Abstract
Use of domestic microwave oven has been suggested as a method of disinfecting a number of dental materials used in dental practice. This study was done to analyse the effect of microwave irradiation on vinyl polysiloxane putty impression material (3M ESPE, Express™ STD) contaminated with test organisms (Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans. 180 square shaped specimens of addition silicon putty material were prepared and divided into 3 groups for three test organisms. The 3 groups were subdivided into 4 subgroups (n = 15) for different exposure parameters (control group 5, 6 and 7 min exposure at 650 W. The specimens were contaminated using standard inoculums of test organism and then were irradiated using domestic microwaves. Broth cultures of the control and test group specimens were plated on selective media culture plates. Colonies formed were counted. Data analyses included Kruskal–Walli’s ANOVA and Mann–Whitney’s tests. Nil values shows complete elimination of C. albicans and P. aeruginosa after 5, 6 and 7 min exposure. Staphylococcus aureus showed colonies with the mean value of 7.6 × 103 ± 2.3 × 103, 4.6 × 103 ± 2.6 × 103 after 5 and 6 min respectively and nil values after 7 min exposure. 5 min exposure caused complete elimination of C. albicans and P. aeruginosa strains, while 7 min exposure eliminated S. aureus completely.
Keywords: Dental material, Sterilization, Micro organism, Culture, Colonies
Introduction
The risk of infections transmitted by saliva and blood is considered a potential occupational hazard in dentistry [1]. One area which has received little attention and which is a potential source of transmitting infection is the handling of dental impressions and casts poured from them. Dental technicians are also at high risk when such contaminated cast are sent to laboratories [2]. So, the dental practitioner has a legal and ethical responsibility to prevent cross infection between patients and staff members, also protecting self from contacting a disease from a patient [3]. Current practice regarding handling and disinfection of dental impressions before sending them to laboratory varies from washing the impressions in water to immersing it in 2 % glutaraldehyde for up to 12 h [1].Recently, the use of domestic microwave oven, to disinfect complete dentures [4], nitrous oxide nasal hoods [5], contact lenses [6], dental casts [7, 8], hard chair side reline resins [9], for resilient liner [10, 11] are proved to causes total sterilization. Use of microwaves to sterilize hydrophilic contact lenses has been successfully demonstrated by using standard domestic microwave of at 700 W for 6 min [6]. These hydrophilic contact lenses are made up of graft copolymers of vinyl pyrrolidone onto silicone rubber [12]. Now silicone elastomeric impression materials are routinely used for making impressions in dental clinics. So, evaluation of effectiveness of domestic microwave for disinfection of impressions made of silicone elastomeric material is needed to facilitate its use to sterilize impressions.
Materials and Methods
180 square shaped (7 × 7 × 7 mm) specimens of addition silicon putty material were prepared, using plaster mold and were sterilized by autoclave (Fig. 1). Three sets of 60 specimens in each set were separated for three test micro organisms, Staphylococcus aureus(S), Pseudomonas aeruginosa (P) and Candida albicans(Ca). Specimens in each set were further divided into four subgroups (n = 15) as SC, SI, SII, SIII, PC, PI, PII, PIII; and CaC, CaI, CaII, CaIII where Group ‘C’contoll (not exposed), group I (5 min exposure) group II (6 min), group III (7 min). Organisms in this study were American type culture collection strains of S. aureus (25923), P. aeruginosa (27853), C. albicans (60193). Standard cultures of bacteria (S. aureus, P. aeruginosa) and yeast (C. albicans) were individually inoculated in brain–heart infusion broth for bacteria and Sabouraud broth for fungus (Fig. 2). The turbidity of the broths were adjusted using a 0.5 Mcfarland standard turbidity tube corresponding to 105 organism/ml in 10 ml of broth. The tubes were incubated at 37 °C for 24 h (Fig. 3). Using sterile forceps, one specimen was transferred in each test tube. 1 ml of appropriate broth and standard inoculums of organism was added in respective test tubes using micropippete (1 ml) and the tubes were incubated. The specimens incubated in the tubes were collected in glass beakers filled with distilled water for microwave exposure (Fig. 4). The beakers were individually exposed to microwaves in a domestic microwave (Kenstar) according to the different exposure parameters. The specimens of each group were transferred into appropriately labelled sterile test tubes. 1 ml of BHI broth for bacterial inoculum and Sabouraud broth if yeast inoculum was taken, in each tube. The tubes were incubated for 30 min at 37 °C. Plates of selective media, BHI Agar for bacteria and Sabouraud agar for yeast were prepared in Petri dishes and were labeled as control, group I and group II and group III for each organisms and cultured in three plates by loop inoculation. Each of the three plates were divided radially into five sections and labeled from 1 to 15, for 15 specimens per group. Broth from the tubes were transferred on the plate in respective sections using 25 μl micropippete. The Petri dishes were incubated at 37 °C for 48 h for fungi and 24 h for bacteria. Colonies on each labeled section of the plates were counted by using a magnifying lens (Fig. 5). Each count was multiplied with the dilution factor (100) and expressed as CFU/ml.
Fig. 1.
Square shaped specimens of addition silicone putty elastomer
Fig. 2.
Inoculation of specimens using standard inoculum of test organisms
Fig. 3.

Incubation of test tubes carrying the t specimens inoculated with test microorganisms
Fig. 4.
After incubation the specimens were collected in a microwave resistant glass beaker filled with distilled water
Fig. 5.
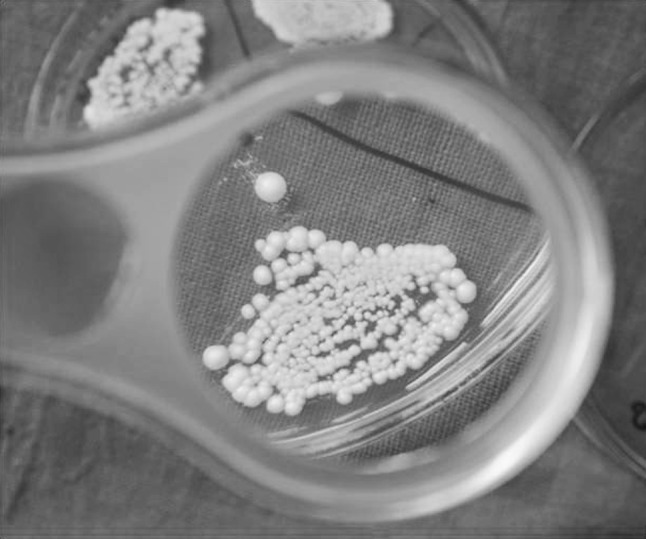
Colonies on each labeled section of the plates were counted by using a magnifying lens
Results
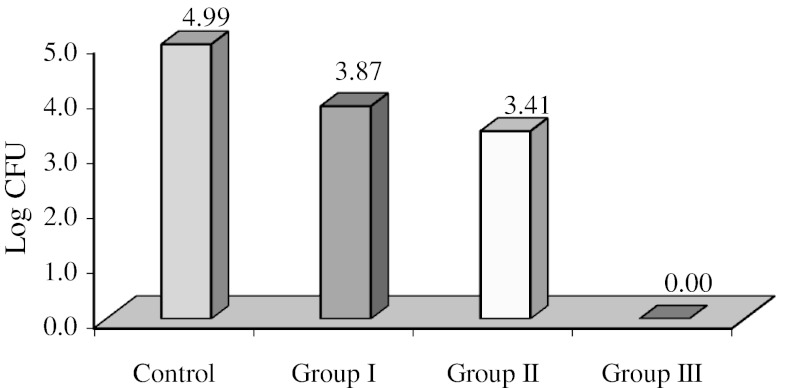
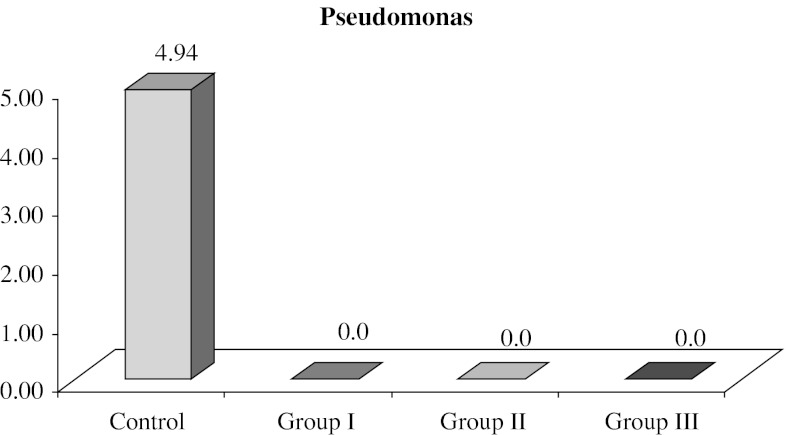
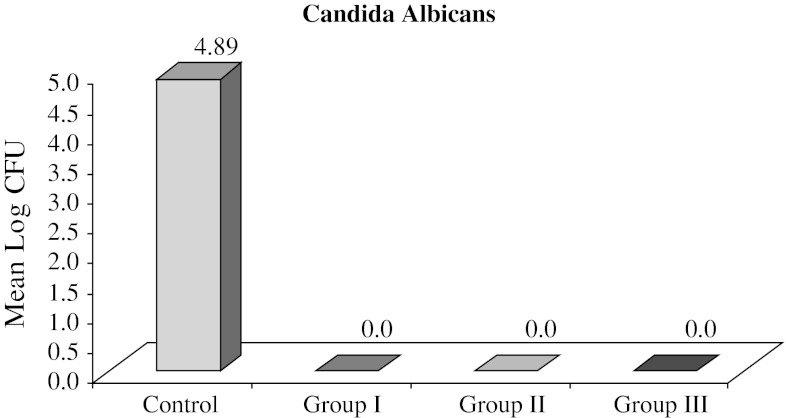
Data obtained represented viable count of the organisms remaining on the specimens after microwave exposure which were measured by counting the number of colonies formed on selective media for the particular organism (Table 1). The mean CFU/ml counts of control and test specimens of all the groups are represented in Table 2. The mean values for SC Group was 1.38 × 105 CFU/ml with a range of 4 × 104 to 4 × 105 CFU/ml, for CaC group 1.07 × 105 CFU/ml with a range of 5 × 104 to 4 × 105 and for PC group 1.15 × 105 with a range of 5 × 104 to 4 × 105 CFU/ml. Nil values of Ca I, Ca II, Ca III, PI, P II, P III groups shows complete elimination of C. albicans and P. aeruginosa after 5 min and subsequent exposure for 6 and 7 min. On the other hand. SI, SII groups showed growth of colonies with the mean value of 7.6 × 103 ± 2.3 × 103 and a range of 5.2 × 103 to 15 × 103 for SI group and mean value of 4.6 × 103 ± 2.6 × 103 in a range of 0–12 × 103 for SII group, while SIII group had nil values, indicating total elimination of S. aureus after 7 min of microwave oven exposure (Table 3) For statistical analysis, these values were converted into log CFU. The log CFU values are tabulated as shown in Table 3 for S. aureus. Statistically significant difference was found between SC and SI group with mean difference of 1.12. The mean difference between SC and SII was 1.58 and the mean difference of 0.46 was found between SI and SII groups (Table 3). Figure 6 gives the mean log CFU count of S. aureus in control group, group I, group II, group III to be 4.99, 3.87 and 3.41 respectively. Figure 7 shows the mean log CFU counts of P. aeruginosa with control group value to be 4.94 and nil values for group I, II and III. Figure 8 gives the mean log CFU values of C. albicans for control group to be 4.89 and zero for the remaining groups.
Table 1.
Viable count of organisms in control and test groups expressed in CFU/ml
| Staphylococcus Aureus “S” | Pseudomonas “P” | Candida Albicans “Ca” | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Group I | Group II | Group III | Control | Group I | Group II | Group III | Control | Group I | Group II | Group III |
| 100,000 | 7,000 | 5,000 | 0 | 100,000 | 0 | 0 | 0 | 50,000 | 0 | 0 | 0 |
| 100,000 | 8,000 | 5,800 | 0 | 50,000 | 0 | 0 | 0 | 50,000 | 0 | 0 | 0 |
| 50,000 | 7,800 | 3,000 | 0 | 90,000 | 0 | 0 | 0 | 80,000 | 0 | 0 | 0 |
| 60,000 | 6,800 | 6,000 | 0 | 80,000 | 0 | 0 | 0 | 60,000 | 0 | 0 | 0 |
| 400,000 | 6,900 | 4,000 | 0 | 60,000 | 0 | 0 | 0 | 50,000 | 0 | 0 | 0 |
| 400,000 | 5,400 | 4,800 | 0 | 60,000 | 0 | 0 | 0 | 60,000 | 0 | 0 | 0 |
| 60,000 | 6,000 | 5,000 | 0 | 50,000 | 0 | 0 | 0 | 50,000 | 0 | 0 | 0 |
| 50,000 | 7,400 | 4,700 | 0 | 50,000 | 0 | 0 | 0 | 80,000 | 0 | 0 | 0 |
| 100,000 | 7,800 | 2,800 | 0 | 60,000 | 0 | 0 | 0 | 50,000 | 0 | 0 | 0 |
| 400,000 | 8,200 | 1,900 | 0 | 60,000 | 0 | 0 | 0 | 70,000 | 0 | 0 | 0 |
| 90,000 | 15,000 | 12,000 | 0 | 100,000 | 0 | 0 | 0 | 60,000 | 0 | 0 | 0 |
| 60,000 | 8,000 | 5,000 | 0 | 60,000 | 0 | 0 | 0 | 400,000 | 0 | 0 | 0 |
| 40,000 | 5,200 | 5,000 | 0 | 400,000 | 0 | 0 | 0 | 100,000 | 0 | 0 | 0 |
| 60,000 | 9,000 | 3,800 | 0 | 400,000 | 0 | 0 | 0 | 50,000 | 0 | 0 | 0 |
| 100,000 | 5,400 | 0 | 0 | 100,000 | 0 | 0 | 0 | 400,000 | 0 | 0 | 0 |
Table 2.
Mean colony forming units per milliliter (CFU/ml) for each organism values are expressed as mean ± SD (range)
| Groups (time of microwave exposure) | No. of samples | Staphylococcus aureus “S” | Candida albicans “Ca” | Pseudomonas aeruginosa “P” |
|---|---|---|---|---|
| Control “C” (not exposed) | 15 | 1.38 × 105 ± 1.3 × 105 (4 × 104 − 4 × 105) | 1.07 × 105 ± 1.2 × 105 (5 × 104 − 4 × 105) | 1.15 × 105 ± 1.2 × 105 (5 × 104 − 4 × 105) |
| Group I (5 min) | 15 | 7.6 × 103 ± 2.3 × 103 (5.2 × 103 − 15.0 × 103) | No growth | No growth |
| Group II (6 min) | 15 | 4.6 × 103 ± 2.6 × 103 (0 –12.0 × 103) | No growth | No growth |
| Group III (7 min) | 15 | No growth | No growth | No growth |
Table 3.
Comparison of log CFU between three groups for Staphylococcus aureus
| Groups (time of exposure in microwave) | Log CFU | Difference between groupsa | ||
|---|---|---|---|---|
| Mean ± SD | Groups compared | Mean difference | p value | |
| Control (no exposed) “SC” | 4.99 ± 0.34 | Control–I “SC”–“SI” | 1.12 | <0.01, S |
| Group I (5 min exposure) “SI” | 3.87 ± 0.11 | Control–II “SC”–“SII” | 1.58 | <0.01, S |
| Group II (6 min exposure “SII” | 3.41 ± 0.96 | I–II “SI”–“SII” |
0.46 | <0.01, S |
| Group III (7 min exposure) “SIII” | 0.0 | |||
Kruskal–Wallis ANOVA, x2 = 35.9
aMann–Whitney test
Fig. 6.
Graph representing mean log CFU count of S. aureus in control group, group I, group II, group III
Fig. 7.
Graph representing mean log CFU count of P. aeruginosa in control group, group I, group II, group III
Fig. 8.
Graph representing mean log CFU count of C. albicans in control group, group I, group II, group III
Discussion
One of the principal potential route of transmission of infection from a patient to a dental technician is via contaminated impressions and casts obtained from them. To avoid contamination of dental office staff and dental technicians, it has been recommended that impressions be disinfected immediately after their removal from mouth [13].The distributors of one microwave oven have claimed that microwave cooking ‘kills all germs and disinfects all materials’ (microwave distributors, Australia, 1974). In theory this claim is unreasonable since microorganisms contain polar molecules which when excited at high frequency, might cause disaggregation of microbial structures [14]. The first article published on use of domestic microwaves for disinfection was by M.D. Rohrer who used it to sterilize contact lenses and recommended 4 min for total sterilization [6].
Two modes of action of microwaves for sterilization is described [4, 6, 9]. This include thermal effect which is conversion of microwave energy into heat by prolonged kinetic motion of polar molecules and non thermal effect by direct interaction of electromagnetic field with the biologic molecule, creating effects that cannot be caused by thermal action alone. The suggested possible mechanism include:
Denaturation of proteins.
Cell wall membrane phenomenon involving loss in selective permeability and molecular resonance resulting in cleavage.
Certain intracellular changes e.g., orientation of cell organelles.
In the present study a domestic microwave (Kenstar) with output power of 2,450 MHz was used. Microwave was programmed at 650 W for exposing the specimens at three time period of 5, 6, 7 min.
Three major problems must be overcomed before microwave oven is operated [15].
Microwaves are generated in a Magnetron and propogated in a straight line along the wave guide in what is called the “dominant mode”. Because of this the field strength is non uniform and non homogeneous throughout the oven creating ‘cold spots’.
During operation of oven, almost all waves are not absorbed and these gets reflected back to the magnetron. If significant amount is reflected back to the source, the magnetron cannot service long.
The microwave oven must also be protected from arcs, which develops when microwave reflecting material like metal is placed in the oven. The arch that is developed can destroy the magnetron as well as the material that is placed in the microwave oven [3].
To avoid ‘cold spots’ most of the domestic microwaves are designed with rotating platform and the same type was used in the present study. The specimens exposed to microwave were immersed in distilled water in a sterile glass beaker, which acted as microwave absorber and also avoiding any arch effect. Placing the specimens in water during microwave exposure provided uniform heating of the specimens. This is considered adequate to kill the organisms within the pores of the material [15].
The selection of microorganisms used in the present study was based on peer-reviewed scientific data regarding concepts of indicator and surrogate pathogen organisms. Gram-positive S. aureus, gram-negative P. aeruginosa and fungus C. albicans, have been recommended as indicator and surrogate pathogens to validate the effectiveness of disinfection procedure [5, 16]. So, the same group of organisms were used in this study.
A viable cell count is a measure of number of living cells capable of multiplying and producing a visible colony of cells in a sample. It is commonly estimated by spreading a known volume of cell suspension onto an agar plate and counting the number of colonies that arise after a period of incubation. The method is based on a premise that each visible colony has been derived from the repeated division of a single cell. In reality, it is accepted that this is not always the case, and so viable counts are expressed in colony forming units (CFU) rather than cells per unit volume [16]. For statistical reliability, the suspension of each group of organisms were diluted adjusting its turbidity matching to 0.5 Mcfarland’s turbidity tube, which corresponds to 105 organisms/ml of suspension. Dilution of suspension is also required to ease the colony counting procedure. Standard procedures used for isolation and growth of organisms by microbiological laboratories were followed in the present study. Precaution was taken to avoid contamination of the specimens and experimental setup. Microbial inoculation was carried out in laminar air flow chamber, which is considered to be a completely sterile chamber.
The present study showed that specimens of the control group produced substantial microbial growth on the plates after 24 h incubation for bacteria and 48 h incubation for fungi at 37 °C. Consistent 1 log range CFU/ml values of the positive controls indicated that the cultivation protocol used were adequate [7]. The mean colony counts of S. aureus (1.38 × 105 CFU/ml) and P. aeruginosa (1.15 × 105 CFU/ml) were greater than those of C. albicans (1.07 × 105 CFU/ml). It has been reported that cell size and surface roughness of dental materials significantly affect the retention of microorganism. Larger yeast cells (5–10 μm) requires larger surface area for retention compared to smaller bacteria (0.5–3 μm)[4, 9].
The results showed that complete sterilization of specimens, inoculated with individual suspensions of three groups of micro-organisms was achieved at 5 min and subsequent exposure for P. aeruginosa and C. albicans and at 7 min for S. aureus, as negative colonies were obtained on respective plate cultures. The variation in the time required to kill the three groups of microorganisms, in the present study was interesting. Roher MD and Bulard RA in their study found similar behavior of S. aureus with positive growth at 5 min of microwave exposure and total elimination after 8 min [3, 6].
Results on sterilization of C. albicans and P. aeruginosa at 5 and 6 min exposure time in microwave, support the experimental protocol advocated by Neppelenbrock et al. [15] (6 min at 650 W) for hard chairside reline resins. Silva et al. [4] (6 min at 650 W) for complete dentures, Baysan et al. (650 W for 5 min) for soft lining materials [10].
Results showed that microwave exposure do have a lethal effect on micro organism that may contaminate elastomeric impression material. So, use of domestic microwave for disinfection and sterilization of elastomeric impressions can be considered as an effective, convenient and quick option. The effect of microwave exposure time tested in this study on the physical and mechanical properties, dimensional accuracy and surface reproduction of the addition silicon elastomeric material needs further investigation.
Conclusions
Within the limitation of the study the following conclusion can be drawn,
Microwave exposure is proved to have a lethal effect on S. aureus, P. aeruginosa and C. albicans grown on addition silicone putty material.
Exposure parameter of 650 W for 5 min caused complete elimination of C. albicans and P. aeruginosa strains, while 650 W for 7 min exposure eliminated S. aureus completely.
References
- 1.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–249. doi: 10.1016/0022-3913(91)90169-W. [DOI] [PubMed] [Google Scholar]
- 2.Owen CP, Goolam R. Disinfection of impression materials to prevent viral cross contamination: a review and a protocol. Int J Prosthodont. 1993;6:480–494. [PubMed] [Google Scholar]
- 3.Rohrer MD, Bulard RA. Microwave sterilization. J Am Dent Assoc. 1985;110:194–198. doi: 10.14219/jada.archive.1985.0250. [DOI] [PubMed] [Google Scholar]
- 4.Silva MM, Vergani CE, Giampaolo ET, Neppelenbroek KH, Spolidorio DMP, Machado AL. Effectiveness of microwave irradiation on the disinfection of complete dentures. Int J Prosthodont. 2006;19:288–293. [PubMed] [Google Scholar]
- 5.Young SK, Graves DC, Rohrer MD, Bulard RA. Microwave sterilization of nitrous oxide nasal hoods contaminated with virus. Oral Surg Oral Med Oral Pathol. 1985;60:581–585. doi: 10.1016/0030-4220(85)90355-X. [DOI] [PubMed] [Google Scholar]
- 6.Rohrer MD, Terry MA, Bulard RA, Graves DC, Taylor EM. Microwave sterilization of hydrophilic contact lenses. Am J Opthalomol. 1986;101:49–57. doi: 10.1016/0002-9394(86)90464-2. [DOI] [PubMed] [Google Scholar]
- 7.Berg E, Nielsen O, Skaug N. High level microwave disinfection of dental gypsum casts. Int J Prosthodont. 2005;18:520–525. [PubMed] [Google Scholar]
- 8.Berg E, Skaug N. Efficacy of high level microwave disinfection of dental gypsum casts: the effects of number and weight of casts. Int J Prosthodont. 2007;20:463–464. [PubMed] [Google Scholar]
- 9.Ewerton G, Pavarina AC, Mima O, Neppelenbroek KH, Vergani CE, Spolidorio DN, Machodo AL. Effect of different exposure times on microwave irradiation on the disinfection of a hard chairside reline resin. J Prosthodont. 2008;17:312–317. doi: 10.1111/j.1532-849X.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- 10.Baysan A, Whiley R, Wright PS. Use of microwave energy to disinfect a long term soft lining material contaminated with Candida albicans or Staphylococcus aureus. J Prosthet Dent. 1998;79:454–458. doi: 10.1016/S0022-3913(98)70161-1. [DOI] [PubMed] [Google Scholar]
- 11.Machado AL, Breeding LC, Puckett AD. Effect of microwave disinfection on the hardness and adhesion of two resilent liners. J Prosthet Dent. 2005;94:183–189. doi: 10.1016/j.prosdent.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Montague R. Soft contact lenses, clinical and applied technologies. New York: Wiley; 1978. pp. 24–25. [Google Scholar]
- 13.Goldstein GR. Evaluation of an ultraviolet disinfection unit. J Prosth Dent. 1987;58:650–654. doi: 10.1016/0022-3913(87)90403-3. [DOI] [PubMed] [Google Scholar]
- 14.Hume WR. Sterilizing dental instruments: evaluation of lubricating oils and microwave radiation. Oper Dent. 1978;3:93–102. [PubMed] [Google Scholar]
- 15.Neppelenbrock KH, Pavarisa AC, Spolidoria DM, Vergani CE, Mima EG, Machode AL. Effectiveness of microwave sterilization on three hard chairside reline resins. Int J Prosthodont. 2003;16:616–620. [PubMed] [Google Scholar]
- 16.Hogg S. Essential microbiology. 1. New York: Wiley; 2005. pp. 90–91. [Google Scholar]