH Clinical Research Center and in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
Abbreviations
- LIPS
luciferase immunoprecipitation systems
- KS
Kaposi Sarcoma
- MSM
men who have sex with men
Footnotes
Competing interest: None.
Ethical approval: IRB-approved protocols at the NIH Clinical Center, National Institutes of Health, Bethesda, MD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batista MD, Ferreira S, Sauer MM, Tomiyama H, Giret MT, Pannuti CS, et al. High human herpesvirus 8 (HHV-8) prevalence, clinical correlates and high incidence among recently HIV-1-infected subjects in Sao Paulo, Brazil. PLoS One. 2009;4:e5613. doi: 10.1371/journal.pone.0005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar RJ, Engels EA, Whitby D, Kedes DH, Goedert JJ. Antibody reactivity to latent and lytic antigens to human herpesvirus-8 in longitudinally followed homosexual men. J Infect Dis. 2003;187:12–8. doi: 10.1086/345866. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS) J Vis Exp. 2009a;32 doi: 10.3791/1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Issa AT, Ching KH, Wyvill KM, Little RF, Iadarola MJ, et al. Distinct profiles of antibodies to Kaposi sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. J Infect Dis. 2010;201:1919–1922. doi: 10.1086/652869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Leahy HP, Groot S, Bishop LR, Miley W, Iadarola MJ, et al. Four-antigen mixture containing v-cyclin for serological screening of human herpesvirus 8 infection. Clin Vaccine Immunol. 2009b;16:621–627. doi: 10.1128/CVI.00474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Carrell D, Miller KG, Judson FD, Meier AS, Pauk JS, et al. HIV serodiscordant sex partners and the prevalence of human herpesvirus 8 infection among HIV negative men who have sex with men: baseline data from the EXPLORE Study. Sex Transm Infect. 2006;82:229–235. doi: 10.1136/sti.2005.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Wald A, Pauk J, Tabet SR, Corey L, Celum CL. Correlates of prevalent and incident Kaposi’s sarcoma-associated herpesvirus infection in men who have sex with men. J Infect Dis. 2002;185:990–993. doi: 10.1086/339605. [DOI] [PubMed] [Google Scholar]
- Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196:199–207. doi: 10.1086/518791. [DOI] [PubMed] [Google Scholar]
- Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011;24:295–301. doi: 10.1097/QCO.0b013e3283486d04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani M, Cordiali-Fei P, Castilletti C, Di Carlo A, Palamara G, Boros S, et al. Incidence of human herpesvirus 8 (HHV-8) infection among HIV-uninfected individuals at high risk for sexually transmitted infections. BMC Infect Dis. 2007;7:143. doi: 10.1186/1471-2334-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guanira JV, Casper C, Lama JR, Morrow R, Montano SM, Caballero P, et al. Prevalence and correlates of human herpesvirus 8 infection among Peruvian men who have sex with men. J Acquir Immune Defic Syndr. 2008;49:557–562. doi: 10.1097/QAI.0b013e31818d5bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney AS, Peters JS, Manzi SM, Kingsley LA, Chang Y, Moore PS. Use of a multiantigen detection algorithm for diagnosis of Kaposi’s sarcoma-associated herpesvirus infection. J Clin Microbiol. 2006;44:3734–3741. doi: 10.1128/JCM.00191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol. 2007;125:120–126. doi: 10.1016/j.clim.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- Mbisa GL, Miley W, Gamache CJ, Gillette WK, Esposito D, Hopkins R, et al. Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods. 2010;356:39–46. doi: 10.1016/j.jim.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbulaiteye SM, Atkinson JO, Whitby D, Wohl DA, Gallant JE, Royal S, et al. Risk factors for human herpesvirus 8 seropositivity in the AIDS Cancer Cohort Study. J Clin Virol. 2006;35:442–9. doi: 10.1016/j.jcv.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343:1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- Uldrick TS, Wang V, O’Mahony D, Aleman K, Wyvill KM, Marshall V, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis. 2011;51:350–8. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D, Marshall VA, Bagni RK, Miley WJ, McCloud TG, Hines-Boykin R, et al. Reactivation of Kaposi’s sarcoma-associated herpesvirus by natural products from Kaposi’s sarcoma endemic regions. Int J Cancer. 2007;120:321–8. doi: 10.1002/ijc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods. 2001;91:109–117. doi: 10.1016/s0166-0934(00)00244-5. [DOI] [PubMed] [Google Scholar]
- Zheng D, Wan J, Cho YG, Wang L, Chiou CJ, Pai S, et al. Comparison of humoral immune responses to Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus using a viral proteome microarray. J Infect Dis. 2011;204:1683–1691. doi: 10.1093/infdis/jir645. [DOI] [PMC free article] [PubMed] [Google Scholar]