Abstract
Background
The thyroid hormones thyroxine (T4) and 3,5,3′-triidothyronine (T3) are essential for regulating a number of biological processes, including growth, neurodevelopment, carbohydrate metabolism, oxygen consumption and protein synthesis. Immunoassays are the current methods for thyroid hormone measurement and suffer from a lack of specificity. Our objective was to simultaneously measure T4 and T3 using isotope dilution tandem mass spectrometry within a single run. To compare the results obtained by this MS/MS method with those obtained by an immunoassay procedure on the same samples (DPC Immulite for T3, Diagnostics Product, and Dade RxL Dimension for T4, Dade-Behring).
Methods
An API-3000 tandem mass spectrometer (SCIEX, Toronto, Canada) equipped with TurboIonSpray and Shimadzu HPLC system was used employing isotope dilution with deuterium-labeled internal standard (l-thyroxin-d2). The method requires 100 µl of serum and involves addition of internal standard, precipitation of proteins with methanol and injection of the supernatant onto a C-18 column. After washing, the switch valve is activated and T4 and T3 eluted using a methanol gradient. T4 and T3 by immunoassay were performed using the Dade RxL Dimension and the DPC Immulite, respectively.
Results and conclusions
An isotope dilution tandem mass spectrometry method for the simultaneous determination of total T4 and T3 in serum is described which is accurate, specific, precise (%CVs 3.5–9.0), simple and fast (< 7 min).
Keywords: Thyroxine, Triiodothyronine, Thyroid Hormones, Tandem mass spectrometry
1. Introduction
The thyroid hormones, thyroxine (T4) and triidothyronine (T3), are important in regulating a number of biological processes, including growth and development, carbohydrate metabolism, oxygen consumption, protein synthesis and fetal neurodevelopment. Synthesis of all circulating T4 and a small percentage of circulating T3 occurs on thyroglobulin molecules located within the thyroid. The bulk of the T3 present in the blood is produced enzymatically via monodeiodination of T4 by specific intracellular deiodinases—enzymes present in the follicular cells and the cells of target tissues [1]. In serum drawn from healthy human subjects, total T4 is present at about 60-fold higher concentration than total T3. T4 acts as a prohormone, as the reservoir for the production of T3, the active hormone. The metabolic activity associated with thyroid hormone (TH) is initiated by T3 binding to specific nuclear receptors within target cells. Thyroid hormone concentrations in blood are essential tests for the assessment of thyroid function.
The presence of circulating iodothyronine-binding autoantibodies that interfere with total T4 and T3 RIAs is a known phenomenon [2–4]. These autoantibodies may give falsely high, or falsely low values of thyroid hormone measurements depending on the assay separation method used, and are often in discordance with the clinical features [2–4]. Direct serum-free T4 and T3 (FT4 and FT3) measurements are a way to compensate for such abnormal binding. However, technically, it is difficult to measure the free hormone concentrations since these are so low. It is easier to measure the total (free and protein-bound) thyroid hormone concentrations; total hormone concentrations are measured at nanomolar levels whereas free hormone concentrations are measured in the picomole range and to be valid, must be free from interference by the much higher total hormone concentrations.
Immunoassays (IAs) are notoriously unreliable with more and more literature published supporting their lack of specificity [5–12]. Table 1 shows the major differences reported by the College of American Pathologists program for proficiency testing of thyroid hormones that clearly illustrates the difference in specificity of the various antibodies used. For example, Table 1 shows mean results between different methods reported in the College of American Pathologists Proficiency Testing (CAP PT) Program can vary by a factor of approximately 2. Some factors such as pregnancy, estrogen therapy or genetics abnormalities in protein binding have also reportedly made IA methods for T4 and T3 diagnostically unreliable [2,3,13,14].
Table 1.
Problems with immunoassays: data acquired for samples from the CAP PT program 2003
| Analyte | Mean CAP result for method giving lowest value |
Mean CAP result for method giving highest value |
|---|---|---|
| Triiodothyronine (ng/dl) |
108.5 | 190.2 |
| 364.8 | 610.1 | |
| Thyroxine | 5.64 | 10.09 |
| (µg/dl) | 1.64 | 3.65 |
| 8.73 | 13.12 |
Currently serum total T4 (TT4) and total serum T3 (TT3) concentrations are most commonly measured by immunoassay methods. Recently some reports of quantitative measurement of T4 and T3 by HPLC, gas chromatography mass spectrometry (GC-MS), liquid chromatography mass spectrometry (LC-MS) or tandem mass spectrometry (LC-MS/MS) were published [15–19]. All those methods required extraction, derivatization and even prior chromatographic separation that are very time-consuming [20,21]. This paper describes an isotope dilution tandem mass spectrometry method for the simultaneous determination of T4 and T3 in serum.
2. Methods
2.1. Chemicals and reagents
Standards of T4 and T3 were from Sigma (St. Louis, MO). A stable deuterium-labeled internal standard, l-thyroxin-d2 was synthesized according to procedures described in the literature [15,16] by Dr. Tomas Class from the Chemistry Department at Georgetown University. HPLC grade methanol was purchased from VWR Scientific. All other chemicals were of analytical grade and purchased from Sigma.
2.2. Solutions and standards
Stock solutions of T3, T4 and internal standard (IS) were prepared separately to obtain concentration of 1 mg/ml for each. 40% ammonium hydroxide (v/v) in methanol was used as a solvent. The analyte stock solutions were diluted with methanol to obtain the spiking solutions. The solutions were stored at 4 °C and could be used for several months. Standards for the calibration curve in the range of 0.325–5 ng/ml for T3 and 12.5–200 ng/ml for T4 were prepared by adding the analyses to 3% human γ-globulin (volume of spiking solution < 2% of final volume). Quality control (QC) samples (Diagnostic Product, Los Angeles, CA) at low, medium and high levels were used. A solution of 50 ng/ml d2-T4 in methanol was used as the internal standard.
2.3. Sample preparation
Serum/plasma samples were thawed at room temperature. IS solution (150 µl) was added to aliquots of 100 µl of the serum or plasma sample. After 30 s of vortex mixing, the samples were stored for 10 min at room temperature to allow complete protein precipitation. The samples were centrifuged for 10 min at 15,000 rpm and 100 µl of supernatant was injected into the LC-MS-MS system.
2.4. LC/MS/MS conditions
An API 3000 tandem mass spectrometer (SCIEX, Toronto, Canada) equipped with TurboIonSpray and Shimadzu HPLC system was used to perform the analysis. Negative ion multiple reaction-monitoring (MRM) mode was used. The transitions to monitor were selected at m/z 650 → 127 for T3, m/z 776 → 127 for T4, m/z 778 → 127 for d2-T4. Nitrogen served as auxiliary, curtain and collision gas. Gas flow rates, source temperature, ion spray voltages and collision energies were optimized for every compound by infusion of 1 µg/ml of the standard solutions in methanol at 20 µl/min and by flow-injection analysis (FIA) at LC flow rate. The main working parameters for the mass spectrometer are summarized in Table 2. Data processing was performed on Analyst 1.3 software package.
Table 2.
Tandem mass spectrometer working parameters
| Parameter | Value |
|---|---|
| Nebulizer gas (NEB) | 8 |
| Curtain gas (CUR) | 10 |
| Collision gas (CAD) | 6 |
| TurboIon Spray Heater gas | 7 l/min |
| TurboIon Spray (IS) voltage | 4500 V |
| Entrance Potential (EP) | 7.5 V |
| Collision cell Exit Potential (CXP) | 5 V |
| Source temperature | 450° |
| Dwell time | 250 m sec |
2.5. LC-MS-MS procedure
The procedure is based on an online extraction/cleaning of the injected samples with subsequent introduction into the mass spectrometer by using a built-in Valco switching valve. One hundred microliters of the deproteinized sample was injected onto a Supelco LC-18-DB (3.3 cm × 3.0 mm, 3.0 µm ID) chromatographic column equipped with a Supelco Discovery C-18 (3.0 mm) Guard column, where it underwent cleaning with 20% (v/v) methanol in 5 mmol/l ammonium acetate pH= 4.0 at flow rate 0.8 ml/min. After 3.5 min of cleaning the switching valve was activated, the column was flushed with water/methanol gradient at flow rate 0.5 ml/min and the samples were introduced into the mass spectrometer. The gradient parameters are shown in Table 3.
Table 3.
Gradient parameters
| Time (min) | Methanol (%) |
|---|---|
| 3.50 | 75 |
| 5.25 | 76 |
| 5.50 | 100 |
| 7.00 | End |
2.6. Immunoassays for T4 and T3
T4 was measured by the Dade RxL Dimension (Dade-Behring Diagnostics, Glasgow, DE) and T3 by the DPC Immulite (DPC) according to the manufacturer’s specifications.
3. Results
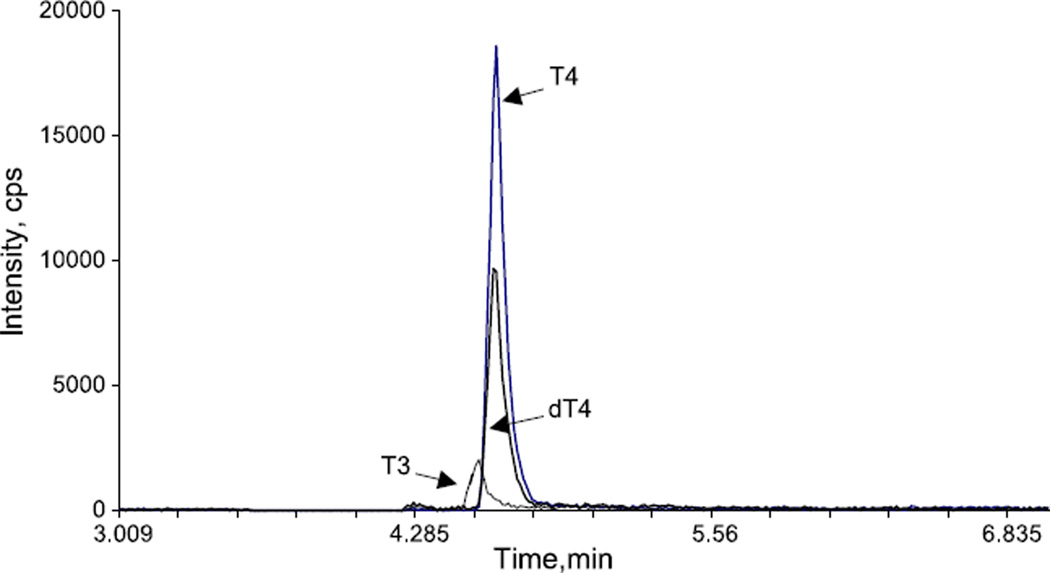
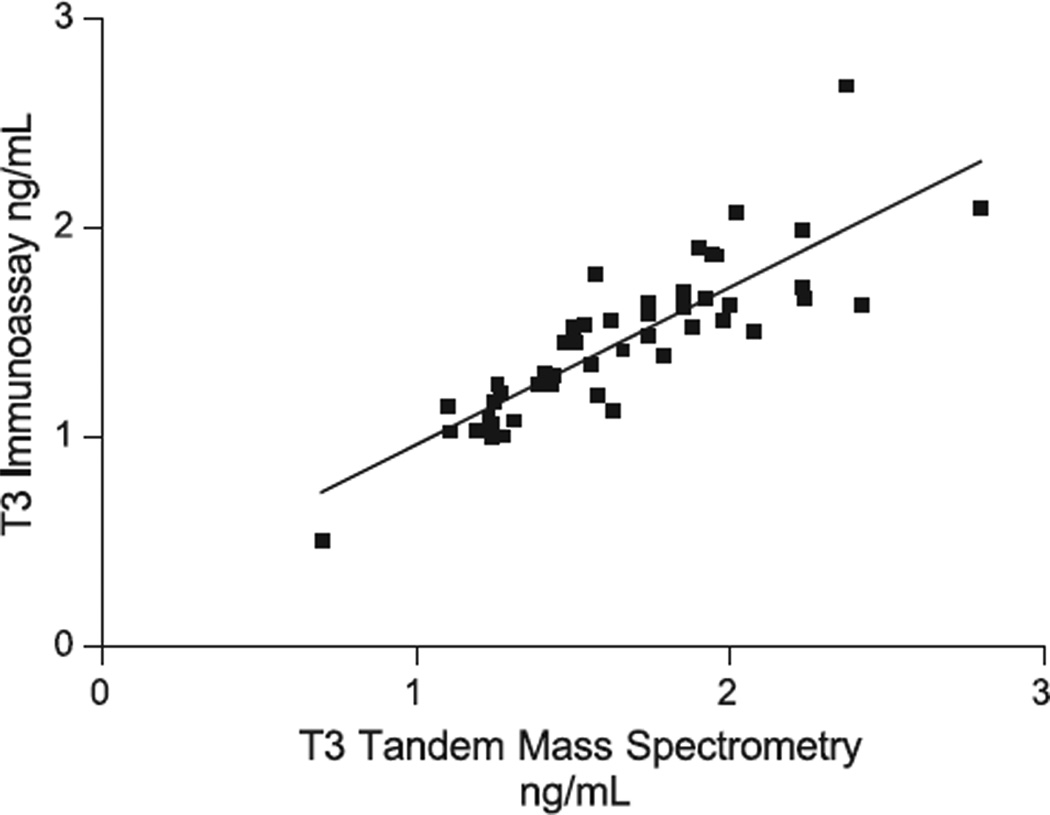
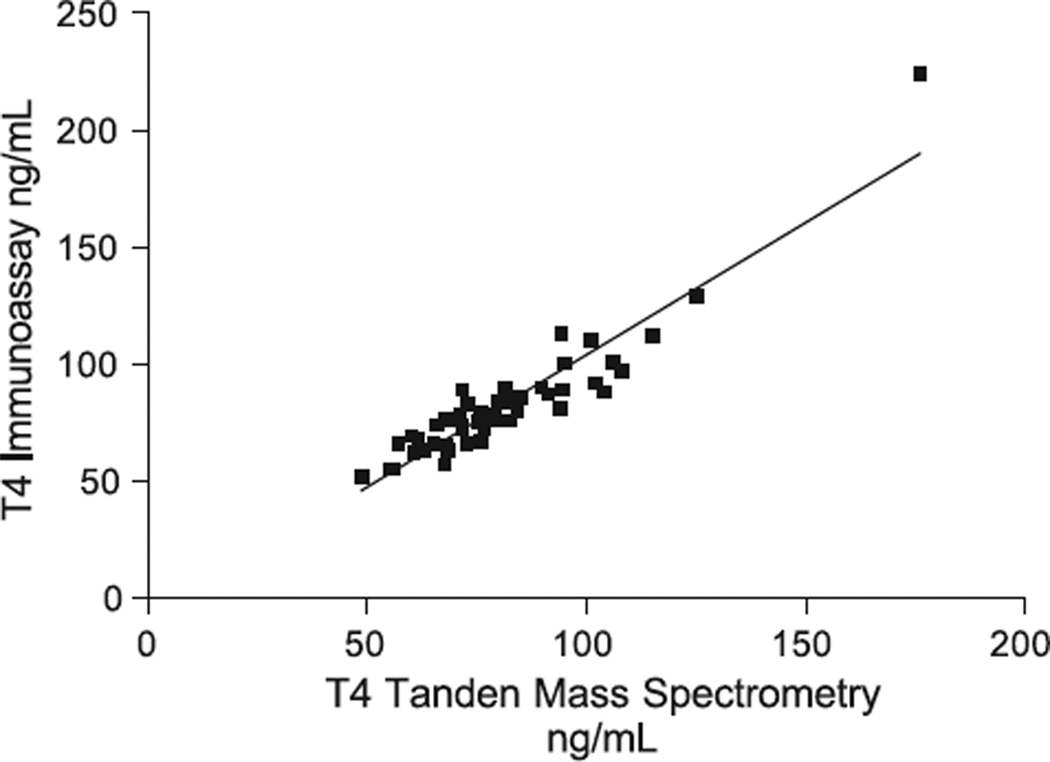
The optimal mass spectrometer working parameters are shown in Tables 2 and 3. Replicate sera were assayed both within- and between-day at several concentrations. The within- and between-day precision data is provided in Tables 4 and 5. Recovery studies for T4 and T3 are shown in Tables 6 and 7. All results shown are the means of 8 replicates. Fig. 1 shows a typical chromatogram. Specimens were tested for T3 and T4 by both immunoassay (T3 DPC Immulite, T4 Dade Behring Dimension RxL) and by tandem mass spectrometry. Linear regression correlations (Prism) are shown in Figs. 2 and 3. The lower limit of quantitation was found to be 0.15 ng/ml for both T3 and T4. Detection limit was around 0.062 ng/ml.
Table 4.
Within-day precision (n = 10)
| Analyte | Control 1 |
Control 2 |
||||
|---|---|---|---|---|---|---|
| Mean (ng/ml) |
S.D. | CV (%) |
Mean (ng/ml) |
S.D. | CV (%) |
|
| T3 | 1.04 | 0.014 | 1.36 | 2.44 | 0.077 | 3.19 |
| T4 | 24.1 | 0.437 | 1.81 | 81.2 | 1.502 | 1.85 |
Table 5.
Between-day precision (n = 20, 1 run per day for 20 days)
| Analyte | Control 1 |
Control 2 |
Control 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (ng/ml) |
S.D. | CV (%) |
Mean (ng/ml) |
S.D. | CV (%) |
Mean (ng/ml) |
S.D. | CV (%) |
|
| T3 | 1.08 | 0.05 | 4.47 | 2.39 | 0.22 | 9.21 | 3.49 | 0.31 | 9.00 |
| T4 | 24.4 | 1.39 | 5.69 | 76.6 | 3.11 | 4.06 | 116.3 | 4.15 | 3.57 |
Table 6.
Recovery of added thyroxine (T4)
| Sample no. | Added (ng/ml) |
Detected mean |
Added amount recovered |
Recovery, % |
|---|---|---|---|---|
| 1 (n = 8) | 0 | 85.9 | NA | NA |
| 10 | 96.7 | 10.8 | 108.0 | |
| 40 | 127.5 | 41.6 | 104.0 | |
| 2 (n = 5) | 0 | 62.6 | NA | NA |
| 10 | 72.1 | 9.5 | 95.0 | |
| 40 | 98.0 | 35.4 | 90.0 | |
| 3 (n = 5) | 0 | 73.8 | NA | NA |
| 10 | 84.7 | 10.9 | 109.0 | |
| 40 | 116 | 42.2 | 105.0 | |
| 4 (n = 5) | 0 | 58.3 | NA | NA |
| 10 | 68.0 | 9.7 | 97.0 | |
| 40 | 95.0 | 36.7 | 92.0 |
NA= not applicable.
Table 7.
Recovery of added triiodothyronine (T3)
| Sample no. | Added (ng/ml) |
Detected mean |
Added amount recovered |
Recovery, % |
|---|---|---|---|---|
| 1 (n = 8) | 0 | 1.88 | NA | NA |
| 0.25 | 2.12 | 0.24 | 96.0 | |
| 1.00 | 2.85 | 0.97 | 97.0 | |
| 2 (n = 5) | 0 | 1.70 | NA | NA |
| 0.25 | 1.96 | 0.26 | 104.0 | |
| 1.00 | 2.76 | 1.06 | 106.0 | |
| 3 (n = 5) | 0 | 1.56 | NA | NA |
| 0.25 | 1.81 | 0.25 | 100.0 | |
| 1.00 | 2.62 | 1.06 | 106.0 | |
| 4 (n = 5) | 0 | 0.49 | NA | NA |
| 0.25 | 0.74 | 0.25 | 100.0 | |
| 1.00 | 1.50 | 1.01 | 101.0 |
NA= not applicable.
Fig. 1.
Tandem mass spectrometric chromatogram for a plasma sample. T4 m/z (776/127); D2T4 m/z (778/127); T3 m/z (650/127).
Fig. 2.
T3 measured by isotope dilution tandem mass spectrometry vs. immunoassay. IA= 0.75 MS + 0.21; r = 0.848; Sy·x = 0.1956; n = 49.
Fig. 3.
T4 measured by Isotope dilution tandem mass spectrometry vs. immunoassay. IA = 1.13 MS – 8.99; r = 0.931; Sy·x = 9.54; n = 50.
4. Discussion
Evidence initially gleaned from both the CAP PT Program and pediatric reference ranges employing different immunoassays indicated the probability of lack of specificity for T4 and T3 immunoassay tests. To adequately assess this phenomenon we developed the isotope dilution tandem mass spectrometric method described in this manuscript. Serum T4 and T3 detection methods have evolved through a variety of technologies since the 1950s. Radioimmunoassay (RIA) methods to detect THs were developed in the 1970s. Serum T4 and T3 concentrations are currently measured by competitive immunoassay methods (IAs) that are mostly non-isotopic and use enzymes, fluorescence or chemiluminescence molecules as signals [22]. Table 1 clearly indicates that current IAs for T4 and T3 lack specificity and give mean results differing by a factor of approximately 2 in CAP PT programs. Total hormone assays necessitate the inclusion of a displacing agent (such as salicylate) to release the hormone from its binding proteins [23]. The displacement of hormone binding from serum proteins by such agents, together with the large sample dilution employed in modern assays, facilitates the binding of hormone to the antibody reagent.
Since T3 is 10-fold lower in concentration compared with T4 in blood it therefore presents both a technical sensitivity and precision challenge despite the use of a higher specimen volume. Although a reliable high-range T3 measurement is critical for diagnosing hyperthyroidism, a reliable normal-range measurement is also important for adjusting antithyroid drug dosage and detecting hyperthyroidism in sick hospitalized patients, in whom a paradoxically normal T3 value may indicate hyperthyroidism.
The correlation coefficient for the T4 comparisons (0.931) is significantly better than for the T3 comparisons (0.848) (Figs. 2 and 3). T3 by tandem mass spectrometry gave slightly higher results than those obtained by the DPC Immulite (Fig. 2). While this is true for children, our preliminary data for non-pregnant and pregnant women indicates a very poor correlation for T3 in both groups (r between 0.407 and 0.574). This is the subject of another manuscript.
The reasons for this are not clear but could include standardization issues, heterophilic antibodies etc. Of importance, we determined that reverse T3, which lacks a daughter ion of 127 m/z, therefore does not interfere in our tandem mass spectrometry method. Applying the tandem mass spectrometric method to CAP PT samples in the K/KN general ligand program again revealed that around 85% of the immunoassay methods gave means on samples which were lower than the means obtained by our tandem mass spectrometry method while 15% had higher means.
In conclusion, correlations between immunoassays and tandem mass spectrometry for T4 proved to be adequate except for the pregnant population, while the data for T3 was far less impressive especially during pregnancy. Recovery studies from several different sera using deuterated T4 as internal standard showed consistent (90–109%) recoveries for both T4 and T3 (Tables 6 and 7). The recovery differences found between samples were surprisingly larger for T4 than for T3. This indicates a lack of need to use deuterated T3 as the T3 internal standard. The isotope dilution tandem mass spectrometric method we developed is rapid (< 7 min), accurate (provides the true result as has been assessed by recovery studies), specific (measures only the analyte it purports to measure), precise (low %CV) and easy to perform. Its role in the routine clinical laboratory is currently being evaluated.
Acknowledgements
This work was supported by an NIH grant, GCRC grant number 5-MO1-RR-13297.
References
- 1.Lum SM, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest. 1984;73(2):570–575. doi: 10.1172/JCI111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakata S, Nakamura S, Miura K. Autoantibodies against thyroid hormones or iodothyronine. Implications in diagnosis, thyroid function, treatment, and pathogenesis. Ann Intern Med. 1985;103(4):579–589. doi: 10.7326/0003-4819-103-4-579. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Peccoz P, Romelli PB, Cattaneo MG, Faglia G, White EL, Barlow JW, Stockigt JR. Evaluation of free thyroxine methods in the presence of iodothyronine-binding autoantibodies. J Clin Endocrinol Metab. 1984;58(4):736–739. doi: 10.1210/jcem-58-4-736. [DOI] [PubMed] [Google Scholar]
- 4.Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124(6):921–923. doi: 10.5858/2000-124-0921-HAMA. [DOI] [PubMed] [Google Scholar]
- 5.Soldin SJ. Digoxin—issues and controversies. Clin Chem. 1986;32(1):5–12. [PubMed] [Google Scholar]
- 6.Soldin SJ, Papanastasiou-Diamandi A, Heyes J, Lingwood C, Olley P. Are immunoassays for digoxin reliable? Clin Biochem. 1984;17(5):317–320. doi: 10.1016/s0009-9120(84)90637-4. [DOI] [PubMed] [Google Scholar]
- 7.Thong B, Soldin SJ, Lingwood CA. Lack of specificity of current anti-digoxin antibodies, and preparation of a new, specific polyclonal antibody that recognizes the carbohydrate moiety of digoxin. Clin Chem. 1985;31(10):1625–1631. [PubMed] [Google Scholar]
- 8.Murthy JN, Davis DL, Yatscoff RW, Soldin SJ. Tacrolimus metabolite cross-reactivity in different tacrolimus assays. Clin Biochem. 1998;31(8):613–617. doi: 10.1016/s0009-9120(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 9.Murthy JN, Yatscoff RW, Soldin SJ. Cyclosporine metabolite cross-reactivity in different cyclosporine assays. Clin Biochem. 1998;31(3):159–163. doi: 10.1016/s0009-9120(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 10.Shen S, Elin RJ, Soldin SJ. Characterization of cross reactivity by carbamazepine 10,11-epoxide with carbamazepine assays. Clin Biochem. 2001;4(2):157–158. doi: 10.1016/s0009-9120(01)00186-2. [DOI] [PubMed] [Google Scholar]
- 11.Ghoshal AK, Soldin SJ. Tacrolimus II assay: is it reliable at low blood concentrations? A comparison with tandem MS/MS. Clin Biochem. 2002;35(5):389–392. doi: 10.1016/s0009-9120(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 12.Soldin SJ, Steele BW, Witte DL, Wang E, Elin RJ. Lack of specificity of cyclosporine immunoassays. Results of a college of american pathologists study. Arch Pathol Lab Med. 2003;127(1):19–22. doi: 10.5858/2003-127-19-LOSOC. [DOI] [PubMed] [Google Scholar]
- 13.Despres N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44(3):440–454. [PubMed] [Google Scholar]
- 14.Sarne DH, Refetoff S, Nelson JC, Linarelli LG. A new inherited abnormality of thyroxine-binding globulin (TBGSan Diego) with decreased affinity for thyroxine and triiodothyronine. J Clin Endocrinol Metab. 1989;68(1):114–119. doi: 10.1210/jcem-68-1-114. [DOI] [PubMed] [Google Scholar]
- 15.Burman KD, Bongiovanni R, Garis RK, Wartofsky L, Boehm TM. Measurement of serum T4 concentration by high performance liquid chromatography. J Clin Endocrinol Metab. 1981;53(5):909–912. doi: 10.1210/jcem-53-5-909. [DOI] [PubMed] [Google Scholar]
- 16.Tai SS, Sniegoski LT, Welch MJ. Candidate reference method for total thyroxine in human serum: use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem. 2002;48(4):637–642. [PubMed] [Google Scholar]
- 17.Thienpont LM, De Brabandere VI, Stockl D, De Leenheer AP. Development of a new method for the determination of thyroxine in serum based on isotope dilution gas chromatography mass spectrometry. Biol Mass Spectrom. 1994;23(8):475–482. doi: 10.1002/bms.1200230804. [DOI] [PubMed] [Google Scholar]
- 18.Thienpont LM, Fierens C, De Leenheer AP, Przywara L. Isotope dilution-gas chromatography/mass spectrometry and liquid chromatography/electrospray ionization-tandem mass spectrometry for the determination of triiodo-l-thyronine in serum. Rapid Commun Mass Spectrom. 1999;13(19):1924–1931. doi: 10.1002/(SICI)1097-0231(19991015)13:19<1924::AID-RCM734>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.De Brabandere VI, Hou P, Stockl D, Thienpont LM, De Leenheer AP. Isotope dilution-liquid chromatography/electrospray ionization-tandem mass spectrometry for the determination of serum thyroxine as a potential reference method. Rapid Commun Mass Spectrom. 1998;12(16):1099–1103. doi: 10.1002/(SICI)1097-0231(19980831)12:16<1099::AID-RCM290>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Ramsden DB, Farmer MJ. Development of a gas chromatographic selected ion monitoring assay for thyroxine (T4) in human serum. Biomed Mass Spectrom. 1984;11(8):421–427. doi: 10.1002/bms.1200110811. [DOI] [PubMed] [Google Scholar]
- 21.Nishinaga A, Cahnmann HJ, Kon H, Matsuura T. Model reactions for the biosynthesis of thyroxine: XII. The nature of a thyroxine precursor formed in the synthesis of thyroxine from diiodotyrosine and its keto acid analog. Biochemistry. 1968;7(1):388–397. doi: 10.1021/bi00841a049. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JC, Wilcox RB. Analytical performance of free and total thyroxine assays. Clin Chem. 1996;42(1):146–154. [PubMed] [Google Scholar]
- 23.Evans SE, Burr WA, Hogan TC. A reassessment of 8-anilino- 1-naphthalene sulphonic acid as a thyroxine binding inhibitor in the radioimmunoassay of thyroxine. Ann Clin Biochem. 1977;14(6):330–334. doi: 10.1177/000456327701400186. [DOI] [PubMed] [Google Scholar]