Abstract
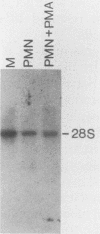
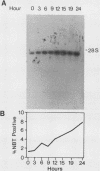
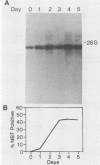
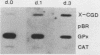
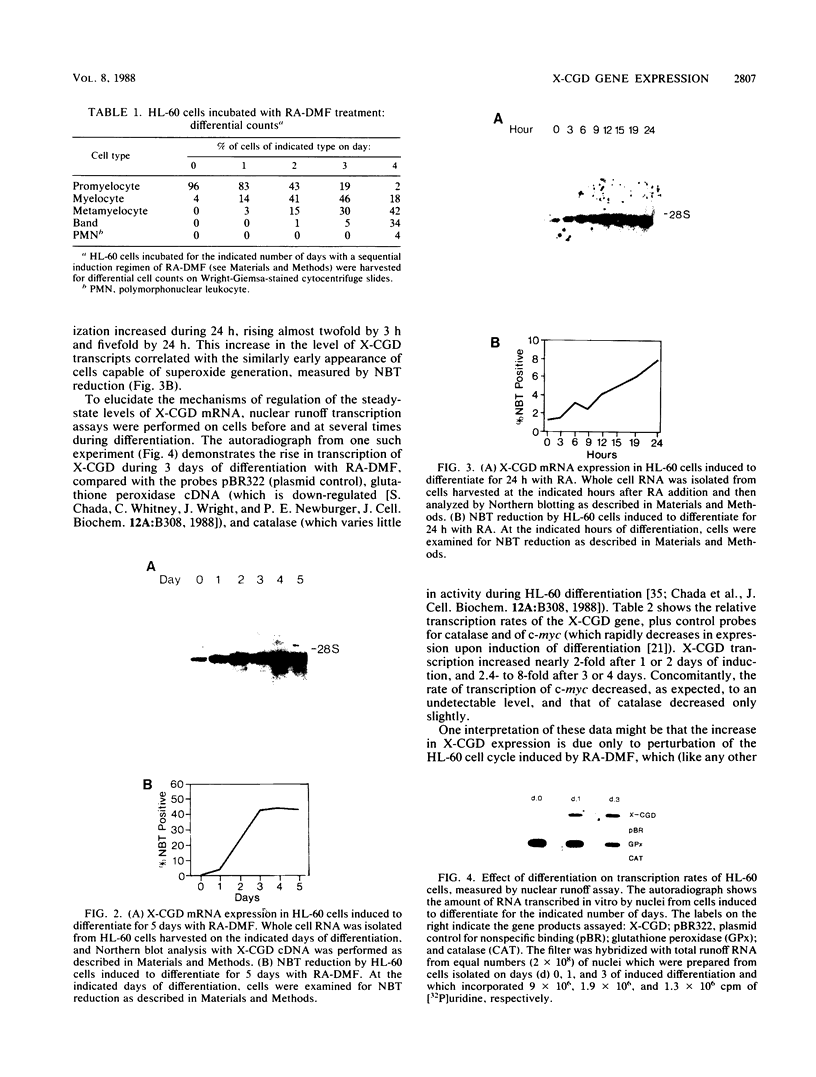
The expression of the X-CGD gene, which encodes the heavy-chain subunit of the phagocyte cytochrome b, was studied during induced myeloid differentiation of HL-60 cells. Incubation of the cells with a combined regimen of retinoic acid and dimethyl formamide resulted in granulocytic morphological differentiation and acquisition of nitroblue tetrazolium reduction, a measure of superoxide generation. During the 5-day course of induced differentiation, the levels of X-CGD mRNA transcripts rose 13-fold, with a 2-fold increase detectable within 3 h of exposure to retinoic acid. Relative transcription rates for the X-CGD gene, determined by nuclear runoff, increased two- to eightfold after 24 to 72 h of induced differentiation. However, the greater change in X-CGD mRNA levels than that in transcription rates implies the involvement of posttranscriptional regulation as well. Fractionation by centrifugal elutriation into phases of the cell cycle showed expression of X-CGD transcripts predominantly in G1 cells before induction and in all phases of the cell cycle 24 h after induction. Thus the rapid increase in X-CGD expression in induced cells reflects the acquisition of functional competence and not the concomitant cessation of proliferation or shift in cell cycle distribution.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Babior G. L., Rosin R. E., McMurrich B. J., Peters W. A., Babior B. M. Arrangement of the respiratory burst oxidase in the plasma membrane of the neutrophil. J Clin Invest. 1981 Jun;67(6):1724–1728. doi: 10.1172/JCI110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981 Jun;57(6):1000–1004. [PubMed] [Google Scholar]
- Collins S. J., Bodner A., Ting R., Gallo R. C. Induction of morphological and functional differentiation of human promyelocytic leukemia cells (HL-60) by componuds which induce differentiation of murine leukemia cells. Int J Cancer. 1980 Feb 15;25(2):213–218. doi: 10.1002/ijc.2910250208. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Dolbeare F., Gratzner H., Pallavicini M. G., Gray J. W. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5573–5577. doi: 10.1073/pnas.80.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsted R. L., Gupta S. K., Glover C. J., Fischkoff S. A., Gallagher R. E. Cell surface membrane protein changes during the differentiation of cultured human promyelocytic leukemia HL-60 cells. Cancer Res. 1983 Jun;43(6):2754–2761. [PubMed] [Google Scholar]
- Fontana J. A., Wright D. G., Schiffman E., Corcoran B. A., Deisseroth A. B. Development of chemotactic responsiveness in myeloid precursor cells: studies with a human leukemia cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3664–3668. doi: 10.1073/pnas.77.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Grosso L. E., Pitot H. C. Transcriptional regulation of c-myc during chemically induced differentiation of HL-60 cultures. Cancer Res. 1985 Feb;45(2):847–850. [PubMed] [Google Scholar]
- Heil M. F., Wu J. M., Chiao J. W. Cell-cycle differences of HL-60 leukemia cells fractionated by centrifugal elutriation. Biochim Biophys Acta. 1985 Apr 22;845(1):17–20. doi: 10.1016/0167-4889(85)90048-5. [DOI] [PubMed] [Google Scholar]
- High K. A., Stolle C. A., Schneider J. W., Hu W., Benz E. J., Jr c-myc gene inactivation during induced maturation of HL-60 cells. Transcriptional repression and loss of a specific DNAse I hypersensitive site. J Clin Invest. 1987 Jan;79(1):93–99. doi: 10.1172/JCI112814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. The NADPH oxidase of human polymorphonuclear leukocytes. Evidence for regulation by multiple signals. J Biol Chem. 1984 May 10;259(9):5768–5775. [PubMed] [Google Scholar]
- Nathan C. F., Tsunawaki S. Secretion of toxic oxygen products by macrophages: regulatory cytokines and their effects on the oxidase. Ciba Found Symp. 1986;118:211–230. doi: 10.1002/9780470720998.ch14. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979 Aug;82(2):315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Speier C., Borregaard N., Walsh C. E., Whitin J. C., Simons E. R. Development of the superoxide-generating system during differentiation of the HL-60 human promyelocytic leukemia cell line. J Biol Chem. 1984 Mar 25;259(6):3771–3776. [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Seger R. A., Tiefenauer L., Matsunaga T., Wildfeuer A., Newburger P. E. Chronic granulomatous disease due to granulocytes with abnormal NADPH oxidase activity and deficient cytochrome-b. Blood. 1983 Mar;61(3):423–428. [PubMed] [Google Scholar]
- Speier C., Newburger P. E. Changes in superoxide dismutase, catalase, and the glutathione cycle during induced myeloid differentiation. Arch Biochem Biophys. 1986 Dec;251(2):551–557. doi: 10.1016/0003-9861(86)90363-2. [DOI] [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]