Abstract
Post-translational protein modifications can play a significant role in immune cell signaling. Recently, we showed that inhibition of transmethylation curtails experimental autoimmune encephalomyelitis, notably by reducing T cell receptor (TCR)-induced activation of CD4+ T cells. Here, we demonstrate that transmethylation inhibition by a reversible S-adenosyl-l-homocysteine hydrolase inhibitor (DZ2002) led to immunosuppression by reducing TLR-, B cell receptor (BCR)- and TCR-induced activation of immune cells, most likely by blocking NF-κB activity. Moreover, prophylactic treatment with DZ2002 prevented lupus-like disease from developing in both BXSB and MRL-Faslpr mouse models. DZ2002 treatment initiated during active disease significantly improved outcomes in both in vivo models, suggesting methylation inhibition as a novel approach for the treatment of autoimmune/inflammatory diseases.
Keywords: Systemic lupus erythematosus, transmethylation, S-adenosyl-L-homocysteine hydrolase, Toll-Like Receptors, lymphocytes
1. INTRODUCTION
Cellular activation and homeostasis require post-translational modifications, with phosphorylation being the most frequently studied. Recent findings, however, indicate an important, but yet undefined role for methylation, particularly in immune cells [1; 2; 3]. In the process of transmethylation, S-adenosylmethionine (SAM), the major cellular methyl donor, contributes methyl groups to various macromolecules via a large family of methyltransferases (MTs). Transmethylation alters its substrates in novel ways. Protein methylation greatly expands the functional repertoire of proteins available to cells, and DNA methylation, particularly at CpG islands, modulates gene expression [4]. Immune cells are especially subject to transmethylation, and the finding that B and T cells consume SAM at a much greater rate than most other cell types [5] has generated interest in identifying post-translational modification sites as potential therapeutic targets for immunological disorders.
Transmethylation involves conversion of SAM to S-adenosyl-L-homocysteine (SAH) and its reversible hydrolysis by S-adenosyl-L-homocysteine hydrolase (SAHase) into adenosine and homocysteine. SAHase is likely an important regulator of these reactions since its blockade results in accumulation of SAH and thereby to the global inhibition of most MT activity [6; 7]. Consequently, inhibiting SAHase activity has become an attractive strategy for preventing cellular transmethylation.
We [8; 9; 10] and others [11; 12; 13] have demonstrated a requirement for methylation in T cell signaling pathways. Despite being appropriately phosphorylated, Vav1, a key T cell receptor (TCR) signaling molecule requires arginine methylation for nuclear localization and induction of IL-2 in vitro [14; 15]. We recently confirmed those findings and further demonstrated that a reversible SAHase inhibitor (DZ2002) could significantly reduce the severity of experimental autoimmune encephalomyelitis (EAE) [10]. We [8] and others have also suggested a key role for methylation in several other immune cell types, including B cells and macrophages [11; 12].
Systemic lupus erythematosus (SLE), the prototypic systemic autoimmune disease, is characterized by autoantibodies to nuclear and cytoplasmic components, immune complex-mediated glomerulonephritis (GN), and early death [16]. Endosomal Toll-like receptors (TLRs; TLR3, TLR7, TLR9) in B cells, plasmacytoid dendritic cells (pDCs), and conventional DCs are thought to play an important role in lupus pathogenesis through the recognition of self nucleic acids and related immune complexes [17]. Anti-nuclear antibody production depends on the trafficking of these endosomal TLRs from the endoplasmic reticulum (ER) to endolysosomes, where recognition occurs [17; 18]. Moreover, the lupus-like disease in male BXSB mice is apparently a consequence of a TLR7 duplication on the Y-chromosome [19].
Here, we show that B cells, like T cells, require transmethylation for BCR-dependent activation. Additionally, we show that TLR signaling in antigen-presenting cells (APCs) also requires SAHase activity, probably through a NF-κB-mediated mechanism. We believe this is the first report demonstrating that TLR-signaling, and the ensuing production of inflammatory mediators such as type I IFNs, is transmethylation-dependent.
2. MATERIALS AND METHODS
2.1 Mice
We obtained 2-month-old male or female MRL-Faslpr, BXSB, and C57BL/6 mice from the Scripps Research Institute breeding colony or the Jackson Laboratory. The appropriate animal research committees at the Torrey Pines Institute, Scripps Research Institute, and Shanghai Institute of Materia Medica approved all procedures.
2.2 In vitro lymphocytes stimulation assays
Purified B cells from MRL-Faslpr and C57BL/6 mice were stimulated with either LPS (20 μg/ml) or anti-IgM (5 μg/ml) plus anti-CD40 (0.5 μg/ml) in the presence of a reversible SAHase inhibitor, methyl 4-(adenin-9-yl)-2-hydroxybutanoate (DZ2002) (100 μM), or an irreversible one, 5′-methylthioadenosine (MTA) (300 μM), for 48 hr, and [3H]thymidine incorporation was assessed.
CD4+ T cells, negatively sorted from DZ2002- or vehicle-treated 4.5-month-old MRL-Faslpr mice, were stimulated with plate-bound anti-CD3 (10 μg/ml) plus soluble anti-CD28 (5 μg/ml) and assessed for cytokine production as described [10].
2.3 Cytokine assessment
In vitro culture supernatants and mouse sera were assessed for cytokine production by ELISA (BioLegend) according to the manufacturer’s instructions.
2.4 TLR stimulation
Thioglycolate-elicited peritoneal monocytes or bone marrow-derived monocytes from MRL-Faslpr or C57BL/6 mice (8 per group) were stimulated with various TLR ligands, including LPS (TLR4) (100 ng/ml), resiquimod (TLR7) (100 ng/ml), poly I:C (TLR3) (50 μg/ml), or CpG (TLR9) (1 μM) in the presence or absence of MTA (100, 300, or 500 μM) or DZ2002 (0-100 μM) for 4-16 hr, and the supernatants were assessed for type I IFN and/or TNF-α production. Plasmacytoid dentritic cells (pDCs) and conventional dendritic cells (cDCs) were differentiated and expanded from MRL-Faslpr bone marrow, using either Flt3-L (200 ng/ml) for 9 days or GM-CSF (20 ng/ml) for 7 days, respectively. An IFN-sensitive luciferase bioassay was used to determine IFN concentration [20] and cyclohexamide-treated TNF-α-sensitive L929 cells were used to determine TNF-α production [20]. Both were calculated by comparison to standard curves.
2.5 NF-κB bioassays
Cell lines EL-4 (mouse CD4+ T cells), THP-1 (human monocytes), and 293A (human kidney cells) were transfected with a NF-κB luciferase reporter plasmid (Superarray) according to the manufacturer’s instructions, pretreated with DZ2002 for 2 hr, and stimulated for 18 hr with either human or mouse TNF-α (50 ng/ml), as appropriate. Luciferase assays were performed and luminescence expressed as relative luciferase units. Controls included non-TNF-α stimulated cells and cells transfected with a plasmid containing a non-inducible negative control sequence.
2.6 Serologic analysis
Total and anti-chromatin serum IgG subclasses were captured on ELISA plates coated with Fc-specific F(ab’) 2 of goat anti-mouse IgG (5 μg/ml; Jackson ImmunoResearch Laboratories). IgG autoantibodies were captured on plates coated with double stranded dsDNA (25 μg/ml following poly-L lysine treatment) or chromatin (3.5 μg/ml). Bound IgG subclasses were visualized with alkaline phosphatase-conjugated goat anti-mouse IgG subclass-specific antibodies (Caltag Laboratories), as described elsewhere [18]. Blood urea nitrogen (BUN) was measured in fresh blood samples with Azostix strips (Bayer) and recorded on a scale of 1-4+, which corresponded to concentrations of 5-15 to 50-90 mg/dl.
2.7 Kidney pathology
OCT-embedded frozen kidney sections were fixed in ice-cold acetone, blocked with 10% horse serum in phosphate buffered saline, stained with anti-IgG-FITC (Vector Laboratories), and scored by comparison of glomerular FITC intensity after equal exposure times. An untreated 5-mo-old female MRL-Faslpr mouse served as a reference for a score of 4. Periodic acid Schiff-stained kidney sections were examined in a blind manner, and severity of GN was rated on a scale of 0-4+ (0 = no pathology, 1 = minimal mesangial thickening, 2 = noticeable increases in both mesangium and glomerular cellularity, 3 = the preceding plus inflammatory exudates and/or capsular adhesions, and 4 = obliteration of glomerular architecture involving ≥70% of glomeruli), as previousl described [21]. Mouse urine protein concentration was measured via the Bradford assay.
2.8 Flow cytometry and BrdU staining
Splenic T and B cell subsets were stained with combinations of antibodies to CD3, CD4, CD8, CD11b, CD19, CD25, CD40, CD44, CD69, CD80, and CD86. For bromodeoxyuridine (BrdU) labeling, mice received drinking water containing 0.8 mg/mL BrdU (Sigma) prepared ever other day for 9 days, as described elsewhere [16]. BrdU-labeled cells were surface-labeled, fixed with paraformaldehyde, permeabilized with saponin, and stained with FITC-conjugated anti-BrdU Ab (Becton Dickinson) [16]. Data were acquired on a FACSCalibur and analyzed with CellQuest analysis software (both from BD Biosciences). Antibodies were purchased from BioLegend or BD PharMingen.
2.9 Statistics
Survival was estimated by the Kaplan-Meier method with comparisons by log-rank tests, and the Student t test was used for group comparisons. P <0.05 was considered significant.
3. RESULTS
3.1 DZ2002 protected MRL-Faslpr mice from early lupus mortality
In preliminary tests, we observed no overt toxicity in C57BL/6 mice injected for up to 9 months with therapeutic doses of DZ2002 (data not shown). The two SAHase inhibitors appeared to be interchangeable, although DZ2002 was less toxic than MTA in vivo. Thus, we selected DZ2002 for subsequent in vitro and in vivo experiments and used MTA only for confirmatory in vitro studies.
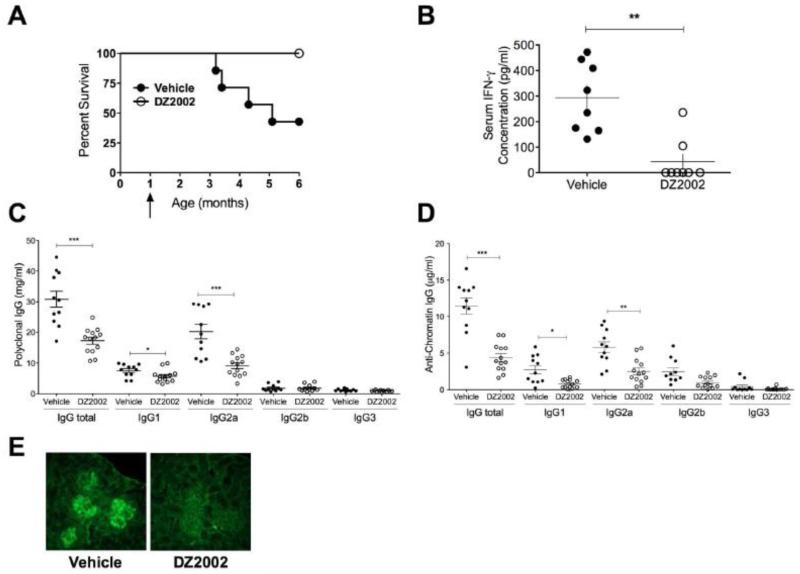
To test whether inhibition of methylation was effective in blocking lupus development, we treated MRL-Faslpr mice with DZ2002 or vehicle starting at 1 month of age until the mortality of the vehicle-treated controls reached 50% (~5 months). At that point, none of the DZ2002-treated mice had died (p = 0.0095) (Fig. 1A). This was associated with markedly reduced serum concentrations of IFN-γ (Fig. 1B), a cytokine that increases dramatically with disease severity in this model [22].
Figure 1.
Prophylactic treatment of MRL-Faslpr mice with DZ2002 reduces lupus manifestations and early mortality.
One-month-old MRL-Faslpr mice (8/group) were treated with DZ2002 (50 mg/kg/day) or vehicle by daily i.p. injection until half the control animals died. (A) 6-month survival curves. p < 0.01, Kaplan-Meier plot with log-rank test comparison. Arrow indicates initiation of DZ2002 treatment. (B) Serum IFN-γ, (C) polyclonal IgG, (D) anti-chromatin IgG concentrations. **p < 0.01, ***p < 0.001, Student t-test. Errors bars represent SEM. (E) Representative anti-IgG FITC-stained fields of kidney sections from vehicle- and DZ2002-treated mice. Typical glomerular immune complex deposits are shown on left control panel. Magnification 40×. (B-E) mice were 4.5 months old.
3.2 DZ2002 reduced kidney pathology
At 6 months of age, vehicle-treated MRL-Faslpr mice showed typical signs of lupus—splenomegaly, lymphadenopathy, glomerular damage, deposition of immune complexes and high BUN concentrations (Fig. 1E,Table 1). DZ2002-treated mice, in contrast, showed reduced glomerulonephritis, minimal immune complex deposition, and mostly normal BUN concentrations. These results indicate that DZ2002 improved survival by inhibiting immune complex deposition and development of kidney pathology.
Table 1. Effect of DZ2002 treatment on lupus disease manifestations in MRL-Faslpr mice.
| Treatment | LN weight (g) | Spleen weight (g) | BUN | GN |
|---|---|---|---|---|
| Vehicle | 3.3 ± 0.37 | 0.39 ± 0.04 | 3.3 ± 0.2 | 3.2 ± 0.26 |
| DZ2002 | 1.4 ± 0.3 | 0.16 ± 0.05 | 1.9 ± 0.13 | 2.0 ± 0.35 |
| p-value | 0.005 | 0.004 | <0.001 | 0.03 |
LN, lymph node; BUN, blood urea nitrogen concentration (scale of 1-4); GN, glomerulonephritis severity (scale of 0-4). Values are means ± SEM.
3.3 Polyclonal Ig and autoantibodies were reduced following DZ2002 treatment
DZ2002-treatment caused significant reductions in IgG1 and IgG2a (the dominant polyclonal subclass in the strain) and a consequent reduction in total polyclonal IgG concentration (Fig. 1C).
Consistent with previous reports (25), the autoantibody response to chromatin in our vehicle-treated MRL-Faslpr mice controls involved mostly IgG2a. Serum concentrations of anti-chromatin IgG1 and IgG2a were significantly reduced in DZ2002-treated mice while concentrations of anti-chromatin IgG2b and IgG3 were both low and appeared unaffected. (Fig. 1D). Taken together, our findings suggests that DZ2002 suppresses systemic autoimmunity by inhibiting both autoantibody and IFN-γ production.
3.4 DZ2002 treatment decreased lymphoaccumulation
MRL-Faslpr mice exhibit massive lymphoid hyperplasia primarily caused by the expansion of apoptosis-defective double-negative T cells (DN; CD3+CD4−8−) [23; 24]. Strikingly, DZ2002 treatment markedly reduced the sizes of secondary lymphoid organs (Table 1) and, numbers of DN as well as activated/memory phenotype (CD44hi) CD4+ T cells (Table 2). Regulatory T cell counts, in contrast, did not differ significantly between the groups (data not shown). SAHase inhibition did not alter the proportion of T cells expressing CD40L, CD80, and CD86 or B cells expressing CD40, CD69 (data not shown), or CD86 (Table 2). However, B cells expressing the activation marker CD80 (B7.1) were reduced by more than half (Table 2).
Table 2. Effect of DZ2002 treatment on lymphocyte abnormalities in MRL-Faslpr mice.
| Treatment | Total cells (× 106) |
T cells (×106) | B cells (× 106) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CD3+ DN |
CD4+ | CD8+ | CD4+CD44hi | CD19+ | CD19+CD80+ | CD19+CD86+ | ||
| Vehicle | 146 ± 8.7 |
55.5 ± 3.1 |
26.3 ± 2.2 |
14.6 ± 2.0 |
18.1 ± 7.8 | 56.7 ± 4.6 |
15.9 ± 5 | 6.5 ± 3.2 |
| DZ2002 | 108 ± 16 |
16.2 ± 0.5 |
16.1 ± 5.2 |
9.7 ± 1.2 |
5.0 ± 2.5 | 64.8 ± 3.2 |
6.0 ± 1.2 | 8.4 ± 2.1 |
| p-value | 0.04 | 0.002 | 0.03 | 0.03 | 0.003 | 0.22 | 0.01 | 0.12 |
DN, double negative. Values are means ± SEM..
In agreement with these findings, levels of in vivo BrdU incorporation was significantly reduced in B cells and all T cell subsets, particularly the DN T cell population (Table 3).
Table 3. Effect of DZ2002 on T and B cell cycling.
| Treatment | DN | DNCD44hi | CD4+ | CD4+CD44hi | CD8+ | CD19+ |
|---|---|---|---|---|---|---|
| Vehicle | 47.8 ± 3.7 | 39.6 ± 1.8 | 44.6 ± 1.5 | 88.8 ± 3.9 | 41.3 ± 1.7 | 30.6 ± 2.4 |
| DZ2002 | 18.4 ± 2.2 | 22.1 ± 2.9 | 23.6 ± 2.0 | 64.7 ± 2.6 | 23.6 ± 3.3 | 17.2 ± 1.7 |
| p-value | 0.01 | 0.01 | 0.003 | 0.01 | 0.01 | 0.02 |
DN, double negative. Values are mean splenocyte percent ± SEM.
3.5 DZ2002 reduced lupus manifestations during active disease
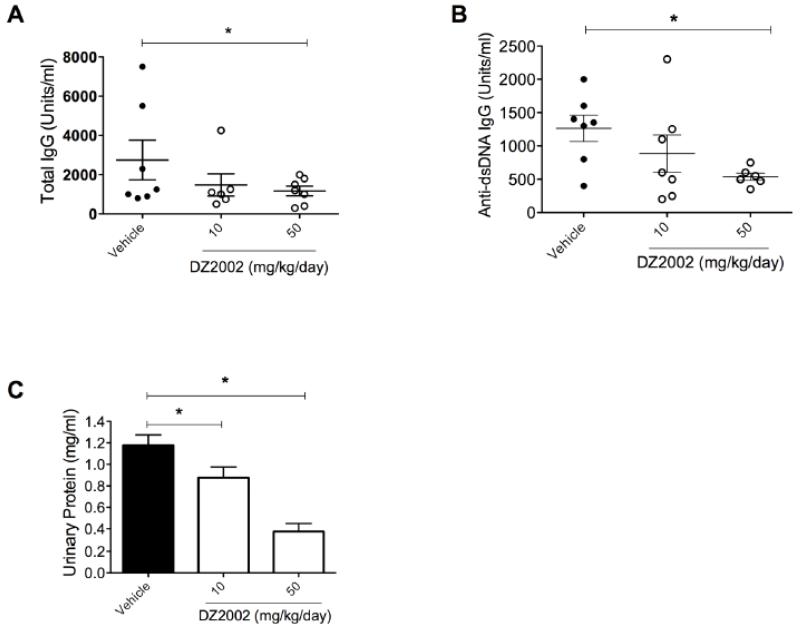
DZ2002 treatment of MRL-Faslpr mice for 30 days starting at 4.5 months of age, when disease has already developed, lowered total IgG and anti-dsDNA autoantibody concentrations in serum and reduced proteinuria in a dose-dependent manner (Fig. 2 A-C). Thus, SAHase inhibition can inhibit disease manifestations under therapeutic conditions.
Figure 2.
Therapeutic treatment of MRL-Faslpr mice reduces lupus biomarkers.
MRL-Faslpr mice (8/group) were treated i.p. with vehicle or DZ2002 (10 or 50 mg/kg/day) starting at 4.5 months of age, which corresponds to intermediate- to advanced–stage lupus. After 30 days, we evaluated (A) total serum IgG, (B) anti-dsDNA autoantibodies, and (C) proteinuria. *p < 0.05, Student t-test. Error bars represent SEM.
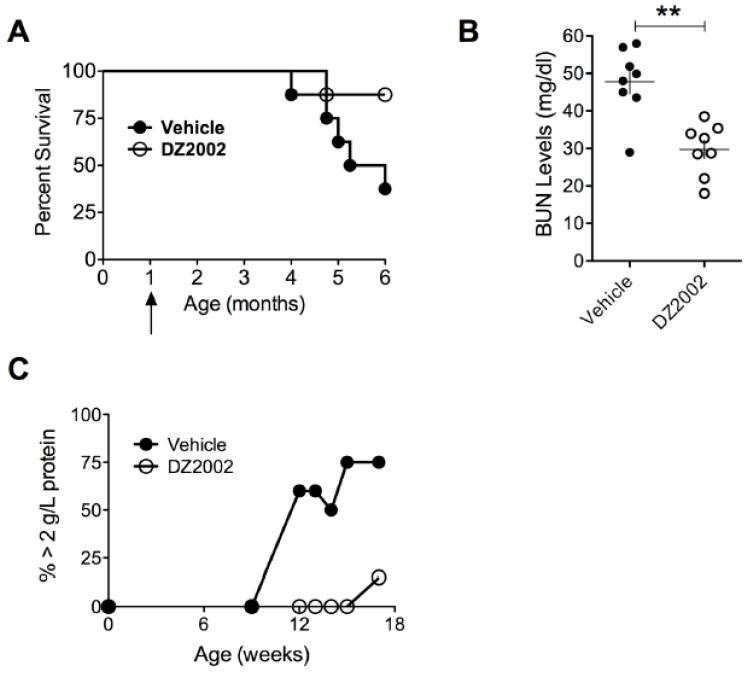
3.6 DZ2002 protected male BXSB mice from a lupus-like disease
SLE is thought to be a TLR-mediated disease with nuclear autoantigens playing a key role by engaging endosomal TLRs [25]. We therefore treated male BXSB mice, who are highly susceptible to lupus because of a duplication of TLR7 on the Y-chromosome [19], with DZ2002 for 6 months. Strikingly, DZ2002 significantly increased survival relative to controls (Fig. 3A). This was associated with decreased autoantibodies to chromatin (data not shown) and reduced levels of BUN and proteinuria (Fig. 3 B-D). Taken together with the MRL-Faslpr data, our studies firmly establish the efficacy of SAHase blockade in reducing lupus manifestations in two animal models with distinct genetic abnormalities, i.e. the MRL-Faslpr model with Fas mutations and the BXSB male model with excess TLR7 signaling.
Figure 3.
Prophylactic DZ2002 treatment of lupus-like disease in the TLR-mediated BXSB model improves survival.
BXSB mice (8/group) were given i.p injections of DZ2002 (50 mg/kg/day) or vehicle for 6 months. (A) Six-month survival curves. p = 0.02, Kaplan-Meier method with log-rank test comparison. Arrow indicates initiation of DZ2002 treatment. (B) BUN at 6.5 months of age. (C) Proteinuria at 9-16 weeks of age. **p < 0.01, Student t-test. Error bars represent SEM.
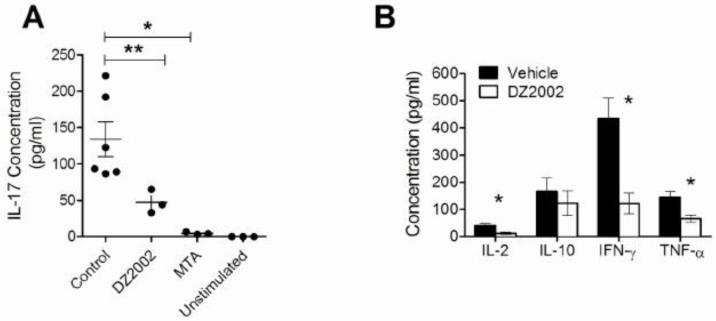
3.7 DZ2002 reduced Th1 and Th17 polarization of isolated CD4+ T cells
Since transmethylation is required for T cell signaling with specificity for the CD4 T cell subset [10; 14], we analyzed CD4 T cell polarization in T cells isolated from previously DZ2002-treated MRL-Faslpr mice. After activation with anti-CD3/28 in the absence of additional inhibitor, the cells continued to show reduced proliferation (data not shown) and less production of IL-2, IFN-γ, and TNF-α, but not IL-10, relative to cells from vehicle-treated mice (Fig. 4B) suggesting, as in our previous study (10), that transmethylation was required for Th1 polarization and that suppression of CD4+ T cell proliferation was maintained ex-vivo without additional DZ2002. In humans, high concentrations of IL-17 have been associated with SLE [26]. Although the potential role of IL-17 in lupus mouse models is unclear, we tested whether Th17 polarization requires transmethylation by activating purified CD4 T cells from MRL-Faslpr with anti-CD3/CD28 in the presence or absence of DZ2002 or MTA. IL-17 production by CD4 T cells was markedly reduced in the presence of either inhibitor (Fig. 4A).
Figure 4.
Effect of SAHase activity on TCR-induced CD4+ T cell polarization.
We stimulated purified T cells from MRL-Faslpr mice in the presence of vehicle-, DZ2002-, or MTA with plate-bound anti-CD3 (10 μg/ml) plus soluble anti-CD28 (5 μg/ml) and evaluated cytokine production in the supernatant. (A) IL-17 concentration (B) IL-2, IL-10, IFN-γ, and TNF-α concentrations. Graphs represent the mean of 3-6 independent experiments. Error bars represent SEM. *p < 0.05, **p < 0.01, Student t-test.
3.8 B cell proliferation required SAHase activity
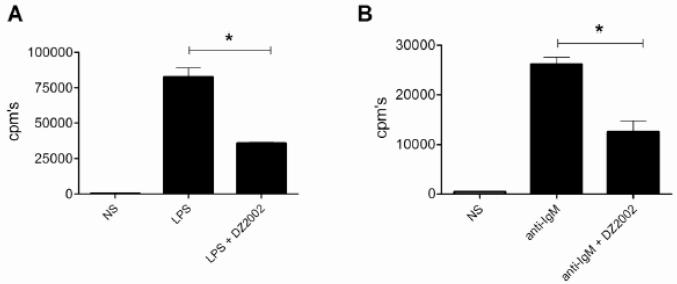
B cells isolated from naïve MRL-Faslpr mice and stimulated in the presence or absence of DZ2002 with either LPS (Fig. 5A) or anti-IgM plus anti-CD40 (anti-IgM/CD40) (Fig. 5B) showed significantly lower proliferation rates than vehicle-treated control cells. We observed similar anti-proliferative effects for MTA (anti-IgM/CD40, 26,150 ± 1,408 controls versus 6,630 ± 520 cpms MTA-treated; p < 0.0001; and LPS, 82,730 ± 6,173 control versus 5,901 ± 607 cpm’s MTA-treated; p < 0.0001). Moreover, we obtained analogous results with both compounds using B cells from non-autoimmune C57BL/6 mice (data not shown). Taken together, these results indicated that in addition to affecting TCR-mediated polarization of CD4+ T cells, transmethylation was also required for B cell receptor (BCR)- and TLR-induced B cell activation.
Figure 5.
Effect of SAHase inhibition on TLR- and BCR-dependent B cell proliferation. Purified B cells from MRL-Faslpr mice were stimulated by (A) LPS (20μg/ml) or (B) anti-IgM (5 μg/ml) plus anti-CD40 (0.5 μg/ml) for 48h in the presence or absence of DZ2002 (100 μg/ml), and proliferation was evaluated by [3H]thymidine incorporation.
cpm’s, mean counts per minute. *p < 0.05, Student t-test. Results are the mean of 3-6 independent experiments. Error bars represent SEM.
3.9 TLR signaling was transmethylation-dependent
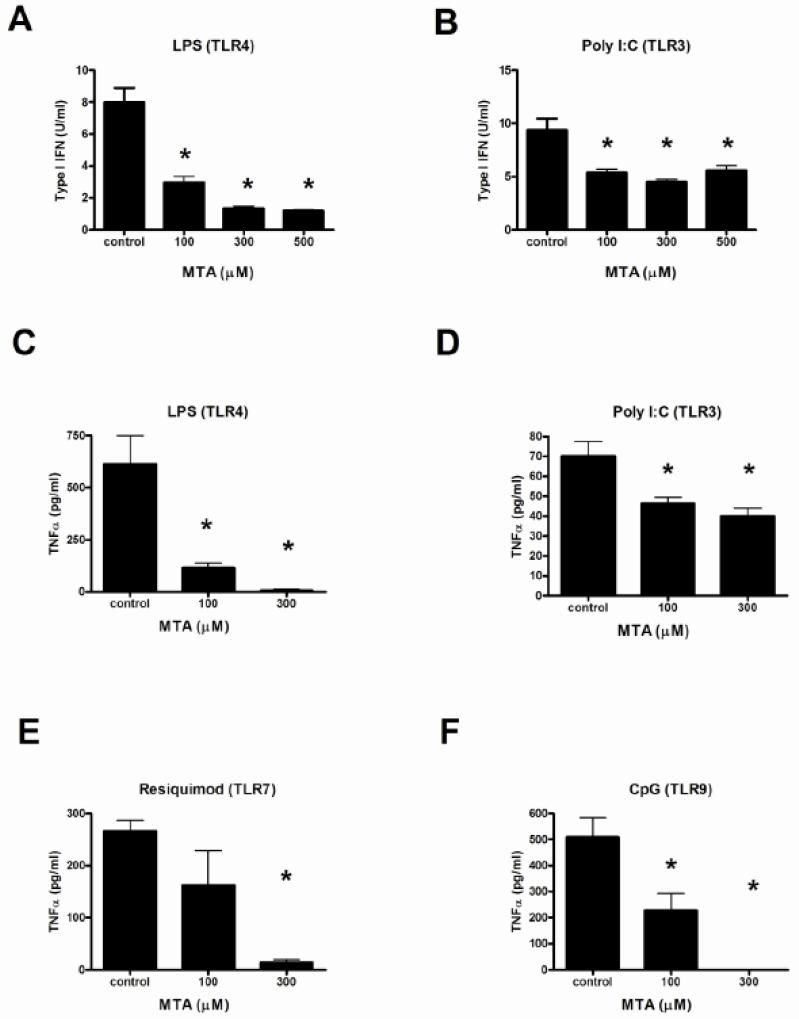
MTA (Fig. 6 A-F) and DZ2002 (data not shown) significantly and dose-dependently reduced the production of type I IFN and TNF-α by thioglycolate-elicited peritoneal macrophages taken from MRL-Faslpr mice and stimulated with additional TLR ligands. In poly I:C-stimulated cells, interestingly, SAHase inhibitors did not reduce type I IFN concentrations by more than 50% even at the highest concentration. TLR-stimulated control cells were identically treated with SAHase inhibitors and tested for toxicity without loss of viability using MTT (data not shown). Comparable inhibitory results were obtained from C57BL/6 mouse peritoneal monocytes as well as bone marrow-derived monocytes (data not shown).
Figure 6.
Effect of SAHase inhibition on TLR-induced cytokine production by macrophages.
Thioglycate-elicited peritoneal macrophages from MRL-Faslpr mice were stimulated with LPS (TLR4, 100 ng/ml) (A and C), poly I:C (TLR3, 50 μg/ml) (B and D), resiquimod (TLR7; 100ng/ml) (E), or CpG (TLR9; 1 μg/ml) (F) in presence or absence of increasing concentrations of MTA (100-500 μg/ml). Secretion of type I IFN (A-B) and TNF-α (C-F) were evaluated by an interferon-sensitive luciferase bioassay or cyclohexamide-treated TNF-α L929 cells, respectively. *p < 0.05, Student t-test. Data represent the mean of 8 mice per group. Error bars represent SEM.
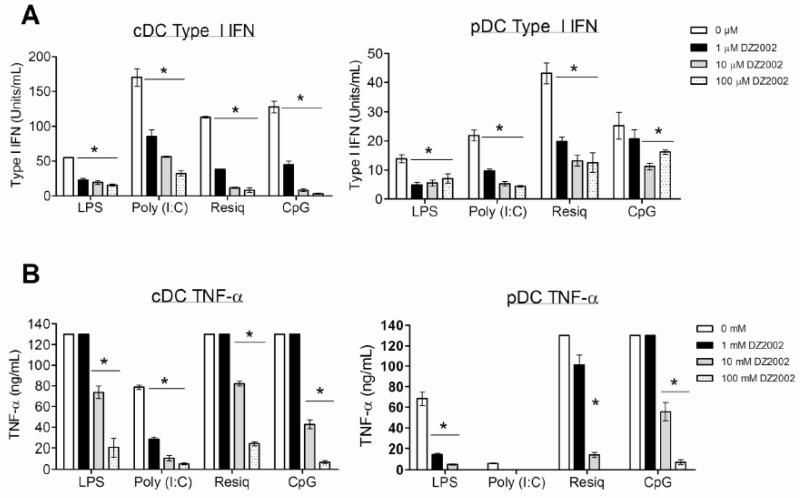
DZ2002- or vehicle-treated pDCs and cDCs (thought to be major producers of type I IFNs [27]) from MRL-Faslpr mice cultured with poly I:C, LPS, resiquimod, or CpG showed significant and dose-dependent reductions in the production of both type I IFNs and TNF-α, particularly to TLR stimuli important in the recognition of nucleic acids (TLRs 3, 7, and 9) (Fig. 7 A-B). TNF-α responses were, in fact, abrogated at the highest DZ2002 concentration in pDCs following LPS, poly (I:C), or resiquimod treatment. Viability was unaffected (data not shown). These results indicated that transmethylation was required for efficient TLR-dependent signaling.
Figure 7.
Effect of SAHase inhibition on TLR-induced cytokine production by dendritic cells.
Bone marrow-derived pDCs or cDCs from MRL-Faslpr mice were stimulated with LPS (TLR4, 100 ng/ml), poly I:C (TLR3, 50 μg/ml), resiquimod (TLR7, 100ng/ml) or CpG (TLR9, 1 μg/ml) in the presence of increasing concentrations of DZ2002 (0-100 μM). Secretion of type I IFN (A) and TNF-α (B) were evaluated by an interferon-sensitive luciferase bioassay or cyclohexamide-treated TNF-α L929 cells, respectively. *p < 0.05, Student t-test. Data represent the mean of 8 mice per group. Error bars represent SEM.
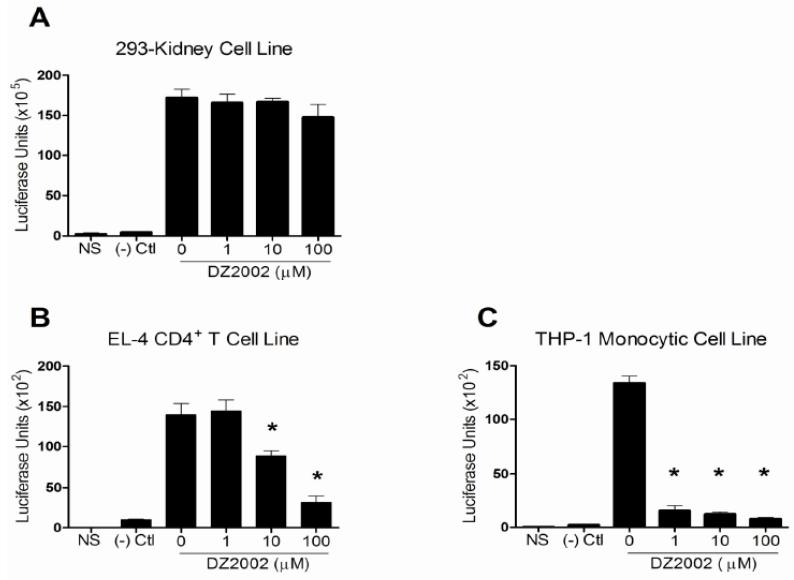
3.10 NF-κB activity in immune cells required SAHase activity
DZ2002 significantly and dose-dependently inhibited NF-κB activation in TNF-α-stimulated EL-4 and THP-1 cells (Fig. 8 A-C). Remarkably, almost complete inhibition of NF-κB was observed in THP-1 cells, even at the lowest concentration, suggesting exquisite sensitivity to loss of SAHase activity. Not surprisingly, DZ2002 did not reduce NF-κB activation in a kidney cell line, corroborating the idea that immunocytes are more sensitive than other cell types to transmethylation inhibition [5]. These results suggest that the signaling molecule(s) negatively affected by SAHase inhibition is, or is proximal to, NF-κB and, in conjunction with our previous T cell findings [10], indicate that the immunosuppressive effects of SAHase inhibition encompass at least three major immune signaling pathways, TCR, BCR, and TLR.
Figure 8.
Effect of SAHase inhibition on NF-κB activation in kidney and immune cells. 293-A (kidney) (A), EL-4 (CD4 T cells) (B), and THP-1 (monocytic) (C) cell lines were transfected with a NF-κB luciferase reporter construct, pre-treated with increasing concentrations of DZ2002 (0-100 μM), stimulated with 50 ng/ml of TNF-α, and NF-κB promoter activity was measured. Controls were non-stimulated cells (NS) and cells transfected with a plasmid containing a non-inducible negative control ((-) Ctl). *p < 0.05, Student t-test. Data represent the mean of 3 independent experiments. Error bars represent SEM.
4. DISCUSSION
In this study of the effects of transmethylation inhibition in two in vivo mouse lupus models—both prophylactically and therapeutically—we have presented what we believe to be direct evidence that transmethylation reactions are critical for TLR signaling and diseases that are reliant upon such signals, including SLE. Moreover, the fact that transmethylation inhibition affected every TLR ligand tested suggests that methylation of a central adaptor molecule in the TLR pathway is required. Additionally, B cell activation was also significantly blocked in the presence of the inhibitors. These data, in conjunction with previous reports citing T cell dependence on these same reactions, indicate that transmethylation inhibitors exert broad effects on several immune system compartments and will likely be efficacious immunosuppressives for a wide-variety of autoimmune/proinflammatory diseases.
Although, our results indicate the importance of transmethylation in the activation of immune cells, little is known about the specific molecule(s) and pathways that require this modification. In this regard, it was recently demonstrated that a conserved arginine in the tail sequence of the Igα subunit of the BCR was constitutively methylated by PRMT1 [28]. In contrast to T cell signaling, this modification was proposed to dampen signals from the BCR by downregulating PI3K activity and thereby limiting the Ca2+ response. These findings run somewhat counter to our B cell activation data, which suggest that total transmethylation inhibition may also affect molecule(s) in the BCR pathway that require methylation for proper functioning, thus counterbalancing constitutive PRMT1-mediated Igα subunit methylation.
Poly I:C-stimulated cells pretreated at even the highest concentrations of MTA or DZ2002 produced type I IFNs. In contrast, similarly-treated cells stimulated with other TLR ligands, including LPS, resiquimod, and CpG, showed nearly complete blockade of cytokine production. We presume that the maintenance of type I IFN production by poly I:C stimulate cells may be attributable in part to apolipoprotein E (ApoE). In fact, whereas reductive lysine methylation of ApoE inhibits its ability to suppress macrophage response to LPS, it has no effect on poly I:C-induced macrophage stimulation [29]. Thus, transmethylation inhibition, by altering the methylation status of ApoE, leads to the inhibition of LPS (and other TLR)-induced macrophage activation without disturbing poly I:C-induced macrophage stimulation. Other mechanisms in the poly I:C pathway may be affected by transmethylation inhibition, however, since TLR3-induced macrophages are inhibited to some degree. Since cDC showed significant inflammatory cytokine reductions in the presence of the SAHase inhibitors following poly I:C stimulation, this effect appears to be restricted to macrophages.
We treated T cell, monocyte, and kidney cell lines with SAHase inhibitors and demonstrated significant NF-κB inhibition only in immunocytes. The data presented here on NF-κB inhibition are in agreement with previous data generated with a different irreversible SAHase inhibitor [30; 31; 32] as well as with our previous findings with DZ2002 in T cells [10]. Our data here are in complete agreement with a recent report demonstrating that DZ2002 was able to inhibit TNF-α- and IFN-γ-induced NF-κB activation, at least in part, by modulating IκBα [33]. Moreover, two recent reports suggested that PRMT2 and PRMT4—two enzymes likely inhibited by global transmethylation inhibitors—are directly involved in NF-κB activation [34; 35]. We also checked IκBα, however, and found no significant perturbations (unpublished data). Nonetheless, these data strongly support the idea that methylation is required for NF-κB activation and suggest that transmethylation inhibitors could be efficient immunosuppressives thru repression of NF-κB activity.
In vitro experiments consistently demonstrate that TLR signaling can enhance activation of both B cells and dendritic cells following antigen receptor-mediated endocytosis of DNA/RNA-containing material and following FcγRIIa-dependent (FcγRIII-dependent in mice) endocytosis of DNA/RNA-containing immune complexes [18]. When lupus-prone mice were examined, however, the effects of TLR9-deficiency on the production of DNA or chromatin autoantibodies and, to a lesser extent, end-organ damage, were mixed. In MRL background Faslpr mice, lack of TLR9 inexplicably had different effects on anti-nuclear antibodies, anti-dsDNA antibodies, and anti-nucleosome antibodies in different studies, yet overall disease was consistently exacerbated [36; 37; 38]. The same was true in TLR9-deficient MRL-Fas+/+ mice [37] and in TLR9-deficient B6-Faslpr mice [39]. Although there is uncertainty about the role of TLR9 in the production of anti-DNA-related antibodies, the majority of studies indicate that TLR9 has an overall disease-suppressing function in mice with active lupus. Here, however, the MRL-Faslpr lupus-like disease was markedly improved by a treatment regimen that, based on our in vitro data, very likely included blockade of TLR9 signaling. Although TLR9 signaling is generally considered beneficial in lupus, we think that our approach blocked other TLR signals, including those known to be detrimental, such as TLR7 [37]. It should also be noted that lupus disease models are necessarily B and T cell-dependent, so in addition to inhibiting TLR signaling, our approach also inhibits CD4+ T [10] and B cell activity (current study). Hence, SAHase inhibition exhibited multiple protective mechanisms in the treatment of lupus.
In contrast to TLR9, TLR7 is involved in promoting lupus, as is evident by the effect of the extra TLR7 gene in the male BXSB mouse [19; 40]. Moreover, MRL-Faslpr mice deficient in TLR7 have reduced antibodies to RNP, less lymphoproliferation, and a modest reduction in composite renal disease score but levels of anti-nucleosome and anti-dsDNA antibodies similar to those of non-deficient mice [37]. Thus, in MRL-Faslpr mice, TLR7 appears to play a major role in the induction of anti-RNA-related antibodies [37]. Accordingly, the case for TLR inhibition in the BXSB disease is more compelling. We demonstrated reduced TLR signaling in the presence of transmethylation inhibitors and reduced lupus disease in this model, which is ideally suited to test whether a TLR-dependent disease process could be slowed by this approach. However, the BXSB disease is also T cell-dependent, so it is possible that some of the beneficial effects seen in these studies were also due to T cell inhibition.
We believe that there are several lines of evidence strongly suggesting that transmethylation plays a critical role in TLR-mediated signaling: 1) B cell proliferation in response to a TLR4 ligand (LPS) was reduced in MTA and DZ2002-treated cells, 2) monocytes treated with several different TLR ligands failed to produce type I IFN or TNF-α in the presence of SAHase inhibitors, 3) mice treated with a SAHase inhibitor are protected from LPS-induced lethality [30; 41], and 4) BXSB mice in the current study were protected from a lupus-like disease.
Although we have not yet identified the kind of methylated macromolecule(s), we assume it is not a nucleic acid because of the rapidity of the suppressive response (two hour pretreatment with the inhibitors). A possible candidate is p53—a protein whose activity appears to be regulated by arginine and lysine methylation [42; 43; 44]. MRL-Faslpr mice treated with nutlin-3a, a small molecule blocking the interaction between p53 and Mdm2 that protects p53 from Mdm2-mediated ubiquitination, leads to prolonged survival and a phenotype similar to that described here after SAHase inhibition [45]. Moreover, nutlin-3a treatment of LPS-stimulated macrophages and neutrophils attenuates NF-κB DNA-binding activity and subsequent production of proinflammatory cytokines [46]. Thus, it is possible that transmethylation inhibition reduces p53 methylation rendering it unable to interact with Mdm2, thus leading to reduced lupus-like disease, likely in a TLR-dependent manner.
As a potential therapeutic approach, the specific TLR affected is not all that critical since all the ones tested to date were negatively affected, particularly those most responsive to nucleic acids, such as TLRs 3, 7, and 9. In this context, we recently analyzed B6-Faslpr and congenic BXSB mice that had an ENU-induced mutation in UNC93b1 (3d) that compromised TLRs 3, 7 and 9 signaling, and they showed significantly reduced lupus manifestations, especially anti-nucleic acid autoantibody production [18].
We conclude from these data that SAHase inhibition may be an effective therapeutic approach to systemic autoimmunity and possibly other immune-mediated diseases that require T and B cells and/or TLRs.
Highlights.
Transmethylation inhibitors block B cell activation.
Transmethylation inhibitors block Toll-like receptor signaling.
Transmethylation inhibitors are efficacious in two separate models of lupus.
ACKNOWLEDGMENTS
This is manuscript number 23,014 from the Department of Immunology and Microbial Science of The Scripps Research Institute. We thank M. Kat Occhipinti and Miriam Bloom for editorial assistance and Tannaz Hasnat for excellent technical assistance. This work was supported in part by funding from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Pike MC, DeMeester CA. Inhibition of phosphoinositide metabolism in human polymorphonuclear leukocytes by S-adenosylhomocysteine. J Biol Chem. 1988;263:3592–9. [PubMed] [Google Scholar]
- [2].Pike MC, Kredich NM, Snyderman R. Requirement of S-adenosyl-L-methionine-mediated methylation for human monocyte chemotaxis. Proc Natl Acad Sci U S A. 1978;75:3928–32. doi: 10.1073/pnas.75.8.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pike MC, Snyderman R. Transmethylation reactions are required for initial morphologic and biochemical responses of human monocytes to chemoattractants. J Immunol. 1981;127:1444–9. [PubMed] [Google Scholar]
- [4].Lawson BR, Eleftheriadis T, Tardif V, Gonzalez-Quintial R, Baccala R, Kono DH, Theofilopoulos AN. Transmethylation in immunity and autoimmunity. Clin Immunol. 2012;143:8–21. doi: 10.1016/j.clim.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].German DC, Bloch CA, Kredich NM. Measurements of S-adenosylmethionine and L-homocysteine metabolism in cultured human lymphoid cells. J Biol Chem. 1983;258:10997–1003. [PubMed] [Google Scholar]
- [6].Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP. S-Adenosylmethionine and methylation. Faseb J. 1996;10:471–80. [PubMed] [Google Scholar]
- [7].Ueland PM, Helland S, Broch OJ, Schanche JS. Homocysteine in tissues of the mouse and rat. J Biol Chem. 1984;259:2360–4. [PubMed] [Google Scholar]
- [8].Fu YF, Wang JX, Zhao Y, Yang Y, Tang W, Ni J, Zhu YN, Zhou R, He PL, Li C, Li XY, Yang YF, Lawson BR, Zuo JP. S-adenosyl-L-homocysteine hydrolase inactivation curtails ovalbumin-induced immune responses. J Pharmacol Exp Ther. 2006;316:1229–37. doi: 10.1124/jpet.105.093369. [DOI] [PubMed] [Google Scholar]
- [9].Fu YF, Zhu YN, Ni J, Zhong XG, Tang W, Re YD, Shi LP, Wan J, Yang YF, Yuan C, Nan FJ, Lawson BR, Zuo JP. A reversible S-adenosyl-L-homocysteine hydrolase inhibitor ameliorates experimental autoimmune encephalomyelitis by inhibiting T cell activation. J Pharmacol Exp Ther. 2006;319:799–808. doi: 10.1124/jpet.106.107185. [DOI] [PubMed] [Google Scholar]
- [10].Lawson BR, Manenkova Y, Ahamed J, Chen X, Zou JP, Baccala R, Theofilopoulos AN, Yuan C. Inhibition of transmethylation down-regulates CD4 T cell activation and curtails development of autoimmunity in a model system. J Immunol. 2007;178:5366–74. doi: 10.4049/jimmunol.178.8.5366. [DOI] [PubMed] [Google Scholar]
- [11].Lambert LE, Frondorf KA, Berling JS, Wolos JA. Effects of an S-adenosyl-L-homocysteine hydrolase inhibitor on murine macrophage activation and function. Immunopharmacology. 1995;29:121–7. doi: 10.1016/0162-3109(94)00051-g. [DOI] [PubMed] [Google Scholar]
- [12].Wolos JA, Frondorf KA, Babcock GF, Stripp SA, Bowlin TL. Immunomodulation by an inhibitor of S-adenosyl-L-homocysteine hydrolase: inhibition of in vitro and in vivo allogeneic responses. Cell Immunol. 1993;149:402–8. doi: 10.1006/cimm.1993.1165. [DOI] [PubMed] [Google Scholar]
- [13].Wolos JA, Frondorf KA, Davis GF, Jarvi ET, McCarthy JR, Bowlin TL. Selective inhibition of T cell activation by an inhibitor of S-adenosyl-L-homocysteine hydrolase. J Immunol. 1993;150:3264–73. [PubMed] [Google Scholar]
- [14].Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O. CD28 costimulatory signal induces protein arginine methylation in T cells. J Exp Med. 2005;202:371–7. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blanchet F, Schurter BT, Acuto O. Protein arginine methylation in lymphocyte signaling. Curr Opin Immunol. 2006;18:321–8. doi: 10.1016/j.coi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [16].Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–71. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Intracellular nucleic acid sensors and autoimmunity. J Interferon Cytokine Res. 2011;31:867–86. doi: 10.1089/jir.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kono DH, Haraldsson MK, Lawson BR, Pollard KM, Koh YT, Du X, Arnold CN, Baccala R, Silverman GJ, Beutler BA, Theofilopoulos AN. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–6. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–72. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- [20].Janssen E, Tabeta K, Barnes MJ, Rutschmann S, McBride S, Bahjat KS, Schoenberger SP, Theofilopoulos AN, Beutler B, Hoebe K. Efficient T cell activation via a Toll-Interleukin 1 Receptor-independent pathway. Immunity. 2006;24:787–99. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [21].Lawson BR, Prud’homme GJ, Chang Y, Gardner HA, Kuan J, Kono DH, Theofilopoulos AN. Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J Clin Invest. 2000;106:207–15. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3:136–41. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gonzalez-Quintial R, Lawson BR, Scatizzi JC, Craft J, Kono DH, Baccala R, Theofilopoulos AN. Systemic autoimmunity and lymphoproliferation are associated with excess IL-7 and inhibited by IL-7Ralpha blockade. PLoS One. 2011;6:e27528. doi: 10.1371/journal.pone.0027528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Theofilopoulos AN, Balderas RS, Shawler DL, Lee S, Dixon FJ. Influence of thymic genotype on the systemic lupus erythematosus-like disease and T cell proliferation of MRL/Mp-lpr/lpr mice. J Exp Med. 1981;153:1405–14. doi: 10.1084/jem.153.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baccala R, Gonzalez-Quintial R, Lawson BR, Stern ME, Kono DH, Beutler B, Theofilopoulos AN. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol. 2009;5:448–56. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- [26].Dolff S, Bijl M, Huitema MG, Limburg PC, Kallenberg CG, Abdulahad WH. Disturbed Th1, Th2, Th17 and T(reg) balance in patients with systemic lupus erythematosus. Clin Immunol. 2011;141:197–204. doi: 10.1016/j.clim.2011.08.005. [DOI] [PubMed] [Google Scholar]
- [27].Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–51. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- [28].Infantino S, Benz B, Waldmann T, Jung M, Schneider R, Reth M. Arginine methylation of the B cell antigen receptor promotes differentiation. J Exp Med. 2010;207:711–9. doi: 10.1084/jem.20091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhu Y, Kodvawala A, Hui DY. Apolipoprotein E inhibits toll-like receptor (TLR)-3- and TLR-4-mediated macrophage activation through distinct mechanisms. Biochem J. 2010;428:47–54. doi: 10.1042/BJ20100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hevia H, Varela-Rey M, Corrales FJ, Berasain C, Martinez-Chantar ML, Latasa MU, Lu SC, Mato JM, Garcia-Trevijano ER, Avila MA. 5′-methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology. 2004;39:1088–98. doi: 10.1002/hep.20154. [DOI] [PubMed] [Google Scholar]
- [31].Law RE, Stimmel JB, Damore MA, Carter C, Clarke S, Wall R. Lipopolysaccharide-induced NF-kappa B activation in mouse 70Z/3 pre-B lymphocytes is inhibited by mevinolin and 5′-methylthioadenosine: roles of protein isoprenylation and carboxyl methylation reactions. Mol Cell Biol. 1992;12:103–11. doi: 10.1128/mcb.12.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Veal N, Hsieh CL, Xiong S, Mato JM, Lu S, Tsukamoto H. Inhibition of lipopolysaccharide-stimulated TNF-alpha promoter activity by S-adenosylmethionine and 5′-methylthioadenosine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G352–62. doi: 10.1152/ajpgi.00316.2003. [DOI] [PubMed] [Google Scholar]
- [33].Kominsky DJ, Keely S, MacManus CF, Glover LE, Scully M, Collins CB, Bowers BE, Campbell EL, Colgan SP. An endogenously anti-inflammatory role for methylation in mucosal inflammation identified through metabolite profiling. J Immunol. 2011;186:6505–14. doi: 10.4049/jimmunol.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Covic M, Hassa PO, Saccani S, Buerki C, Meier NI, Lombardi C, Imhof R, Bedford MT, Natoli G, Hottiger MO. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-kappaB-dependent gene expression. EMBO J. 2005;24:85–96. doi: 10.1038/sj.emboj.7600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ganesh L, Yoshimoto T, Moorthy NC, Akahata W, Boehm M, Nabel EG, Nabel GJ. Protein methyltransferase 2 inhibits NF-kappaB function and promotes apoptosis. Mol Cell Biol. 2006;26:3864–74. doi: 10.1128/MCB.26.10.3864-3874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–31. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- [38].Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–42. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- [39].Lartigue A, Courville P, Auquit I, Francois A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–54. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- [40].Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–5. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ara AI, Xia M, Ramani K, Mato JM, Lu SC. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–66. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D. Regulation of p53 activity through lysine methylation. Nature. 2004;432:353–60. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- [43].Durant ST, Cho EC, La Thangue NB. p53 methylation--the Arg-ument is clear. Cell Cycle. 2009;8:801–2. doi: 10.4161/cc.8.6.7850. [DOI] [PubMed] [Google Scholar]
- [44].Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, La Thangue NB. Arginine methylation regulates the p53 response. Nat Cell Biol. 2008;10:1431–9. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- [45].Allam R, Sayyed SG, Kulkarni OP, Lichtnekert J, Anders HJ. Mdm2 promotes systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol. 2011;22:2016–27. doi: 10.1681/ASN.2011010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J Immunol. 2009;182:5063–71. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]