Abstract
Objective
To establish and estimate incidence of contagious bovine pleuropneumonia (CBPP), using abattoir survey as a diagnostic tool in slaughtered cattle in Northern Tanzania.
Methods
A total of 4 460 cattle were slaughtered in five abattoirs in 3 northern zone regions (Arusha, Kilimanjaro and Tanga) during the period of January to May 2004. They were examined ante-mortem for ‘pneumonia signs’, and ‘characteristic contagious bovine pleuropneumonia (CBPP) lung lesions’.
Results
Forty-one (0.91%) of the slaughtered cattle, the majority of which were Tanzania short horn zebu, had gross lung lesions suggestive of CBPP. The prevalence of lesions was significantly (P<0.05) higher in Karatu abattoir compared to others. No animal was detected to have lesion in Bomang' ombe abattoir. The most observed pneumonic signs included labored breathing (90%), dry cough (57%) and mucopurulent nasal discharge (47%). The gross characteristic CBPP pathological lesion, frequently encountered was left lung lesion (47%), pinkish lung (71%) and pleural adhesion (98%). Epidemiological reports show that the CBPP reported outbreaks increased from 19 in 2002, 65 in 2003 and 18 in 2004 (January-March). The corresponding number of reported deaths increased from 137 in 2002, 269 in 2003 and 77 in 2004 (January-March).
Conclusions
It's concluded from this study that CBPP is a problem in spite of the extensive awareness and vaccination campaigns. Nevertheless, a continued surveillance programme including routine checks of all cattle carcasses at the abattoir and subsequent epidemiological investigation of suspected cases are recommended.
Keywords: Abattoir, Bovine pleuropneumonia, Prevalence estimates, Slaughtered cattle, Tanzania
1. Introduction
Contagious bovine pleuropneumonia (CBPP), an OIE listed disease, is the second most important trans-boundary animal disease (cattle) after rinderpest[1]. Unlike rinderpest however, CBPP is not dramatic; it is insidious in nature with the majority of the cases remaining sub-clinical[2]–[4]. CBPP is an infectious and highly contagious disease of cattle and water buffaloes and is considered to be amongst the most important infectious disease of these animals[5],[6]. CBPP is characterized by the presence of sero-fibrinous interlobular oedema and hepatization giving a marbled appearance to the lung in acute to sub-acute cases and capsulated lesions (sequestra) in the lungs of some chronically infected cattle. The causative agent of CBPP is Mycoplasma mycoides subspecies mycoides small colony variant[5]–[8]. Affected animals have difficulty in breathing due to damage to the lungs; they loose condition and a proportion of them die. All ages of cattle are susceptible, but young cattle develop joint swelling rather than lung infections. Many cattle show no disease signs despite being infected[9]. CBPP represents a major constraint to cattle production in Africa and is regarded as the most serious infectious animal disease affecting cattle on the continent[2],[3].
CBPP was first introduced in Tanzania in 1916 and was eradicated in 1964[10]. The disease re-emerged in the country in 1990 and since then it has spread widely, threatening the entire national cattle herd. The disease threatens 11.9% of the cattle in in the northern zone regions (Arusha, Manyara, Kilimanjaro and Tanga), Tanzania[10]. The impact of CBPP is felt at both national and local levels, affecting both trade and the local economy. Direct losses result from mortality, reduced milk yields, and the costs of vaccination, antibiotics, veterinary services and disease surveillance. Indirect costs are mainly due to the chronic nature of the disease and include loss of weight and working ability, reduced fertility, losses due to quarantine and reduced cattle trade. This last aspect is particularly important at a regional/national/international level since the presence of CBPP severely restricts cattle trade whilst effectively closing the EU/USA-export markets. Farmers also incur social costs, as cattle cannot be used for functions associated with marriage, transport and loaning. Abattoir survey, as a tool for disease survey and investigation is known to provide essential information that can be utilized for research and disease control purposes[11],[12]. Moreover, diagnosis and evaluation of diseases such as cysticercosis, liver fluke and CBPP through abattoir surveys can be made much reliably than other conventional diagnostic methods.
The purpose of this study was to evaluate the magnitude of CBPP incidences in the slaughtered cattle. This study was restricted to identifying the most frequently encountered pre-slaughter pneumonia signs, observation of ‘CBPP characteristic like lesion’ and estimating the prevalences of CBPP in slaughtered cattle from selected abattoirs/slaughter-slabs in the northern districts of Tanzania.
2. Materials and methods
2.1. Study abattoirs
The study was carried out in five abattoirs, two in Tanga region (Muheza and Tanga), one in Kilimanjaro region (Bomang' ombe) and two in Arusha region (Sakina and Karatu). The criteria for selection included availability of abattoir or slaughter facility, qualified meat inspector and willingness to join the study.
Muheza and Tanga abattoir receives trade livestock mostly from Kiteto, Handeni and Kondoa Districts; also from within Muheza and Tanga districts. Bomang' ombe abattoir receives trade livestock mostly from Hai, Mbulu and Simanjiro Districts and from Shinyanga regions. Sakina abattoir receives trade livestock mostly from Arumeru, Karatu, Mbulu, Monduli, Babati, Simanjiro and Hanang Districts and from Shinyanga, Singida, Dodoma and Tabora regions. Karatu abattoir receives trade livestock from Karatu and Mbulu districts and sometimes from Shinyanga region.
Cattle presented to the abattoirs came from livestock traders and individual livestock keepers.
Breeds of cattle often submitted include Tanzania short horn zebu and less often, crosses of dairy stock.
2.2. Data collection and pre-slaughter examination of animals
Determination of age was done through dentition observation, the slaughtered stock were grouped into three age groups, thus younger than 6 months, 6-18 months and above 18 months old.
The questionnaire was designed in one page and had four major components: abattoir profile, source/destination of slaughtered stocks, pre-slaughter pneumonia signs and post mortem features. To maximize and improve the precision of responses, more closed ended questions were used after pre-testing. Each abattoir was visited (by respective abattoir meat inspector) daily for a month, between the period of January and May 2004. Records of numbers slaughtered, source of slaughter stock and the lesion(s) observed were noted down in the questionnaire by meat inspectors in these abattoirs. All animals presented for slaughter were physically observed a day or shortly prior to slaughter. Inspection of the animals was made while at rest or in motion for any obvious signs of disease. Special attention was made to the respiratory related signs like breathing pattern (labored/distressed), coughing (dry/moist), standing posture (nostril dilated, neck extended).
2.3. Post slaughter examination of animals
Post slaughter examination involved visual examination of all carcasses and organs including palpation and incision of tissues/organs as described by Phiri AM[11]. Gross pathological lesions on each diseased lung were recorded. Samples (sera and tissue) for detailed laboratory investigation could not be collected due to the logistic and capacity of the local laboratory reasons.
2.4. Data analysis
Monthly prevalence of CBPP was calculated using the following formula: Proportion of the stock affected by one or more lung lesion(s) to the total (monthly) number of slaughtered stock. Data files for the studied parameters were edited, developed and analyzed using both Epi-Info version 6.04d and Statistix[14].
3. Results
A total of 4 460 cattle were slaughtered and examined in the selected abattoirs during the survey period. The average number (mean±SE) of cattle slaughtered per day by abattoir was Muheza 9.70±0.50, Bomang' ombe 5.40±0.35, Tanga 29.00±0.42, Karatu 7.40±0.19 and Sakina 75.80±0.21. Of the slaughtered and examined cattle, 0.91% cattle had lesions suggestive of CBPP. Four thousands and four hundred nineteen (99.09%) showed no gross lesion suggestive of CBPP. The slaughter of cattle aged between 0-6 months was not recorded in all abattoirs. The proportions of slaughters and stocks with CBPP in each category of each variable investigated during the study are shown in Tables 1 and 2. The prevalence of lesions was significantly (P<0.05) higher in Karatu compared to other abattoirs. Age of the slaughtered animals was not significantly associated with CBBP like lesions (P>0.05).
Table 1. Prevalence of CBPP by abattoir location.
| Abattoir | Number of slaughtered | Number of affected [n (%)] |
| Tanga | 898 | 4 (0.44) |
| Muheza | 292 | 2 (0.68) |
| Bomang' ombe | 163 | 0 (0.00) |
| Sakina | 2 884 | 5 (0.17) |
| Karatu | 223 | 30 (13.40) |
| Overall | 4 460 | 41 (0.91) |
Table 2. Prevalence of CBPP by age category.
| Age category (Months) | Number of slaughtered | Number of affected [n (%)] |
| <6 | 0 | 0 (0.00) |
| 6-18 | 17 | 2 (11.7) |
| >18 | 4 443 | 39 (0.88) |
| Overall | 4 460 | 41 (0.91) |
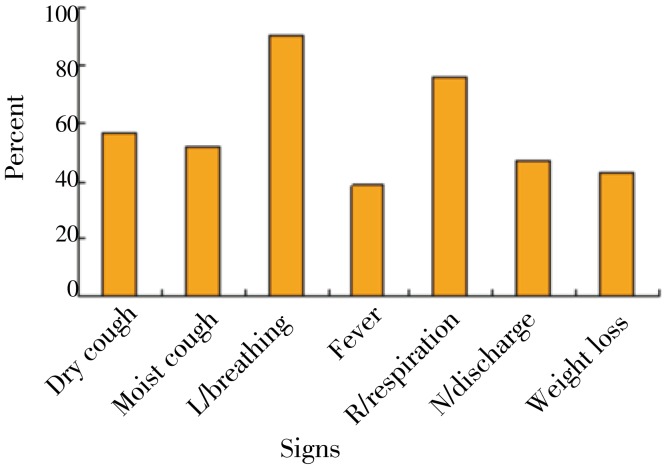
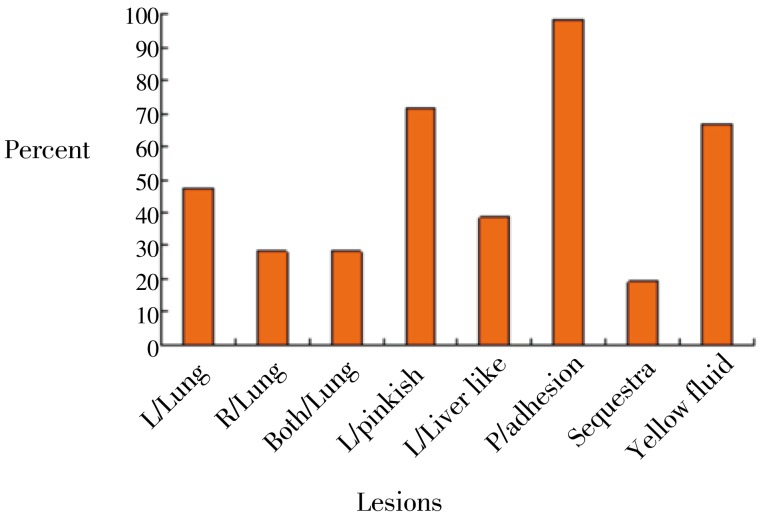
The results in Figure 1 shows that of the 41 cattle affected, 57%, 90% and 47% had dry cough, labored breathing and mucopurulent nasal discharge, respectively. The results in Figure 2 shows that out of 41 cases of lung infection, 28% and 47% affected the right and left lungs, respectively while 28% was bilateral. Pleural adhesion and presence of yellowish fluid in the thoracic cavity was observed in 98% and 89% respectively of all cattle that had lung lesions. Sequestra formation was associated with 20% of all animals slaughtered.
Figure 1. The most observed ante-mortem ‘pneumonia like, signs in bovine during Jan-May 2004.
L/breathing: Labored breathing; N/discharge: Mucopurulent nasal discharge.
Figure 2. The most observed post-mortem lung lesion in Bovine: Jan-May 2004.
L/Lung: Left lung; R/Lung: Right lung.
4. Discussion
From the pre and post slaughter signs and lesions recorded in this survey, there is indication that CBBP was present in the study area as seen 41 (0.91%) positive cases. Epidemiological data available also support this observation. In 2001, Tanzania reported 166 outbreaks involving 5 721 cases, of which 3 301 cattle died of the disease[10]. In the northern regions, the disease was reported in 7 districts in 2002, 13 districts in 2003 and 5 districts (Jan-March) in 2004 with Simanjiro accounting for 64% of all the reported outbreaks. In all these outbreaks, 346 cattle were recorded to have died of the disease[14],[15]. The estimated prevalence of 0.91% is slightly higher than findings of 0.01% in Nigeria[16], however lower than the prevalence of 3.60% reported in Zambia[11]. Although the overall prevalence of CBBP was low, stratifications of prevalence by location and age category reveals that Karatu abattoir and animals aged 6-18 months to have recorded significantly high number of CBBP infected cattle than any other abattoirs and other age categories. The reason for high prevalence rate might be difficult to explain, however, detailed tracing of source of cattle indicate majority to originate from Matala (33.3%), Karatu (26.9%) and Mbulu (23.3%) areas which are thought to be CBPP endemic areas. These areas warrant close attention. The control of CBPP is not given the adequate attention as rinderpest probably because of the insidious nature of CBPP. Majority of the cases remain sub clinical and affected animals becoming carriers due to the encapsulation of the lesions in the lungs and uphazard, uncoordinated vaccination activities[17],[18]. The present study was only restricted to few abattoirs where animals are brought for slaughter from within the studied districts. Therefore, this cannot be treated as a true representation of the northern districts of Tanzania. However, this survey gives an insight to the status of CBPP in some districts and warrants measures for their control.
It is concluded from this study that CBPP remain a problem in the northern districts and probably Tanzania as a whole. Coordinated territorial efforts to eradicate the disease through improvements of cattle movement control, strengthening epidemio-surveillance networks and vaccination should be intensified. Tracing the source of infected cattle detected at abattoirs and enforcement of strict rules for livestock movement can aid in the control of the disease in such areas.
Acknowledgments
Ministry of Livestock Development (MoLD) through PACE/CBPP Unit (Grant No: EU/EDF/PACE/8 ACP TPS 032) financed this work. The author wish to extend thanks to Mr. J. Lupatu (Tanga), A. Mlemba (Muheza), S. Manyika (Hai), F. Mcharo (Karatu) and M. Constantine (Sakina) for virtually doing the meat inspection and recording the data. Thanks are also extended to staff at VIC for their valuable comments and encouragement.
Comments
Background
CBPP is an economically significant transboundary animal disease in Africa. Its diagnosis relies on the use of more than one technique in order to improve accuracy. The current study sought to establish the prevalence of CBPP in northern regions of Tanzania using pre-slaughter and post-slaughter observations of animals presentated at four local abattoirs.
Research frontiers
It is the first attempt to establish prevalence of CBPP in Northern Tanzania via abattoir surveillance. Of 4 460 cattle slaughtered low prevalence of CBPP was demonstrated with 0.91% of carcasses inspected showing lesions suggestive of CBPP.
Related reports
Article describes attempts to estimate prevalence of CBPP in a zone of Tanzania following methods that have previously been used in Zambia to determine common causes of carcass and offal condemnations. In order to improve precision of results, pre-tested and close-ended questionnaires were used to record findings on abattoir profile, source of cattle, and observations of pre-slaughter pneumonic signs. Post mortem features were based on visual examination, palpation and incisions.
Innovations and breakthroughs
Abattoir surveillance of CBPP has demonstrated low level of CBPP prevalence in Northern Tanzania, an area that has often been presumed to be endemic with this disease but for which credible evidence has not been presented.
Applications
The methods and results of the present study could be used to estimate prevalence of CBPP in other regions of Tanzania and other parts of Africa. However, this should be combined with other diagnostic techniques such as pathogen (mycoplasma) isolation and identification and serological tests.
Peer review
The article presents evidence of low levels of CBPP prevalence in an area of Tanzania that has often been wrongly assumed to be endemic with the disease. Use of close ended questionnaires is thought to have contributed to precision of results.
Footnotes
Foundation Project: Supported by Ministry of Livestock Development (MoLD) through PACE/CBPP Unit (Grant No: EU/EDF/PACE/8 ACP TPS 032).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Tambi NE, Maina WO, Ndi C. An estimation of the economic impact of contagious bovine pleuropneumonia in Africa: Review. Rev Sci Tech. 2006;25(3):999–1011. [PubMed] [Google Scholar]
- 2.Amanfu W. Contagious bovine pleuropneumonia (lung sickness) in Africa. Onderstepoort J Vet Res. 2009;76(1):13–17. [PubMed] [Google Scholar]
- 3.Morobela Raborokgwe C. Contagious bovine pleuropneumonia in Botswana: Experience with control, eradication, prevention and surveillance. Vet Ital. 2011;47(4):397–405. [PubMed] [Google Scholar]
- 4.Kassaye D, Molla W. Seroprevalence of contagious bovine pleuropneumonia at export quarantine centers in and around Adama, Ethiopia. Trop Anim Health Prod. 2012;45(1):275–279. doi: 10.1007/s11250-012-0212-3. [DOI] [PubMed] [Google Scholar]
- 5.Schnee C, Heller M, Jores J, Tomaso H, Neubauer H. Assessment of a novel multiplex real-time PCR assay for the detection of the CBPP agent Mycoplasma mycoides subsp. mycoides SC through experimental infection in cattle. BMC Vet Res. 2011;7:47. doi: 10.1186/1746-6148-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tardy F, Gaurivaud P, Manso-Silván L, Thiaucourt F, Pellet MP, Mercier P, et al. et al. Extended surveillance for CBPP in a free country: Challenges and solutions regarding the potential caprine reservoir. Prev Vet Med. 2011;101(1–2):89–95. doi: 10.1016/j.prevetmed.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Vilei EM, Frey J. Detection of Mycoplasma mycoides subsp. mycoides SC in bronchoalveolar lavage fluids of cows based on a TaqMan real-time PCR discriminating wild type strains from an lppQ(-) mutant vaccine strain used for DIVA-strategies. J Microbiol Methods. 2010;81(3):211–218. doi: 10.1016/j.mimet.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer A, Shapiro B, Muriuki C, Heller M, Schnee C, Bongcam-Rudloff E, et al. et al. The origin of the ‘Mycoplasma mycoides cluster’ coincides with domestication of ruminants. PLoS One. 2012;7(4):e36150. doi: 10.1371/journal.pone.0036150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scacchia S, Tjipura-Zaire G, Lelli R, Sacchini F, Pini A. Contagious bovine pleuropneumonia: humoral and pathological events in cattle infected by intubation and by exposure to infected animals. Vet Ital. 2011;47(4):407–413. [PubMed] [Google Scholar]
- 10.Tanzania Ministry of Livestock and Fisheries Development . Livestock sector development strategy. United Republic of Tanzania: Government Printers, Dar-es Salaam; 2010. pp. 35–36. [Google Scholar]
- 11.Phiri AM. Common conditions leading to cattle carcass and offal condemnations at 3 abattoirs in the western province of Zambia and their zoonotic implications to consumers. J S Afr Vet Assoc. 2006;77(1):28–32. doi: 10.4102/jsava.v77i1.336. [DOI] [PubMed] [Google Scholar]
- 12.Cadmus SI, Adesokan HK. Causes and implications of bovine organs/offal condemnations in some abattoirs in Western Nigeria. Trop Anim Health Prod. 2009;41(7):1455–1463. doi: 10.1007/s11250-009-9334-7. [DOI] [PubMed] [Google Scholar]
- 13.Norusis MJ. A guide to data analysis. Upper Saddle River, NewJersey: Prentice-Hall; 1998. pp. 4–7. [Google Scholar]
- 14.Anonymous . Annual report. Dar-es-Salaam, Tanzania: Department of Veterinary Services, Ministry of Livestock Development and Fisheries; 2009. pp. 18–30. [Google Scholar]
- 15.Anonymous . Annual report. Arusha, Tanzania: Veterinary Investigation Centre; 2011. p. 15. [Google Scholar]
- 16.Alawa CBI, Etukudo-Joseph I, Alawa JN. A 6-year survey of pathological conditions of slaughtered animals at Zango abattoir in Zaria, Kaduna State, Nigeria. Trop Anim Health Prod. 2012;43(1):127–131. doi: 10.1007/s11250-010-9664-5. [DOI] [PubMed] [Google Scholar]
- 17.Office International Des Epizooties (OIE) Manual of diagnostic tests and vaccines for terrestrial animals (Mammals, birds and bees) 6th ed. Paris: Office International Des Epizooties; 2008. pp. 712–724. [PubMed] [Google Scholar]
- 18.Ezanno P, Lesnoff M. A metapopulation model for the spread and persistence of contagious bovine pleuropneumonia (CBPP) in African sedentary mixed crop-livestock systems. J Theoretical Biol. 2009;256:493–503. doi: 10.1016/j.jtbi.2008.10.001. [DOI] [PubMed] [Google Scholar]