Abstract
OBJECTIVES:
Chronic paracoccidioidomycosis can diffusely affect the lungs. Even after antifungal therapy, patients may present with residual respiratory abnormalities due to fungus-induced lung fibrosis.
METHODS:
A cross-sectional analysis of 50 consecutive inactive, chronic paracoccidioidomycosis patients was performed using high resolution computed tomography, pulmonary function tests, ergospirometry, the six-minute walk test and health-related quality of life questionnaires.
RESULTS:
Radiological abnormalities were present in 98% of cases, the most frequent of which were architectural distortion (90%), reticulate and septal thickening (88%), centrilobular and paraseptal emphysema (84%) and parenchymal bands (74%). Patients typically presented with a mild obstructive disorder and a mild reduction in diffusion capacity with preserved exercise capacity, including VO2max and six-minute walking distance. Patient evaluation with the Saint-George Respiratory Questionnaire showed low impairment in the health-related quality of life, and the Medical Research Council questionnaire indicated a low dyspnea index. There were, however, patients with significant oxygen desaturation upon exercise that was associated with respiratory distress compared with the non-desaturated patients. The initial counterimmunoelectrophoresis of these patients was higher and lung emphysema was more prominent; however, there were no differences in the interstitial fibrotic tomographic abnormalities, tobacco exposure, functional responses, exercise capacity or quality of life.
CONCLUSIONS:
Inactive, chronic paracoccidioidomycosis patients show persistent and disseminated radiological abnormalities by high resolution computed tomography, short impairments in pulmonary function and low impacts on aerobic capacity and quality of life. However, there was a subset of individuals whose functional impairment was more severe. These patients present with higher initial serology and more severe emphysema, stressing the importance of adequate treatment associated with tobacco exposure cessation.
Keywords: Paracoccidioidomycosis, Lung, Pulmonary Function, Quality Of Life, Computed Tomography
INTRODUCTION
In 1908, Adolph Lutz first described the human Paracoccidioides brasiliensis infection (1). This thermally dimorphic fungus is acquired by inhalation of the infective forms of the organism and causes notable, systemic mycosis (2-4). The disease, termed paracoccidioidomycosis (PCM), is the most common systemic mycosis affecting non-immunocompromised hosts in South America and Brazil, which account for more than 80% of all reported cases (5-8). Additionally, cases outside these areas continue to be reported and are generally associated with long periods of latency, representing endogenous reactivation of the infectious focus that was previously acquired in endemic regions (4). PCM is subdivided into the following two groups that are differentiated by their time course and the age of the host: juvenile and chronic forms. The juvenile form has an acute or subacute clinical course with a predominantly lymphatic distribution. The chronic or reactivation form has a more insidious course in which the lung is the most frequently involved organ, with lesser involvement of the reticuloendothelial and lymphatic systems (3,4,9). The cure rate is high when adequate treatment is readily administered, and mycological eradication can be achieved with sulfonamides, azole compounds or amphotericin (9-11). However, in pulmonary parenchyma, even after treatment, P. brasiliensis induces chronic damage that leads to the development of lung fibrosis, which is most likely due to persistent antigenic stimulus that elicits a continuous inflammatory response (12). This process can result in a severe restriction of respiratory function and a decline in work capacity, thereby affecting patient quality of life (4,13,14). In fact, in more than 50% of cases in which the patient receives an adequate course of therapy, pulmonary fibrosis is radiographically documented at the end of the treatment period (15). However, the true incidence and severity of radiographical and functional disability of the patient following treatment for this endemic mycosis remain unknown.
The objective of this study was to characterize both the residual pulmonary involvement in PCM by high resolution computed tomography (HRCT) and the sequelae related to lung function, exercise capacity and quality of life in the inactive chronic form of PCM.
PATIENTS AND METHODS
From July 2008 to July 2010, all PCM patients seen in the outpatient clinic of the Pulmonary and Infectious Diseases Divisions of the Hospital das Clinicas at the University of Sao Paulo were invited to participate in the study. The patients were included if the PCM diagnosis was confirmed by the identification of P. brasiliensis yeast cells in biopsies and/or other clinical specimens and/or serological diagnosis. The patients were enrolled if they met the criteria for inactive disease as determined by a treatment length of at least 6 months, negative mycological examinations, resolution of skin and mucosal lesions, low anti-P. brasiliensis antibody titers by counterimmunoelectrophoresis (CIE) (titers <1:4 or a fall in at least four dilutions) and a lack of radiological activity on chest radiographs (9,16,17). The exclusion criteria were pulmonary co-infections, such as tuberculosis, histoplasmosis or other chronic infections, or lung neoplasia. From a cohort of 81 PCM patients, 72 met the inclusion criteria, and 22 were excluded. Of the 22 excluded patients, 10 had the juvenile form, seven had tuberculosis co-infections, three were tracheotomized, one had microstomia and one refused. Of the 50 patients included in the study, 47 were men ranging in age from 35 to 78 years, with a mean age of 56.9 years. All were previously diagnosed based on mycological examination, including direct visualization and/or culture of smears of lesions and/or biopsies.
HRCT scans were obtained using 1 or 2 mm collimation at 10 mm intervals (Multislice Philips Brilliance CT40, Cleveland, United States). Two investigators (CF and CRRC) blinded to the clinical history of the patients retrospectively read the CT scans, and when necessary, final decisions were reached by consensus. The findings were analyzed with regard to distribution in the lung parenchyma, (central, peripheral or both) and upper (above the level of the tracheal carina), middle (between the level of the carina and inferior pulmonary veins) and lower zones (below the level of the inferior pulmonary veins) or a combination of zones (2,18).
For the pulmonary function tests (PFT), all measurements were obtained using the recommended standards (19-22). Spirometry was performed using a calibrated pneumotachograph (Medical Graphics Corporation, MGC, St. Paul, MN, USA, 2005), whereas lung volumes and CO diffusing capacity (DLCO) were obtained with a body plethysmograph (Elite Dx, Elite SeriesTM, MGC). The following variables were considered: FVC (forced vital capacity), FEV1 (expiratory forced volume in the first second), IC (inspiratory capacity), TLC (total lung capacity), RV (residual volume), MVV (maximal voluntary ventilation) and DLCO. The predicted values were derived based on the Brazilian population (19-21). For the cardiopulmonary exercise test (CPET), a ramp symptom-limited CPET was performed on a cycle (Corival, Lode B.V. Medical Technology, Groningen, The Netherlands) consisting of a 2 min period of rest and 2 min period of warm-up, followed by an incremental work-rate period, which was increased from 10 to 20 W/min. Oxygen saturation (SpO2) by pulse oximetry (NONIN-ONYX, model 9500, Plymouth, MN, USA) and electrocardiography (Welch Allyn CardioPerfect, Inc, NY) were monitored continuously (23). The following variables were recorded (CardiO2 System, MGC): work rate, VO2, minute-ventilation (VE), carbon dioxide output (VCO2), tidal volume (VT), respiratory rate (RR), respiratory exchange rate (RER) and heart rate (HR). The predicted values for CPET were calculated based on the Brazilian population (23). The six-minute walk test (6MWT) was performed according to the ATS guidelines (24), and the parameters analyzed were the distance walked (meters), SpO2 minimum maintained for at least 10 seconds and the difference between the basal saturation (at rest) and minimal saturation achieved during the test (SpO2 basal – SpO2 min). The distance was analyzed based on the predictive equations for the adult Brazilian population (25).
A standard questionnaire was administered by a pulmonologist to obtain information on age, sex, body mass index, smoking habits and possible respiratory co-infections. Two specific respiratory questionnaires, the Saint George Respiratory Questionnaire (SGRQ) (26,27), and Medical Research Council (MRC) Dyspnea Questionnaire (28,29) were used. The SGRQ is a standardized airway disease-specific questionnaire divided into three subscales: symptoms, activity and impacts. The scores were calculated using algorithms as recommended (P.W. Jones, St George's Hospital Medical School, London, UK, personal communication). For each subscale and the overall questionnaire, scores ranged from zero, indicating no impairment, to 100, indicating maximum impairment. Values above 10 reflected an altered quality of life for that specific area (26,27). The MRC breathlessness scale is a measurement of disability in patients with chronic obstructive pulmonary disease. It comprises five statements that describe nearly the entire range of respiratory disability from none (Grade 1) to almost complete incapacity (Grade 5) (28,29).
Patients were then divided into two groups based on the severity of exercise gas exchange disability by desaturation in the 6MWT. Group A demonstrated desaturation, and Group B had no desaturation. Desaturation was defined as a fall >4% of the resting SpO2 value (30). We chose the 6MWT because it is a simple, efficient and low-cost tool used to evaluate the performance of individuals during submaximal exercise and can be even more sensitive than maximal incremental cycle testing for the detection of oxygen desaturation (24,30).
Statistical analysis was performed with GraphPad Prism Version 5.0 for Windows (GraphPad Software, San Diego CA, USA). Parametric variables are expressed as the mean and standard deviation (SD), and nonparametric variables are expressed as the median and interquartile range (IQ). Correlations were performed using either Pearson or Spearman tests, depending on the distribution of the variables. Comparisons between groups were performed with a Student's T test or Mann-Whitney test when appropriate. A chi-squared analysis was used to examine categorical variables, and a Fisher exact test was used for small samples. Statistical significance was assumed for p-values<0.05.
Ethics Statement
Our research was approved by our two Ethics Committees, Comissão de Pesquisa do Departamento de Cardiopneumologia and Comissão de Ética para a Análise de Projetos de Pesquisa – CAPPesq da Diretoria Clínica do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, on June 09, 2007, under approval number 870/06. All patients in our study signed an informed consent form, and all clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki.
RESULTS
The clinical and epidemiological data for the patients and their serum titers on CIE at the time of diagnosis and the time of our evaluation are shown in Table 1. All but one of the patients were smokers. The antifungal drugs used by the patients were itraconazole (26%), sulfamethoxazole-trimethoprim (16%), sulfadiazine (12%) and ketoconazole (8%). The remaining 38% used two or more of these drugs for treatment. Patients were not HIV co-infected and had no autoimmune disease or referred use of immunosuppressant drugs.
Table 1.
Clinical and epidemiological data of Group A (desaturation >4% in 6MWT) and Group B (desaturation <4% in 6MWT).
| n = 50 | GROUP A n = 18 | GROUP B n = 32 | p (A × B) | |
| Age in years | 56.9±9.7 | 59.1±2.2 | 55.6±1.7 | 0.21 |
| Gender male (%) | 92% | 89% | 94% | 0.62 |
| Body mass index | 24.0±4.8 | 24.7±1.4 | 23.6±0.6 | 0.50 |
| Tobacco exposure (%) | 98% | 100% | 97% | 1.00 |
| Tobacco exposure in pack - years | 35(27-50) | 30(20-42) | 37(30-53) | 0.25 |
| Length of treatment in months | 42±34 | 38.2±37.2 | 44±31.7 | 0.56 |
| Time between onset of treatment and evaluation (years) | 5.9±3.8 | 5.9±3.8 | 5.9±3.9 | 0.99 |
| Initial CIE | 1:64 (1:16-1:256) | 1:128 (1:32-1:256) | 1:32 (1:8-1:128) | 0.01 |
| CIE at evaluation | 1:2(0-1:4) | 1:2(0-1:5) | 1:1.5(1-1:4) | 0.62 |
The data are reported as either the median values (IQ) or as the mean±SD. IQ = interquartile, SD = standard deviation, CIE = counterimmunoelectrophoresis.
Twenty-seven patients remained on antifungal medications because it is a common practice in the infectious diseases outpatient unit to use low-dose sulfamethoxazole-trimetropin as a maintenance treatment to prevent relapses of the chronic forms of infections. These patients represented 40% in Group A (desaturation in 6 MWT) and 60% in Group B (p = 0.34).
The serum CIE titer at diagnosis was significantly higher in Group A compared with the patients without exercise desaturation in Group B. The other clinical and epidemiological parameters were not different between these two groups.
Radiological Findings
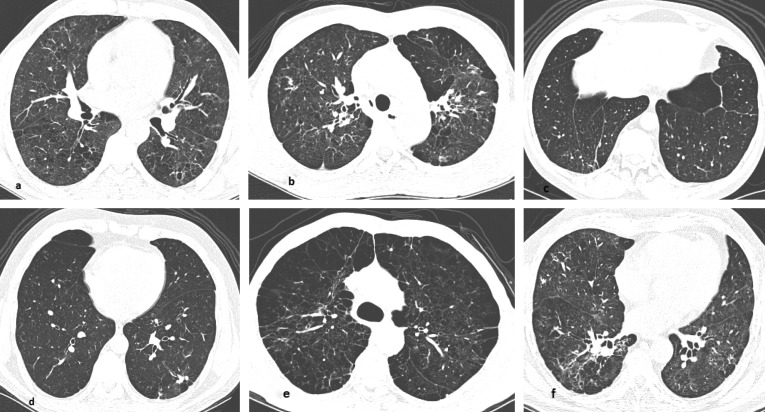
Abnormal pulmonary findings on HRCT were observed in all but one patient, and multiple abnormalities were often present simultaneously (median of eight abnormalities/patient). CT findings included architectural distortion (90%, n = 45), interlobular septal thickening and reticulate (88%, n = 44), centrilobular or paraseptal emphysema (82%, n = 41), bronchial thickening (82%, n = 41), parenchymal bands (74%, n = 37), areas of cicatricial emphysema (66%, n = 33), nodules <3 cm (62%, n = 31) and ground glass opacities (46%, n = 23). Pleural thickening was observed in 10 (20%) patients, and mediastinal lymphadenopathy in only three cases. Thirty-eight patients presented with mosaic perfusion patterns with expiratory maneuvers. The major tomographic findings were predominantly diffuse in their distribution (84%), were most prominent in the superior and medium zones and had a combination of central and peripheral locations in 80% of cases. When predominance was apparent, these findings were most common in the upper and middle lung zones. The most frequent abnormalities are shown in Figure 1 (A-F). Notably, a comparison between Group A and B revealed no difference in the number of interstitial fibrotic CT abnormalities, which were architectural distortion, interlobular septal thickening and reticulate and parenchymal bands. However, when emphysema quantification scores were compared, they were higher (more prominent emphysema) in Group A than Group B (Figure 2), p = 0.0009).
Figure 1.
The patterns of pulmonary alterations found on HRCT. (A) Upper lobes showing septal thickening and peripheral reticulate. (B) Upper lobes showing architectural distortion. (C) Lower lobes showing parenchymal bands. (D) Lower lobes showing non-calcified nodules in the left lower lobe. (E) Upper lobes showing centrilobular and paraseptal emphysema. (F) Lower lobes showing peribronchovascular thickening.
Figure 2.
Figure showing that emphysema quantification by CT was significantly higher in the most severely impaired PCM patients compared to the least severely impaired PCM patients.
Pulmonary function tests (PFT)
Lung function test results are shown in Table 2. Seven patients (14%) presented with normal PFT. An obstructive defect, defined as FEV1/FVC<0.70, was observed in 70% (35) of patients (31). Of the latter, 85% (n = 30) presented with only a mild obstruction (FEV1>60%), and only one patient presented with a severe obstruction (FEV1<40%). The residual volume/total lung capacity was slightly increased, suggesting air trapping without pulmonary hyperinflation. Only one patient presented with a pure restrictive pattern, showing a TLC of 61% of the predicted value. Diffusing capacity was impaired in 43 (86%) patients (mean DLCO: 60.6% of the predicted value). Nine patients presented with a severe (DLCO<40%), 15 with a moderate (40%<DLCO<60%) and 19 with a mild reduction (DLCO>60%) in diffusion capacity. There was no bronchodilator response (data not shown). A comparison between groups showed that Group A had significantly reduced gas exchange and ventilatory strength than Group B (Table 2).
Table 2.
Lung function measurements of Group A (desaturation >4% in 6 MWT) and Group B (desaturation <4% in 6MWT).
| n = 50 | Group A | Group B | p-value | |
| FVC (L) | 4.00±0.82 | 3.75±0.2 | 4.13±0.1 | 0.14 |
| % predicted | 94.8±12.1 | 91.0±3.2 | 97.0±1.8 | 0.12 |
| FEV1 (L) | 2.60±0.68 | 2.38±0.1 | 2.72±0.1 | 0.09 |
| % predicted | 77.6±17.0 | 72.1±3.9 | 80.2±2.9 | 0.10 |
| FEV1/FVC | 0.65±0.11 | 63.3±2.8 | 66.16±1.7 | 0.39 |
| % predicted | 81.7±13.4 | 79.3±3.5 | 83.0±2.1 | 0.17 |
| FEF 25-75% (L/s) | 1.6±0.93 | 1.42±0.2 | 1.73±0.1 | 0.26 |
| % predicted | 52.1±28.4 | 45.9±6.2 | 55.5±5.1 | 0.24 |
| TLC (L) | 6.80±0.20 | 6.14±0.3 | 6.47±0.1 | 0.40 |
| % predicted | 100.4±12.9 | 98.8±3.9 | 101.3±1.9 | 0.58 |
| RV (L) | 2.40±0.73 | 2.41±0.2 | 2.39±0.1 | 0.95 |
| % predicted | 121.2±34.5 | 122.4±9.9 | 120.6±5.6 | 0.87 |
| RV/TLC | 0.38±0.07 | 0.38±0.02 | 0.37±0.01 | 0.58 |
| % predicted | 117.2±23.4 | 120.5±7 | 115.2±3 | 0.51 |
| DLCO (mL/min/mmHg) | 20.4±6.6 | 16.7±1.3 | 22.5±1.10 | 0.002 |
| % predicted | 60.6±19.9 | 49.2±3.8 | 66.9±3.4 | 0.001 |
| MVV (L) | 98.7±29 | 86.5±20.5 | 105.8±4.7 | 0.14 |
| % predicted | 72.5±20.2 | 65.2±3.9 | 76.5±3.9 | 0.04 |
The data are reported as the means±SD. SD = standard deviation, FVC = forced vital capacity, FEV1 = forced expiratory volume in 1 s, FEF25-75% = forced expiratory flow from 25 to 75% of the FVC, TLC = total lung capacity, RV = residual volume, DLCO = carbon monoxide diffusing capacity, MVV = maximal voluntary ventilation.
Exercise evaluation
The maximal exercise performance was preserved in the PCM patients, with a VO2max value of 80.8%±18.5 of the predicted value. At exercise cessation, there was a large ventilatory reserve that was accomplished without oxygen desaturation, showing a preserved performance of the respiratory system under effort. Cardiac behavior was characterized by a normal oxygen pulse (VO2/HR = 11.0±2.8 mL.min-1), and the significant cardiac reserve suggested that there were no cardiac limitations. Of the 50 patients, only one had ECG abnormalities that were compatible with coronary disease, which was not confirmed by an invasive posterior coronary study. When Groups A and B were examined, no significant difference was found between the patients for either VO2max or oxygen pulse; however, Group A had a lower ventilatory reserve with higher hyperventilation (augmented VE/VCO2). In summary, Group A presented with more serious respiratory impairments during exercise compared with Group B (Table 3).
Table 3.
Maximal cardiopulmonary and 6MWT tests for Group A (desaturation >4% in 6MWT) and Group B (desaturation <4% in 6MWT).
| n = 50 | Group A | Group B | ||
| Cardiopulmonary test | p-value | |||
| VO2 max (% predicted) | 80.8±18.5 | 79.5±3.9 | 81.6±3.6 | 0.70 |
| RER | 1.18 (1.12-1.26) | 1.18 (1.12-1.24) | 1.18 (1.11-1.27) | 0.89 |
| Ventilatory reserve (%) | 40.4±17 | 21.2±5.2 | 39.4±4.3 | 0.01 |
| VE/VCO2 | 36.0±8 | 38.3±1.5 | 34.2±1.2 | 0.04 |
| VO2/HR (mL.min-1) | 11.0±2.8 | 10.0±0.7 | 11.3±0.4 | 0.13 |
| HR (% predicted) | 82.4±13 | 86.9±2.5 | 80.3±2.4 | 0.07 |
| SpO2 | ||||
| • Initial | 97 (96-98) | 96.2±0.36 | 97.0±0.3 | 0.11 |
| • Final | 94 (92-96) | 91.7±1 | 94.7±0.4 | 0.04 |
| 6MWT | p-value | |||
| Walked distance (meters) | 492 (456-520) | 431 (354-480) | 477 (306-492) | 0.71 |
| SpO2 | ||||
| • Initial | 96±1.6 | 95.9±0.3 | 95.9±0.28 l | 0.96 |
| • Final | 93±4 | 89.1±1.0 | 94.8±0.3 | <0.0001 |
| HR (% predicted) | 62.8±15 | 69.8±2.1 | 63.0±1.3 | 0.01 |
The data are reported as either the median values (IQ) or the means±SD. IQ = interquartile, ST = standard deviation, VO2 max = maximal oxygen consumption, VE/VCO2 = ventilatory equivalent ratio for carbon dioxide, VO2/FC = oxygen pulse, HR = heart rate, SpO2 = peripheral oxygen saturation.
In the 6MWT, patients achieved a normal distance with a median of 492 meters, which was 131% of the predicted value, without desaturation. The distance achieved was similar between groups. However, for the submaximal test (very large cardiac reserve at exercise cessation), 18 (36%) of the patients presented with a decrease >4% in SpO2, which was indicative of an abnormal arterial-alveolar gradient that was previously shown for PCM in other studies (32,33) (Table 3).
Health-related quality of life (HRQOL)
The Saint George Respiratory Questionnaire scores are presented in Table 4. Patients presented with high (worse) scores in the symptoms and activity scales but low overall impairments in health-related quality of life. For the MRC Dyspnea Questionnaire, the patients had a mean value of 1, which corresponds with “not troubled by breathlessness except on strenuous exercise” (28). Even in Group A, the more severe impairments did not translate into a worse quality of life, as measured by either the SGRQ or MRC Dyspnea Questionnaire. There was a strong correlation between the two scores, with p<0.0001 and r = 0.65.
Table 4.
Saint George Respiratory Questionnaire scores for Group A (desaturation >4% in 6 MWT) and Group B (desaturation <4% in 6MWT).
| Symptoms | n = 50 | Group A | Group B | p (A × B) |
| % total (IQ) | 17.8 (3.7-34.9) | 18.2 (6-36) | 16.9 (0.5-34.4) | 0.61 |
| Activity | ||||
| % total (IQ) | 29.5 (5.8-41.8) | 26.4 (0-41.8) | 29.5 (6.0-41.8) | 0.76 |
| Impacts | ||||
| % total (IQ) | 3.2 (0-12.9) | 3.5 (0-12.9) | 3.1 (0-14.5) | 1.00 |
| Overall ± SD | ||||
| % total±ST | 16.2±13.8 | 24.7±10.4 | 14.5±13.9 | 0.9 |
The data are reported as either the median values (IQ) or the means±SD. IQ = interquartile, ST standard deviation.
Correlation studies
The initial CIE titers inversely correlated with the DLCO (p = 0.001, r = -0.44) and positively correlated with impairments the in HRQOL (p = 0.021, r = 0.33). However, there was no correlation between the CIE and any of the other parameters analyzed in the population under study.
DISCUSSION
In contrast with previous investigations of treated PCM patients, we found a consistently higher incidence of radiological involvement, with 98% of patients presenting with at least one CT abnormality. The most common abnormalities were architectural distortion and interlobular septal thickening and reticulate, which were most likely related to dissemination of the fungi through the lymphatics and subsequent fibrosis formation (13). The finding of peribronchovascular interstitial thickening diffusely distributed throughout the pulmonary zones in 62% of the patients is another abnormality related to the pattern of fungal dissemination previously described in histopathology studies (2). In contrast, centrilobular and paraseptal emphysema were observed in 82% of individuals, which reflects the high tobacco exposure in our population, a feature regularly described in cohorts of chronic PCM (8). Finally, different from the pre-treatment CT scan findings (2,18,34), consolidation and cavitations were very rare, and no reversed halo sign was observed, confirming the inactivity of the mycosis.
It has been shown by thoracic roentgenogram that fungus-induced lung fibrosis in the form of residual lesions occurs in up to 53% of treated patients (15), which has led to experimental studies involving pentoxifylline as a complementary treatment for pulmonary PCM due to its immunomodulatory and anti-fibrotic properties (35). However, this radiologic approach may not be beneficial for an even greater proportion of individuals with interstitial pulmonary abnormalities because thoracic roentgenogram is not sufficiently sensitive to evaluate these lesions. In addition, chest X-rays are not sufficiently sensitive to confirm the possible presence of lung emphysema (15). Indeed, HRCT is currently the standard approach to confirm lung parenchymal involvement (18,36,37). As expected, using HRCT, we found a greater proportion of lung residual abnormalities than previously reported, confirming that PCM can lead to even further radiological involvement of respiratory tissue than previously thought.
Despite these findings, the pulmonary functional impairments were not that limiting. On average, the patients presented with a mild obstructive disorder associated with a mild reduction in diffusion capacity and slight reduction in the maximal voluntary ventilation. Indeed, most of the patients were classified as having mild obstructive defects, and only one patient presented with severe obstruction. This contrasts with earlier studies in which more than 50% of the patients evaluated presented with a moderate to severe obstructive defect (38), the alveolar-arterial gradient was altered in all patients (39,40), and cor pulmonale was present in almost 25% of the cases (40). This discrepancy may be related to the more restricted access to health care facilities in Brazil at the time these studies were conducted, which delayed proper diagnosis and treatment. In contrast, in our patients, the DLCO was severely impaired in only 18% of patients. A plausible explanation for these functional findings of mild obstruction with more severe DLCO impairment is the presence of interstitial fibrotic disease associated with pulmonary emphysema, as described for Combined Pulmonary Fibrosis and Emphysema (CPFE). In this condition, there are subnormal spirometry exams, despite severe impairment in DLCO, that are most likely secondary to overinflation and an increase in pulmonary compliance due to the loss of elasticity in areas with emphysema that are balanced by the loss of volume and decreased compliance caused by fibrosis (41).
When our patients performed maximal and submaximal exercises, their performances were normal. Furthermore, we could not find any ventilatory or cardiac limitations. This behavior was also reflected in the quality of life measurements. On average, the patients presented with low quality of life impairments, as measured by the Saint George Respiratory Questionnaire. The mean score of 16 can be interpreted as altered because it is higher than 10 (29), but compared with the general population, it remained within the 80th percentile limit (26), indicating that the HRQOL was not impaired in a severe manner. Indeed, in the MRC questionnaire, the patients had a mean value of 1.
There was, however, a substantial percentage (36%) of patients who presented with significant gas exchange dysfunction that was confirmed by desaturation (decrease ≥4%) in the 6MWT. This proportion of impaired patients is smaller than previously thought, but this finding suggests that a proportion of PCM patients will present with some degree of respiratory distress during exercise. Notably, both groups had comparable interstitial fibrotic alterations regarding lung CTs, type and length of treatments, age and tobacco exposure. Patients with exercise desaturation (Group A) presented with more severe emphysema than Group B, even though they had similar tobacco exposure. One likely explanation for this finding is the higher pre-treatment serology titers in Group B, denoting a putative higher fungal burden and subsequently stronger inflammatory response. In fact, the initial serology was inversely correlated with the DLCO and positively correlated with impairment in the health-related quality of life questionnaire. This aspect warrants further evaluation.
It is also conceivable that in some patients, the diagnosis was delayed; therefore, at the time treatment began, the fibrosis was already at an advanced stage. Similarly, differences in the host-parasite relationship due to distinct host immune responses and regulation among the patients could also explain the different outcomes (42,43). Finally, genetic differences in the response to tobacco exposure leading to more severe emphysema in face of comparable tobacco exposure could explain why a subgroup of patients developed higher levels of emphysema (44). Also, the influence of genetic background on emphysema severity could have been accentuated by the concomitant pulmonary insult caused by the fungi. This is of particular interest because emphysema was the major tomographic finding associated with more intense impairments in aerobic capacity and gas exchange.
Our study has several limitations. We could not assess the patients at the onset of antifungal therapy, and information regarding the clinical course of the disease was retrospectively assessed. Notably, these data are particularly difficult to interpret because the CF of the mycosis has a very insidious and often subclinical course, and at the time of diagnosis, the patients usually exhibit marked clinical-radiological dissociation (4). Tobacco exposure may indeed be a confounding variable. However, obtaining a group of patients without tobacco exposure is not feasible when studying the chronic form of PCM because a smoking habit is almost universal in individuals with this form of mycosis (2,8,45). Additionally, patients used different classes of drugs for treatment, which can be a confounding factor for the final endpoints, but currently, there are no studies demonstrating the improved efficacy of one antifungal drug compared with others (9,10,46,47).
In conclusion, we showed that patients with the chronic form of PCM and high exposure to tobacco displayed several residual lesions on the lung CT scans in association with emphysema. However, these residual lesions did not predict severe pulmonary functional impairments or a decreased HRQOL. A subset of patients, however, persisted with gas exchange impairments during exercise and higher respiratory distress and should be identified by the simple and inexpensive 6MWT. The main factors involved with the respiratory impairments were emphysema, as shown by the HRCT scan, and higher initial serology titer, as shown by the CIE and higher initial serology titer. This stresses the value of adequate and early specific treatments associated with tobacco cessation. The factors involved in this more severe evolution of PCM, however, remain unclear and require further investigation.
Footnotes
No potential conflict of interest was reported.
REFERENCE
- 1.Lutz A. Uma mycose pseudococcidica localisada na boca e observada no Brazil. Contribuicao ao conhecimento das hyphoblastomycoses americanas. Bras Med. 1908;(22):121–4. [Google Scholar]
- 2.Funari M, Kavakama J, Shikanai-Yasuda MA, Castro LG, Bernard G, Rocha MS, et al. Chronic pulmonary paracoccidioidomycosis (South American blastomycosis): high-resolution CT findings in 41 patients. AJR Am J Roentgenol. 1999;173(1):59–64. doi: 10.2214/ajr.173.1.10397100. [DOI] [PubMed] [Google Scholar]
- 3.Bethlem EP, Capone D, Maranhao B, Carvalho CR, Wanke B. Paracoccidioidomycosis. Curr Opin Pulm Med. 1999;5(5):319–25. doi: 10.1097/00063198-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Queiroz-Telles F, Escuissato DL. Pulmonary paracoccidioidomycosis. Semin Respir Crit Care Med. 2011;32(6):764–74. doi: 10.1055/s-0031-1295724. [DOI] [PubMed] [Google Scholar]
- 5.Blotta MH, Mamoni RL, Oliveira SJ, Nouer SA, Papaiordanou PM, Goveia A, et al. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am J Trop Med Hyg. 1999;61(3):390–4. doi: 10.4269/ajtmh.1999.61.390. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho ZF, Silva D, Lazéra M, Petri V, Oliveira RM, Sabroza PC, et al. Paracoccidioidomycosis mortality in Brazil (1980-1995) Cad Saúde Pública. 2002;18(5):1441–54. doi: 10.1590/s0102-311x2002000500037. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues Gda S, Severo CB, Oliveira Fde M, Moreira Jda S, Prolla JC, Severo LC. Association between paracoccidioidomycosis and cancer. J Bras Pneumol. 2010;36(3):356–62. doi: 10.1590/s1806-37132010000300014. [DOI] [PubMed] [Google Scholar]
- 8.Bellissimo-Rodrigues F, Machado AA, Martinez R. Paracoccidioidomycosis epidemiological features of a 1,000-cases series from a hyperendemic area on the southeast of Brazil. Am J Trop Med Hyg. 2011;85(3):546–50. doi: 10.4269/ajtmh.2011.11-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shikanai-Yasuda MA, Telles Filho Fde Q, Mendes RP, Colombo AL, Moretti ML. [Guidelines in paracoccidioidomycosis] Rev Soc Bras Med Trop. 2006;39(3):297–310. doi: 10.1590/s0037-86822006000300017. [DOI] [PubMed] [Google Scholar]
- 10.Shikanai-Yasuda MA, Benard G, Higaki Y, Del Negro GM, Hoo S, Vaccari EH, et al. Randomized trial with itraconazole, ketoconazole and sulfadiazine in paracoccidioidomycosis. Med Mycol. 2002;40(4):411–7. doi: 10.1080/mmy.40.4.411.417. [DOI] [PubMed] [Google Scholar]
- 11.Tercarioli GR, Bagagli E, Reis GM, Theodoro RC, Bosco Sde M, Macoris SA, et al. Ecological study of Paracoccidioides brasiliensis in soil: growth ability, conidia production and molecular detection. BMC Microbiol. 2007;7:92. doi: 10.1186/1471-2180-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restrepo S, Tobon A, Trujillo J, Restrepo A. Development of pulmonary fibrosis in mice during infection with Paracoccidioides brasiliensis conidia. J Med Vet Mycol. 1992;30(3):173–84. [PubMed] [Google Scholar]
- 13.Tuder RM, el Ibrahim R, Godoy CE, De Brito T. Pathology of the human pulmonary paracoccidioidomycosis. Mycopathologia. 1985;92(3):179–88. doi: 10.1007/BF00437631. [DOI] [PubMed] [Google Scholar]
- 14.Lemle A, Wanke B, Miranda JL, Kropf GL, Mandel MB, Mandel S. Pulmonary function in paracoccidioidomycosis (South American blastomycosis). An analysis of the obstructive defect. Chest. 1983;83(5):827–8. doi: 10.1378/chest.83.5.827. [DOI] [PubMed] [Google Scholar]
- 15.Tobon AM, Agudelo CA, Osorio ML, Alvarez DL, Arango M, Cano LE, et al. Residual pulmonary abnormalities in adult patients with chronic paracoccidioidomycosis: prolonged follow-up after itraconazole therapy. Clin Infect Dis. 2003;37(7):898–904. doi: 10.1086/377538. [DOI] [PubMed] [Google Scholar]
- 16.Del Negro GM, Pereira CN, Andrade HF, Palacios SA, Vidal MM, Charbel CE, et al. Evaluation of tests for antibody response in the follow-up of patients with acute and chronic forms of paracoccidioidomycosis. J Med Microbiol. 2000;49(1):37–46. doi: 10.1099/0022-1317-49-1-37. [DOI] [PubMed] [Google Scholar]
- 17.de Camargo ZP. Serology of paracoccidioidomycosis. Mycopathologia. 2008;165(4-5):289–302. doi: 10.1007/s11046-007-9060-5. [DOI] [PubMed] [Google Scholar]
- 18.Souza AS, Jr, Gasparetto EL, Davaus T, Escuissato DL, Marchiori E. High-resolution CT findings of 77 patients with untreated pulmonary paracoccidioidomycosis. AJR Am J Roentgenol. 2006;187(5):1248–52. doi: 10.2214/AJR.05.1065. [DOI] [PubMed] [Google Scholar]
- 19.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397–406. doi: 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 20.Neder JA, Andreoni S, Castelo-Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res. 1999;32(6):703–17. doi: 10.1590/s0100-879x1999000600006. [DOI] [PubMed] [Google Scholar]
- 21.Neder JA, Andreoni S, Peres C, Nery LE. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfer factor) Braz J Med Biol Res. 1999;32(6):729–37. doi: 10.1590/s0100-879x1999000600008. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Neder JA, Nery LE, Castelo A, Andreoni S, Lerario MC, Sachs A, et al. Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: a randomised study. Eur Respir J. 1999;14(6):1304–13. doi: 10.1183/09031936.99.14613049. [DOI] [PubMed] [Google Scholar]
- 24.Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. ATS statement: guidelines for the six-minute walk test. [DOI] [PubMed] [Google Scholar]
- 25.Soares MR, Pereira CA. Six-minute walk test: reference values for healthy adults in Brazil. J Bras Pneumol. 2011;37(5):576–83. doi: 10.1590/s1806-37132011000500003. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer M, Villasante C, Alonso J, Sobradillo V, Gabriel R, Vilagut G, et al. Interpretation of quality of life scores from the St George's Respiratory Questionnaire. Eur Respir J. 2002;19(3):405–13. doi: 10.1183/09031936.02.00213202. [DOI] [PubMed] [Google Scholar]
- 27.Sousa TC, Jardim JRB, Jones PW. Validação do Questionário do Hospital Saint George na Doença Respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Bras Pneumol. 2000;16:119–25. [Google Scholar]
- 28.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovelis D, Segretti NO, Probst VS, Lareau SC, Brunetto AF, Pitta F. Validation of the Modified Pulmonary Functional Status and Dyspnea Questionnaire and the Medical Research Council scale for use in Brazilian patients with chronic obstructive pulmonary disease. J Bras Pneumol. 2008;34(12):1008–18. doi: 10.1590/s1806-37132008001200005. [DOI] [PubMed] [Google Scholar]
- 30.Poulain M, Durand F, Palomba B, Ceugniet F, Desplan J, Varray A, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest. 2003;123(5):1401–7. doi: 10.1378/chest.123.5.1401. [DOI] [PubMed] [Google Scholar]
- 31.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available at: http://www.goldcopd.org/. Accessed 26 April 2012. Journal [serial on the Internet]. Date [cited 2011: Available from: http://www.goldcopd.org/
- 32.Lemle A, Wanke B, Mandel MB. Pulmonary localization of paracoccidioidomycosis: lung function studies before and after treatment. Rev Inst Med Trop Sao Paulo. 1983;25(12):73–8. [PubMed] [Google Scholar]
- 33.Afonso JE, Nery LE, Romaldini H, Bogossian M, Ribeiro-Ratto O. [Pulmonary function in paracoccidioidomycosis (South American blastomycosis)] Rev Inst Med Trop Sao Paulo. 1979;21(6):269–80. [PubMed] [Google Scholar]
- 34.Marchiori E, Zanetti G, Escuissato DL, Souza AS, Jr, Meirelles Gde S, Fagundes J, et al. Reversed Halo Sign: High-Resolution CT Scan Findings in 79 Patients. Chest. 2012;141(5):1260–6. doi: 10.1378/chest.11-1050. [DOI] [PubMed] [Google Scholar]
- 35.Naranjo TW, Lopera DE, Diaz-Granados LR, Duque JJ, Restrepo AM, Cano LE. Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm Pharmacol Ther. 2010;24(1):81–91. doi: 10.1016/j.pupt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Marchiori E, Valiante PM, Mano CM, Zanetti G, Escuissato DL, Souza AS, Jr, et al. Paracoccidioidomycosis: high-resolution computed tomography-pathologic correlation. Eur J Radiol. 2009;77(1):80–4. doi: 10.1016/j.ejrad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Barreto MM, Marchiori E, Amorim VB, Zanetti G, Takayassu TC, Escuissato DL, et al. Thoracic Paracoccidioidomycosis: Radiographic and CT Findings. Radiographics. 2012;32(1):71–84. doi: 10.1148/rg.321115052. [DOI] [PubMed] [Google Scholar]
- 38.Lemle A, Vieira LO, Milward GA, Miranda JL. Lung function studies in pulmonary South American blastomycosis. Correlation with clinical and roentgenologic findings. Am J Med. 1970;48(4):434–42. doi: 10.1016/0002-9343(70)90042-2. [DOI] [PubMed] [Google Scholar]
- 39.Campos EP, Cataneo AMJ. Função pulmonar na evolução de 35 pacientes com paracoccidioidomicose. Rev Inst Med Trop Sao Paulo. 1986;28(5):330–6. doi: 10.1590/s0036-46651986000500008. [DOI] [PubMed] [Google Scholar]
- 40.Campos EP, Padovani CR, Cataneo AMJ. Paracoccidioidomicose: estudo radiológico e pulmonar de 58 casos. Rev Inst Med Trop Sao Paulo. 1991;33(4):267–76. [PubMed] [Google Scholar]
- 41.Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest. 2012;141(1):222–31. doi: 10.1378/chest.11-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benard G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia. 2008;165(4-5):209–21. doi: 10.1007/s11046-007-9065-0. [DOI] [PubMed] [Google Scholar]
- 43.Calich VL, Vaz CA, Burger E. Immunity to Paracoccidioides brasiliensis infection. Res Immunol. 1998;149(4-5):407–17. discussion 99–500. doi: 10.1016/s0923-2494(98)80764-5. [DOI] [PubMed] [Google Scholar]
- 44.Kim WJ, Hoffman E, Reilly J, Hersh C, Demeo D, Washko G, et al. Association of COPD candidate genes with computed tomography emphysema and airway phenotypes in severe COPD. Eur Respir J. 2011;37(1):39–43. doi: 10.1183/09031936.00173009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos WA, Silva BM, Passos ED, Zandonade E, Falqueto A. Associação entre tabagismo e paracoccidioidomicose: um estudo de caso-controle no Estado do Espírito Santo, Brasil. Cad Saúde Pública. 2003;19(1):245–53. doi: 10.1590/s0102-311x2003000100027. [DOI] [PubMed] [Google Scholar]
- 46.Stamm AM, Dismukes WE. Current therapy of pulmonary and disseminated fungal diseases. Chest. 1983;83(6):911–7. doi: 10.1378/chest.83.6.911. [DOI] [PubMed] [Google Scholar]
- 47.Menezes VM, Soares BG, Fontes CJ. Drugs for treating paracoccidioidomycosis. Cochrane Database Syst Rev. 2006;(2):CD004967. doi: 10.1002/14651858.CD004967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]