Abstract
Objective
To test Candonocypris novaezelandiae (Baird) (C. novaezelandiae), sub-class Ostracoda, obtained from the Nile, Egypt for its predatory activity on snail, Biomphalaria alexandrina (B. alexandrina), intermediate host of Schistosoma mansoni (S. mansoni) and on the free-living larval stages of this parasite (miracidia and cercariae).
Methods
The predatory activity of C. novaezelandiae was determined on B. alexandrina snail (several densities of eggs, newly hatched and juveniles). This activity was also determined on S. mansoni miracidia and cercariae using different volumes of water and different numbers of larvae. C. novaezelandiae was also tested for its effect on infection of snails and on the cercarial production.
Results
C. novaezelandiae was found to feed on the eggs, newly hatched and juvenile snails, but with significant reduction in the consumption in the presence of other diet like the blue green algae (Nostoc muscorum). This ostracod also showed considerable predatory activity on the free-living larval stages of S. mansoni which was affected by certain environmental factors such as volume of water, density of C. novaezelandiae and number of larvae of the parasite.
Conclusions
The presence of this ostracod in the aquatic habitat led to significant reduction of snail population, infection rate of snails with schistosme miracidia as well as of cercarial production from the infected snails. This may suggest that introducing C. novaezelandiae into the habitat at schistosome risky sites could suppress the transmission of the disease.
Keywords: Candonocypris novaezelandiae, Schistosoma mansoni, Biomphalaria alexandrina, Biological control, Schistosomiasis
1. Introduction
Schistosomiasis is a serious public health problem in Egypt and several other countries in the tropical and subtropical regions of Africa, Asia and South America[1]. Planorbid snails play a major role as intermediate hosts of the schistosome parasites and consequently in the transmission of this disease. It was claimed that current population-based schistosomiasis treatment programs are the first step to reduce the global burden of Schistosoma-related disease[2]; however, they might not dramatically reduce parasite transmission in highly endemic areas. The real health benefits of transmission control are needed to be reconsidered and attention should be given to more aggressive and, ultimately, more affordable parasite elimination strategies. The next generation of schistosomiasis control can be optimized using new monitoring tools and effective transmission containment.
Snail control strategies are considered a priority for the reduction of transmission. One of the main methods of snail control comprises the application of chemical molluscicides which could give only a temporary relief[3]. Another method is the biological control using specific organisms to eliminate or minimize the population of the vector snails without negative impact on the environment. In this respect, many organisms were reported to be variably successful biocontrol agents such as viruses, fungi, bacteria and protozoa. Predators belonging to almost all groups of animal kingdom such as leeches, insects and their larvae, crustaceans, fish and mammals are effective biocontrol agents[4]–[7]. Microcrustaceans and fish have been observed to eat cercariae of some species, although the possibility that predation represents a significant source of mortality for cercariae has been largely unexplored[8]. Although such variety of organisms could be considered as potential biocontrol agents especially under certain conditions in habitats, the ideal one is still missing.
Ostracods are one of the most successful crustacean groups with approximately 8 000 living species. They are found in practically every aquatic environment including freshwater lakes, ponds and streams. C. novaezelandiae is found in the River Nile and several streams in Egypt. It is found in many cases associated with aquatic plants like Eichhornia crassipes. At the same time, aquatic plants furnish a good habitat for the schistosome vector snails. A review of the literature shows that evaluation of any ostracod as a biocontrol agent in Schistosoma transmission is not found. Ostracods naturally feed on dead organic material, suspended particles and microscopic plants, and some are predators. Cypris spp. are not known to be specifically able to feed on parasites or snails. In the present study, an ostracod, namely, Candonocypris novaezelandiae (Baird) (C. novaezelandiae) was tested as a potential bicontrol agent against schistosomiasis transmission in Egypt by its potential predation on the snail intermediate host Biomphalaria alexandrina (B. alexandrina) and against the free-living larval stages of the parasites as well as reducing the snail infection with the parasite.
2. Materials and methods
2.1. Ostracod, snail and schistosome
The materials used in this study were large numbers of the ostracod C. novaezelandiae (Baird) (sub-class Ostracoda, class Maxillopoda, phylum Arthropoda), the schistosome vector snail B. alexandrina Ehrenberg (Planorbidae) and the free larvae (miracidia and cercariae) of Schistosoma mansoni Sambon (Schistosomatidae) (S. mansoni). The original sample of C. novaezelandiae individuals (about 200) was obtained from the Nile in Giza Governorate and maintained in the laboratory. They were found to belong to two species of ostracods. About 50 individuals of C. novaezelandiae were picked and carried to a special aquarium where they were maintained under suitable laboratory conditions of temperature, light and algae food to reproduce forming a large colony. The snails, their egg masses and the free larval stages of the parasite (miracidia and cercariae) were obtained from the big colonies at the Schistosome Biological Supply Centre of Theodor Bilharz Research Institute.
2.2. Predatory test for snail eggs
To study the effect of eggs density on the predatory activity of C. novaezelandiae, a number of Petri dishes containing 50 mL dechlorinated water were provided each with 100 C. novaezelandiae and different numbers of B. alexandrina eggs (1-20 egg masses containing 22-371 eggs). Three replicates were set up and all Petri dishes were maintained at (25±2) °C in the laboratory and examined after 24 h using a stereomicroscope. The number of consumed or injured eggs was counted and recorded in each case.
2.3. Predatory test for snails
The predatory activity of C. novaezelandiae on B. alexandrina snails was tested on both newly hatched and juvenile snails (1 and 3 mm shell height respectively). In the first case, 11 Petri dishes with each containing 50 mL dechlorinated water and 100 C. novaezelandiae were provided with different numbers of snails (5-50) and three replicates were set up. The Petri dishes were examined after 24 h, and the number of consumed or injured snails was counted and recorded in each case. The predatory activity on juvenile snails was studied using 50 snails in Petri dish and three replicates were set up. Dead snails were counted daily for 5 d and the mortality rate was calculated in each case.
To elucidate the effect of presence of other diet on the predatory activity of C. novaezelandiae, 9 Petri dishes were set up, each containing 50 mL dechlorinated water, 100 C. novaezelandiae and 100 B. alexandrina eggs. Among them, 3 Petri dishes were provided with excess of blue green algae (Nostoc muscorum), another 3 with excess of boiled lettuce leaves and the third group (3 Petri dishes) was left as control. This means that 3 replicates were set up and the eggs were examined daily for 3 d using a stereomicroscope and the consumed eggs were counted in each case.
2.4. Predatory test for the free-living larval stages of S. mansoni
The effect of C. novaezelandiae on the survival of S. mansoni miracidia and cercariae was tested using three different combinations. Thus, in one combination, different volumes of dechlorinated water (50, 100 and 500 mL) with 100 miracidia or cercariae and 10 C. novaezelandiae were used. In another combination, different numbers of free larvae (100, 200 and 300) in the same volume of water (50 mL) and 10 C. novaezelandiae were set up. In the third combination, different numbers of C. novaezelandiae (10-70) in the same volume of water (50 mL) and the same number of larvae (100) were used. In all cases, C. novaezelandiae were maintained with the free larvae (miracidia and cercariae) for 1 h after which the survived organisms were counted using a stereomicroscope. The miracidia and cercariae were used within 1 h after hatching or shedding respectively. Three replicates were performed in each case.
2.5. Predatory test for snails infected by Schistosoma miracidia
Four Petri dishes were provided, each with 25 B. alexandrina snails of close size (4-5 mm shell height) and 10 mL dechlorinated water. The snails were exposed in mass to 10-12 miracidia/snail for 2 h. Three of the Petri dishes were provided with 10, 50 and 100 C. novaezelandiae and the 4th was maintained without C. novaezelandiae as control. Three replicates were performed in each case and all groups were maintained under the same laboratory conditions. All miracidially exposed snails were maintained and fed according to the routine standards in the laboratory. Starting from day 30 post miracidial exposure, the surviving snails were examined individually for cercarial shedding by exposing them for 1 h to 40 W florescent lamp, 40 cm far, at (25±2) °C. Snails that proved to be positive were isolated. Mortality and infection rates of snails during the incubation period were determined.
Six small beakers each containing 10 mL dechlorinated tap water and five cercarially shedding snails were set up. Fifity C. novaezelandiae were added to each one of three beakers and the other three were maintained without C. novaezelandiae as control. The emerged cercarial suspension was poured each hour in a graduated Petri dish and replaced by an equal volume of water and equal numbers of C. novaezelandiae. A few drops of Bouin fluid were added to each cercarial suspension to kill, fix and colour them and cercariae were counted using a stereomicroscope. The hourly cercarial production was determined for 6 h in the presence or absence of the ostracod to elucidate the effect on shedding pattern.
2.6. Statistical analysis
Results are expressed as mean±SD or number of snails. Comparison between the three different groups was analyzed using the ANOVA test followed by the post-Hoc Sheffe test. The data were considered significant if P<0.05, highly significant if P<0.01 and very highly significant if P<0.001. Results of two groups were compared using the non-parametric Wilcoxon-Mann-Whitney U-test, while the Chi-square test was used to compare categorical data. Statistical analysis was performed with the aid of the SPSS computer program (version 18 for windows).
A logistic regression model was constructed to evaluate the effects of C. novaezelandiae (r=0.57).
Logistic regression model:
y=exp[-4.185 818-(0.024 9×Number of cercariae)-(0.746 4×Number of miracidia)-(1.003 9×Number of eggs)]
3. Results
3.1. Predatory activity of C. novaezelandiae on B. alexandrina snail eggs
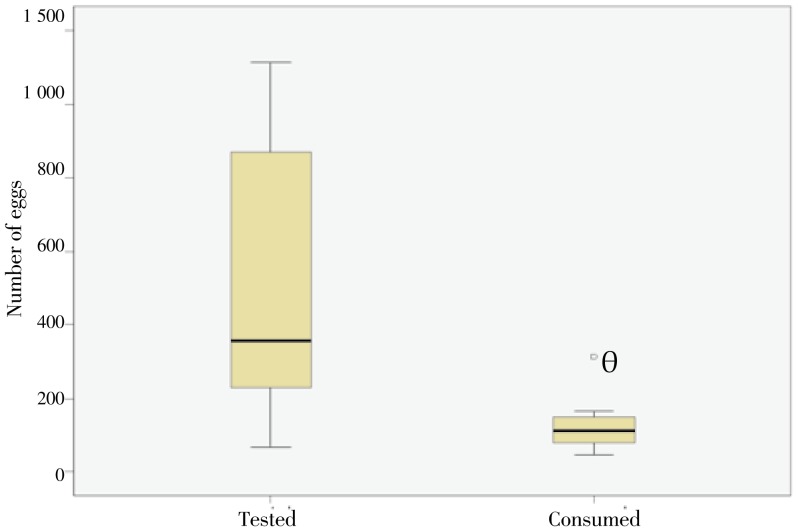
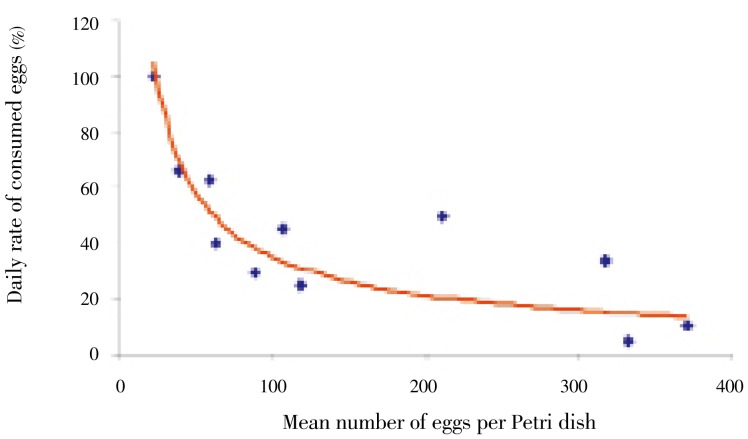
C. novaezelandiae individuals were found to feed on snail egg masses and were observed to gather to feed on a single egg mass. The median numbers of eggs tested and consumed were 354 and 111, respectively, showing a consumption rate of 31.4% (Figure 1). The daily number of eggs consumed by 100 C. novaezelandiae maintained in the same volume of water (50 mL) was found to vary with the availability of eggs. However, the rate of consumed eggs was significantly less (P<0.01) if the number of eggs was larger (Figure 2).
Figure 1. Box plots of the total number of eggs tested and that of eggs consumed.
The horizontal line inside each box represents the median, while the top and bottom of boxes represent the 25th and 75th percentiles, respectively. Vertical lines from the ends of the box encompass the extreme data points. θ represents the outliers.
Figure 2. Effect of condensation of B. alexandrina eggs on predatory capacity of C. novaezelandiae.
Daily rate of consumed eggs was calculated using number of eggs consumed by 100 C. novaezelandiae.
3.2. Predatory activity of C. novaezelandiae on B. alexandrina snails
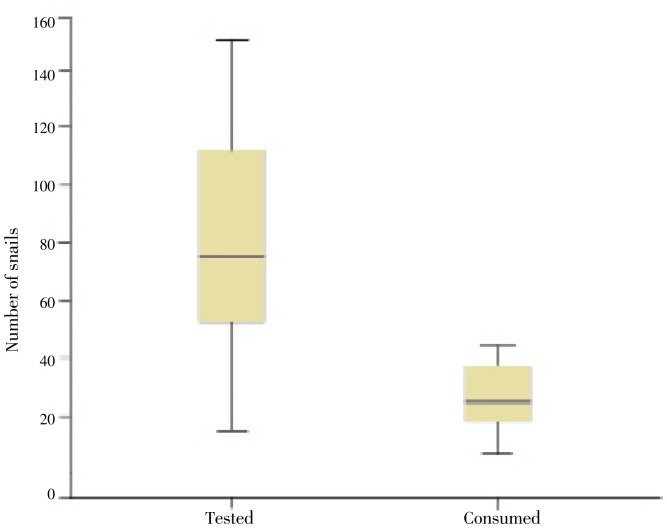
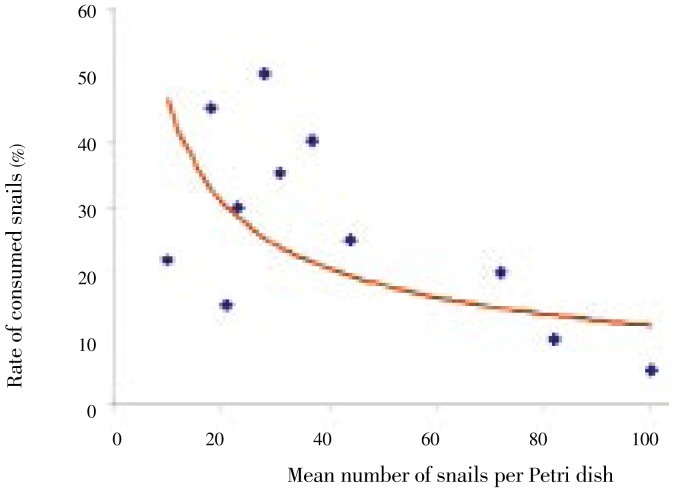
The results showed that predation of C. novaezelandiae on hatched snails had a similar pattern to that on eggs. The number of newly hatched snails consumed by 100 C. novaezelandiae after 1 d was 2-15. The rate of predation (Figures 3 and 4) was significantly (P=0.001) high when the number of available snails was low, and it decreased gradually with the increase of snails. Moreover, it was observed that C. novaezelandiae could prey on juvenile snails and almost all available snails were consumed in the duration of the experiment. It was also found that the cumulative rate of consumption of juvenile snails by 100 C. novaezelandiae was 16.70%, 46.70%, 60.83% and 96.70% in 5 successive days. The daily predation was non-significant.
Figure 3. Box plots of the total number of B. alexandrina snails tested and that of snails consumed.
The horizontal line inside each box represents the median, while the top and bottom of boxes represent the 25th and 75th percentiles, respectively. Vertical lines from the ends of the box encompass the extreme data points.
Figure 4. Effect of density of newly hatched B. alexandrina snails on predatory activity of C. novaezelandiae.
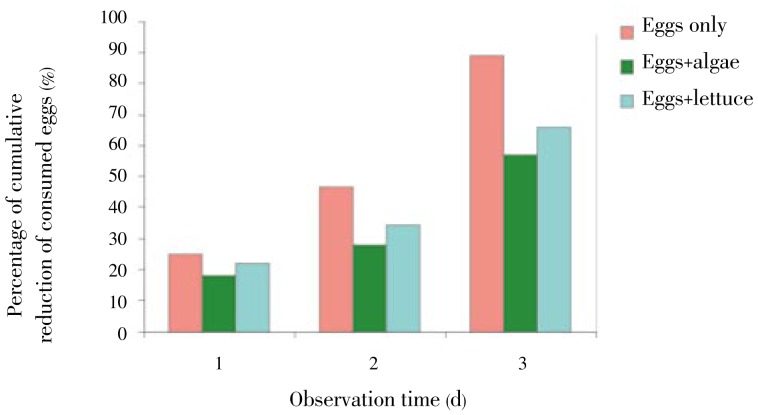
3.3. Effect of presence of other diet on the predatory activity of C. novaezelandiae on snail eggs
During the 3-day observation, both lettuce and algae showed significant reduction (P<0.01) in predation of C. novaezelandiae on snail eggs. Comparing both types of food, the algae made more slight reduction than the lettuce (P<0.05) (Figure 5).
Figure 5. Predatory activity of C. novaezelandiae on B. alexandrina eggs in presence of diet.
3.4. Predatory effect of C. novaezelandiae on the free larval stages of S. mansoni
The present results showed that C. novaezelandiae could prey on the free-living larvae of S. mansoni (miracidia or cercariae), an activity that was found to be dependent on water volume, number of available schistosome organisms and number of C. novaezelandiae. The consumption decreased with increase of water volume and increased with increase of C. novaezelandiae or schistosome larval organisms, statistically significant (F=20.227, P<0.001). C. novaezelandiae were found sufficient to consume all cercariae and miracidia in 50 mL water in 1 h under the present conditions respectively (Table 1). Comparing the predatory effect on miracidia vs. that on cercariae, it was found that increase of water volume and number of schistosome organisms were more significantly effective on miracidia than on cercariae (P<0.01), while increase in number of C. novaezelandiae had almost the same effect on both schistosome larval stages.
Table 1. Predatory activity of C. novaezelandiae on free larvae of S. mansoni.
| Parameter | Value | Miracidia | Cercariae |
| Volume of watera (mL) | 50 | 44.3±8.5 | 50.0±7.0 |
| 100 | 31.3±3.8 | 54.3±9.3 | |
| 500 | 11.3±1.2 | 33.3±6.5 | |
| Number of organisms (miracidia or cercariae)b | 100 | 42.0±13.1 | 42.0±6.2 |
| 200 | 80.7±9.3 | 58.7±16.3 | |
| 300 | 94.0±12.8 | 72.3±8.7 | |
| Number of C. novaezelandiaec | 10 | 35.0±7.9 | 56.7±4.2 |
| 20 | 58.7±6.5 | 72.3±3.5 | |
| 30 | 89.7±6.8 | 90.0±2.6 | |
| 40 | 82.3±3.5 | 90.7±2.5 | |
| 50 | 88.3±1.5 | 95.3±1.5 | |
| 60 | 96.0±2.0 | 100.0 | |
| 70 | 100.0 | - |
a: using 10 C. novaezelandiae and 100 organisms; b: using 10 C. novaezelandiae and 50 mL water; c: using 100 organisms and 50 mL water.
3.5. Effect of C. novaezelandiae on infection of B. alexandrina snails with S. mansoni miracidia
The results (Table 2) showed that snail mortality increased significantly (P<0.05) in the presence of larger numbers of C. novaezelandiae during exposure period in comparison with snails not associated with C. novaezelandiae (maybe because of possible damage exerted on snails) as well as in comparison with snails exposed to miracidia in presence of few number of C. novaezelandiae. Regarding the infection of snails, the presence of 10 C. novaezelandiae only with 25 B. alexandrina snails in 10 mL of water during the miracidial exposure did not affect the infection rate significantly. However, with the increasing number of C. novaezelandiae individuals, the infection rate of snails significantly decreased (P<0.001). This reducing effect was almost similar for 50 and 100 C. novaezelandiae.
Table 2. Effect of different numbers of C. novaezelandiae on infection of B. alexandrina snails with S. mansoni miracidia.
| Number of C. novaezelandiae | Mortality of snails (%) | Survival of snails (%) | Infection percentage of snails (%) |
| 10 | 28.0±6.1 | 54 | 55.5±8.0 |
| 50 | 40.0±4.6 | 45 | 20.0±3.6 |
| 100 | 56.0±12.2 | 33 | 18.2±3.0 |
| Control | 24.0±6.1 | 57 | 63.2±8.9 |
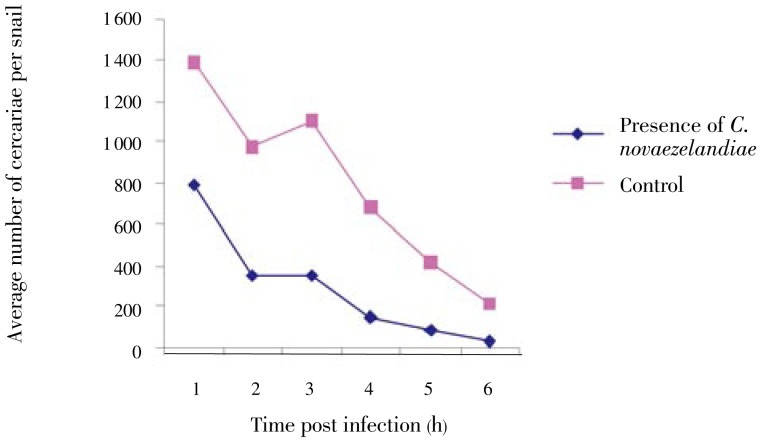
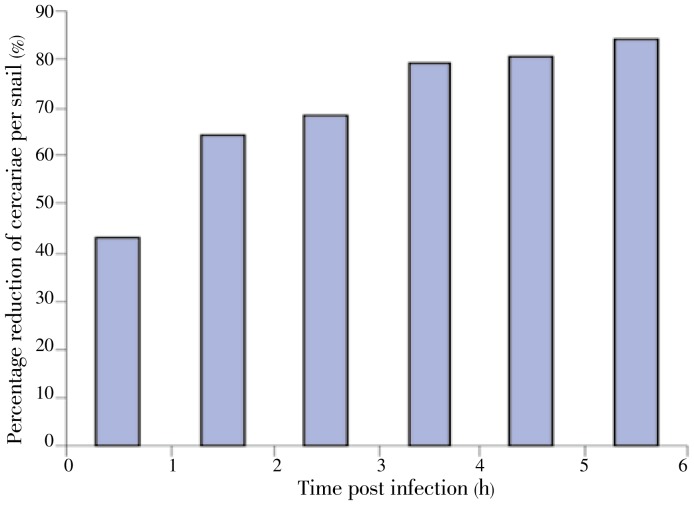
3.6. Effect of C. novaezelandiae on cercarial shedding
In the control group, the average number of released cercariae/infected B. alexandrina snail was 1 392.5 during the 1st hour of exposure to light. This number considerably gradually decreased in the following hours and became distinctly lower starting from the 4th hour to reach about 212.5 cercariae/snail in the 6th hour. The presence of C. novaezelandiae in the medium of the shedding snails proved to cause significant reducing effect on the cercariae production throughout the observation period (Figures 6 and 7), being more significant after 6 h (F=10.354, P<0.001). Using the Scheffe test to compare cercarial production in presence and absence of C. novaezelandiae, this result was confirmed (P<0.01).
Figure 6. Effect of C. novaezelandiae on pattern of cercarial shedding of infected B. alexandrina.
Figure 7. Effect of C. novaezelandiae on cercarial production of infected B. alexandrina.
4. Discussion
The present study demonstrated variable predatory activities of the ostracod C. novaezelandiae on B. alexandrina snails, the intermediate host of S. mansoni in Egypt, as well as on the free living stages of the parasite. Such effect was found to depend on certain environmental factors such as water volume, density of snails, larval stages of the parasite as well as density of C. novaezelandiae individuals. However, the availability of other diet in the habitat like blue green algae or vegetation (lettuce in the study) slightly reduced consumption of snail eggs. Moreover, the presence of C. novaezelandiae in the habitat during the exposure of snails to schistosome miracidia significantly reduced mortality and infection rate of snails and consequently the following production of cercariae. Ostracod has not been evaluated as a possible biocontrol agent in Schistosoma transmission. Therefore, the present study on C. novaezelandiae should draw attraction to a new method of biocontrol of schistosomiasis transmission. It is thought that this ostracod does not affect the natural habitats, since high densities are not associated with significant negative effects. The application of this ostracod would be achieved by introducing large numbers in schistosome risky sites. This organism could be produced in large scale by cultivation, which should be carried out under controlled optimal conditions. It could be introduced into the natural system at the proper time, considering the water volume, temperature and season. However, final evaluation of the present ostracod as a biocontrol agent against schistosomiasis will be arrived at after testing under simulated natural conditions.
Other crustacea like Daphnia magna (Copepoda) was found to consume miracidia and consequently reduce the infection rate of vector snails as well as production of cercariae[9]. Previously, Christensen et al found that Daphnia pulex significantly protected Lymnaea truncatula snails from infection with Fasciola hepatica[10].
Similarly, predation of snail eggs by sciomyzid larvae (Diptera: Sciomyzidae) was claimed by the researchers[11]–[13]. Recently, it is reported on larval feeding preference in the case of the sciomyzid fly Sepedon scapularis[5]. The present results showed that predation was affected by certain environmental factors, which is agreed with the results of scholars who claimed that snail predation by Psychoda alternata (Diptera: Psychodidae) larvae is influenced by the morphology and behavior of the mollusc which makes it more or less vulnerable[14]. The predation by Psychoda larvae on juvenile B. alexandrina snails showed that this activity on the free-swimming schistosome stages (miracidia and cercariae) depends on some factors such as water volume and density of larvae. They also found that experimental infection of Biomphalaria glabrata (B. glabrata) snails with schistosome miracidia and the total periodic cercarial production were significantly reduced by Psychoda in comparison with the control group. The prevalence of infection by S. mansoni in B. glabrata was significantly higher in snails that were devoid of Chaetogaster limnaei (Oligochaeta: Annelida), indicating that such oligochaete may protect the host from trematode infection[7],[15]. The reduction of the cercarial production from infected snails by C. novaezelandiae may be explained by the predatory effect of this organism on the cercariae. Another explanation may be that this is due to the mechanical effect C. novaezelandiae may exert on body wall of infected snails.
In conclusion and as universally agreed, the best possible method for schistosomiasis control may be the attack upon the vector snails and free-living larvae of the parasite. Therefore, the present results showing that introduction of C. novaezelandiae in a risky transmission sites of schistosomiasis could contribute markedly to this concept.
Acknowledgments
The work was supported by the Academy of Scientific Research and Technology in Egypt, Program of the National Strategy for Biotechnology and Genetic Engineering (Grant No. 41/2005). The authors thank Prof. Dr. Farouk R. Melek, Natural Compounds Department, National Research Center, Giza, Egypt, for his advice and help during the performance of this work.
Comments
Background
Schistosomiasis is a serious public health problem in the tropics and subtropical countries, including Egypt. The accepted control measure is to reduce the populations of snail vectors and free-living larvae of Schistosoma spp. This original paper based on results obtained from laboratory-based experiment highlighted the potential of utilizing C. novaezelandiae as a biological agent to break the transmission of S. mansoni.
Research frontiers
The currently used chemical molluscicides are not environment friendly, and they provide only temporary relief in snail control. Since no ideal biological predators have been reported to control the intermediate host for S. mansoni transmission, this paper reported the potential of using C. novaezelandiae as a biological agent not only for the B. alexandrina snail, but also revealed that it predated on the free-living larval stages of S. mansoni.
Related reports
Gawish et al. (2006) reported the positive effect of another crustacea, Daphnia magna in reducing the infection rate of B. alexandrina snail and also the production of free-swimming stages of S. mansoni. This study tested similar effect but utilized a different predator, namely, C. novaezelandiae.
Innovations and breakthroughs
Reports on biological control of snail populations are scarce and new ones are unavailable. This is the first paper that reported the potential of an ostracod, C. novaezelandiae, as a biological agent for control of S. mansoni transmission.
Applications
Chemical molluscicides are not environment friendly. If C. novaezelandiae proves to be effective in field study, then the potential would be huge, especially because the predator also feed on the free-swimming larvae of S. mansoni.
Peer review
In general, this is a well designed and laboratory-based study on the effectiveness of C. novaezelandiae in reducing the populations of both the intermediate snail stage and the free-swimming larvae of S. mansoni. It would be interesting to know the effect in a field study.
Footnotes
Fundation Project: Supported by the Academy of scientific Research and Technology in Egypt, Program of the National Strategy for Biotechnology and Genetic Engineering (Grant No. 41/2005).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.WHO Expert Committee Prevention and control of schistosomiasis and soil transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 2.King CH, Sturrock RF, Kariuki C, Hamburger J. Transmission control for schistosomiasis-why it matters now. Trends Parasitol. 2006;22:575–582. doi: 10.1016/j.pt.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Aditya G, Raut SK. Predation potential of the water bugs Sphaerodema rusticum on the sewage snails Physa acuta. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2002;97:531–534. doi: 10.1590/s0074-02762002000400015. [DOI] [PubMed] [Google Scholar]
- 4.Ismail M. The miracidiophagic and cercariophagic activity of the fish Gambusia affinis and Oreochromis niloticus and their effect on the infection of Biomphalaria alexandrina by Schistosoma mansoni. Egypt J Aqu Biol. 2003;7:87–98. [Google Scholar]
- 5.Maharaj R, Naidoo I, Appleton CC. Susceptibility of schistosome host snails to predation by sciomyzid flies (Diptera: Sciomyzidae) in South Africa. African J Aqu Sci. 2005;30:175–178. [Google Scholar]
- 6.Yousif F, Nashid N, Roushdy MZ, Tadros M, Ayoub M. Factors affecting predation of the freshwater leech Helobdella punctatolineata (Glossiphoniidae) on the schistosome vector snail Biomphalaria alexandrina (Planorbidae) under laboratory conditions. A- Biotic factors. Egypt J Zool. 2006;46:175–187. [Google Scholar]
- 7.Tadros M, El Bardicy S, Gawish F, Yousif F. Experimental studies on effect of Chaetogaster limnaei limnaei (Oligochaeta: Annelida) on Schistosoma mansoni transmission in aquatic habitats. New Egypt J Med. 2009;40:198–204. [Google Scholar]
- 8.Schotthoefer AM, Labak KM, Beasley VR. Ribeiroia ondatrae are consumed by aquatic invertebrate predators. J Parasitol. 2007;93:1240–1243. doi: 10.1645/GE1129R.1. [DOI] [PubMed] [Google Scholar]
- 9.Gawish F, El-Khayat H, Ismail N. Predatory activity of Daphnia magna on the free swimming stages of Schistosoma mansoni and on infection of Biomphalaria alexandrina with Schistosoma mansoni miracidia. Egypt J Zool. 2006;47:119–129. [Google Scholar]
- 10.Christensen NO, Nansen P, Frandsen F. Interference with Fasciola hepatica snail finding by various aquatic organisms. Parasitol. 1977;74:285–290. doi: 10.1017/s0031182000047909. [DOI] [PubMed] [Google Scholar]
- 11.Manguin S, Vala JC. Prey consumption by larvae of Tetanocera ferruginea (Diptera: Sciomyzidae) in relation to number of snail prey species available. Entomol Soc Am. 1989;82:588–592. [Google Scholar]
- 12.Maharaj R, Appleton CC, Miller RM. Snail predation by larvae of Sepedon scapularis Adams (Diptera: Siomyzidae), a potential biocontrol agent of snail intermediate hosts of schistosomiasis in South Africa. Med Veterin Entomol. 1992;6:183–187. doi: 10.1111/j.1365-2915.1992.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 13.Foote BA, Keiper JB. The snail killing flies if Ohio (Insecta: Diptera: Sciomyzidae) Kirtlandia. 2004;54:43–88. [Google Scholar]
- 14.El Bardicy S, Tadros M, Yousif F, Hafez S. Predatory activity of Psychoda alternata Say (Diptera: Psychodidae) larvae on Biomphalaria glabrata and Lymnaea natalensis snails and the free- living larval stages of Schistosoma mansoni. Australian Bas Appl Sci J. 2009;3:4503–4509. [Google Scholar]
- 15.Rodgers JK, Sandland GJ, Joyce SR, Minchella DJ. Multi-species interactions among a commensal (Chaetogaster limnaei limnaei), a parasite (Schistosoma mansoni), and an aquatic snail host (Biomphalaria glabrata) Parasitol J. 2005;91:709–712. doi: 10.1645/GE-421R. [DOI] [PubMed] [Google Scholar]