Abstract
Objective
To assess the prevalence of anemia in children with urinary schistosomiasis, malaria and concurrent infections by the two diseases.
Methods
Urine and blood samples were collected from 387 children (216 males and 171 females) to examine urinary schistosomiasis and malaria and to determine hemoglobin concentration at Hassoba and Hassoba Buri village in Amibara woreda, Afar region, Ethiopia.
Results
The overall prevalence of urinary schistosomiasis and Plasmodium falciparum malaria was 24.54% and 6.20% respectively. Only 2.84% of children carried concurrent infections of both parasites. There was high percentage of anemic patients (81.81%) in the coinfected cases than in either malaria (33.3%) or schistosomiasis (38.94%) cases. There was significantly low mean hemoglobin concentration in concurrently infected children than non-infected and single infected (P<0.05). The mean hemoglobin concentration between Plasmodium falciparum and S. haematobium infected children showed no significant difference (P>0.05). The level of hemoglobin was negatively correlated with the number of S. haematobium eggs/10 mL urine (r=-0.6) and malaria parasitemia (r=-0.53).
Conclusions
The study showed that anemia is higher in concurrently infected children than non-infected and single infected. Furthermore, level of hemoglobin was negatively correlated with the number of S. haematobium eggs and malaria parsitemia. Therefore, examination of hemoglobin status in patients co-infected with malaria and schistosomiasis is important to reduce the risk of anemia and to improve health of the community.
Keywords: Urinary schistosomiasis, Plasmodium falciparum, Anemia, Malaria, Ethiopia
1. Introduction
Malaria and helminth infection are the most prevalent parasitic diseases in developing countries and their epidemiologic co-existence is frequently observed, particularly in Ethiopia. In many regions of Sub-Saharan Africa, intestinal helminth infections overlap geographically with Plasmodium falciparum (P. falciparum) malaria where much of the morbidity associated with both disease results from anemia[1]–[5]. Anemia is one of the most wide spread and common health conditions affecting individuals living in tropics[6].
The effects of infection with a single helminth or malaria species on the risk of anemia are well documented[7],[8]. Schistomes cause anemia by chronic blood loss, as eggs penetrate the wall of the bowl (in intestinal schistosomiasis) and the urinary tract (in urinary schistosomiasis)[6],[9]. Malaria cause anemia by destruction and removal of parasitized red blood cells and shortening of the life span of non-parasitized red cells, and decreasing the rate of erythrocyte production in bone marrow[7]. Although the distinct mechanisms by which malaria and schistosomes used to reduce hemoglobin (Hb) levels are well documented, their combined presence and interaction to enhance the risk of anemia received little attention.
Therefore, the scarcity of reports on the interaction of malaria and schistosomes infection in the risk of anemia prompted investigation of the situation in S. haematobium and malaria endemic locality of Ethiopia. The research findings could provide data on the role of malaria and schistosomiasis co-infection in Hb concentration and to propose strategies to protect those groups that might be at risk of developing anemia due to co-infection by urinary schistosomiasis and malaria.
2. Materials and methods
2.1. The study area
The study area was identified based on the distribution of urinary schistosome and malaria species. Accordingly Amibara Woreda (district) in the middle awash valley of Afar region is selected for the study area. Amibara is one of the 29 woredas in the Afar region of Ethiopia and is part of the administrative zone 3. The rainfall distribution varies from year to year, but the average mean annual rainfall is about 575 mm. In general, arid and semi-arid climatic environment is the typical characteristics of the district. The district is known for long to be infested by Bulinus abysinicus, snail intermediate host for S. haematobium[10],[11]. The study area is endemic both in malaria and urinary schistosomiasis.
2.2. The study population
A total of 387 study subjects (school children) aged between 5 and 15 years (corresponding to the age group at risk for urinary schistosomiasis) were included in the study. Children aged between 5 and 15 years, had no history of S. haematobium or P. falciparum drug administration in the two weeks prior to screening, absence of any other serious chronic infection, had ability to give blood and urine samples were included in the study.
2.3. Urine analysis
Urine samples were analysed using the centrifugation method as described by Okanla[12]. Briefly, the samples were left to stand on the bench for about 30 min. Following this, the urine in each sample was drawn off leaving the last 10 mL in the bottle. The content of each bottle was shaken to suspend the sediment and was transferred into a 20 mL centrifuge tube. The tubes were centrifuged at 1 000 r/min for 5 min. The supernatant was discarded and the residue was put on a clean glass slide and examined under 10× objective lens of the microscope. Intensity of infection was estimated according to the number of eggs per 10 mL urine.
2.4. Determination of haemoglobin concentration
Finger-prick samples were collected and used to fill the microcuvette by touching the cuvette tip on the middle until completely filled with the drop of blood. The loaded microcuvette was then inserted into the holder of a portable, battery-operated HemoCue Hb 201 analyzer (HemoCue AB, Angel Holm, Sweden) and analysed.
2.5. Examination of blood for malaria parasite
Finger-pricked blood was taken to prepare thick and thin blood films. Thick blood film was directly stained using Giemsa method for 30 min and malaria parasite was observed under microscope. Thin blood film was fixed with methanol for a few seconds and then stained for 30 min. Stained film was rinsed with running tap water for 10 seconds and then allowed to dry. The slide was examined under light microscope (100× oil-immersion objective). Parasitaemia was calculated per 200 white blood cells (WBC) assuming 8 000 WBC/µL of blood[13].
2.6. Treatment
Children who were positive for urinary schistosomiasis and malaria were treated based on the recommended drug regimen. A single dose of praziquantel (40 mg/kg body weight) was given to treat urinary schistosomiasis and recommended anti-malarial drug coartem (artemether-lumefantrine) was given to P. falciparum positive children. Children with severe anemia were referred to the nearby health post and clinic for treatment and further follow up.
2.7. Statistical analysis
Data was analysed using SPSS software (version 13.0, Chicago, IL, USA) and SISA software. Chi-square was used to determine association. Difference between means was analysed by ANOVA, and values were considered to be statistically significant when P values are less than 0.05.
3. Results
3.1. Description of study participant
A total of 387 study participants were included in this study, 216 (55.8%) were males and 171 (44.20%) were females. The overall prevalences of urinary schistosomiasis and P. falciparum malaria were 24.54% and 6.20%, respectively. Only 2.84% of children carried concurrent infections of both parasites. The mean age of all the study participants was (10.0±3.1) years with minimum 5 and maximum 15 years. There was no statistically significant differences (P>0.05) between the mean age of male and female study participants. The mean Hb level of male was (12.14±2.20) g/dL and female was (12.07±1.70) g/dL. There was no significant difference in mean Hb concentrations between the male and female study participants (P>0.05).
3.2. Anemia prevalence in the study participants
The overall prevalence of anemia in the study participant (infected and non-infected) was 31.8%, based on WHO cutoff value for anemia and anemia definition[14]. From the total study participants who are coinfected with P. falciparum and S. haematobium, 81.82% are anemic and the remaining 18.18% have normal Hb level (>11g/dL). Out of the 24 malaria positive individuals, 7 (29.20%) have less than 11 g/dL Hb concentration (Table 1). Furthermore, anemia was prevalent in 37 (38.95%) study participants who are infected by S. haematobium (Table 1).
Table 1. Overall prevalence of anemia in children with malaria, urinary schistosomiasis or concurrent infection in children from Hassoba and Hassoba Buri villages, Afar region, Ethiopia, Nov-Dec, 2008.
| Hemoglobin concentration (g/dL) | Age (year) | No. of children | Hemoglobin concentration (g/dL) |
||
| Hb<7 | Hb<11 | Hb>11 | |||
| Malaria | 9.67±3.24 | 24 | 1 (4.20%) | 7 (29.20%) | 16 (66.60%) |
| Schistosomiasis | 10.77±2.73 | 95 | 0 (0.00%) | 37 (38.95%) | 58 (61.05%) |
| Co-infected | 9.18±3.57 | 11 | 4 (36.36%) | 5 (45.46%) | 2 (18.18%) |
Values of age are shown in mean±SD.
3.3. Relationships between S. haematobium egg counts and Hb level in children
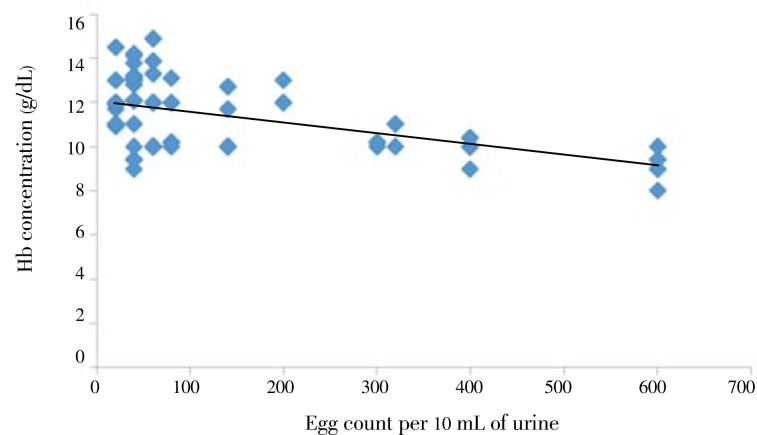
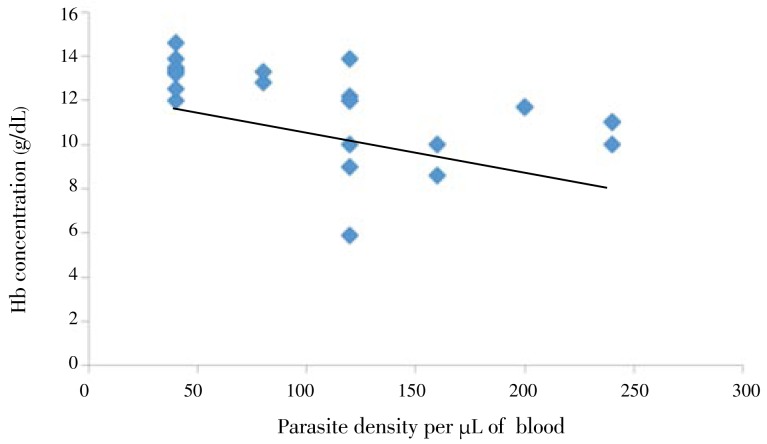
The level of hemoglobin in children was negatively correlated (r=-0.6 and P<0.01) with the number of S. haematobium eggs/10 mL urine. As the number of eggs per 10 mL urine increase, the Hb concentration significantly decreases (Figure 1). Furthermore, parasitemia was negatively correlated (r=-0.53) with hemoglobin level of the children (Figure 2).
Figure 1. Relationship between egg count and Hb concentration from Hassoba and Hassoba Buri villages, Afar region, Ethiopia, Nov.-Dec., 2008.
Figure 2. Relationship between parasite density and Hb concentration from Hassoba and Hassoba Buri villages, Afar region, Ethiopia, Nov.-Dec., 2008.
3.4. Hemoglobin concentration comparison in concurrent infections, single infection and non-infected children
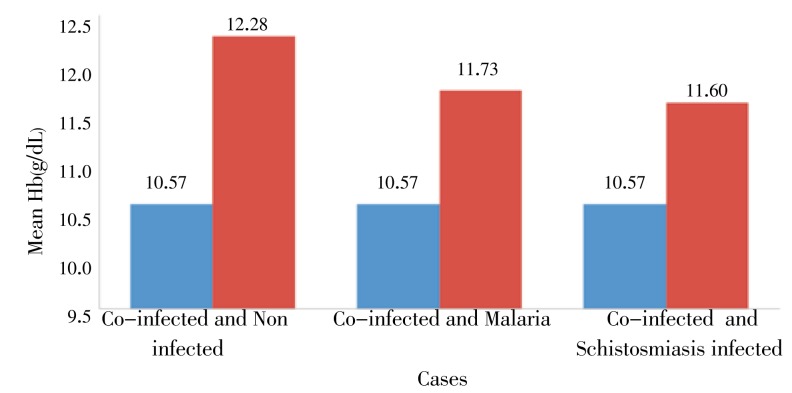
The mean hemoglobin level of all concurrently infected children was (10.57±1.10) g/dL. One way ANOVA showed that, the mean hemoglobin level in concurrently infected children was significantly lower than non infected group (P=0.03). As compared to the non infected and single infected cases, there was significantly low mean hemoglobin concentration in concurrently infected children (P<0.05). However, although the mean hemoglobin concentration of children with concurrent infection (10.57±1.10) g/dL was lower than those infected with malaria (11.73±2.02) g/dL or schistosomiasis (11.60±1.61) g/dL, the difference was not statistically significant (P>0.05) in both cases (Figure 3).
Figure 3. Mean Hb concentration in non infected, co-infected, malaria infected and schistosomiasis infected children from Hassoba and Hassoba Buri villages, Afar region, Ethiopia, Nov.-Dec., 2008.
4. Discussion
In the present study, the overall prevalence of S. haematobium in children was found to be 24.54%, which indicates decreased rate of infection compared with previous studies in the area. For instance, Jemaneh et al[10], reported high prevalence (70%) of urinary schistosomiasis using filtration method in Amibara irrigation scheme (Hassoba). Aemero also had reported 46% prevalence of S. haematobium in Hassoba village using urine dipstick method[11]. Furthermore, Ayele et al. reported 47.6% prevalence of urinary schistosomiasis by urine filtration method among Hassoba village school children[15]. Several factors that might have contributed to the decreased prevalence of urinary schistosomiasis in the area include improvement in sanitation, safe water supply, awareness about the disease and repeated chemotherapy. This may resulted in decreased frequency of using schistosome infected river water for bathing, drinking and for other purposes. In addition, repeated year to year chemotherapy of schoolchildren after epidemiological survey had an effect on prevalence reduction.
This study revealed that, there is a significantly negative correlation between egg count/intensity and hemoglobin concentration. This agrees with the work of Friedman et al.[6] who reported risk of anemia are correlated with infection intensity and Okafor and Elenwo[18] showed that the magnitude of S. haematobium egg counts are significantly related to hemoglobin concentration[16]. In addition, Stephenson et al reported that the mean hemoglobin level in children is lower with high S. haematobium infection and malaria positives[17]. A negative linear relationship was also found between low hemoglobin concentration and prevalence of mixed-infection[18].
In this study, the hemoglobin concentration in concurrently infected children showed significant difference compared to the non-infected group, S. haematobium infected, and P. falciparum infected. This is in agreement with the study of Okafor and Elenwo[18], who reported children who are concurrently infected with malaria and urinary schistosomiasis were found to have lower Hb concentration relative to those with single infection and uninfected one. Although the basis for malaria mediated anaemia has been elucidated by several investigators[7],[19]–[22], the mechanism by which schistosomiasis cause anemia is not well understood, but a logical explanation lies on the ability of schistosomes eggs to penetrate the wall of the bowl (in intestinal schistosomiasis) and the urinary tract (in urinary schistosomiasis)[6]. Therefore, their combined presence and interaction to enhance the risk of anemia may be responsible for the low hemoglobin concentration in concurrently infected children than those infected with either malaria or schistosomiasis.
In the present study, it was also been shown that although there was a negative correlation between parasitemia and hemoglobin concentration, the correlation was not statistically significant. In contrast, Kitua et al.[20] showed a significant increase in the prevalence of anaemia with increase in malaria parasite density. The finding of the present study substantiated by the fact that, there is national malaria control program in Ethiopia. This includes effective malaria control such as early diagnosis and antimalarial drug treatment to reduce malaria morbidity and acute infections. Therefore, early diagnosed and treatment of patient before they develop high parasitemia may be the reason for contradictory report on the effect of parasitemia on hemoglobin concentration.
The present study does not adjust for the effect of other determinants such as hematological features that might play key roles in affecting the level of anemia, thus the effect of these factors could not be discussed in this paper.
Acknowledgments
We are very grateful to the study participants for their cooperation. The study was financially supported by Addis Ababa University and Swedish International Development Cooperation Agency (SIDA/ SAREC) with Grant No. SS-TR/004/2007.
Comments
Background
Schistosomiasis and malaria are fatal parasitic infections that are prevalent in Ethiopia. Both diseases cause anemia but existing reports were scanty on the association between anemia and children who were co-infected with both parasites. This original research performed in Amibara Woreda, Ethiopia revealed that there was statistically significant reduction in hemoglobin level in children with the co-infection as compared to those who were healthy or infected with either one of the parasites.
Research frontiers
Schistosomiasis and malaria are among the fatal neglected diseases that afflict the poor in Ethiopia. This study highlighted the significant reduction of hemoglobin levels among children who were co-infected with the parasites. It is pertinent for health care workers to be aware of the importance of hemoglobin deficiency among the infected children in their effort to reduce the mortality and morbidity rates related to the infections.
Related reports
Friedman et al. (2005), McDevitt et al. (2004) and Sousa-Figueiredo et al. (2012) reported on the effect of single infection (either by Schistosoma spp. or Plasmodium spp.) on the reduction of hemoglobin level of infected individuals.
Innovations and breakthroughs
There are scarce reports on the correlation between schistosomiasis and malaria co-infection and hemoglobin level. This manuscript is among the few reported
Applications
Awareness among the health workers on the significant reduction of hemoglobin level among co-infected children will provide extra evidence for them to initiate the reduction of mortality and morbidity rates associated with the fatal infections.
Peer review
This is a well-designed study on the correlation between urinary schistosomiasis/malaria co-infection on anemia in an endemic district in Ethiopia.
Footnotes
Fundation Project: Supported by Addis Ababa University and Swedish International Development Cooperation Agency (SIDA/SAREC), Grant No. SS-TR/004/2007.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2001;64:36–44. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 2.Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, Kabatereine NB, et al. et al. Plasmodium-helminth coinfection and its sources of heterogeneity across East Africa. J Infect Dis. 2012;205(5):841–852. doi: 10.1093/infdis/jir844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mboera LE, Senkoro KP, Rumisha SF, Mayala BK, Shayo EH, Mlozi MR. Plasmodium falciparum and helminth coinfections among schoolchildren in relation to agro-ecosystems in Mvomero District, Tanzania. Acta Trop. 2011;120(1–2):95–102. doi: 10.1016/j.actatropica.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Diallo TO, Remoue F, Gaayeb L, Schacht AM, Charrier N, De Clerck D, et al. et al. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS One. 2010;15(9):e12764. doi: 10.1371/journal.pone.0012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adegnika AA, Kremsner PG. Epidemiology of malaria and helminth interaction: a review from 2001 to 2011. Curr Opin HIV AIDS. 2012;7(3):221–224. doi: 10.1097/COH.0b013e3283524d90. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: The relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.McDevitt MA, Xie J, Gordeuk V, Bucala R. The anemia of malaria infection: role of inflammatory cytokines. Curr hematol Rep. 2004;3:97–106. [PubMed] [Google Scholar]
- 8.Kloos H, Lemma A, DE Sole G. Schistosoma mansoni distribution in Ethiopia: a study in medical geography. Ann Trop Med Parasitol. 1978;72:461–470. doi: 10.1080/00034983.1978.11719346. [DOI] [PubMed] [Google Scholar]
- 9.Sousa-Figueiredo JC, Gamboa D, Pedro JM, Fançony C, Langa AJ, Soares Magalhães RJ, et al. et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One. 2012;7(4):e33189. doi: 10.1371/journal.pone.0033189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemaneh L, Tedla S, Birrie H. The use of reagent strip for detection of urinary schistosomiasis infection in the middle awash valley, Ethiopia. East Afr Med J. 1994;71:679–689. [PubMed] [Google Scholar]
- 11.Aemero A. Studies on the association between salmonelosis and schistosomiasis in the middle Awash and Ziway, Ethiopia [dissertation] 2004 AAU. [Google Scholar]
- 12.Okanla EO. Schistosomiasis: influence of parental occupation and rural or urban dwelling on prevalence. Nig J Pure Appl Sci. 1991;6:154–159. [Google Scholar]
- 13.Cheesebrough M. District laboratory practice in tropical countries. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 14.WHO . Iron deficiency anemia assessment,prevention,and control-Aguide for programme managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 15.Ayele B, Erko B, Legese M, Hailu A, Medhin G. Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia. Parasite. 2008;15:69–75. doi: 10.1051/parasite/2008151069. [DOI] [PubMed] [Google Scholar]
- 16.Beasley NM. The impact of population level deworming on the hemoglobin levels of school children in Tanga, Tanzania. Trop Med Int Health. 1999;4:744–750. doi: 10.1046/j.1365-3156.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson SL, Letham CM, Kurz MK, Kinoti NS, Oduori LM, Crompton TW. Relationships of Schistosoma haematobium, hook worm and malaria infection and metrifonate treatment to hemoglobin level in Kenya school children. Am J Trop Med Hyg. 1985;34:519–528. doi: 10.4269/ajtmh.1985.34.519. [DOI] [PubMed] [Google Scholar]
- 18.Okafor EJ, Elenwo AC. Hemoglobin in status of children with mixed infection of malaria and urinary schistosomiasis in Odau Community, Rivers State, Nigeria. J Agr Soc R. 2007;7:56–62. [Google Scholar]
- 19.Rowe A, Obeiro J, Newbold CI, Marsh K. Plasmodium falciparum resetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitua AY, Smith TA, Alonso PL, Urassa H, Masanja H, Kimario J, et al. et al. The role of low level Plasmodium falciparum parasitaemia in anaemia among infants living in an area of intense and perennial transmission. Trop Med Health. 1997;4:325–333. doi: 10.1111/j.1365-3156.1997.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 21.Carlson J, Helmby H, Hill AVS, Brewster D, Greenwood BM, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 22.Tekeste Z, Petros B. The ABO blood group and Plasmodium falciparum malaria in Awash, Metehara and Ziway areas, Ethiopia. Malaria J. 2010;9:280. doi: 10.1186/1475-2875-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]