Abstract
Objective
To distinguish the difference among the Clinacanthus nutans (Burm. f.) Lindau (C. nutans) and Clinacanthus siamensis Bremek (C. siamensis) by assessing pharmacognosy characteristics, molecular aspect and also to evaluate their anti-herpes simplex virus (HSV) type 1 and type 2 activities.
Methods
Macroscopic and microscopic evaluation were performed according to WHO Geneva guideline. Stomatal number, stomatal index and palisade ratio of leaves were evaluated. Genomic DNA was extracted by modified CTAB method and ITS region was amplified using PCR and then sequenced. Dry leaves were subsequently extracted with n-hexane, dichloromethane and methanol and antiviral activity was performed using plaque reduction assay and the cytotoxicity of the extracts on Vero cells was determined by MTT assay.
Results
Cross section of midrib and stem showed similar major components. Leaf measurement index of stomatal number, stomatal index and palisade ratio of C. nutans were 168.32±29.49, 13.83±0.86 and 6.84±0.66, respectively, while C. siamensis were 161.60±18.04, 11.93±0.81 and 3.37±0.31, respectively. The PCR amplification of ITS region generated the PCR product approximately 700 bp in size. There were 34 polymorphisms within the ITS region which consisted of 11 Indels and 23 nucleotide substitutions. The IC50 values of C. nutans extracted with n-hexane, dichloromethane and methanol against HSV-1 were (32.05±3.63) µg/mL, (44.50±2.66) µg/mL, (64.93±7.00) µg/mL, respectively where as those of C. siamensis were (60.00±11.61) µg/mL, (55.69±4.41) µg/mL, (37.39±5.85) µg/mL, respectively. Anti HSV-2 activity of n-hexane, dichloromethane and methanol C. nutans leaves extracts were (72.62±12.60) µg/mL, (65.19±21.45) µg/mL, (65.13±2.22) µg/mL, respectively where as those of C. siamensis were (46.52±4.08) µg/mL, (49.63±2.59) µg/mL, (72.64±6.52) µg/mL, respectively.
Conclusions
The combination of macroscopic, microscopic and biomolecular method are able to authenticate these closely related plants and both of them have a potency to be an anti-HSV agent.
Keywords: Clinacanthus nutans, Clinacanthus siamensis, Microscopic analysis, Biomolecular analysis, Internal transcribed spacer, Herpes simplex virus, Plaque reduction assay
1. Introduction
Medicinal plants are the source of a large number of chemical compounds used as drug in primary health care of the world's population since prehistoric times. Clinacanthus nutans (Burm. f.) Lindau (C. nutans) and Clinacanthus siamensis Bremek. (C. siamensis) are medicinal plants in family Acanthaceae which can be found in Thailand[1]. The fresh leaves have been used for relieving of insect bites and skin rashes, and extracts from fresh leaves have been traditionally used for treatment of herpes simplex virus (HSV) infection in primary health care[2],[3]. Since a number of experiments have reported for their antiviral activities, C. nutans (Thai name: Phaya Yo) is suggested to be used as antiviral agent against HSV. C. siamensis is known in Thailand by the name of “Lin ngu hao”. It is found throughout Thailand and used as traditional medicine to relieve painful swelling. Several phytochemical studies on C. nutans have been reported but none on C. siamensis. Acyclovir (ACV) is an effective drug used for HSV infection. The widespread use of ACV may lead to drug resistant[4],[5]. Screening the HSV inhibitory agents from herbal medicine that are reputed to have this efficacy may be useful in order to obtain an alternative agent for inhibiting the HSV infection and the patients may not have to spend expensively for the antiviral drug.
Both C. nutans and C. siamensis are in the same genus Clinacanthus, and some of their morphologies are similar to each other. Traditionally authentication of medicinal plant relies upon morphological, physical and chemical inspection. Pharmacognosy evaluation using multidisciplinary characteristics such as macroscopic, microscopic and chemical fingerprinting can be used for plant authentication. However, many closely related species have similar morphology and chemical analysis may be limited by the amount of material, growth conditions as well as many other variables such as the storage condition and the harvest processing. Therefore, additional DNA based methods have been developed for medicinal plants authentication and DNA markers have now become a popular mean for identification and authentication of plant species due to the less effects caused by age, physiological condition and environment factors. Moreover, small amount of sample is sufficient for analysis and can be detected at any phase of plant development[6]. Sequence variation of the internal transcribe spacer (ITS) region in the nuclear DNA has been applied widely for phylogenetic evaluation at species level and can be developed to be a pattern for identification of herbal plants. Even more, this method can be further applied for investigation the adulteration of herbal drugs[7].
Due to their similar morphologies and the reports about identification and efficacies on anti-HSV-1 and HSV-2 activity of these two medicinal plants are quite limit. Hence, the aim of this present study has been conducted using pharmacognotic characteristic and sequence variation in the ITS region in order to distinguish between C. nutans and C. siamensis and assessing their in vitro antiviral activity against HSV-1 and HSV-2.
2. Materials and methods
2.1. Plants
C. nutans (Burm. f.) Lindau and C. siamensis Bremek. were collected from different sources of Thailand. The plants were authenticated by the expert-Associate Prof. Dr. Nijsiri Ruangrungsi and deposited at College of Public Health Sciences, Chulalongkorn University.
2.2. Macroscopic and microscopic evaluation
The mature branch including flower of C. nutans and C. siamensis was collected for macroscopic observation and illustrated on drawing paper with pencil and black ink pen. Microscopic evaluation was carried out under the appropriate magnitude using a photomicroscope attached with digital camera (Canon Power Shot A640). The stem and midrib from mature leaf were crossed section into thin pieces, mounted onto a slide and then observed under microscope. The recorded images were illustrated in a size proportional to the original. Leaf measurement was followed the method described in Pulok with some modifications[8]. The mature leaves were clearly bleached by boiling in sodium hypochlorite solution (sodium hypochlorite: water, 1:1, v/v). Then, cut the small piece of leaf on the center next to the midrib and peeled the lower side of the leaf then, evaluated under microscope. The stomatal number was counted within a 1 mm2 for 30 fields, and stomatal index was calculated. To determine the palisade ratio, after bleaching process, the fraction of leaf was placed uppermost on the slide, evaluated under microscope and counted the round light green palisade cells in four continuous boundary epidermal cells then, calculated for the proportion of palisade cells in one epidermal cell. The stomatal number, stomatal index and palisade ratio were presented as mean±SD.
2.3. Molecular evaluation
Genomic DNA of individual young leaves of plant samples were extracted by modified CTAB method described by Dellaporta and used as DNA template for PCR amplification[9]. The ITS region (including ITS1-5.8s -ITS2) was amplified using the universal ITS5 forward primer (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) and ITS4 reverse primer (5′-TCC TCC GCT TAT TGA TAT GC-3′)[10]. PCR amplification was carried out in 20 µL reaction mixture containing approximately 1-2 µL of genomic DNA, 1×PCR Buffer (100 mmol/L KCl, 20 mmol/L Tris-HCl, pH 8.0), 2.5 mmol/L MgCl2, 100 µmol/L dNTPs, 0.2 µmol/L of each primers and 0.5 units/µL of Taq polymerase (Promega). The amplified was carried out under the following conditions: initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C denaturation for 30 seconds, 55 °C annealing for 30 seconds, and 72 °C extension for 30 seconds, then followed by final extension at 72 °C for 5 min. Five microliter of PCR products were evaluated by 1.5% agarose gel electrophoresis, stained with ethidium bromide and visualized under UV transilluminator. The PCR products were then purified by QIAquick PCR purification kit (QIAGEN) prior sequencing. The ITS sequences from sence and antisence were analyzed using CLC DNA Workbench 6.0.
2.4. Anti-herpes simplex virus type 1 and type 2 activities evaluation
Ten grams of dried powdered leaves of C. nutans and C. siamensis was subsequently extracted first with n-hexane (400 mL), then dichloromethane (400 mL) and finally with methanol (400 mL) until exhausted in soxhlet extraction apparatus. All the three fractions were evaporated at the temperature not exceeding 40 °C to give the corresponding n-hexane fraction, dichloromethane fraction and methanol fraction. The crude extracts were dissolved in dimethylsulfoxide (DMSO) in the stock solution of 1 000 µg/mL then two-fold serially diluted with minimum essential media (MEM) to give the desire concentration ranging from 100 µg/mL to 12.5 µg/mL. Acyclovir (ACV, Sigma) was use as the positive control.
Herpes simplex virus type 1 (KOS) and type 2 (Baylor 186) and Vero cells were obtained from Department of Biochemistry and Microbiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University. Vero cells were cultured in MEM supplemented with 10% (v/v) fetal bovine serum and 1% antibiotics.
The plaque reduction assay was performed by modified method described by Phrutivorapongkul et al[11]. Vero cells (105 cells/mL) in 96-well tissue culture plate in triplicate were infected with 30 plaque forming unit (PFU)/25 µL per well of HSV-1 (KOS) or HSV-2 (Baylor 186). After 1 h inoculation at room temperature for virus adsorption, the overlay media containing various concentrations of the plant extracts were added. The infected cultures were further incubated at 37 °C for 2 d. The infected cells were fixed and stained with 5% (v/v) formalin and 0.03% (v/v) methylene blue solution. The numbers of plaques were counted and the percent inhibition was determined compared with the control. The 50% inhibitory concentration (IC50) was determined from the curve relating the plaque number to the concentration of the extract compared to acyclovir.
The cytotoxicity assay of plant extracts was evaluated. The culture medium containing the extract at various concentrations ranging from 100-1 600 µg/mL were added in Vero cells monolayer in 96-well tissue culture plate and cells were further grown at 37 °C for 2 d. After incubation, the viability of cells was evaluated by MTT assay[12],[13]. The percentage of cell viability was evaluated by comparing to the number of cell control. The concentration of the extract reducing cell viability by 50% (50% cytotoxicity concentration, CC50) was determined. The selectivity index (SI) was also determined by the ratio between the CC50 and IC50 value. The higher of SI indicated the potential of safer therapy of the given extract.
3. Results
3.1. Macroscopic and microscopic evaluation
The morphological evaluation of C. nutans and C. siamensis was illustrated and described in Figure 1. C. nutans is a shrub with short hair branches. Leaves are pale green, simple, opposite, narrowly elliptic oblong with acute apex size 2.5-13.0 cm long and 0.5-1.5 cm wide. There are 6-7 pairs of side veins and quite prominent below. The stem is straight green with white internodes and vertical strips throughout the entire stem. Flowers are in dense cymes at the top of the branches, often terminating drooping horizontal branches but themselves erect. Each flower has glandular-pubescent calyx, corolla glandular-pubescent, about 3.5 cm, dull red with green base and yellow streaks on lower lip. There are 2 stamens appressed against the upper lip.
Figure 1. Branch and flower illustration of C. nutans and C. siamensis.

a: C. nutans; b: C. siamensis.
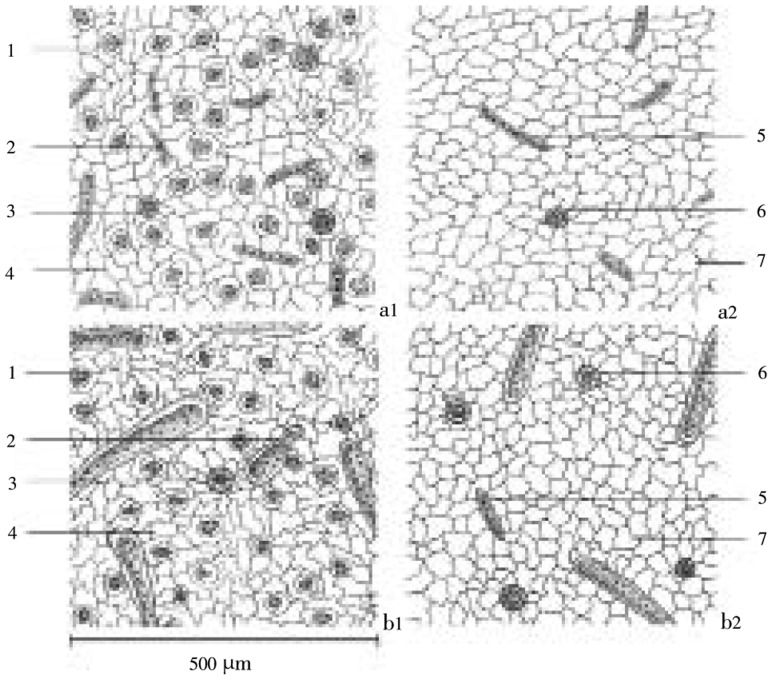
Microscopically, the stomata of both plants could be found only on the lower epidermis of the leaf with other components (glandular trichomes, lithocysts). The plant stomata was classified as diacytic type which the stomata was accompanied by two subsidiary cells at right angle to it and the epidemic cell components were illustrated in Figure 2. The average stomatal number, stomatal index in the area of 1 mm2 and palisade ratio of C. nutans were 168.32±29.49, 13.83±0.86 and 6.84±0.66, respectively while C. siamensis were 161.60±18.04, 11.93±0.81 and 3.37±0.31, respectively. The stem cross section of C. nutans and C. siamensis revealed that there were lithocysts interposed in the cortical parenchyma which lies beneath epidermis. Groups of collenchyma lies above parenchyma of cortex and pisiform parenchyma which is located above the phloem tissues. The xylem fibers align gather in group and were interposed longitudinally with parenchyma ray. There was xylem vessels with a wide lumen interposed sparsely in the xylem fibers. The cells in the ground tissue were polygonal shape in various sizes (Figure 3).
Figure 2. Epidermis of the leaf of C. nutans and C. siamensis with 20× magnification.
a: C. nutans; b: C. siamensis; a1, b1: lower epidermis; 1: diaccytic type stoma; 2: lithocyst; 3: glandular trichome; 4: epidermal cell; a2, b2: upper epidermis; 5: lithocyst; 6: glandular trichome; 7: epidermal cell.
Figure 3. Stem cross section of C. nutans and C. siamensis.
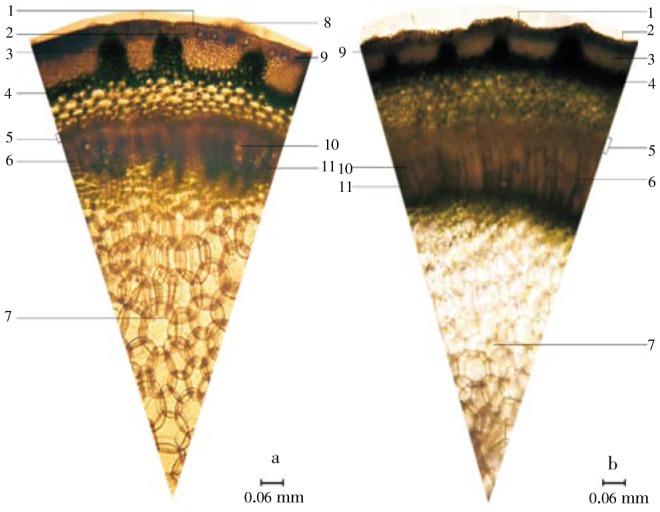
a: C. nutans; b: C. siamensis; 1: epidermis; 2: lithocyst; 3: group of collenchyma; 4: parenchyma of cortex; 5: phloem tissues; 6: parenchyma ray; 7: ground tissue; 8: multicellular trichome; 9: cortical parenchyma; 10: xylem vessel; 11: xylem fiber.
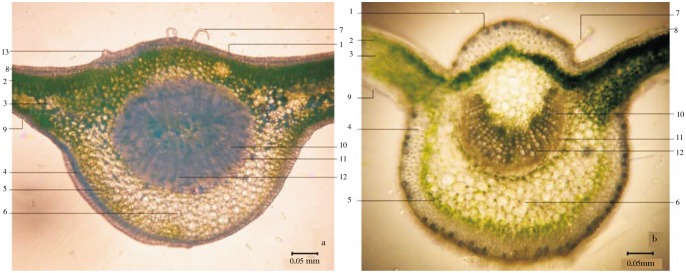
Midrib cross section of C. nutans and C. siamensis revealed multicellular and glandular trichomes on the epidermis which was interposed sparsely with lithocysts. Collenchyma located next to the epidermis. Palisade mesophyll and chlorenchyma lied next to the collenchyma. Xylem vessels arranged longitudinally and were interposed by parenchyma ray in the central portion of the polygonal shape parenchyma. Phloem tissue lied semicircular next to the xylem vessels in the central portion of the parenchyma (Figure 4).
Figure 4. Midrib cross section of C. nutans and C. siamensis.
a: C. nutans; b: C. siamensis; 1: lithocyst; 2: palisade mesophyll; 3: spongy mesophyll; 4: collenchyma; 5:chlorenchyma; 6: parenchyma; 7: multicellular trichome; 8: epidermis; 9: stoma; 10: xylem vessel; 11: phloem tissue; 12: parenchyma ray; 13: glandular trichome.
3.2. Molecular evaluation
Universal PCR primers designed from highly conserved regions flanking the ITS region was used for PCR amplification and generated the PCR product approximately 700 bp in size. Sequence analysis of C. nutans from 3 different locations (CN-MU, CN-CU, CN-NS) showed 98% homology and there were 7 and 11 single nucleotide polymorphism (SNP) within the ITS1 and ITS2 region, respectively. Where those of C. siamensis (CS-MU, CS-CU, CS-CS) showed 97% homology and there were 7 and 15 SNPs within the ITS1 and ITS2 region were observed. Sequence comparison of ITS region between C. nutans and C. siamensis showed 97% homology and 68% GC content. There were 34 polymorphisms within the ITS region which consisted of 11 Indels and 23 nucleotide substitutions.
3.3. Anti-herpes simplex virus type 1 and type 2 activities
Three extracts were isolated successively from the leaves of C. nutans and C. siamensis ranging from the least polar to the most polar (n-hexane, dichloromethane and methanol, respectively). The crude extracts yield of C. nutans and C. siamensis obtained from n-hexane were 0.36 g (3.58% w/w) and 0.16 g (1.59% w/w), respectively, dichloromethane crude extract of C. nutans and C. siamensis were 0.16 g (1.59% w/w) and 0.24 g (2.39 % w/w) respectively and methanol crude extract of C. nutans and C. siamensis were 1.72 g (17.15% w/w) and 1.70 g (16.95 % w/w) of dry weight of plant material, respectively.
To determine the anti-HSV-1 and HSV-2 activities, Vero cells were infected with 30 PFU/25 µL of HSV-1 (KOS) or HSV-2 (Baylor186). After incubation, the infected cells were fixed and stained and then the numbers of plaques were counted. Percent inhibition of plaque formation and therapeutic index were determined as shown in Table 1.
Table 1. IC50, SI and CC50 of C. nutans and C. siamensis extracts against HSV-1 and HSV-2.
| Extracts |
C. nutans |
C. siamensis |
|||||||||||
| IC50 (µg/mL, mean±SD) |
SI |
CC50 (µg/mL) | IC50 (µg/mL, mean±SD) |
SI |
CC50 (µg/mL) | ||||||||
| HSV-1 | HSV-2 | HSV-1 | HSV-2 | HSV-1 | HSV-2 | HSV-1 | HSV-2 | ||||||
| n-hexane | 32.05±3.63 | 72.62±12.60 | >50.36 | >22.50 | >1 600 | 60.00±11.61 | 46.52±4.08 | >27.31 | >34.53 | >1 600 | |||
| Dichloromethane | 44.50±2.66 | 65.19±21.45 | 19.57 | 14.09 | 869 | 55.69±4.41 | 49.63±2.59 | 3.50 | 3.92 | 194 | |||
| Methanol | 64.93±7.00 | 65.13±2.22 | >24.84 | >24.59 | >1 600 | 37.39±5.85 | 72.64±6.52 | >43.52 | >22.14 | >1 600 | |||
All extractions of the two species at concentration of 100 µg/mL presented the anti HSV-1 and HSV-2 activities with more than 50% inhibition of plaque formation (30 PFU/25 µL). The lowest IC50 values of C. nutans was (32.05±3.63) µg/mL of n-hexane extract that inhibited HSV-1 with selectivity index>50.36 and (65.13±2.22) µg/mL of methanol extract that inhibited HSV-2 with selectivity index>24.59 while those of C. siamensis was (37.39±5.85) µg/mL of methanol extract that inhibited HSV-1 with selectivity index>43.52 and (46.52±4.08) µg/mL of n-hexane extract that inhibited HSV-2 with selectivity index>34.53. Compared to acyclovir which was used as a positive control in plaque reduction assay expressed the IC50 value at (0.09±0.02) µg/mL against HSV-1 and (0.43±0.04) µg/mL against HSV-2 (data not shown). Fifty percent cytotoxicity concentration (CC50) of the extracts to Vero cells was also investigated. Among all extracts, dichloromethane extract of the both plants exhibited the highest value (869 and 194 µg/mL for C. nutans and C. siamensis, respectively). However, the highest IC50 value that could inhibit the viral activity possessed the cytotoxicity in Vero cells.
4. Discussion
Human beings use plants as medicine for the treatment of various diseases for thousands of year. It is essential to use the right herbal material for the correct pharmacological effect. Correct identification and quality control of the starting material is essential for herbal preparation. There are many methods for medicinal plant authentication. Morphological assessment is an effective tool for determining the identity of plant material as it is fast and inexpensive. However, it is subjective and depends on experience of the examiner when closely related species are involved. Since most herbal medicines are used as processed material and crude extracts, various methods have been developed for separation and identification. The use of chemical techniques to standardize and quality control has some limitation because of their variable sources and chemical complexity. Therefore, additional of DNA technologies have been applied for medicinal plant authentication and combinations of various methods have been employed for herbal drug technology development. The present study deals with the discrimination between the two closely related plants in genus Clinacanthus by using macroscopic, microscopic and molecular methods. According to the results, macroscopic and microscopic analysis of these two closely related plants revealed the similar morphology and cell components. However, the leave measurement index (stomatal number, stomata index and palisade ratio) which are the important property for species identification showed different constant numbers. Similar to the previously pharmacognostic study indicated that the value of leave measurement of C. siamensis leave differ from C. nutans especially, the value of palisade ratio[14]. There have been previous reports using leave measurement index for identification of medicinal plants[15],[16]. In addition, molecular markers have been utilized in medicinal plants identification due to their powerful in elucidating the identity of the substitutes and adulterants of medicinal materials that often introduced intentionally or accidentally. Identifying of specific regions in the DNA can be used for different level of authentication as the genetic composition is unique for each species and is not affected by physical and environmental factors[17],[18]. The ITS region is widely used in plant taxonomy and molecular phylogeny because it has a high degree of variation even between closely related species[19]. According to the results, the ITS regions between C. nutans and C. siamensis are similar to those previously published, with sequence lengths of approximately 700 bp. Typically, published ITS1 sequences were approximately 300 bp, while ITS2 were 400-600 bp with the conservative 5.8S rDNA sequences. Due to the limitation in published ITS sequence of C. nutans and C. siamensis, comparable with that of traditional classification is still doubtful and need to be further investigated in number. However, ITS sequences are capable of providing many variable information, which plays a more important role in the authentications of medicinal herbs. The ITS sequences of C. siamensis showed considerable variations due to the high level of intra-individual variation. The sequence of the ITS region in this study were almost identical between C. nutans and C. siamensis (97-99% similarity) indicating closely related species and ITS region may not be suitable molecular marker for differentiation between C. nutans and C. siamensis. Not only the sequence variation of the ITS region was suitable for identification of various medicinal plants[17]–[19], but other coding and spacer regions of nuclear, chloroplast and mitochondrial genes such as rbcL, matK and trnK gene have been also explored and used as molecular markers for authentication[20]–[22]. It is generally accepted that multi-locus combinations will be required for species discrimination[23]. DNA based techniques have been extensively applied in traditional Chinese medicine as well as Asian traditional medicine such as Ginseng[17],[24], especially in case of the identification of substituted or adulterated with other species that morphologically and phytochemically indistinguishable. In Thai medicinal plants, molecular markers were also extensively used for the identification of an important medicinal plants remedy[25].
HSV infection is commonly found worldwide. ACV and other nucleoside derivatives have been used for treatment of HSV infections. A large number of anti-HSV agents from medicinal plants have been previously reported in several studies[26]–[28], it was found that natural products from medicinal plant extracts are good and potential sources for promising anti-HSV drug including C. nutans and C. siamensis. Twenty medicinal plant extracts from Thailand have been evaluated for their anti-HSV activity, 11 out of 20 inhibited more than 50% plaque formation of HSV-1[29]. Methanolic extracted of C. siamensis was shown to have more than 90% antiviral activity against HSV-1 and HSV-2 at the concentration 50 µg/mL[30]. Moreover a large number of bioactive constituents of the extracts from C. nutans and C. siamensis leave, like polyphenolic, glycoside, terpenes, were found to be promising anti-HSV agents[30]–[32]. Three chlorophyll derivatives isolated from the chloroform extract of C. nutans leaves were individually tested for anti-HSV activity and the results indicated that these compounds have anti herpes simplex activity in pre-viral entry step[33]. There were various assays to study on antiviral activities of medicinal plants, Plaque reduction assay has always been used for in vitro antiviral activities. In this study, the result indicates that n-hexane, dichloromethane and methanol extracts of C. nutans and C. siamensis dry leaves can inhibit the plaque forming of both HSV-1 and HSV-2. The obtained results revealed that methanolic extract of C. nutans exhibit only a slight anti-HSV-1 activity (IC50=64.93 µg/mL) compare to the n-hexane (IC50=32.05 µg/mL) and selectivity index, determined by the CC50 to IC50 against HSV-1 was >50.36. By contrast, methanolic extract of C. siamensis exhibited the strongest activity against HSV-1 (IC50=37.39 µg/mL) with selectivity index value >43.52 while the n-hexane exhibit only a slight anti-HSV-1 activity (IC50=60.00 µg/mL). Comparison to the anti-HSV-2 activity, n-hexane, dichloromethane and methanol extracts of C. nutans exhibited slight activity (72.62, 65.19, 65.13 µg/mL, respectively) whereas n-hexane and dichloromethane exhibited better activity (46.52 and 49.63 µg/mL) than methanolic extract (72.64 µg/mL) of C. siamensis. Many variables can affect the IC50 value obtained in the test, these include host cell line, viral inoculums titer, strain of virus, incubation time. Control HSV strains with known IC50 value must be include in each test. By comparing the ability of C. nutans and ACV to inactivate HSV-2, the results showed that the ethanolic extracts of C. nutans was able to inhibit plaque formation by HSV-2 in baby hamster kidney cell line and it was further tested for clinical trial in patients with genital herpes, in which the result exhibited that using the extract, the lesion could completely heal within 7 days which is much greater than the placebo group[34]. In contrast to the study done by YooSook, organic solvent extracts of C. nutans did not show anti HSV-2 activity[28]. The results in study of HSV-1 resistant to either ACV or foscarnet from C. siamensis ethanolic extract suggested that C. siamensis ethanolic extract had effect to the herpes simplex virus ACV-resistant AR3 with IC50 at 62.5 µg/mL.
In conclusion, macroscopic, microscopic and biomolecular method are able to authenticate the closely related plants; C. nutans and C. siamensis. Accordingly, these medicinal plants can be a source for isolation of anti-HSV compounds and the extraction of these medicinal plants exhibit antiviral activity against both HSV-1 and HSV-2, suggesting that these Thai medicinal plants have a potential to be an antiviral agent.
Acknowledgments
PK was scholarly supported by the scholarship by The Office of the Higher Education Commission, Thailand, under the Strategic Scholarships for Graduate Programs, University of Phayao. This research was partially supported by CU Graduate School Thesis grant, Teaching Assistant Fellowship, and The Herbal Remedies and Alternative Medicine of Special Task Force for Activating Research (STAR) under 100 Years of Chulalongkorn University Fund (5200601), Thailand. The authors would like to thanks College of Public Health Sciences, for facilities support and Mr. Nirun Vipunngeun for his assistance with plant drawing.
Comments
Background
Both C. nutans and C. siamensis are closely related species and have similar morphology. Assessing their identities by using pharmacognostic, molecular aspects and their antiviral activity are interesting.
Research frontiers
Studies are being performed in order to identify the characteristics of two closely related medicinal plants; C. nutans and C. siamensis by using many aspects in pharmacognostic, molecular and biological activity for identification.
Related reports
According to the previous investigation, there are some information about these two medicinal plants. However, comparison of their characteristics by using several aspects still limited.
Innovations and breakthroughs
This study aims to distinguish the similar characteristics of two closely related medicinal plants using difference characteristics such as macroscopic, microscopic, DNA sequence and biological activity in order to differentiate these two plants.
Applications
This study provides the information regarding to the comparison of two closely related plants species and their potential to be an anti-viral agent from Thai medicinal plant.
Peer review
This is a good study providing the scientific interesting information and development of the natural product for antiviral drug. The result obtained from this study indicated that combination of pharmacognostic and molecular data are suitable for identification and these Thai herbal medicines have a potential for development of antiviral agent deriving from the natural product.
Footnotes
Foundation Project: Supported by Office of the Higher Education Commission, Thailand, University of Phayao and CU Graduate School Thesis Grant, Chulalongkorn University (Grant No. 5200601).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Smitinand T. Thai plant names. 2nd ed. Bangkok: Prachachon Co. Ltd; 2001. p. p. 140. [Google Scholar]
- 2.Wirotesangthong M, Rattanakiat S. Anti-herpes simplex virus type 2 activities of some Thai medicinal plants. Thai J Pharm Sci. 2006;30:19–27. [Google Scholar]
- 3.Wirotesangthong M, Nagai T, Yamada H, Amnuoypol S, Mungmee C. Effects of Clinacanthus siamensis leaf extract on influenza virus infection. Microbiol Immunol. 2009;53:66–74. doi: 10.1111/j.1348-0421.2008.00095.x. [DOI] [PubMed] [Google Scholar]
- 4.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus esistance to acyclovir and penciclovir after two decades of antivirus therapy. Clin Microbiol Reviews. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirotesangthong M, Rattanakiat S, Matsui K, Lipipun V. Characterization of herpes simplex virus type 1 selected in culture for resistance to acyclovir or foscarnet. Thai J Pharm Sci. 2007;31:36–44. [Google Scholar]
- 6.Sucher NJ, Carles MC. Genome-based approaches to the authentication of medicinal plants. Planta Med. 2008;74:603–623. doi: 10.1055/s-2008-1074517. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki Y, Komatsu K, Nagumo S. Rapid detection of Panax ginseng by loop-mediated isothermal amplification and its application to authentication of ginseng. Biol Pharm Bull. 2008;31:1806–1808. doi: 10.1248/bpb.31.1806. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee PK. Quality control of herbal drugs. New Delhi: Business Horizon; 2007. pp. 160–178. [Google Scholar]
- 9.Weigel D, Glazebrook J. Dellaporta miniprep for plant DNA isolation. Cold Spring Harb Protoc. 2009 doi: 10.1101/pdb.prot4765. [DOI] [PubMed] [Google Scholar]
- 10.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis Michael A., editor. PCR protocols: A guide to methods and applications. University of Michigan: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 11.Phrutivorapongkul A, Lipipun V, Ruangrungsi N, Kirtikara K, Nishikawa K, Maruyama S, et al. et al. Studies on the chemical constituents of stem bark of Millettia leucantha: Isolation of new chalcones with cytotoxic, anti-herpes simplex virus and anti-inflammatory activities. Chem Pharm Bull. 2003;51(2):187–190. doi: 10.1248/cpb.51.187. [DOI] [PubMed] [Google Scholar]
- 12.Chansriniyom C, Ruangrungsri N, Lipipun V, Kumamoto T, Ishikawa T. Isolation of acridone alkaloids and N-[(4-monoterpenyloxy) phenuylethyl]-substituted sulfur-containing propanamide derivatives from Glycosmis parva and their anti-herpes simplex virus activity. Chem Pharm Bull. 2009;57(11):1246–1250. doi: 10.1248/cpb.57.1246. [DOI] [PubMed] [Google Scholar]
- 13.Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA, et al. et al. Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharm. 2005;99:43–47. doi: 10.1016/j.jep.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 14.Jamtaweekul J. Chulalongkorn University; 2001. Pharmacognostic evaluation of Clinacanthus siamensis leaves. Thesis, Program in pharmacy in pharmacognosy. [Google Scholar]
- 15.Roonyamarai W, Rungsihirunrat K, Vipunngeun N, Ruangrungsi N. Microscopic and molecular analyses of selected Morinda species in Thailand. Asian J Trad Med. 2011;6(3):118–126. [Google Scholar]
- 16.Abere TA, Onwukaeme DN, Eboka CJ. Pharmacognostic evaluation of the leaves of Mitracarpus scaber Zucc (Rubiaceae) Trop J Pharm Res. 2007;6(4):849–853. [Google Scholar]
- 17.Feng T, Liu S, He XJ. Molecular authentication of the traditional Chinese medicinal plant Angelica sinensis based on internal transcribed spacer of nrDNA. Electron J Biotechnol. 2010;13(1):9–10. [Google Scholar]
- 18.Xue HG, Zhou SD, He XJ, Yu Y. Molecular authentication of the traditional Chinese medicinal plant Euphorbia pekinensis. 2007;73(1):91–93. doi: 10.1055/s-2006-951769. [DOI] [PubMed] [Google Scholar]
- 19.Lin SY, Geun PW, Woung KO, Kwan HS. The internal transcribed spacer rDNA specific markers for identification of Zanthoxylum piperitum. Afr J Biotechnol. 2010;9(37):6027–6039. [Google Scholar]
- 20.Liu T, Yan H, Guo X. Discrimination of the medicinal plant Fallopia multiflora and its adulterants by diagnostic polymerase chain reaction (PCR) J Med Plant Res. 2011;5(15):3461–3465. [Google Scholar]
- 21.Li M, Cao H, But PPH, Shaw PC. Identification of herbal medicinal materials using DNA barcodes. J System Evol. 2011;49(3):271–283. [Google Scholar]
- 22.Fan LL, Zhu S, Chen HB, Yang DH, Cai SQ, Komatsu K. Molecular analysis of Stemona plants in China based on sequences of four chloroplast DNA regions. Biol Pharm Bull. 2009;32(8):1439–1446. doi: 10.1248/bpb.32.1439. [DOI] [PubMed] [Google Scholar]
- 23.CBOL Plant Working Group A DNA barcode for land plants. Proc Natl Acad Sci USA. 2009;106(31):12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YE, Ahn CH, Kim BB, Yoon ES. Development of species specific AFLP-derived SCAR marker for authentication of Panax japonicas C.A. Meyer. Biol Pharm Bull. 2008;31:135–138. doi: 10.1248/bpb.31.135. [DOI] [PubMed] [Google Scholar]
- 25.Manissorn J, Sukrong S, Ruangrungsi N, Mizukami H. Molecular phylogenetic analysis of Phyllanthus species in Thailand and the application of polymerase chain reaction-restriction fragment length polymorphism for Phyllanthus amarus identification. Biol Pharma Bull. 2010;33(10):1723–1727. doi: 10.1248/bpb.33.1723. [DOI] [PubMed] [Google Scholar]
- 26.Kurokawa M, Nagasaka K, Hirabayashi T, Uyama S, Sato H, Kageyama T, et al. et al. Efficacy of traditional herbal medicines in combination with acyclovir against herpes simplex virus type 1 infection in vitro and in vivo. Antivirus Res. 1995;27(1–2):19–37. doi: 10.1016/0166-3542(94)00076-k. [DOI] [PubMed] [Google Scholar]
- 27.Chuanasa T, Phromjai J, Lipipun V, Likhitwitayawuid K, Suzuki M, Pramyothin P, et al. et al. Anti-herpes simplex virus (HSV-1) avtivity of oxyreseveratrol derived from Thai medicinal plant: mechanism of actionherapeutic efficacy on the cutaneous HSV-1 infection in mice. Antiviral Res. 2008;80(1):62–70. doi: 10.1016/j.antiviral.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Yoosook C, Panpisutchai Y, Chaichana S, Santisuk T, Reutrakul V. Evaluation of anti-HSV 2 activity of Barleria lupulina and Clinacanthus nutans. J Ethnopharmacol. 1999;67(2):179–187. doi: 10.1016/s0378-8741(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 29.Lipipun V, Kurokawa M, Suttisri R, Taweechotipatr P, Pramyothin P, Hattori M, et al. et al. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antiviral Res. 2003;60:175–180. doi: 10.1016/s0166-3542(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 30.Lertsupwichit W. Chulalongkorn University; 2003. Chemical constituents of Clinacanthus siamensis leaves. Thesis, Program in Pharmacy in Pharmacognosy. [Google Scholar]
- 31.Tuntiwachwuttikul P, Pootaeng-on Y, Phansa P, Taylor WC. Cerebrosides and a monoacylmonogalactosylglycerol form Clinacanthus nutans. Chem Pharm Bull. 2004;53:27–32. doi: 10.1248/cpb.52.27. [DOI] [PubMed] [Google Scholar]
- 32.Wanikiat P, Panthong A, Sujayanon P, Yoosook C, Rossi AG, Reutrakul V. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J Ethnopharmacol. 2008;116:234–244. doi: 10.1016/j.jep.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Sakdarat S, Shuyprom A, Pientong C, Ekalaksananan T, Thongchai S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorg Med Chem. 2009;17(5):1857–1860. doi: 10.1016/j.bmc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 34.Jayavasu C, Balachandra K, Sangkitporn S, Bunjob M, Chavalittumrong P. Clinical trial in the treatment of genital Herpes patients with Clinacanthus nutans extract. Com Dis J. 1992;18(3):152–161. [Google Scholar]