Abstract
Neuroblastoma is a childhood tumor that arises from immature neuroblasts of the sympathetic nervous system. Krüpple‐like factor 4 (KLF4) is a transcription factor, the precise function of which in neuroblastoma is unclear. We examined the effects of KLF4 overexpression and apigenin (APG) treatment in human malignant neuroblastoma SK‐N‐DZ and IMR‐32 cell lines. KLF4 overexpression in both SK‐N‐DZ and IMR‐32 cell lines was confirmed by laser scanning immunofluorescent confocal microscopy and Western blotting. We found that 100 nM KLF4 plasmid and 25 μM APG synergistically inhibited the growth of SK‐N‐DZ and IMR‐32 cells. We also found increase in KLF4 expression in response to treatment with various concentrations of APG. Combination of KLF4 plasmid and APG treatment significantly increased the amounts of apoptosis in both cell lines when compared with control vector or single treatment. We also noticed that the combination therapy decreased expression of the anti‐apoptotic proteins Bcl‐2 and Mcl‐1, increased expression of the pro‐apoptotic proteins Bax, Noxa, and Puma, upregulated p53, and caused activation of caspase‐3 for cleavage of the inhibitor of caspase‐activated DNase (ICAD) leading to completion of apoptosis machinery. Further, combination of KLF4 overexpression and APG treatment was highly effective in inhibiting migration of both neuroblastoma cell lines and was associated with down regulation of matrix metalloproteinases (MMPs) such as MMP‐2 and MMP‐9. Collectively, our results from this investigation strongly suggest that KLF4 functions as a tumor suppressor and potentiates the anti‐cancer activities of APG in two different human malignant neuroblastoma cell lines.
Keywords: Apoptosis, Apigenin, Combination therapy, KLF4, Neuroblastoma
Highlights
-
►
Krüpple‐like factor 4 (KLF4) acts as a tumor suppressor in malignant neuroblastoma.
-
►
Apigenin (APG) is capable of inducing apoptosis in malignant neuroblastoma.
-
►
KLF4 overexpression and APG treatment worked synergistically in neuroblastoma.
-
►
KLF4 potentiated the anti‐cancer effects of APG in different neuroblastoma cells.
1. Introduction
Neuroblastoma is the most common extracranial solid tumor in childhood and it arises from nervous tissue of sympathetic ganglia (Brodeur, 2003; Maris, 2010). Depending upon biological features, this cancer demonstrates heterogeneity, ranging from spontaneous regression to aggressive form of disease and death (Brodeur, 2003; Maris et al., 2007; Maris, 2010). About 50% of neuroblastomas occur in children younger than two years old, and 75% occur in children less than 4 years old. Prognosis for patients diagnosed with malignant neuroblastoma remains very dismal, despite the use of variety of treatment modalities such as surgery, radiation, and chemotherapy (Maris et al., 2007; Matthay et al., 2012). Due to very limited efficacy of current chemotherapeutic drugs, there is urgent need to develop specific combination treatment strategies targeting specific signaling molecules involved in the pathogenesis of malignant neuroblastoma.
Krüppel‐like factor 4 (KLF4) is a member of the KLF zinc‐finger‐containing transcription factor family and KLF4 plays a critical role in regulation of multiple processes including cell proliferation, apoptosis, tissue homeostasis, and growth inhibition in normal tissue (Shields et al., 1996; Rowland et al., 2005; Wei et al., 2005; Wang et al., 2010). Previous studies have suggested that it can have both tumor suppressor and oncogenic function in tissue greatly depending on context (Rowland et al., 2005; Yori et al., 2011; Yu et al., 2011). Tumor suppressor function of KLF4 has been reported in several human cancers including colon, lung, esophageal, gastric, and bladder cancers due to its loss of expression. Ectopic overexpression of KLF4 has been shown to be associated with inhibition of cell proliferation, apoptosis induction, cell cycle arrest; whereas the inactivation of KLF4 expression has been shown to promote tumor progression. A recent study showed that the enforced expression of KLF4 by transfecting with adenovector‐mediated gene transfer suppressed tumor growth in in vivo models of lung cancers (Hu et al., 2009). Another study reported that KLF4 overexpression via RNA activation (RNAa) significantly inhibited the cell survival and proliferation in prostate cancer cells and altered the levels of expression of cell cycle‐related genes (Wang et al., 2010). However, very little is known about the levels of expression of KLF4 in neuroblastoma cells and its function in regulating the growth and proliferation of this pediatric tumor.
Apigenin (APG) is a naturally occurring non‐toxic flavone that is abundantly present in common fruits and vegetables. Various research reports have shown that APG remarkably possesses anti‐cancer and anti‐inflammatory properties, which have been found to be predominantly effective in preventing growth of human cancers including squamous cell carcinoma, leukemia, breast, lung, and prostate cancers (Fotsis et al., 1997; Patel et al., 2007; Xu et al., 2011; Chan et al., 2012). Epidemiological studies have suggested that flavonoids play a crucial role in diminishing the risk of cancers; hence, they have received considerable attention for developing as promising cancer preventive and chemotherapeutic agents. In our laboratory, we have previously shown that APG induces apoptosis in neuroblastoma cell lines via mitochondrial pathway with activation of down stream caspase cascade (Karmakar et al., 2009; Mohan et al., 2011a). A recent study showed that APG activated apoptosis in human leukemia cells by down regulating Akt and activating JNK (Budhraja et al., 2012).
In this study, we have used human malignant neuroblastoma SK‐N‐DZ and IMR‐32 cells as cell culture models to investigate the effects of KLF4 overexpression and concurrent APG treatment. We found that combination of KLF4 overexpression and APG treatment significantly inhibited the viability of cells, induced apoptosis, and suppressed the cell migration so as to control the growth of malignant neuroblastoma cells.
2. Materials and methods
2.1. Neuroblastoma cell lines and culture conditions
The human neuroblastoma cell lines SK‐N‐DZ and IMR‐32 were purchased from American Type Culture Collection (ATCC, Manassas, VA). The IMR‐32 cell line is derived from an abdominal mass occurring in a 13‐month‐old Caucasian male, whereas the SK‐N‐DZ cell line is derived from a bone marrow metastasis from a child with poorly differentiated embryonal neuroblastoma. The SK‐N‐DZ cells were cultured in RPMI 1640 medium while the IMR‐32 cells were cultured in DMEM medium, both supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and 1% streptomycin (GIBCO/BRL, Grand Island, NY). Cells were grown in 75‐cm2 flasks (Corning Corporation, Corning, NY) and maintained in a fully‐humidified incubator containing 5% CO2 at 37 °C. APG (biological source – Parsley) was procured from Sigma–Aldrich (St. Louise, MO) in powder form and dissolved in dimethyl sulphoxide (DMSO) at concentration of 27 mg/ml as indicated by the supplier.
2.2. Transfection of cells with a KLF4‐overexpresing plasmid vector
KLF4 overexpression vector (pcDNA3.1/KLF4‐HisB) and control vector (pcDNA3.1/HisB) were used for transfection studies. All transfection experiments were performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cells were seeded in 24‐well plates for 24 h and allowed to grow till they reached 80–90% confluency. Plasmid DNA and Lipofectamine reagent were separately diluted in opti‐MEM medium without serum, mixed together, and incubated for 30 min. Then, combination of plasmid and reagent was added to each well containing cells, mixed gently, and incubated in CO2 incubator at 37 °C for 24 h. To determine the expression of KLF4 after transfection in SK‐N‐DZ and IMR‐32 cells, we performed immunofluorescent staining and observed the cells under laser scanning confocal microscope. In brief, cells were grown on coverslips, washed with phosphate buffered saline (PBS, pH 7.4), fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton X‐100 containing 2% bovine serum albumin (BSA). After fixation, cells were stained with fluorescein isothiocyanate (FITC) conjugated KLF4 antibody (Abcam, Cambridge, MA) diluted in PBS and staining procedure was carried out according to manufacturer's instruction. Nuclei were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Thermo Scientific, Rockwood, TN) that could strongly bind to A‐T rich regions in DNA, showing blue fluorescence. After mounting with the coverslips on the microscopic slide, cells were examined using the Zeiss LSM510 META laser scanning confocal microscope (Zeiss, Germany). Data sets were merged using Zeiss ZEN confocal imaging software.
2.3. MTT colorimetric assay to determine residual cell viability
Dose–response studies were conducted using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) colorimetric assay and the residual cell viability was measured after transfection/treatment with various amount of KLF4 plasmid and APG as single agents and their respective combinations. The yellow colored tetrazolium salt of MTT was reduced in metabolically active cells to form insoluble purple formazan crystals. Then, formazan crystals were dissolved by addition of isopropyl alcohol and absorbance was measured at 570 nm wavelength using a plate reader (BioTek, Winooski, VT). Cell viability data from the MTT assay were then subjected to analysis by Compusyn software (Combosyn, Paramus, NJ) to generate a combination index (CI) (Chou, 2006; Mohan et al., 2009, 2011b). Conventionally, CI > 1 indicates antagonism, CI = 1 indicates additive effect, and CI < 1 indicates synergism.
2.4. Annexin V‐fluorescein isothiocyanate (FITC)/propidium iodide (PI) double staining and flow cytometry
Annexin V‐FITC/PI double staining is a widely used method to quantitatively determine the percentage of cells undergoing apoptosis within a population. Staining procedure was performed using Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA) according to manufacturer's instructions, as previously described (Mohan et al., 2011c). Annexin V is a Ca2+‐dependent phospholipid‐binding protein that has a high affinity for phosphatidylserine (PS), and binds to cells with exposed PS. FITC conjugated Annexin V serves as a sensitive probe for flow cytometric analysis of apoptotic cells. In brief, cells were washed twice with cold PBS, and resuspended in 1× binding buffer. Five microliter of Annexin V‐FITC and 5 μl of PI were added to the samples, mixed gently, and incubated in dark for 15 min. After adding 400 μl binding buffer, samples were analyzed using a flow cytometer (Beckman Coulter, Brea, CA).
2.5. In vitro cell migration assay
To investigate the inhibitory effect of KLF4 overexpression on migration of neuroblastoma cells, we performed in vitro cell migration assay using 6‐well transwell of pore size 8 μm in diameter and assessed the migratory ability of cells in response to chemoattractant (Mohan et al., 2011c). Cells (1 × 104) were seeded on the upper chamber wells of transwell plates after treatment with KLF4 plasmid and APG alone and in combination. Matrigel (BD Biosciences) was used as a chemoattractant and placed in the lower chamber. The chamber was incubated at 37 °C for 24 h. After incubation, the non‐migrated cells were discarded and the upper wells were washed with PBS, the filters were scraped with a plastic blade and the cells were fixed in 4% formaldehyde in PBS and subjected to Diff‐quick staining. Four fields were viewed under a light microscope and the cells were counted.
2.6. Western blotting
Western blotting was carried out, as we previously described (Mohan et al., 2009, 2011b). After transfection and treatment, cells were trypsinized, washed with PBS, lysed by resuspending in lysis buffer. After sonication, protein samples were extracted and protein concentrations were measured with BSA as standard using modified Bradford assay. Protein samples were resolved on the 4–20% SDS‐polyacrylamide gels by electrophoresis and resolved proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA). Membranes were blocked with 5% non‐fat dry milk in Tris‐buffered saline–Tween (0.1%) and probed with primary IgG antibody. After overnight incubation with primary IgG antibody, membranes were incubated with horseradish peroxidase‐linked bovine anti‐rabbit secondary IgG antibody for 2 h. Immunodetection was carried out using enhanced chemiluminescence (ECL) and exposing the blots to X‐OMAT AR films.
2.7. Reverse transcription‐polymerase chain reaction (RT‐PCR)
RT‐PCR was carried out to determine the levels of expression of mRNA of matrix metalloproteinases (MMPs) such as MMP‐2 and MMP‐9 after transfection with KLF4 vector or/and treatment with APG in SK‐N‐DZ and IMR‐32 cells. Custom designed gene‐specific primer sequences (Eurofin MWG/Operon, Huntsville, AL) were used for PCR amplification of the MMP‐2 gene (forward primer: 5′‐ACC TGG ATG CCG TCG TGG AC‐3′ and reverse primer: 5′‐GTG GCA GCA CCA GGG CAG C‐3′; product size: 447 bp) and MMP‐9 gene (forward primer: 5′‐CAC TGT CCA CCC CTC AGA GC‐3′ and reverse primer: 5′‐GCC ACT TGT CGG CGA TAA GG‐3′; product size: 263 bp), and also glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene (forward primer: 5′‐CCA CCC ATG GCA AAT TCC‐3′ and reverse primer: 5′‐CAG GAG GCA TTG CTG ATG AT‐3′; product size: 304 bp). The primers were transcribed with 300 ng of total RNA using a single‐step RT‐PCR kit (Invitrogen, Carlsbad, CA) on a PCR cycler (Eppendorf, Westbury, NY) with the following program: synthesis of cDNA at 49 °C for 30 min, inactivation of reverse transcriptase at 94 °C for 3 min, PCR amplification (denaturation at 94 °C for 1 min, annealing at 52 °C for 45 s, and extension at 72 °C for 2 min) for 35 cycles. The RT‐PCR products were resolved on 2% agarose gels by electrophoresis, stained with ethidium bromide (1 μg/ml), destained the background with water, visualized on a UV (303 nm) transilluminator, and photographed digitally using the UVDI Compact Digimage System (Major Science, Saratoga, CA). Expression of GAPDH, a housekeeping gene, was used as a loading control.
2.8. Statistical analysis
Results obtained from specific experiments were analyzed using Minitab 16 Statistical software (Minitab, State College, PA). Data were expressed as mean ± standard error of mean (SEM) of separate experiments (n > 3) and compared by one‐way analysis of variance (ANOVA) followed by the Fisher's post‐hoc test. Difference between control (CTL, the untreated group) and a treatment was considered significant at *P < 0.05. Difference between an individual treatment and combination of two treatments was considered significant at #P < 0.05.
3. Results
3.1. Transfection of KLF4 plasmid in neuroblastoma cells
KLF4 is a zinc‐finger transcription factor, which plays a crucial role in tumor biology. However, the molecular mechanism of action of KLF4 in malignant neuroblastoma requires more studies. In the current investigation, we transfected human malignant neuroblastoma cells with a KLF4 overexpressing plasmid and then stained the cells with FITC conjugated KLF4 antibody to monitor the levels of expression of KLF4 in the transfected cells (Figure 1). Immunostaining and confocal microscopic images showed that SK‐N‐DZ and IMR‐32 cells prominently expressed KLF4 after transfection with the KLF4 plasmid (Figure 1A). Cell nucleus was stained with DAPI. Cells transfected with empty vector were considered as control that hardly showed expression of KLF4. We further confirmed the differential KLF4 expression in KLF4 plasmid transfected cells and control vector transfected cells by Western blotting. Consistent with KLF4 expression after immunostaining, Western blotting also showed high expression of KLF4 in KLF4 plasmid transfected cells, whereas control vector transfected cells showed little or no expression of KLF4 (Figure 1B).
Figure 1.
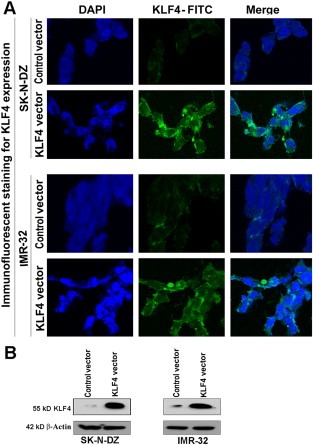
Overexpression of KLF4 in human malignant neuroblastoma SK‐N‐DZ and IMR‐32 cells. (A) Cells were transfected with control vector (pcDNA3.1/HisB) or KLF4 plasmid vector (pcDNA3.1/KLF4‐HisB). After transfection (24 h), immunofluorescent staining was performed with FITC conjugated KLF4 antibody to examine cells using the Zeiss LSM510 META laser scanning confocal microscope. Nuclei were stained with the nuclear stain DAPI. (B) Western blotting to further confirm the expression of KLF4 in cells transfected with KLF4 plasmid vector. Expression of β‐actin was used as a loading control.
3.2. KLF4 overexpression and APG treatment inhibited cell viability
Next, we tested whether the enforced expression of KLF4 in neuroblastoma cells might inhibit cell proliferation and reduce cell viability (Figure 2). The effects of KLF4 overexpressing plasmid and APG alone and their combination on the viability of neuroblastoma cells were examined after transfecting the cells with KLF4 plasmid (10, 50, and 100 nM) and treatment with APG (10, 25, and 50 μM). Cells incubated in the medium without any treatment were considered as control (CTL). Dose–response studies were carried out using the MTT assay. We noticed that KLF4 overexpression and APG treatment significantly reduced cell viability of both SK‐N‐DZ and IMR‐32 cell lines in a dose‐dependent manner, whereas little effect was observed on the viability of untreated cells (Figure 2A and B). Data obtained from MTT assay was subjected to analysis by Compusyn software to determine the CI values. It appeared that 100 nM KLF4 and 25 μM APG in combination could show synergistic effect (CI < 1) in reducing cell viability in both neuroblastoma cell lines. We also performed Western blotting to show the change in KLF4 expression after treatment with various concentrations (5, 10, 25, and 50 μM) of APG. We found increase in KLF4 expression due to treatment with increasing doses of APG in both neuroblastoma cell lines; however, KLF4 expression showed no significant difference in cells treated with 25 and 50 μM doses of APG (Figure 2C).
Figure 2.
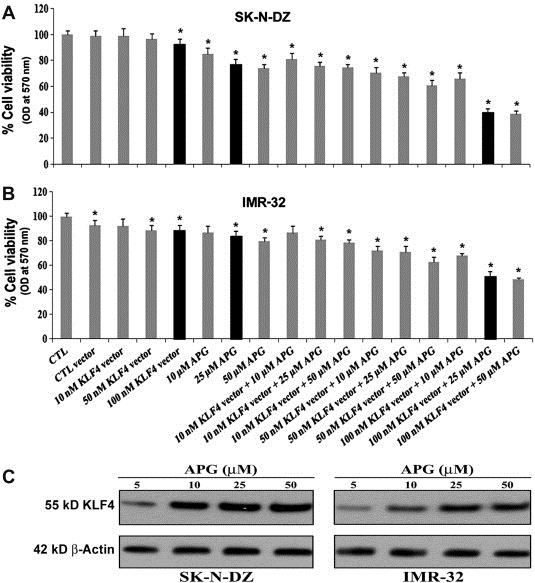
Reduction in residual cell viability of SK‐N‐DZ and IMR‐32 cells after KLF4 overexpression and APG treatment. SK‐N‐DZ (A) and IMR‐32 (B) cells were transfected with various amount of KLF4 plasmid vector (10, 50, and 100 nM), APG (10, 25, and 50 μM), and their respective combinations. The cell viability was measured by the MTT assay, as described in the Materials and methods. The absorbance was measured at 570 nm wavelength using a plate reader. Based on the CI values, as determined by Compusyn software, combination of 100 nM KLF4 vector and 25 μM APG (black bars) showed the best synergistic efficacy for the highest reduction in residual cell viability of SK‐N‐DZ and IMR‐32 cells. (C) The change in KLF4 expression after treatment with varying concentrations (5, 10, 25, and 50 μM) of APG was determined by Western blotting in both neuroblastoma cell lines. Expression of β‐actin was used as a loading control.
3.3. KLF4 overexpression and APG treatment induced apoptosis in neuroblastoma cells
To determine whether reduction in cell viability after KLF4 overexpression and APG treatment was due to induction of apoptosis, we examined the cell lines for presence of apoptosis via in situ Wright staining and Annexin V assay (Figure 3). Combination of both agents showed significantly increase in number of apoptotic cells, when compared with either KLF4 overexpression or APG treatment alone. Morphological features of apoptosis were clearly visible in cells due to KLF4 overexpression and APG treatment (Figure 3A). To quantify amounts of apoptosis, Annexin V assay was performed and data indicated minimal apoptosis in untreated cells and cells transfected with control vector (Figure 3B). The data from Annexin V assay confirmed that reduction in cell viability following KLF4 overexpression and APG treatment in neuroblastoma cells was due to induction of apoptosis (A4 quadrant). Induction of apoptosis by combination of KLF4 overexpression and APG treatment was significantly higher than that by KLF4 overexpression or APG treatment alone (Figure 3C). Therefore, the results from both Wright staining and Annexin V assay confirmed the occurrence of apoptosis in malignant neuroblastoma cells after combination of KLF4 overexpression and APG treatment.
Figure 3.
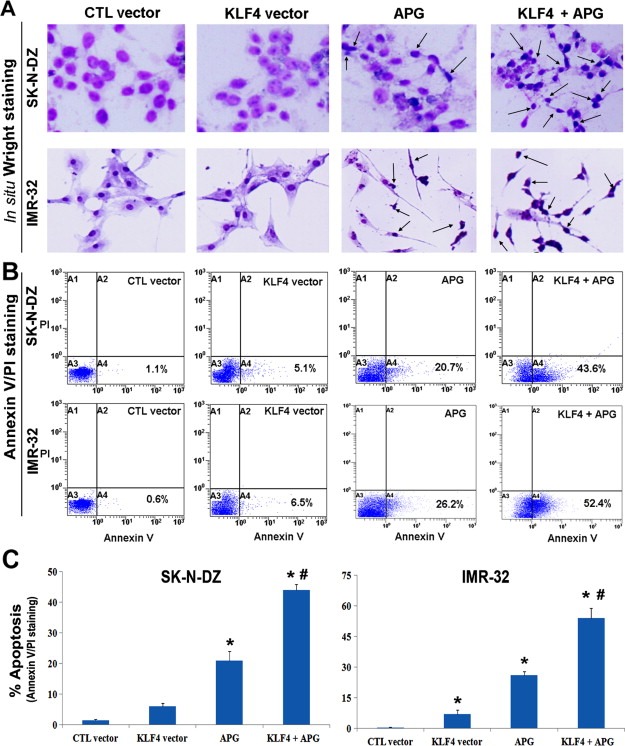
Morphological and biochemical detection of apoptosis in SK‐N‐DZ and IMR‐32 cells. Treatments (24 h): 100 nM control (CTL) vector, 100 nM KLF4 vector, 25 μM APG, 100 nM KLF4 vector + 25 μM APG. (A) In situ Wright staining was performed to show the morphological features of apoptosis in both neuroblastoma cell lines after individual and combination treatments. Arrows were used to indicate some of the apoptotic cells in different treatments. (B) Flow cytometric analysis of Annexin V‐FITC/PI stained cells for biochemical detection of apoptotic cells (in A4 quadrant) after different treatments. (C) Bar diagram to demonstrate the amounts of apoptosis after different treatments.
3.4. KLF4 overexpression resulted in upregulation Bax and down regulation Bcl‐2 leading to increase in Bax:Bcl‐2 ratio
Next, we examined whether KLF4 overexpression played a critical role in modulating the expression of the apoptosis regulatory molecules Bcl‐2 and Bax (Figure 4). With or without Bcl‐2 knockdown by transfection with Bcl‐2 short hairpin RNA (shRNA) plasmid (Santa Cruz Biotechnology, Santa Cruz, CA), we performed KLF4 plasmid transfection in SK‐N‐DZ and IMR‐32 cells. Western blotting showed that combination of Bcl‐2 shRNA + KLF4 overexpression induced upregulation of the pro‐apoptotic protein Bax in both neuroblastoma cell lines, when compared with individual treatments and control vector (Figure 4A). However, the level of the anti‐apoptotic protein Bcl‐2 expression was very prominently down regulated after combination of Bcl‐2 shRNA + KLF4 overexpression (Figure 4A). Interestingly, KLF4 overexpression was associated with a decrease in Bcl‐2 expression. Furthermore, we estimated the Bax:Bcl‐2 ratio after treatment with control vector, individual protein expression vectors, and the combination of both in these two neuroblastoma cell lines (Figure 4B). We noticed that combination of Bcl‐2 shRNA + KLF4 overexpression significantly increased the Bax:Bcl‐2 ratio, thereby tilting the balance of cell fate towards induction of apoptosis.
Figure 4.
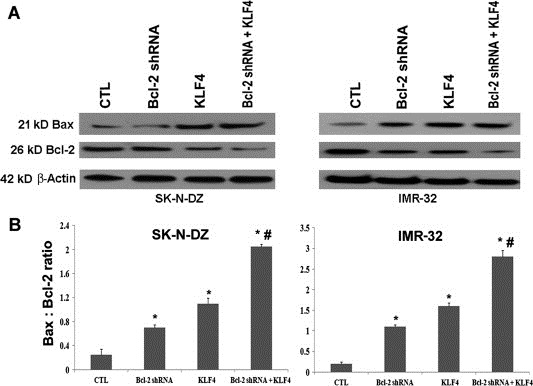
Regulation of expression of Bax and Bcl‐2 proteins in SK‐N‐DZ and IMR‐32 cells by KLF4. Treatments (24 h): control (CTL) cells, 100 nM Bcl‐2 shRNA, 100 nM KLF4 vector, and 100 nM Bcl‐2 shRNA + 100 nM KLF4 vector. (A) Western blotting was performed to examine the changes in the expression of Bax and Bcl‐2 and after the treatments. Almost uniform expression of β‐actin served as loading control. (B) Bar diagram to show the Bax:Bcl‐2 ratio in SK‐N‐DZ and IMR‐32 cells after the treatments.
3.5. KLF4 overexpression and APG treatment induced apoptosis by down regulating Mcl‐1 and upregulating Noxa, Puma, and p53
The induction of apoptosis is predetermined by alterations in the expression of crucial pro‐ and anti‐apoptotic proteins of the Bcl‐2 family. The anti‐apoptotic proteins such as Mcl‐1 protect mitochondrial membrane rupture whereas pro‐apoptotic proteins such as Noxa and Puma trigger release the apoptogenic proteins from mitochondria. We performed Western blotting to determine the levels of expression of these Bcl‐2 family proteins (Mcl‐1, Noxa, and Puma) after KLF4 overexpression and APG treatment alone and in combination (Figure 5). Results showed that combination therapy remarkably down regulated expression of Mcl‐1 in both cell lines, suggesting that Mcl‐1 protein synthesis was blocked and existing pool of protein was rapidly degraded (Figure 5). We also found that levels of expression of the pro‐apoptotic proteins Noxa and Puma were considerably upregulated after combination therapy (Figure 5). Noxa and Puma, which are pro‐apoptotic proteins and transcriptional targets of p53, significantly contribute to p53‐mediated apoptosis. To confirm whether or not p53 was upregulated, we performed Western blotting and found remarkable increase in its expression in malignant neuroblastoma cells after combination therapy. These findings suggested that down regulation of the anti‐apoptotic protein Mcl‐1, upregulation of the pro‐apoptotic proteins Noxa and Puma, and also increase in p53 could instigate the activation of down stream caspase cascade to demise malignant neuroblastoma cells via induction of apoptosis.
Figure 5.
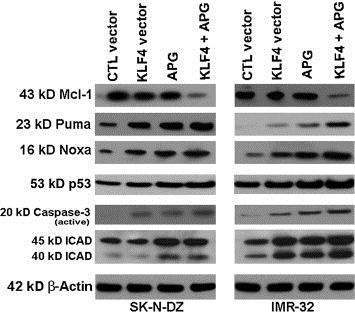
Regulation of Mcl‐1, Puma, Noxa, p53, caspase‐3, and ICAD by KLF4 and APG in SK‐N‐DZ and IMR‐32 cells. Treatments (24 h): 100 nM control (CTL) vector, 100 nM KLF4 vector, 25 μM APG, 100 nM KLF4 vector + 25 μM APG. Western blotting was performed to show alterations in expression of several Bcl‐2 family proteins and other molecules in the apoptosis signaling pathway. Almost uniform expression of β‐actin served as a loading control.
3.6. KLF4 overexpression and APG treatment triggered caspase‐3 activation and cleaved inhibitor of caspase‐activated DNase (ICAD)
Caspases are family of cysteine proteases that play vital role in cleaving key cellular proteins leading to DNA fragmentation and to ensure completion of apoptosis. Caspase‐activated DNase (CAD) is usually bound with its inhibitor (ICAD), and when caspase‐3 is activated, it cleaves ICAD leading to release of CAD, which then enters into the nucleus for fragmentation of genomic DNA. We assessed the levels of expression of active caspase‐3 and ICAD fragment after combination of KLF4 overexpression and APG treatment in both neuroblastoma cell lines (Figure 5). We found that combination therapy drastically increased the levels of expression of active subunit of caspase‐3 in SK‐N‐DZ and IMR‐32 cells (Figure 5). We performed Western blotting to examine the expression of full length and truncated ICAD. We noticed an increase in the level of truncated ICAD, which suggested the cleavage of ICAD after the combination therapy. The caspase‐3 activation and ICAD degradation very prominently indicated the induction of molecular mechanisms of apoptosis in the cells.
3.7. KLF4 overexpression and APG treatment inhibited the migration of malignant neuroblastoma cells
We assessed the ability of migration of malignant neuroblastoma cells following KLF4 plasmid transfection, APG treatment, and combination of both (Figure 6). In vitro migration assay showed that KLF4 overexpression or APG treatment alone did reduce the migration of cells to some extents when compared with the cells without any treatment; however, combination therapy dramatically inhibited the migration of SK‐N‐DZ and IMR‐32 cells (Figure 6A). Quantitative estimation of migrating cells showed that combination of KLF4 overexpression and APG treatment significantly suppressed the cell migration when compared with individual agents in both neuroblastoma cell lines (Figure 6B).
Figure 6.
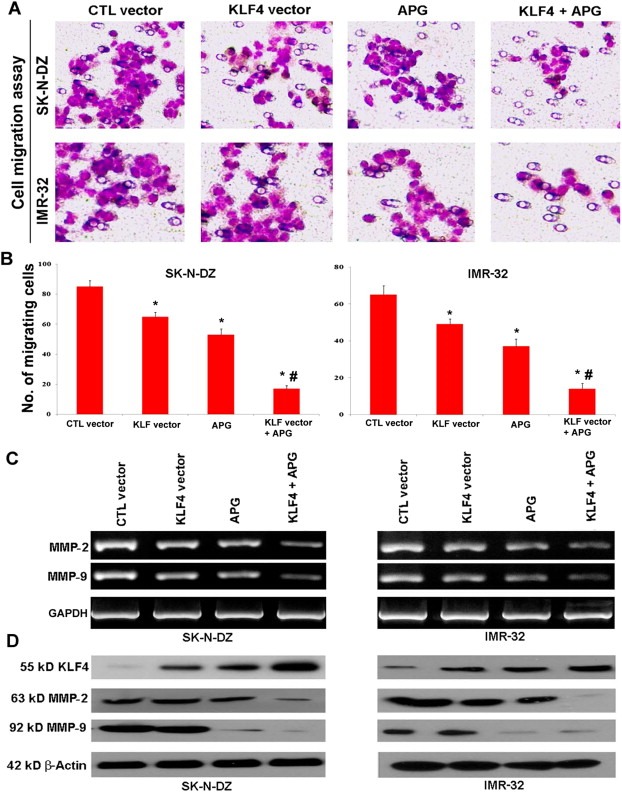
KLF4 overexpression and APG treatment reduced cell migration in SK‐N‐DZ and IMR‐32 cells. Treatments (24 h): 100 nM control (CTL) vector, 100 nM KLF4 vector, 25 μM APG, 100 nM KLF4 vector + 25 μM APG. (A) In vitro cell migration assay to investigate the inhibitory effect of KLF4 on migration of SK‐N‐DZ and IMR‐32 cells. (B) Bar diagram to show the quantitative estimation of migrated cells after different treatments. (C) RT‐PCR to show regulation of transcription of MMP‐2 and MMP‐9 by KLF4 in both neuroblastoma cell lines. GAPDH expression was used as a loading control. (D) Western blotting to show levels of expression of KLF4, MMP‐2, and MMP‐9 in both neuroblastoma cell lines. Almost uniform expression of β‐actin served as a loading control.
3.8. KLF4 overexpression and APG treatment inhibited the expression of MMPs
To understand the cell signaling pathways involved in inhibition of cell migration after KLF4 vector transfection and APG treatment, we performed RT‐PCR and Western blotting and determined the levels of expression of prominent cell migration molecules (MMPs such as MMP‐2 and MMP‐9) at mRNA and protein levels (Figure 6C and D). We performed RT‐PCR and noticed that combination of KLF4 and APG clearly decreased the mRNA expression of MMP‐2 and MMP‐9 in both cell lines (Figure 6C), indicating their transcriptional down regulation by the combination therapy. We also performed Western blotting and found that combination therapy dramatically increased expression of KLF4 but drastically decreased expression of both MMP‐2 and MMP‐9 (Figure 6D). These results suggested that this combination therapy very efficiently inhibited the migration of malignant neuroblastoma cells by suppressing the mRNA and protein expression of the molecules (MMP‐2 and MMP‐9) that otherwise would promote cell migration.
4. Discussion
To investigate an important role for the KLF4 transcription factor in controlling the growth of human malignant neuroblastoma, we transfected SK‐N‐DZ and IMR‐32 cells with the KLF4 plasmid. Transfected cells were subjected to immunofluorescent staining with FITC conjugated KLF4 antibody followed by laser scanning confocal microscopy and also Western blotting, showing the overexpression of KLF4 in SK‐N‐DZ and IMR‐32 cells. Then, we examined the effects of combination of KLF4 overexpression and APG treatment on cell proliferation, apoptosis, and cell migration in these malignant neuroblastoma cells. The important findings from this investigation showed that combination of KLF4 overexpression and APG treatment worked synergistically to inhibit cell viability, induce apoptosis by down regulating the anti‐apoptotic proteins Bcl‐2 and Mcl‐1, upregulating the pro‐apoptotic proteins Noxa and Puma, and increasing the tumor suppressor protein p53 leading to subsequent activation of caspase‐3, and reduction in cell migration by down regulating MMP‐2 and MMP‐9.
Increasing evidence suggests that KLF4 either function as an oncogene or a tumor suppressor depending on the genetic context and cellular settings (Shields et al., 1996; Rowland et al., 2005; Yu et al., 2011). Oncogenic properties of KLF4 are established by its ability to reprogram fibroblast into pluripotent stem cells in cooperation with Oct3/4, Sox2, and c‐Myc (Takahashi and Yamanaka, 2006). The tumor suppressor role of KLF4 is well established in a variety of human cancers including gastric, colon, and pancreatic cancers. Studies have shown that KLF4 as tumor suppressor inhibits cell proliferation by inducing cell cycle arrest at G1/S phase and by promoting p53‐dependent activation of p21Cip1 (Chen et al., 2001; Yoon et al., 2003; Wang et al., 2010). However, the role of KLF4 in tumor progression or tumor suppression in malignant neuroblastoma and molecular mechanisms associated with these processes largely remain elusive. The flavonoid APG is present in abundant quantity in plants and vegetables and has been shown to possess significant anti‐carcinogenic and anti‐cancer properties (Fotsis et al., 1997; Patel et al., 2007; Xu et al., 2011). These studies showed that APG exhibited considerable growth inhibitory and apoptosis‐inducing properties in breast cancer cell lines and also reduction in cell growth, cell cycle arrest, and increase in apoptosis in colon cancer cell lines. Our current results indicate that APG dose‐dependently (upto 25 μM) can increase KLF4 expression in human malignant neuroblastoma cells. In our current investigation, we for the first time demonstrated a role for KLF4 as a tumor suppressor in human malignant neuroblastoma SK‐N‐DZ and IMR‐32 cells and elucidated several signaling events in response to KLF4 overexpression and APG treatment for induction of apoptosis and inhibition of cell migration.
Apoptosis is triggered by the imbalance between the pro‐apoptotic and anti‐apoptotic proteins of the Bcl‐2 family that determines the mitochondrial response to apoptosis stimuli. Mcl‐1 is a multi‐domain anti‐apoptotic protein that is localized in mitochondria for inhibition of apoptosis by antagonizing the functions of pro‐apoptotic proteins (Andersen and Kornbluth, 2012). Mcl‐1 interacts with its antagonist Noxa and the balance between Mcl‐1 and Noxa can be the underlying mechanism in regulating cell death (Alves et al., 2006; Mei et al., 2007; Albershardt et al., 2011). We noticed that down regulation of Mcl‐1 was accompanied by upregulation of Noxa after KLF4 overexpression and APG treatment, suggesting that Mcl‐1/Noxa axis could get disrupted in cells resulting in significant apoptosis. Puma (p53‐mediated regulator of apoptosis) is another potent pro‐apoptotic BH3‐only protein that has been shown to play an essential role in induction of apoptosis. Studies have reported that Puma interacts with Mcl‐1 and assists in stabilization of Mcl‐1 leading to inhibition of apoptosis (Mei et al., 2005; Day et al., 2008). In this investigation, we found increase in Puma expression after KLF4 overexpression and APG treatment, suggesting the activation of pro‐apoptotic function of Puma leading to cell death. The expression of Puma and Noxa in response to apoptotic stimuli is dependent of expression of p53, a transcription factor and tumor suppressor protein, which maintains the genomic integrity. Upregulation of p53 along with overexpression of Noxa and Puma suggested that Noxa and Puma served as the key p53‐dependent activators of intrinsic apoptotic pathways in human malignant neuroblastoma cells after KLF4 overexpression and APG treatment.
A previous study showed that H2O2 stimulated KLF4 overexpression for apoptosis in leukemia cells via involvement of Bcl‐2 family proteins (Li et al., 2010). However, the molecular mechanism underlying any role of KLF4 in regulation of Bcl‐2 family members and its interaction with the anti‐apoptotic Bcl‐2 protein remains poorly understood. We knocked down Bcl‐2 expression in SK‐N‐DZ and IMR‐32 cells by transfection with Bcl‐2 shRNA followed by co‐transfection with KLF4 vector. Our data showed that Bcl‐2 knockdown and KLF4 overexpression together caused upregulation of Bax and drastic down regulation of Bcl‐2, thereby increasing the Bax:Bcl‐2 ratio for induction of apoptosis in human malignant neuroblastoma cells.
The MMPs are zinc‐dependent endopeptidases, which are responsible for degradation of extracellular matrix components and they play a central role in diverse physiological processes such as cell proliferation, migration, adhesion, angiogenesis, and apoptosis (Gianelli et al., 1997; Hadler‐Olsen et al., 2011). MMP‐2 belongs to gelatinase subfamily of MMPs and it activates another gelatinase MMP‐9 and their overexpression has been reported in a number of neoplasms including breast, ovarian, gastric, and cervical cancers (Wang et al., 2006; Song et al., 2009). Our in vitro cell migration assay showed significant reduction in cell migratory activity after KLF4 overexpression and APG treatment. We also noticed that KLF4 overexpression and concurrent APG treatment significantly down regulated MMP‐2 and MMP‐9 at both mRNA and protein levels in malignant neuroblastoma cell lines, suggesting the impairment of transcription and translation of MMP‐2 and MMP‐9 leading to prevention of tumor cell migration.
In conclusion, this study demonstrated an important role for KLF4 in controlling the growth of different human malignant neuroblastoma cells. Overexpression of KLF4 in combination with APG treatment induced apoptosis and suppressed the cell migration via modulating key signaling molecules. Further investigation may elucidate the prominent role for KLF4 in controlling growth of malignant neuroblastoma in vivo and consider it as a promising tumor suppressor in the future therapeutic strategy for treatment of this deadly cancer.
Acknowledgements
This work was supported in part by a grant (R01 NS65456) from the National Institutes of Health (Bethesda, MD, USA) and another grant (SCIRF‐11‐002) from the South Carolina Spinal Cord Injury Research Foundation (Columbia, SC, USA).
Mohan Nishant, Ai Walden, Chakrabarti Mrinmay, Banik Naren L., Ray Swapan K., (2013), KLF4 overexpression and apigenin treatment down regulated anti‐apoptotic Bcl‐2 proteins and matrix metalloproteinases to control growth of human malignant neuroblastoma SK‐N‐DZ and IMR‐32 cells, Molecular Oncology, 7, doi: 10.1016/j.molonc.2012.12.002.
References
- Albershardt, T.C. , Salerni, B.L. , Soderquist, R.S. , Bates, D.J. , Pletnev, A.A. , Kisselev, A.F. , Eastman, A. , 2011. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein Noxa. J. Biol. Chem. 286, 24882–24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, N.L. , Derks, I.A. , Berk, E. , Spijker, R. , van Lier, R.A. , Eldering, E. , 2006. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity 24, 703–716. [DOI] [PubMed] [Google Scholar]
- Andersen, J.L. , Kornbluth, S. , 2012. Mcl-1 rescues a glitch in the matrix. Nat. Cell. Biol. 14, 563–565. [DOI] [PubMed] [Google Scholar]
- Brodeur, G.M. , 2003. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3, 203–216. [DOI] [PubMed] [Google Scholar]
- Budhraja, A. , Gao, N. , Zhang, Z. , Son, Y.O. , Cheng, S. , Wang, X. , Ding, S. , Hitron, A. , Chen, G. , Luo, J. , Shi, X. , 2012. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo. Mol. Cancer Ther. 11, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, L.P. , Chou, T.H. , Ding, H.Y. , Chen, P.R. , Chiang, F.Y. , Kuo, P.L. , Liang, C.H. , 2012. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim. Biophys. Acta 1820, 1081–1091. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Johns, D.C. , Geiman, D.E. , Marban, E. , Dang, D.T. , Hamlin, G. , Sun, R. , Yang, V.W. , 2001. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J. Biol. Chem. 276, 30423–30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, T.C. , 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–681. [DOI] [PubMed] [Google Scholar]
- Day, C.L. , Smits, C. , Fan, F.C. , Lee, E.F. , Fairlie, W.D. , Hinds, M.G. , 2008. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J. Mol. Biol. 380, 958–971. [DOI] [PubMed] [Google Scholar]
- Fotsis, T. , Pepper, M.S. , Aktas, E. , Breit, S. , Rasku, S. , Adlercreutz, H. , Wahala, K. , Montesano, R. , Schweigerer, L. , 1997. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 57, 2916–2921. [PubMed] [Google Scholar]
- Gianelli, G. , Falk-Marziller, J. , Schiraldi, O. , Stetler-Stevenson, W.G. , Quaranta, V. , 1997. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277, 225–228. [DOI] [PubMed] [Google Scholar]
- Hadler-Olsen, E. , Fadnes, B. , Sylte, I. , Uhlin-Hansen, L. , Winberg, J.O. , 2011. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 278, 28–45. [DOI] [PubMed] [Google Scholar]
- Hu, W. , Hofstetter, W.L. , Li, H. , Zhou, Y. , He, Y. , Pataer, A. , Wang, L. , Xie, K. , Swisher, S.G. , Fang, B. , 2009. Putative tumor-suppressive function of Krüppel-like factor 4 in primary lung carcinoma. Clin. Cancer Res. 15, 5688–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar, S. , Davis, K.A. , Choudhury, S.R. , Deeconda, A. , Banik, N.L. , Ray, S.K. , 2009. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 388, 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Zhao, J. , Li, Q. , Yang, W. , Song, Q. , Li, W. , Liu, J. , 2010. KLF4 promotes hydrogen-peroxide-induced apoptosis of chronic myeloid leukemia cells involving the Bcl-2/Bax pathway. Cell Stress Chaperones 15, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris, J.M. , Hogarty, M.D. , Bagatell, R. , Cohn, S.L. , 2007. Neuroblastoma. Lancet 369, 2106–2120. [DOI] [PubMed] [Google Scholar]
- Maris, J.M. , 2010. Recent advances in neuroblastoma. N. Engl. J. Med. 362, 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay, K.K. , George, R.E. , Yu, A.L. , 2012. Promising therapeutic targets in neuroblastoma. Clin. Cancer Res. 18, 2740–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, Y. , Du, W. , Yang, Y. , Wu, M. , 2005. Puma/Mcl-1 interaction is not sufficient to prevent rapid degradation of Mcl-1. Oncogene 24, 7224–7237. [DOI] [PubMed] [Google Scholar]
- Mei, Y. , Xie, C. , Xie, W. , Tian, X. , Li, M. , Wu, M. , 2007. Noxa/Mcl-1 balance regulates susceptibility of cells to camptothecin-induced apoptosis. Neoplasia 9, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan, N. , Karmakar, S. , Choudhury, S.R. , Banik, N.L. , Ray, S.K. , 2009. Bcl-2 inhibitor HA14-1 and genistein together adeptly down regulated survival factors and activated cysteine proteases for apoptosis in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Brain Res. 1283, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan, N. , Banik, N.L. , Ray, S.K. , 2011. Combination of N-(4-hydroxyphenyl) retinamide and apigenin suppressed starvation-induced autophagy and promoted apoptosis in malignant neuroblastoma cells. Neurosci. Lett. 502, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan, N. , Banik, N.L. , Ray, S.K. , 2011. Synergistic efficacy of a novel combination therapy controls growth of Bcl-xL bountiful neuroblastoma cells by increasing differentiation and apoptosis. Cancer Biol. Ther. 12, 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan, N. , Karmakar, S. , Banik, N.L. , Ray, S.K. , 2011. SU5416 and EGCG work synergistically and inhibit angiogenic and survival factors and induce cell cycle arrest to promote apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-BE2 cells. Neurochem. Res. 36, 1383–1396. [DOI] [PubMed] [Google Scholar]
- Patel, D. , Shukla, S. , Gupta, S. , 2007. Apigenin and cancer chemoprevention: progress, potential and promise. Int. J. Oncol. 30, 233–245. [PubMed] [Google Scholar]
- Rowland, B.D. , Bernard, R. , Peepe, D.S. , 2005. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell. Biol. 7, 1074–1082. [DOI] [PubMed] [Google Scholar]
- Shields, J.M. , Christy, R.J. , Yang, V.W. , 1996. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J. Biol. Chem. 271, 20009–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H. , Li, Y. , Lee, J. , Schwartz, A.L. , Bu, G. , 2009. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 69, 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Yamanaka, S. , 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Zhang, Z.G. , Zhang, R.L. , Gregg, S.R. , Hozeska-Solgot, A. , LeTourneau, Y. , Wang, Y. , Chopp, M. , 2006. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J. Neurosci. 26, 5996–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Place, R.F. , Huang, V. , Wang, X. , Noonan, E.J. , Magyar, C.E. , Huang, J. , Li, L.C. , 2010. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 70, 10182–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, D. , Gong, W. , Kanai, M. , Schlunk, C. , Wang, L. , Yao, J.C. , Wu, T.T. , Huang, S. , Xie, K. , 2005. Drastic down-regulation of Krüppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 65, 2746–2754. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Xin, Y. , Diao, Y. , Lu, C. , Fu, J. , Luo, L. , Yin, Z. , 2011. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS One 6, 29169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, H.S. , Chen, X. , Yang, V.W. , 2003. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J. Biol. Chem. 278, 2101–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yori, J.L. , Seachrist, D.D. , Johnson, E. , Lozada, K.L. , Abdul-Karim, F.W. , Chodosh, L.A. , Schiemann, W.P. , Keri, R.A. , 2011. Krüppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia 13, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F. , Li, J. , Chen, H. , Fu, J. , Ray, S. , Huang, S. , Zheng, H. , Ai, W. , 2011. Krüppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene 30, 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]


