Abstract
The rate of water exchange in lanthanide complexes is often overlooked as an important parameter in the design of responsive MR imaging agents. Most often, the number of inner-sphere water coordination sites or the rotational mobility of the complex are considered as the central theme while water exchange is either assumed to be “fast enough” or entirely ignored. On the other hand, relaxation and shift theories predict that water exchange rates may indeed be the key parameter one should consider in any new molecular design. In this short review, the impact of water exchange rates on three classes of lanthanide-based MRI contrast agents, T1-based relaxation agents, T2 exchange line-broadening agents and chemical exchange saturation transfer (CEST) agents, is illustrated and discussed.
Introduction
The design of lanthanide-based responsive MR contrast agents is most often based either on a change in q, the number of inner-sphere water molecules, or a change in molecular reorientation as governed by the rotation correlation time, τR. The rate of water exchange between the inner-sphere coordination position and bulk solvent, kex = 1/τM, is often overlooked, perhaps for a couple of reasons. First, water exchange rates are not easily measured, especially for an agent in vivo. Second, water exchange rates in lanthanide complexes are not easily predicted or described using simple chemical principles, so fine-tuning exchange rates to the optimal values as predicted by theory is problematical. Nevertheless, one can easily argue that water exchange rates may indeed be the key parameter one should consider in any new molecular design (Figure 1). In this short review, the impact of water exchange rates on three classes of lanthanide-based MRI contrast agents, T1-based relaxation agents, T2 exchange line-broadening agents and chemical exchange saturation transfer (CEST) agents, are discussed from basic theoretical predictions and experimental examples. The take home lesson is that choosing an imaging agent platform or complex that has an optimal water exchange rate may be critical for successful implementation of such agents into clinical practice.
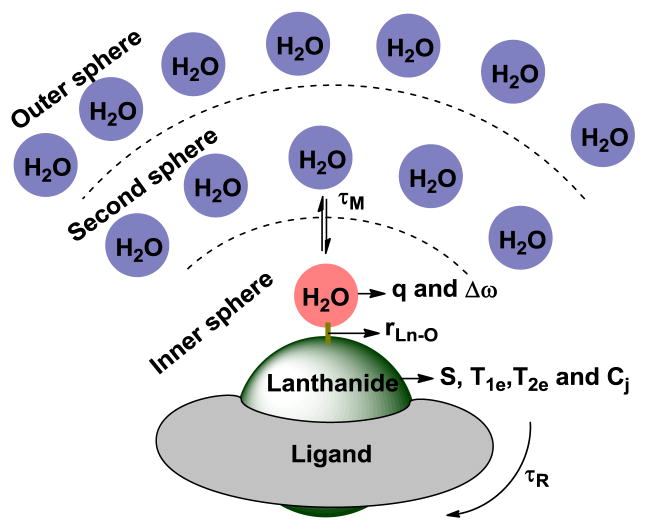
Figure 1.
Key physical parameters that influence the T1, T2 and CEST properties of lanthanide complexes
The first report of a “smart” MR agent [1] was based on an increase in q that parallels enzyme-assisted cleavage of a β-galactose residue that had restricted water access to the Gd3+. This landmark paper stimulated intense interest among the scientific community and other types of responsive agents soon followed. Most of the later designs were based on either an increase in q (the Meade design [1]) or change in molecular rotation (an increase in τR [2]). Almost always, little regard was given to the rate of water exchange in either the pre-activated or activated complex. This was also true for the entire family of first generation Gd3+ complexes approved for clinical use as MRI contrast agents. Long after the first agents had been approved for clinical use, Merbach and colleagues demonstrated that water exchange in these q = 1 complexes, unlike in Gd(H2O)93+, occurs via a dissociative mechanism [3,4] and, as a result, the rates of water exchange were considerably slower than had been earlier assumed [5]. As we will illustrate, this has a relatively small impact on the r1 relaxivity of a low molecular weight and rapidly tumbling gadolinium complex but has a substantial impact as one begins to slow molecular motion by binding such chelates to larger macromolecules in an effort to increase r1.
Why is the rate of water exchange so important?
T1-based relaxation agents
In order for water protons to be efficiently relaxed by Gd3+, water from bulk solvent must be in rapid exchange with water molecules reaching the inner-sphere of Gd3+ (Figure 1). Figure 2 shows plots of r1IS (inner-sphere contribution) versus bound water lifetime (τM) as predicted by Solomon-Bloembergen-Morgan (SBM) theory [6] for a q = 1 Gd3+ complex tumbling at several different molecular rotational correlation times at two different field strengths, 0.47T, a common field for table-top relaxometers used in research and 3T, a common clinical imaging field. These plots illustrate several important points. First, it is widely appreciated that the value of r1 depends upon the molecular tumbling correlation time, τR (as indicated by the six colored curves in each plot). Second, it is also widely appreciated that the maximum value of r1 for a Gd3+-based agent undergoing slow molecular rotation is smaller at higher magnetic field. This is clearly seen by the maximum value of r1 that is reached at 0.47T versus 3T (Figure 2). Finally, the one quantity most often overlooked or ignored is that none of these maximal r1 values can be achieved if water exchange is either too slow or too fast. If τM is too short (< 1 ns), r1 is limited because each water molecule isn’t bound to the paramagnetic center long enough to become fully relaxed. Conversely, if τM is too long (> 1 μs), r1 is also limited because fewer water molecules reach the inner-coordination sphere of Gd3+ per unit time to have the maximum possible impact on the T1 of bulk water protons. Consequently, there exists an optimal value of τM that insures r1 will be maximal for any value of τR at any imaging field strength. This has substantial implications when considering the design of an optimal responsive agent.
Figure 2.
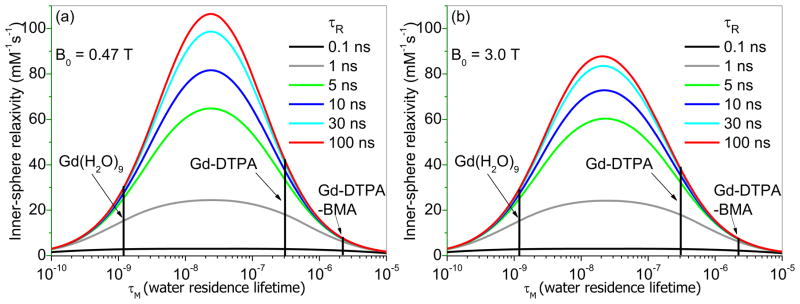
Simulated curves showing the effect of water residence lifetime (τM) of inner-sphere bound water on the r1IS for a Gd3+ complex with the rotational correlation time (τR) variations at 0.47 T (a) and 3.0 T (d), respectively. Parameters used for SBM simulation include rGd-O = 3.1 Å, q = 1 and T1e = 5 ns.
Assume, for the moment, that you have plans to develop a new responsive MR agent where the design does not involve a change in q, but instead relies upon a change in τR. Furthermore, you choose either a DOTA-type or DTPA-type ligand to chelate the Gd3+ and ignore water exchange or assume it is less important than a change in τR. The plots in Figure 2a illustrate that these assumptions would lead to a less than optimal change in r1 as molecular rotation is slowed. For example, the r1 values of GdDTPA and GdDTPA-BMA tumbling in solution as small molecules (τR ~ 0.1 ns) are nearly the same (both around 4 mM−1s−1) even though their τM values differ by ~7-fold (300 ns versus 2222 ns, respectively). Yet, if one takes these two molecules and slows their molecular tumbling rate by binding to a large molecule (or restrict their motion in some other way), the r1IS of GdDTPAslow-rotation-limit would reach a value of ~45 mM−1s−1 while the r1IS of GdDTPA-BMAslow-rotation-limit would reach only ~8 mM−1s−1. This was nicely illustrated experimentally by comparisons of Gd-MS-325 (a derivative of DTPA) versus Gd -MS-325-BMA (a derivative of DTPA-BMA) when bound to human serum albumin (HSA) where the DTPA derivative achieved an r1 of 42 mM−1s−1 while the DTPA-BMA derivative only achieved an r1 of 13 mM−1s−1 [2].
While the importance of water exchange rates in optimizing r1 has been discussed in many comprehensive review articles [2,7–9], this parameter is likely less often considered by chemical designers because our ability to fine-tune water exchange rates in lanthanide complexes is rather limited. Raymond, et al., recognized the importance of creating lanthanide complexes with much faster water exchange rates in order to achieve higher relaxivity MR contrast agents, and have reported over the past several years many Gd3+ complexes derived from tris-HOPO-type ligands [10–14], all of which display faster water exchange than typical polyaza-polycarboxylate ligands such as DOTA or DTPA. These systems are interesting because many of them not only have an expanded number of inner-sphere water coordination positions (up to q = 3) but those inner-sphere water molecules also exchange with solvent water molecules much faster than water molecules in Gd3+ complexes with polyaza-polycarboxlate-type ligands. The rate of water exchange as measured by 17O NMR for one of the more water soluble HOPO complexes, Gd(TREN-bis-1,2-HOPO-TAM-N3) (q = 2), was found to be 5.1 × 108 s−1 (corresponding to a τM of 2 ns [13]), about 100-fold faster than the rate in GdDOTA. This enhanced water exchange rate plus the slightly higher molecular weight of this molecule results in an impressive r1 of ~10 mM−1s−1 at 20 MHz, a value that is substantially higher than the r1 values of GdDOTA or GdDTPA. If one slows molecular rotation of this complex even further by attaching this chelate to a larger macromolecular structure, the curves in Figure 2a would predict that r1 would likely increase to no more than ~45 mM−1s−1 because water exchange in this complex is actually too fast to for optimal τM (lies on the fast exchange side of the curve in Figure 2a). Thus, it is fair to conclude that a complex is yet to be designed with the correct water exchange rate that would allow the agent to reach the highest possible maximum inner sphere relaxivity (slightly over 100 mM−1s−1 at 20 MHz) upon slowing molecular rotation. Among other systems reported, GdPCP2A (τM = 60 ns and q = 2) and GdAAZTA (τM = 90 ns and q = 2) come closest to having optimal water exchange rates. These faster water exchange complexes, like the HOPO systems, do have improved r1 values of 7–8 mM−1s−1 at 20 MHz[15,16]. More importantly, further reducing the tumbling rate of GdAAZTA by creating a fatty acid derivative of AAZTA that binds with HSA [17] results in an even more dramatic increase in r1 to 84 mM−1s−1 at 20 MHz, by far the highest relaxivity reported so far for a slowly tumbling paramagnetic molecular system [17]. Nevertheless, in order for these model complexes to be generally useful for binding or attachment to a macromolecule, a convenient bifunctional version of these two complexes would be advantageous. These data illustrate that it would be desirable to create responsive T1 agents by starting with complexes having a bound water lifetime (τM) near the optimal value of 24 ns.
Recent in vivo applications of responsive Gd3+ agents
Several new Gd3+-based MRI agents that either target to specific biological structures such as fibrin [18–22] and collagen [23,24] or respond to a specific biological event such as release of sequestered zinc ions [25,26] have recently been demonstrated in vivo. Each molecular design was based on an anticipated increase in r1 with slowing of molecular rotation as the agent binds to or reacts with its specific biological target. For these three specific examples, the water exchange rates of the pre-activated and activated complexes were either unknown or assumed. Nevertheless, sufficient changes in T1 occur upon activation of these individual complexes to allow for in vivo detection. One example is illustrated in Figure 3. Here, the bis-amide complex, GdDOTA-diBPEN, was found to bind two equivalents of Zn2+ and the resulting ternary complex then, and only then, binds to a specific site on albumin which slows molecular rotation and increases r1 by ~4-fold [25]. Given that the water exchange rates in DOTA-bis-amide complexes such as this are known to be slower than in GdDOTA (which is already sub-optimal), the fact that we observed image enhancement due to release of Zn2+ from pancreatic β-cells in response to glucose stimulated insulin secretion (GSIS) was fortuitous [26]. This also means that if we can improve the water exchange properties of this system without destroying the HSA binding attributes of the agent, then we may be able to detect subtle biological changes such as this in vivo using a relatively modest amount of imaging agent, perhaps as little as 10 μM.
Figure 3.
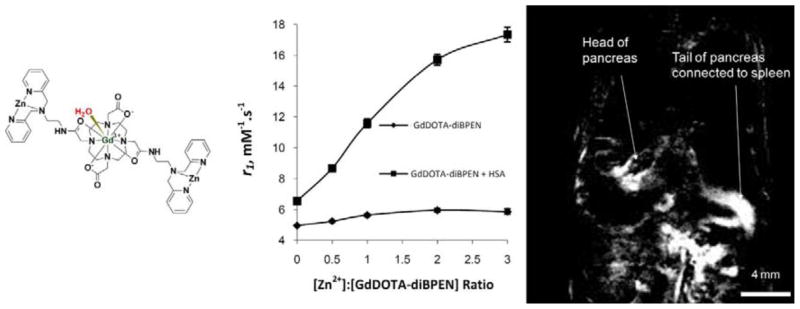
Structure of GdDOTA-diBPEN-(Zn2+)2 (left). The r1 of this complex increases only slightly upon addition of Zn2+ ions in the absence of HSA but increases substantially (to ~17 mM−1s−1 at 23 MHz) in the presence of HSA (center). Difference MR image of a mouse abdomen (pixel intensities post- minus pre-injection of glucose and the Zn2+ responsive agent) showing highlighted regions of the pancreas (right).
Paramagnetic chemical exchange saturation transfer (paraCEST) agents
CEST contrast arises from transfer of saturated spins from one labile proton pool to another, typically from either an endogenous pool of protons such as –NH or –OH protons or protons of an exogenous CEST agent to bulk water protons. The fundamental requirement for CEST is that the frequency difference (Δω) between the two exchanging proton pools must be sufficiently different to allow selective RF saturation of one pool without affecting the spins in the second pool. Even though the frequency differences between most endogenous labile protons and water protons are relatively small at clinical imaging field strengths, CEST imaging of biologically informative pools is beginning to make an impact in clinical medicine [27]. Given the broad impact that Gd3+ complexes have made and continue to make in clinical medicine, it was logical to consider the possibility of using the hyperfine shift characteristics of other Ln3+ complexes to create responsive CEST agents [28]. The major advantage of using such paramagnetic complexes as CEST agents is that the labile proton pools, either a Ln3+-bound water molecule or exchangeable –NH protons on the ligand, experience much larger frequency separations (Δω) from bulk water protons, so they are more easily saturated without inadvertent off-resonance saturation of bulk water itself. This means that CEST contrast can be turned on-and-off by the operator without having a significant impact on the water signal. Like T1-based relaxation agents, contrast arising from a paramagnetic CEST agent also critically depends on the rate of water exchange but, in this case, the exchange rate must be slower than the frequency difference (kex ≤ Δω) for CEST to be observed. Assuming that the pool of bound water protons can be fully saturated, there exists an optimal τM that yields the most intense CEST signal (biggest “dip” in the plots shown in Figure 4a). In this case, CEST contrast depends not only upon the agent concentration (as with Gd3+ agents) but also upon the intensity of the saturation pulse (B1) used to initiate CEST contrast. The data of Figure 4a, generated using the well-known NMR Bloch equations modified for chemical exchange [29,30], illustrate that the optimal Ln3+-bound water lifetime differs for each value of B1. But, unlike Gd3+ agents, the CEST intensity does not depend upon molecular rotation, so the size of the CEST agent does not play a role here. What is important to realize, however, is that the rates of water exchange acceptable for CEST cover a completely different range of water exchange rates (10−5–10−2 s) compared to those required for optimal r1 (Figure 2).
Figure 4.
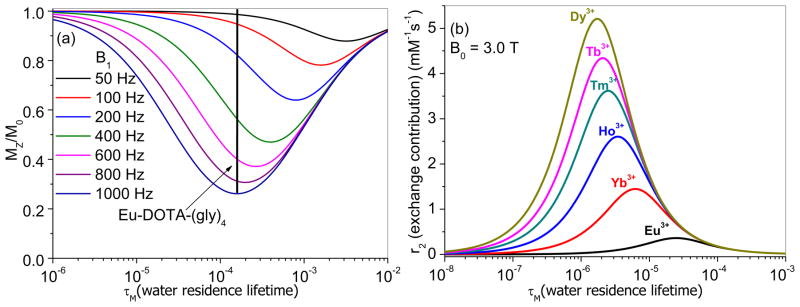
Simulated curves showing the effect of water residence lifetime (τM) on CEST contrast (Mz/M0) and r2exch contrast. (a) The colored curves are for different saturation powers (B1) ranging from 50–1000 Hz. The parameters used in Bloch equation simulation include Cbound protons = 40 mM, Cbulk protons = 111 M, T1bulk = 2.5 s, T1bound water = 0.2 s, and T2bound water = 0.1 s. (b) r2exch (the contribution to the bulk water linewidth due to chemical exchange) for various lanthanide complexes with different bound water chemical shifts (Δω) at 3T. Note that the CEST plot is independent of field strength while the r2exch plot varies strongly with magnetic field.
One of the most widely studied paraCEST imaging agents, EuDOTA-(gly)4−, has a bound water lifetime of ~156 μs near room temperature (shown by the vertical line in Figure 4a) [30]. This means that the water exchange rate for this agent is near optimal for CEST imaging if one could use a B1 of 800–1000 Hz for activation but is too fast for more acceptable B1 power levels of 100–200 Hz. Again, the importance of water exchange rates in designing a responsive CEST agent is just as dramatic here as it is for Gd3+-based T1 agents.
The curves in Figure 4a illustrate several other important points. First, the CEST signal or water contrast is larger (smaller Mz/M0) for complexes undergoing more rapid water exchange. This is somewhat analogous to Gd3+ agents where it was shown that r1 gets larger for faster water exchange complexes until the optimal bound water lifetime is reached at 24 ns. Here, CEST contrast is also larger for faster exchanging systems because more bulk water reaches the inner coordination sphere of the Eu3+ complex (or other lanthanide ions) per unit time where the proton spins become saturated by the applied radio frequency (RF) pulse. One could, in principle, shift these curves even further toward left (to allow for faster exchanging species) by using higher powered pulses for CEST activation. Unfortunately, this is not feasible because the RF power that one can realistically apply to tissue is limited either by power levels available on an imaging system or more likely by the potential of sample heating. In practice, an allowable B1 (for safety reasons) for in vivo CEST imaging may be no higher than ~100–200 Hz. Given that the optimal water exchange rate for CEST (the minimum point in each curve of Figure 4a) as predicted by Bloch theory is equal to 2πB1 [29], lanthanide complexes will need to be discovered that have bound water lifetimes on the order of 0.8 – 1.6 ms. Given that many new types of lanthanide complexes have been discovered over the past several years that display an extremely wide range of water exchange lifetimes (Figure 5) [7,31,32], one should be optimistic that other types of complexes will be discovered that yield the largest CEST signal at power levels considered safe for human imaging.
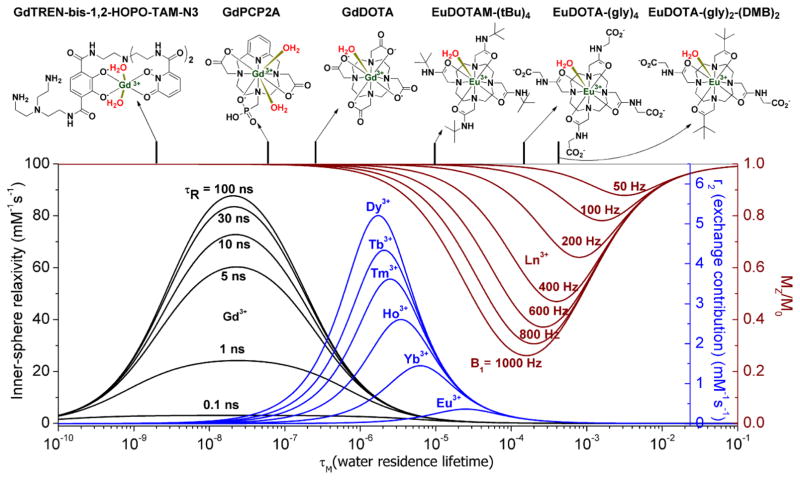
Figure 5.
Simulated curves showing the influence of inner-sphere bound water residence lifetimes (τ M) on r1IS, r2exch, and CEST for various lanthanide complexes as a function of τR, Δω, and B1, respectively. The theoretical curves shown were calculated for 3T. The reported τM values for GdTREN-bis-1,2-HOPO-TAM-N3 [13], GdPCP2A[15], GdDOTA [37], EuDOTAM-(tBu)4 [32], EuDOTA-(gly)4 [30], EuDOTA-(gly)2-(DMB)2 (where DMB = 3,3-dimethylbutan-2-one) [38] are placed along the upper abscissa to illustrate the wide range of water exchange rates that are possible.
T2 exchange line-broadening agents
It was recently observed that the intensity of the water proton signal in kidney images is dramatically quenched in the presence of certain paraCEST agents [33]. This was subsequently traced to an additional water line-broadening effect due to the presence of a complex with a highly shifted water molecule in slow-to-intermediate exchange with bulk water. This additional line-width contribution, called T2exch, is most easily detected in kidney images because this is the site of clearance for most low molecular weight and water soluble imaging agents. This effect is less evident in images of other organs but must be considered for optimal implementation of responsive paraCEST agents in vivo. The impact of water exchange on the transverse relaxation rate (r2exch) depends on two parameters, τM and Δω, as described analytically by the Swift-Connick equation [34]. Simulated Swift-Connick plots for six different lanthanide ions with bound water chemical shifts (Δω) ranging from 50–720 ppm are shown in Figure 4b. These bound water chemical shifts (Δω) are those observed or predicted in high resolution NMR experiments for a series of LnDOTA-(gly)4− complexes [35]. Of course, Δω for other ligands will differ slightly because the magnitude of the hyperfine shift depends upon the geometry of the complex, but in general, one should observe similar trends for other ligands. Most importantly, these Swift-Connick plots illustrate that the magnitude of r2exch for any lanthanide complex once again heavily depends upon the bound water lifetime, τM. At 3T, the effects of r2exch will be significant only for Ln3+ complexes with bound water lifetimes in the range of ~0.1 μs to ~0.1 ms. At higher imaging fields, such as those normally used for small animal imaging, these plots would be shifted toward faster exchanging systems and increase in intensity. But, as with T1 and CEST agents, r2exch has little impact on water line-widths for complexes where τM is either very fast or very slow. Nevertheless, for highly shifting ions such as Dy3+, the line broadening effects of r2exch can be substantial and perhaps even rival the r2 values of some iron oxide nanoparticles [36]. This effect opens yet another opportunity to create new types of responsive agents that turn an image dark (like iron oxide) when activated.
To summarize the concepts described above, the impact of water exchange rates on r1IS, r2exch and CEST agents are compared in a single plot in Figure 5 along with the structures and water residence lifetimes of a limited series of lanthanide complexes. This illustrates that lanthanide complexes having bound water lifetimes varying nearly six orders of magnitude (2 ns to 1 ms) have already been described, most of them in the last 10 years! Plots such as this can provide useful insights that may not be readily apparent otherwise. For example, if one prepares a new Gd3+ complex with the intention of using it as a T1 agent and finds that water exchange is too slow for that purpose, say 2–3 μs, inspection of the plot in Figure 5 shows that one could replace the Gd3+ in this complex with Dy3+ and be assured that this complex would act as a very efficient r2exch agent. The plot also illustrates a very important point about paramagnetic CEST agents. If one were successful in developing an optimal CEST agent for use with a low power B1 activation pulse of 50–100 Hz (τM = 2–3 ms), then one could be assured that the r2exch contribution to the water line-width would be insignificant regardless of which lanthanide ion was used. Slow water exchange complexes optimized for CEST at low B1 are the most challenging systems to design at this time but the knowledge we have gained about how to fine-tune water exchange rates in lanthanide complexes and the scientific interest in developing useful biologically responsive imaging agents will continue to drive this field forward.
Highlights.
The rates of water exchange in lanthanide complexes is often overlooked in the design of new imaging agents
There exists an optimal water exchange rate for T1-based agents, T2 line-broadening agents (i.e., resulting from chemical exchange), and paramagnetic CEST agents
The optimal water exchange rate for each class of imaging agent varies over several orders of magnitude
Lanthanide complexes have been reported that have water exchange rates that vary by nearly six orders of magnitude, from 2 ns to 1 ms.
Acknowledgments
The authors thank the NIH (CA115531, RR02584, EB004582, DK098062 and DK095416), ADA (17-12-MN-76), CPRIT (RP101243) and Robert A. Welch Foundation (AT-584) for research support during the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- *1.Moats RA, Fraser SE, Meade TJ. A “smart” magnetic resonance imaging agent that reports on specific enzymatic activity. Angew Chem Int Ed Engl. 1997;36:726–728. This landmark paper demonstrated that an increase in q for a Gd3+ complex in response to a stimulus is a good strategy to use in the design “smart” MR agents. [Google Scholar]
- 2.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 3.Micskei K, Helm L, Brucher E, Merbach AE. Oxygen-17 NMR study of water exchange on gadolinium polyaminopolyacetates [Gd(DTPA)(H2O)]2− and [Gd(DOTA)(H2O)]− related to NMR imaging. Inorg Chem. 1993;32:3844–3850. [Google Scholar]
- 4.Micskei K, Powell DH, Helm L, Brucher E, Merbach AE. Water exchange on gadolinium (aqua)(propylenediamine tetraacetate) complexes [Gd(H2O)8]3+ and [Gd(PDTA)(H2O)2]− in aqueous solution: a variable-pressure, -temperature and -magnetic field oxygen-17 NMR study. Magn Reson Chem. 1993;31:1011–1020. [Google Scholar]
- 5.Geraldes CFGC, Sherry AD, Cacheris WP, Kuan K-T, Brown RD, Koenic SH, Spillers M. Number of inner-sphere water molecules in Gd3+ and Eu3+ complexes of DTPA-amide and -ester conjugates. Magn Reson Med. 1988;8:191–199. doi: 10.1002/mrm.1910080209. [DOI] [PubMed] [Google Scholar]
- 6.Merbach AE, Tóth É, editors. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. Wiley; 2001. [Google Scholar]
- 7.Caravan P. Protein-targeted gadolinium-based Magnetic Resonance Imaging (MRI) contrast agents: design and mechanism of action. Acc Chem Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 8.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 9.Caravan P, Zhang Z. Structure - relaxivity relationships among targeted MR contrast agents. Eur J Inorg Chem. 2012;2012:1916–1923. doi: 10.1002/ejic.201101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jocher CJ, Botta M, Avedano S, Moore EG, Xu J, Aime S, Raymond KN. Optimized relaxivity and stability of [Gd(H(2,2)-1,2-HOPO)(H2O)]− for use as an MRI contrast agent1. Inorg Chem. 2007;46:4796–4798. doi: 10.1021/ic700399p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson MK, Botta M, Nicolle G, Helm L, Aime S, Merbach AE, Raymond KN. A highly stable gadolinium complex with a fast, associative mechanism of water exchange. J Am Chem Soc. 2003;125:14274–14275. doi: 10.1021/ja037441d. [DOI] [PubMed] [Google Scholar]
- 12.Werner EJ, Avedano S, Botta M, Hay BP, Moore EG, Aime S, Raymond KN. Highly Soluble Tris-hydroxypyridonate Gd(III) Complexes with Increased Hydration Number, Fast Water Exchange, Slow Electronic Relaxation, and High Relaxivity1. J Am Chem Soc. 2007;129:1870–1871. doi: 10.1021/ja068026z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner EJ, Kozhukh J, Botta M, Moore EG, Avedano S, Aime S, Raymond KN. 1,2-Hydroxypyridonate/Terephthalamide complexes of gadolinium(III): synthesis, stability, relaxivity, and water exchange properties. Inorg Chem. 2008;48:277–286. doi: 10.1021/ic801730u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Churchill DG, Botta M, Raymond KN. Gadolinium(III) 1,2-hydroxypyridonate-based complexes: toward MRI contrast agents of high relaxivity1. Inorg Chem. 2004;43:5492–5494. doi: 10.1021/ic049028s. [DOI] [PubMed] [Google Scholar]
- 15.Aime S, Botta M, Frullano L, Geninatti Crich S, Giovenzana G, Pagliarin R, Palmisano G, Sirtori FR, Sisti M. [GdPCP2A(H2O)2]−: a paramagnetic contrast agent designed for improved applications in magnetic resonance imaging. J Med Chem. 2000;43:4017–4024. doi: 10.1021/jm000983a. [DOI] [PubMed] [Google Scholar]
- 16.Aime S, Calabi L, Cavallotti C, Gianolio E, Giovenzana GB, Losi P, Maiocchi A, Palmisano G, Sisti M. [Gd-AAZTA]−: a new structural entry for an improved generation of MRI contrast agents. Inorg Chem. 2004;43:7588–7590. doi: 10.1021/ic0489692. [DOI] [PubMed] [Google Scholar]
- 17.Gianolio E, Giovenzana GB, Longo D, Longo I, Menegotto I, Aime S. Relaxometric and modelling studies of the binding of a lipophilic Gd-AAZTA complex to fatted and defatted human serum albumin. Chemistry. 2007;13:5785–5797. doi: 10.1002/chem.200601277. [DOI] [PubMed] [Google Scholar]
- 18.Overoye-Chan K, Koerner S, Looby RJ, Kolodziej AF, Zech SG, Deng Q, Chasse JM, McMurry TJ, Caravan P. EP-2104R: a fibrin-specific gadolinium-Based MRI contrast agent for detection of thrombus. J Am Chem Soc. 2008;130:6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 19.Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, Parsons EC., Jr Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Invest Radiol. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 20.Nair SA, Kolodziej AF, Bhole G, Greenfield MT, McMurry TJ, Caravan P. Monovalent and bivalent fibrin-specific MRI contrast agents for detection of thrombus. Angew Chem Int Ed Engl. 2008;47:4918–4921. doi: 10.1002/anie.200800563. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Kolodziej AF, Greenfield MT, Caravan P. Heteroditopic binding of magnetic resonance contrast agents for increased relaxivity. Angew Chem Int Ed Engl. 2011;50:2621–2624. doi: 10.1002/anie.201007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uppal R, Catana C, Ay I, Benner T, Sorensen AG, Caravan P. Bimodal thrombus imaging: simultaneous PET/MR imaging with a fibrin-targeted dual PET/MR probe--feasibility study in rat model. Radiology. 2011;258:812–820. doi: 10.1148/radiol.10100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed Engl. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 24.Caravan P, Das B, Deng Q, Dumas S, Jacques V, Koerner SK, Kolodziej A, Looby RJ, Sun WC, Zhang Z. A lysine walk to high relaxivity collagen-targeted MRI contrast agents. Chem Commun. 2009:430–432. doi: 10.1039/b819098d. [DOI] [PubMed] [Google Scholar]
- 25.Esqueda AC, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Ratnakar J, Lubag AJ, Sherry AD, De Leon-Rodriguez LM. A new gadolinium-based MRI zinc sensor. J Am Chem Soc. 2009;131:11387–11391. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Noninvasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc Natl Acad Sci U S A. 2011;108:18400–18405. doi: 10.1073/pnas.1109649108. This paper describes the first in vivo use of a MRI contrast agent for sensing Zn2+ release from pancreatic beta-cells in response to glucose stimulated insulin secretion from mouse pancreas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65:927–948. doi: 10.1002/mrm.22761. A comprehensive review of diaCEST MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Winter P, Wu K, Sherry AD. A novel europium(III)-based MRI contrast agent. J Am Chem Soc. 2001;123:1517–1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- *29.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005;53:790–799. doi: 10.1002/mrm.20408. This paper describes the basic theory of chemical exchange in paramagnetic systems and reports a algorithm for fitting CEST spectra to Bloch theory. [DOI] [PubMed] [Google Scholar]
- 30.Dixon WT, Ren J, Lubag AJ, Ratnakar J, Vinogradov E, Hancu I, Lenkinski RE, Sherry AD. A concentration-independent method to measure exchange rates in PARACEST agents. Magn Reson Med. 2010;63:625–632. doi: 10.1002/mrm.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem Rev. 2010;110:2960–3018. doi: 10.1021/cr900284a. A comprehensive review of paraCEST MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mani T, Tircso G, Togao O, Zhao P, Soesbe TC, Takahashi M, Sherry AD. Modulation of water exchange in Eu(III) DOTA-tetraamide complexes: considerations for in vivo imaging of PARACEST agents. Contrast Media Mol Imaging. 2009;4:183–191. doi: 10.1002/cmmi.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soesbe TC, Merritt ME, Green KN, Rojas-Quijano FA, Sherry AD. T2 exchange agents: a new class of paramagnetic MRI contrast agent that shortens water T2 by chemical exchange rather than relaxation. Magn Reson Med. 2011;66:1697–1703. doi: 10.1002/mrm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swift TJ, Connick RE. NMR-relaxation mechanisms of O17 in aqueous solutions of paramagnetic cations and lifetime of water molecules in first coordination sphere. J Chem Phys. 1962;37:307–321. [Google Scholar]
- 35.Zhang SR, Sherry AD. Physical characteristics of lanthanide complexes that act as magnetization transfer (MT) contrast agents. J Solid State Chem. 2003;171:38–43. [Google Scholar]
- 36.Soesbe T, Wu Y, Sherry A. Advantages of paramagnetic chemical exchange saturation transfer (CEST) complexes having slow-to-intermediate water exchange properties as responsive MRI agents. NMR in Biomedicine. 2012 doi: 10.1002/nbm.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell DH, Dhubhghaill OMN, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. Structural and dynamic parameters obtained from 17O NMR, EPR, and NMRD studies of monomeric and dimeric Gd3+ complexes of interest in magnetic resonance imaging: an integrated and theoretically self-consistent approach1. J Am Chem Soc. 1996;118:9333–9346. [Google Scholar]
- 38.Green KN, Viswanathan S, Rojas-Quijano FA, Kovacs Z, Sherry AD. Europium(III) DOTA-derivatives having ketone donor pendant arms display dramatically slower water exchange. Inorg Chem. 2011;50:1648–1655. doi: 10.1021/ic101856d. [DOI] [PMC free article] [PubMed] [Google Scholar]