Abstract
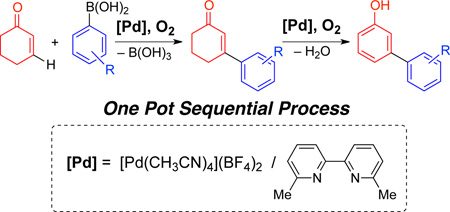
A new dicationic PdII catalyst, employing a 6,6'-dimethylbipyridine ligand, has been identified that is capable of promoting both aerobic oxidative Heck coupling and dehydrogenation reactions of cyclohexenones. These reactions may be combined in a one-pot sequence to enable the straightforward synthesis of meta-substituted phenols.
Keywords: Palladium, Heck Coupling, Cyclohexenone, Phenol, Dehydrogenation, Aerobic Oxidation
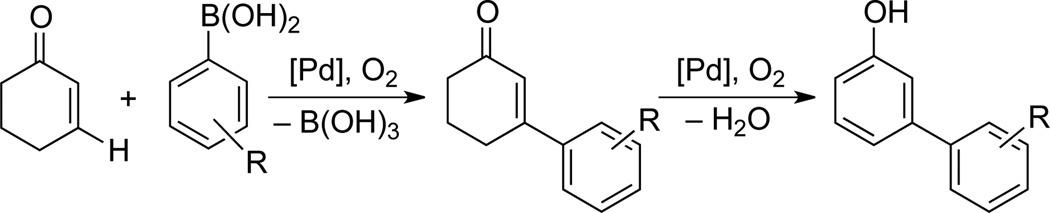
Phenol derivatives are common and important structural motifs in bioactive natural products and pharmaceuticals,[1] and the selective synthesis of substituted phenols is facilated by the strong ortho/para-directing effect of the hydroxyl group. The same directing effect, however, limits access to analogous meta-substituted derivatives. In recent years, considerable efforts have targeted C–H functionalization reactions that enable preparation of meta-substituted arenes via steric[2] or directing-group[3] control over the site selectivity. The overall efficiency of these methods is often limited by functional group interconversions or installation/removal of directing groups needed to access the final product.[4] Moreover, in molecules with more than one electronically or sterically active substituent, competition between the directing groups can lead to product mixtures. Following our recent development of Pd-catalyzed aerobic dehydrogenation reactions of ketones,[5,6,7] we envisioned that meta-substituted phenols could be accessed efficiently via an aerobic oxidative Heck/dehydrogenation sequence with cyclohexenone (Scheme 1).[8] Cyclohexenone is a convenient and inexpensive phenol precursor, and the proposed strategy exploits the intrinsic regioselectivity of additions to electron-deficient alkenes to enable functionalization of the "meta" C–H bond. Here, we describe a new Pd catalyst and reaction conditions compatible with this sequence, and we showcase their utility in the synthesis of a pharmaceutically active phenol derivative.
Scheme 1.
Strategy for the synthesis of meta-substituted phenols.
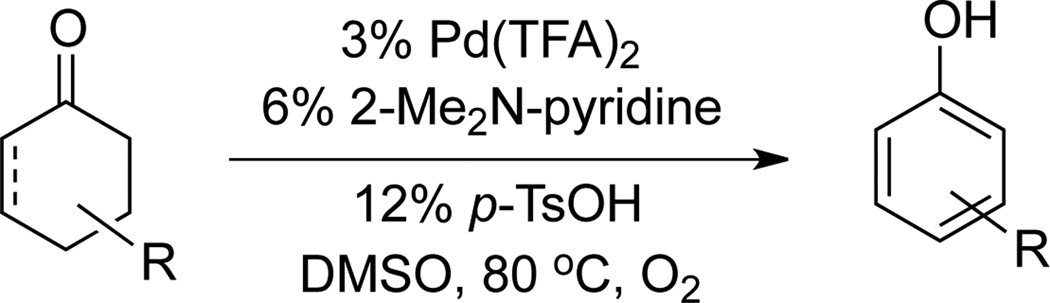
The proposed sequence in Scheme 1 faces several challenges. The oxidative Heck reaction must be more facile than the dehydrogenation step in order to avoid direct conversion of the cyclohexenone starting material to unsubstituted phenol. Furthermore, while aerobic oxidative Heck reactions have extensive precedent with terminal alkenes,[9, 10] analogous reactions with cyclohexenone tend to be more difficult.[11,12] With this substrate, the PdII-enolate must isomerize to place the Pd atom on the opposite side of the ring in order to undergo β-hydride elimination (Scheme 2.[13] Finally, the catalyst and conditions must be compatible with both reactions in the sequence. The only general method for dehydrogenation of cyclohexenones to phenols employs a strong-acid additive (p-TsOH; Scheme 3),[5a] which interferes with oxidative Heck reactions.[14]
Scheme 2.
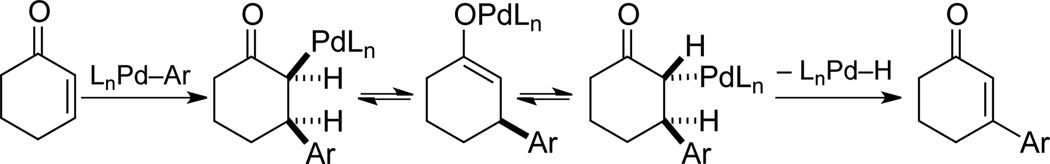
Mechanistic steps highlighting the requirement for isomerization of the PdII-enolate intermediate in Heck reactions of cyclohexenone.
Scheme 3.
Previously reported aerobic dehydrogenation conditions for the synthesis of phenols.
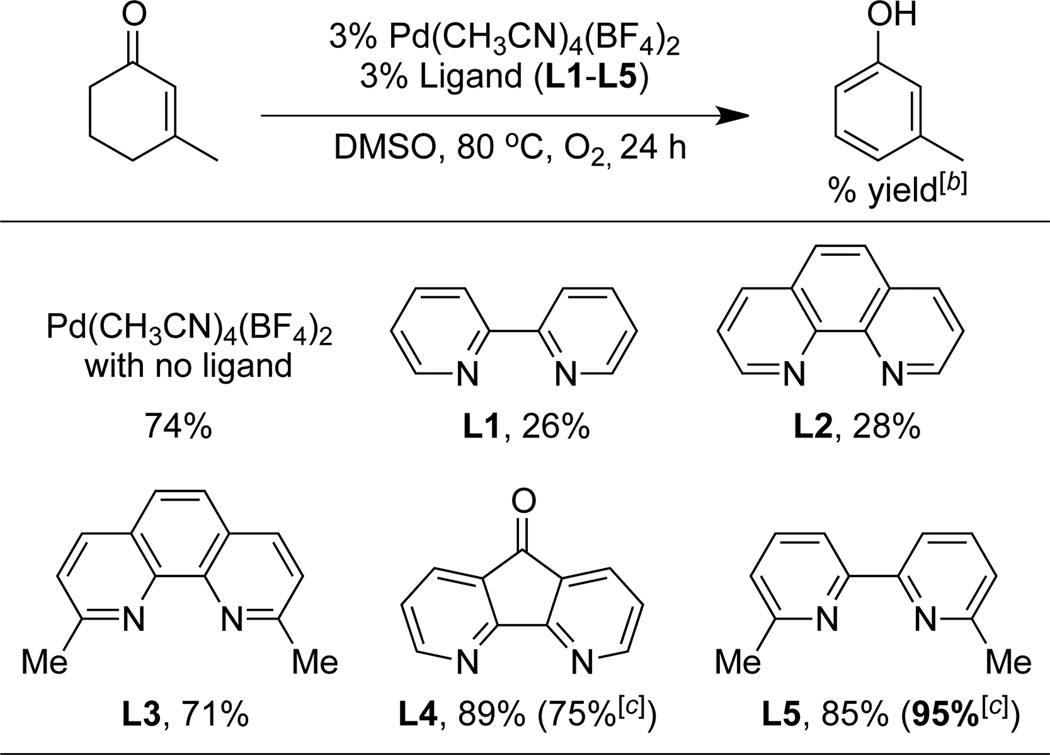
Our initial studies targeted the identification of non-acidic reaction conditions for aerobic dehydrogenation of 3-methylcyclohexenone. Upon screening diverse PdX2 sources, ligands, additives and solvents (see Supp. Info. for full screening data), we found that the dicationic PdII complex [Pd(CH3CN)4](BF4)2 was particularly effective as a catalyst (Table 1). Formation of Pd black and gradual loss of catalytic activity during the reaction prompted us to test ancillary ligands to stabilize the catalyst. Most of the ligands tested inhibited the reaction (cf. Table 1 and Supp. Info.); however, 4,5-diazafluorenone L4[15] and 6,6'-dimethyl-2,2'-bipyridine L5 enabled good product yields to be obtained. While screening of numerous additives, including Brønsted bases, CuII and AgI salts, and quinones showed little beneficial effect, nearly quantitative yield of the phenol product (95%) was obtained when 9 mol % AMS (anthraquinone-2-sulfonic acid sodium salt) was included in the reaction with ligand L5.[16] The optimal result was obtained upon addition of water (20 vol %) to enhance the solubility of AMS.
Table 1.
Dehydrogenation of 3-Cyclohexenones: Screening Results.[a]
 |
Reactions were performed on 1.0 mmol scale in DMSO (0.2 mL), 1 atm O2.
GC yield.
H2O (0.05 mL) and AMS (0.09 mmol) were added. Reaction time: 6.5 h. See Supporting Information for details.
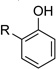
The optimized conditions proved to be effective with a number other substituted cyclohexenones, including those with heteroatom substituents (Table 2). These neutral reaction conditions revealed some advantages over the previously reported conditions in Scheme 3. For example, 6-phenylcyclohexanone underwent dehydrogenation to o-phenyl phenol in only 33% yield under the previous conditions, but this product is obtained in excellent yields under the present conditions (entries 1 and 2). The successful reaction of 3-arylcyclohexenones, prepared via oxidative Heck reactions with cyclohexenone (entries 9–11), provided a useful starting point for the investigation of oxidative Heck and tandem oxidative Heck/dehydrogenation reactions.
Table 2.
Dehydrogenation of Substituted Cyclohexenones,[a]
 | ||||
|---|---|---|---|---|
| Entry | Cyclohexenone | Phenol | Time [h] |
Yield [%][b] |
| 1 |  |
 |
10 | 90[c] |
| 2 | 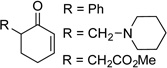 |
 |
10 | 96[c] |
| 3 | 36 | 46 | ||
| 4 | 6.5 | 63 | ||
| 5 | 10 | 87 | ||
| 6 | 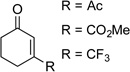 |
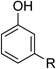 |
24 | 77 |
| 7 | 24 | 73 | ||
| 8 | 12 | 68 | ||
| 9 |  |
 |
10 | 89 |
| 10 | 10 | 52[c] | ||
| 11 | 10 | 83 | ||
Reactions were performed on 1.0 mmol scale in DMSO/H2O (v/v 0.2/0.05 mL).
Isolated yield.
PdII/L5/AMS = 5%/5%/15%.
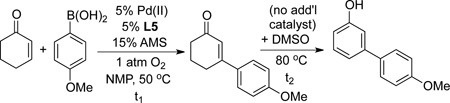
Preliminary experiments showed that this catalyst was quite effective for the oxidative Heck coupling of 4-methoxyphenylboronic acid and cyclohexenone. Moveover, the reaction could take place at 50 °C, a temperature at which no conversion of cyclohexenone to phenol was observed. In DMSO, the oxidative Heck reaction proceeded in 65% yield. Upon heating of this reaction mixture to 80 °C, nearly complete in situ conversion to the 3-aryl phenol was observed (i.e., 64% yield of the phenol; Table 3, entry 1). Several other solvents, including DMF, N-methylpyrrolidone (NMP) and 1,4-dioxane, proved to be better for the oxidative Heck reaction (entries 2–7); however, they proved less effective for the tandem sequence (e.g., entry 2). Further studies revealed that an effective one-pot sequence could be achieved by performing the oxidative Heck reaction in NMP at 50 °C, followed by addition of DMSO and heating to 80 °C for the dehydrogenation step. This protocol enabled a good yield of the phenol to be obtained (84%, entry 12).
Table 3.
Optimization of Conditions for the Oxidative Heck and one-pot Oxidative Heck/Dehydrogenation Reactions.[a]
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Oxidative Heck | Oxidative Heck/ Dehydrogenation |
|||||
| Solvent | Conc. [M][b] |
t1 [h] |
Yield [%][c] |
Solvent | t2 [h] |
Yield [%][d] |
|
| 1 | DMSO | 1.3 | 8 | 65 | - | 24 | 64 |
| 2 | DMF | 1.3 | 3 | 95 | - | 24 | 25[e] |
| 3 | NMP | 1.3 | 3 | 90 | - | - | - |
| 4 | Dioxane | 1.3 | 3 | 93 | - | - | - |
| 5 | CH3CN | 1.3 | 3 | 73 | - | - | - |
| 6 | DMF | 2.5 | 3 | 96 | - | - | - |
| 7 | DMF | 5.0 | 3 | 80 | - | - | - |
| 8 | DMF | 2.5 | 3 | - | DMSO | 24 | 54 |
| 9 | NMP | 2.5 | 3 | - | DMSO | 24 | 72 |
| 10 | NMP | 3.1 | 3 | - | DMSO | 24 | 82 |
| 11[f] | NMP | 3.1 | 3 | - | DMSO | 32 | 82 |
| 12[f,g] | NMP | 3.1 | 4 | 95 | DMSO | 32 | 84 |
Reaction conditions: Heck reactions [cyclohexenone (0.75 mmol), boronic acid (0.25 mmol), Pd(II) = [Pd(CH3CN)4](BF4)2, 50 °C, 3–8 h]; Dehydrogenation: temp. increased to 80 °C, 24–32 h (for entries 8–15, 0.2 mL DMSO added).
Conc. of boronic acid.
GC yields.
1H NMR yields.
65% of Heck product recovered.
10% AMS used.
0.5 mmol cyclohexenone.
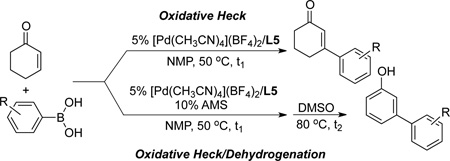
[Pd(CH3CN)4](BF4)2/L5 proved to be very effective as a standalone catalyst for oxidative Heck reactions with cyclohexenone. Good yields of the 3-arylcyclohexenones were obtained with diverse arylboronic acids (Table 4). Reactions with the electron-rich arylboronic acids typically led to higher yields than electron-deficient substrates as the latter substrates were more susceptible to the formation of homocoupling products. Halogenated arylboronic acids (X = F, Cl, Br) were tolerated in the oxidative Heck reaction, with yields ranging from 68% to 86% (entries 4–6 and 18). The same arylboronic acids were then employed in the one-pot oxidative Heck/dehydrogenation to afford the 3-substituted phenol derivatives. In most cases, the phenol yields correlate closely with the yields of the 3-aryl cyclohexenones in the independent oxidative Heck reaction.
Table 4.
Oxidative Heck and One-Pot Oxidative Heck/Dehydrogenation Reactions to Prepare Substituted Cyclohexenones and Phenols.[a]
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | Oxidative Heck | Oxidative Heck/ Dehydrogenation |
|||
| t1 [h] |
Yield[b] [%] |
t2 [h] |
Yield[c] [%] |
|||
| 1 | Me | 4 | 97 | 32 | 91 | |
| 2 | t-Bu | 4 | 98 | 36 | 85 | |
| 3 | MeO | 4 | 94 | 32 | 80 | |
| 4 | Cl | 4 | 85 | 32 | 68 | |
| 5 | Br | 4 | 65 | 32 | 41 | |
| 6 | F | 4 | 86 | 32 | 87 | |
| 7 | OH | 4 | 85 | 32 | 64 | |
| 8 | Ph | 13 | 62 | 36 | 63 | |
| 9 | COOMe | 4 | 86 | 32 | 74 | |
| 10 | CF3 | 4 | 56 | 32 | 40 | |
| 11 | CN | 13 | 41 | 36 | 35 | |
| 12 | H | 4 | 76 | 32 | 75 | |
| 13 |  |
MeO | 13 | 83 | 36 | 47 |
| 14 | Me | 13 | 89 | 36 | 76 | |
| 15 | NO2 | 13 | 48 | 36 | 42 | |
| 16 | CF3 | 4 | 68 | 32 | 60 | |
| 17 |  |
Me | 13 | 73 | 36 | 58 |
| 18 | F | 13 | 75 | 36 | 66 | |
Reactions conditions: cyclohexenone (0.5 mmol), arylboronic acid (0.25 mmol) in NMP (0.2 mL for Heck and 0.08 mL for one-pot) at 50 °C for Heck reactions. For the one-pot reactions, DMSO was then added and the reactions were continued at 80 °C for 32 or 36 hours. See Supporting Information for details.
Isolated yields of 3-substituted cyclohexenones.
Isolated yields of phenols.
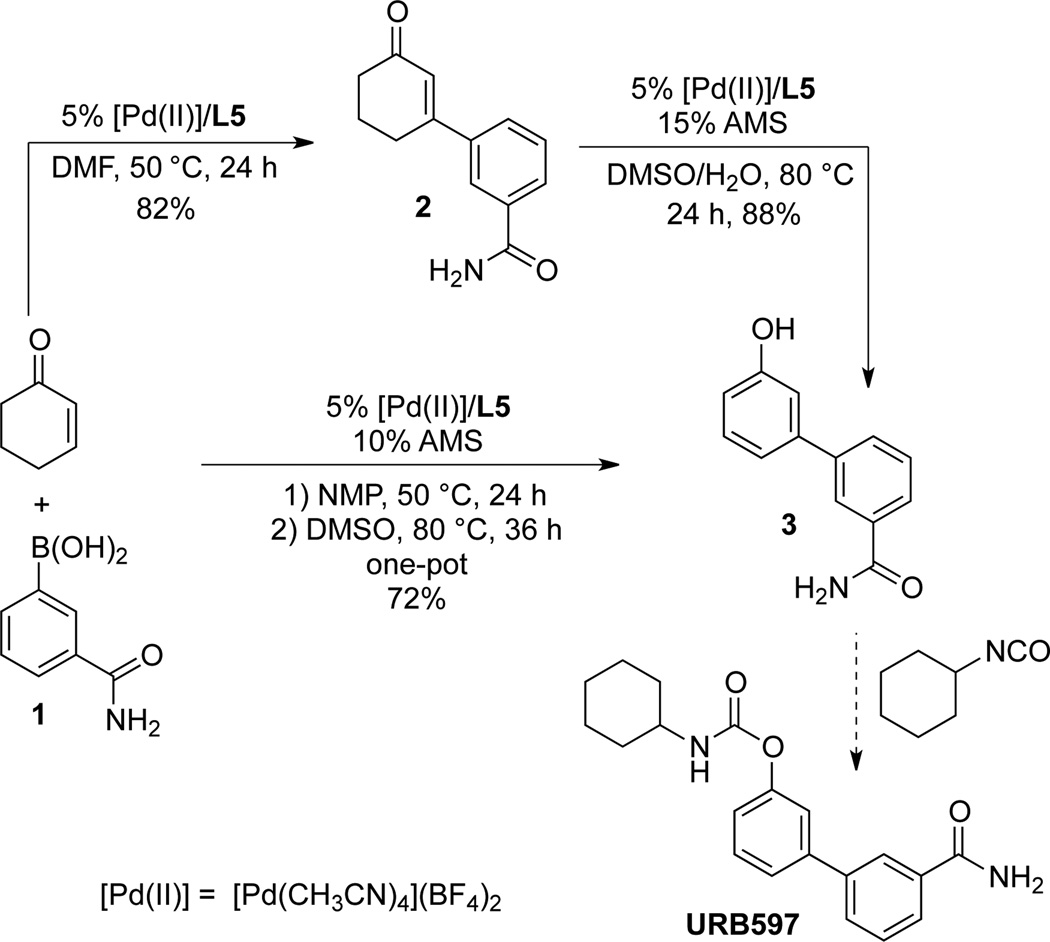
In order to demonstrate the potential utility of the aerobic oxidative Heck/dehydrogenation sequence and further test its functional group compatibility, we investigated the synthesis of URB597 from cyclohexenone and the commercially available benzamide-derived boronic acid 1 (Scheme 4). URB597 is a potent inhibitor of fatty acid amide hydrolase (FAAH) and an important focus of efforts to treat pain, anxiety and depression.[17,18] The phenol intermediate 3 was prepared via stepwise oxidative Heck coupling of 1 and cyclohexenone, followed by catalytic dehydrogenation of the isolated intermediate 2, and in a direct, one-pot process. The [Pd(CH3CN)4](BF4)2/L5 catalyst was employed for each of these steps, and both pathways led to the phenol product 3 in good yield (approx. 72%, in each case).
Scheme 4.
Application of one-pot oxidative Heck/dehydrogenation reactions in the synthesis of URB597.
The results above highlight a new catalyst system that mediates both aerobic oxidative Heck reactions with cyclohexenone and aerobic dehydrogenation of cyclohexenones. The one-pot sequence developed for these reactions represents an efficient strategy for the preparation of meta substituted of phenols, which should be advantageous or highly competitive with other approaches based on C–H functionalization of an aromatic ring.
Supplementary Material
Footnotes
We thank Dr. Doris Pun for assistance in product purification. Financial support was provided by the NIH (R01 GM100143), Mitsubishi Chemical Corporation, and Shanghai Institute of Organic Chemistry (postdoctoral fellowship for CWZ).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For example, see, Tyman JHP. Synthetic and Natural Phenols. New York: Elsevier; 1996. Hoarau C, Pettus TRR. Synlett. 2003:127. doi: 10.1055/s-2003-36234. Dewick PM. Medicinal Natural Products: A Biosynthetic
- 2.a) Cho J-Y, Iverson CN, Smith MR., III J. Am. Chem. Soc. 2000;122:12868. [Google Scholar]; b) Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J. Am. Chem. Soc. 2002;124:390. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]; c) Ishiyama T, Takagi J, Hartwig JF, Miyaura N. Angew. Chem. 2002;114:3182. doi: 10.1002/1521-3773(20020816)41:16<3056::AID-ANIE3056>3.0.CO;2-#. Angew. Chem. Int. Ed.2002, 41, 3056; [DOI] [PubMed] [Google Scholar]; d) Cho J-Y, Tse MK, Holmes D, Maleczka RE, Smith MR., III Science. 2002;295:305. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]; e) Maleczka RE, Jr, Shi F, Holmes D, Smith MR., III J. Am. Chem. Soc. 2003;125:7792. doi: 10.1021/ja0349857. [DOI] [PubMed] [Google Scholar]; f) Zhang YH, Shi BF, Yu JQ. J. Am. Chem. Soc. 2009;131:5072. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]; b) Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Angew. Chem. 2011;123:483. doi: 10.1002/anie.201004704. Angew. Chem. Int. Ed.2011, 50, 463; [DOI] [PubMed] [Google Scholar]; c) Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. J. Am. Chem. Soc. 2011;133:19298. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]; d) Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For other recent advances that enable efficient preparation of substituted phenols, see: Anderson KW, Ikawa T, Tundel RE, Buchwald SL. J. Am. Chem. Soc. 2006;128:10694. doi: 10.1021/ja0639719. Tlili A, Xia N, Monnier F, Taillefer M. Angew. Chem. 2009;121:8881. doi: 10.1002/anie.200903639. Angew. Chem. Int. Ed.2009, 48, 8725; Zhang YH, Yu JQ. J. Am. Chem. Soc. 2009;131:14654. doi: 10.1021/ja907198n. Maurer S, Jiang YW, Ma DW, Liu W, Zhang XJ. Synlett. 2010:976. Zou YQ, Chen JR, Liu XP, Lu LQ, Davis RL, Jørgensen KA, Xiao WJ. Angew. Chem. 2012;124:808. doi: 10.1002/anie.201107028. Angew. Chem. Int. Ed.2012, 51, 784; Yu CW, Chen GS, Huang CW, Chern JW. Org. Lett. 2012;14:3688. doi: 10.1021/ol301523q.
- 5.a) Izawa Y, Pun D, Stahl SS. Science. 2011;333:209. doi: 10.1126/science.1204183. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Diao T, Stahl SS. J. Am. Chem. Soc. 2011;133:14566. doi: 10.1021/ja206575j. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Diao T, Wadzinski TJ, Stahl SS. Chem. Sci. 2012;3:887. doi: 10.1039/C1SC00724F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For precedents and leading references, see: Horning EC, Horning MG. J. Am. Chem. Soc. 1947;69:1359. Hirao T, Mori M, Ohshiro Y. J. Org. Chem. 1990;55:358. Shvo Y, Arisha AHI. J. Org. Chem. 1998;63:5640. Moriuchi T, Kikushima K, Kajikawa T, Hirao T. Tetrahedron Lett. 2009;50:7385. For a review, see: Muzart J. Eur. J. Org. Chem. 2010:3779.
- 7.For related, recently reported dehydrogenation methods, see: Hajra A, Wei Y, Yoshikai N. Org. Lett. 2012;14:5488. doi: 10.1021/ol302568b. Kandukuri SR, Oestreich M. J. Org. Chem. 2012;77:8750. doi: 10.1021/jo301088f. Imahori T, Tokuda T, Taguchi T, Takahata H. Org. Lett. 2012;14:1172. doi: 10.1021/ol300145g. Girard SA, Hu X, Knauber T, Zhou F, Simon M, Deng GJ, Li CJ. Org. Lett. 2012;14:5606. doi: 10.1021/ol3027279. Simon M, Girard SA, Li CJ. Angew. Chem. 2012;124:7655. doi: 10.1002/anie.201200698. Angew. Chem. Int. Ed.2012, 51, 7537; Xie Y, Liu S, Liu Y, Wen Y, Deng GJ. Org. Lett. 2012;14:1692. doi: 10.1021/ol3002442.
- 8.For examples of sequential reactions involving dehydrogenation steps, see: Ohmura T, Kijima A, Suginome M. J. Am. Chem. Soc. 2009;131:6070. doi: 10.1021/ja901095h. Xia X-F, Shu X-Z, Ji K-G, Yang Y-F, Shaukat A, Liu X-Y, Liang Y-M. J. Org. Chem. 2010;75:2893. doi: 10.1021/jo100133z. Yip KT, Nimje RY, Leskinen MV, Pihko PM. Chem. Eur. J. 2012;18:12590. doi: 10.1002/chem.201201988. Moon Y, Kwon D, Hong S. Angew. Chem. 2012;124:11495. doi: 10.1002/anie.201206610. Angew. Chem. Int. Ed.2012, 51, 11333; Leskinen MV, Yip KT, Valkonen A, Pihko PM. J. Am. Chem. Soc. 2012;134:5750. doi: 10.1021/ja300684r.
- 9.For important examples, see: Andappan MMS, Nilsson P, von Schenck H, Larhed M. J. Org. Chem. 2004;69:5212. doi: 10.1021/jo049434t. Andappan MMS, Nilsson P, Larhed M. Chem. Commun. 2004:218. doi: 10.1039/b311492a. Yoon CH, Yoo KS, Yi SW, Mishra RK, Jung KW. Org. Lett. 2004;6:4037. doi: 10.1021/ol0483192. Enquist PA, Lindh J, Nilsson P, Larhed M. Green Chem. 2006;8:338. Yoo KS, Yoon CH, Jung KW. J. Am. Chem. Soc. 2006;128:16384. doi: 10.1021/ja063710z. Lindh J, Enquist P, Pilotti Å, Nilsson P, Larhed M. J. Org. Chem. 2007;72:7957. doi: 10.1021/jo701434s. Yoo KS, Park CP, Yoon CH, Sakaguchi S, O'Neill J, Jung KW. Org. Lett. 2007;9:3933. doi: 10.1021/ol701584f. Ruan J, Li X, Saidi O, Xiao J. J. Am. Chem. Soc. 2008;130:2424. doi: 10.1021/ja0782955. Delcamp JH, Brucks AP, White MC. J. Am. Chem. Soc. 2008;130:11270. doi: 10.1021/ja804120r. Werner EW, Sigman MS. J. Am. Chem. Soc. 2010;132:13981. doi: 10.1021/ja1060998. Zheng CW, Wang D, Stahl SS. J. Am. Chem. Soc. 2012;134:16496. doi: 10.1021/ja307371w.
- 10.For a review, see: Karimi B, Behzadnia H, Elhamifar D, Akhavan PF, Esfahani FK, Zamani A. Synthesis. 2010:1399.
- 11.For examples of oxidative Heck reactions with cyclohexenone, see ref. 9e and the following: Krishna TR, Jayaraman N. Tetrahedron. 2004;60:10325. Fall Y, Doucet H, Santelli M. Tetrahedron. 2009;65:489. Gottumukkala AL, Teichert JF, Heijnen D, Eisink N, Van Dijk S, Ferrer C, Minnaard AJ, Van Den Hoogenband A. J. Org. Chem. 2011;76:3498. doi: 10.1021/jo101942f.
- 12.Other methods to synthesize 3-aryl cyclohexenones usually need pre-functionalized 3-substituted cyclohexenones for coupling partner. Please see selected examples: Zimmerman HE, Nesterov EE. J. Am. Chem. Soc. 2003;125:5422. doi: 10.1021/ja028631b. Matsuo J, Aizawa Y. Chem. Commun. 2005:2399. doi: 10.1039/b502134k. Quasdorf KW, Riener M, Petrova KV, Garg NK. J. Am. Chem. Soc. 2009;131:17748. doi: 10.1021/ja906477r.
- 13.Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 14.For example, preliminary experiments revealed substantial biaryl product formation, arising from homocoupling of the boronic acid, or non-oxidative conjugate addition to the enone.
- 15.a) Campbell AN, White PB, Guzei IA, Stahl SS. J. Am. Chem. Soc. 2010;132:15116. doi: 10.1021/ja105829t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Campbell AN, Meyer EB, Stahl SS. Chem. Commun. 2011:10257. doi: 10.1039/c1cc13632a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For previous use of AMS as a cocatalyst in a Pd-catalyzed oxidation reaction, see: Sheldon RA, Sobczak JM. J. Mol. Catal. 1991;68:1.
- 17.Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Nat. Med. 2002;9:76. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]; b) Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. J. Pharmacol. Exp. Ther. 2005;313:352. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]; c) Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. CNS Drug Reviews. 2006;12:21. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For previous syntheses, see: Mor M, Rivara S, Lodola A, Plazzi PV, Tarzia G, Duranti A, Tontini A, Piersanti G, Kathuria S, Piomelli D. J. Med. Chem. 2004;47:4998. doi: 10.1021/jm031140x.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.