Abstract
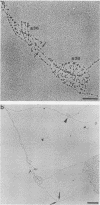
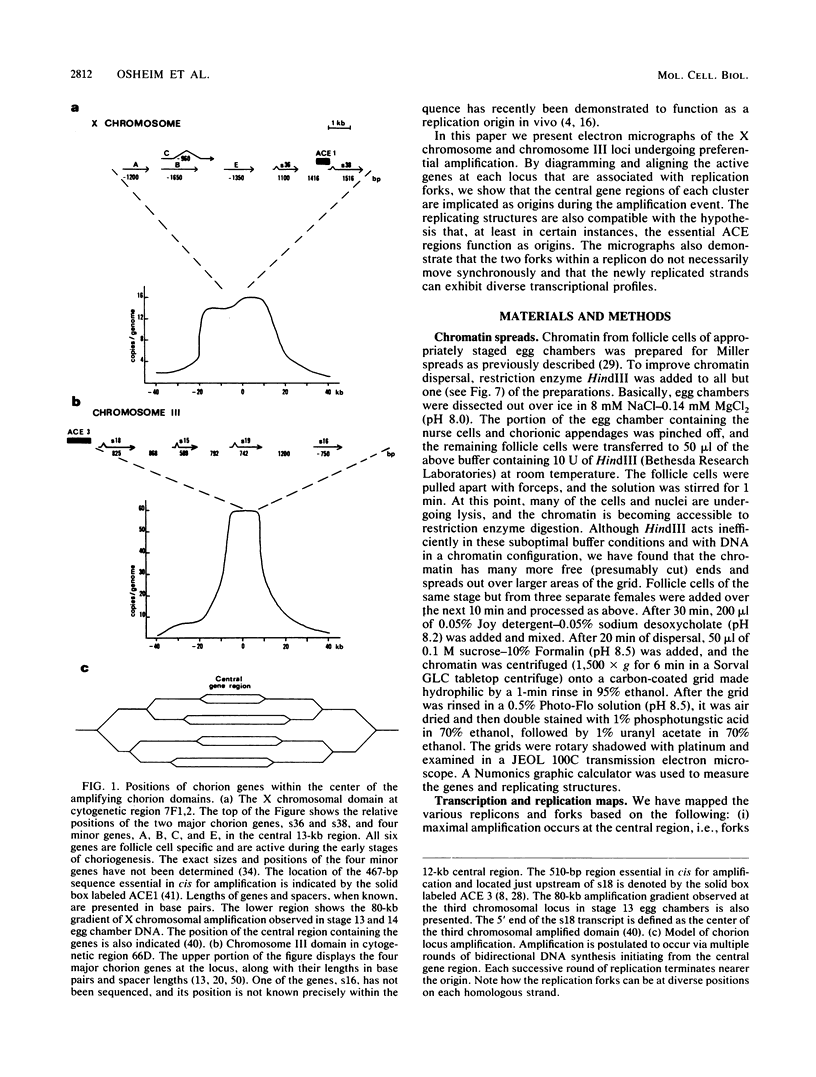
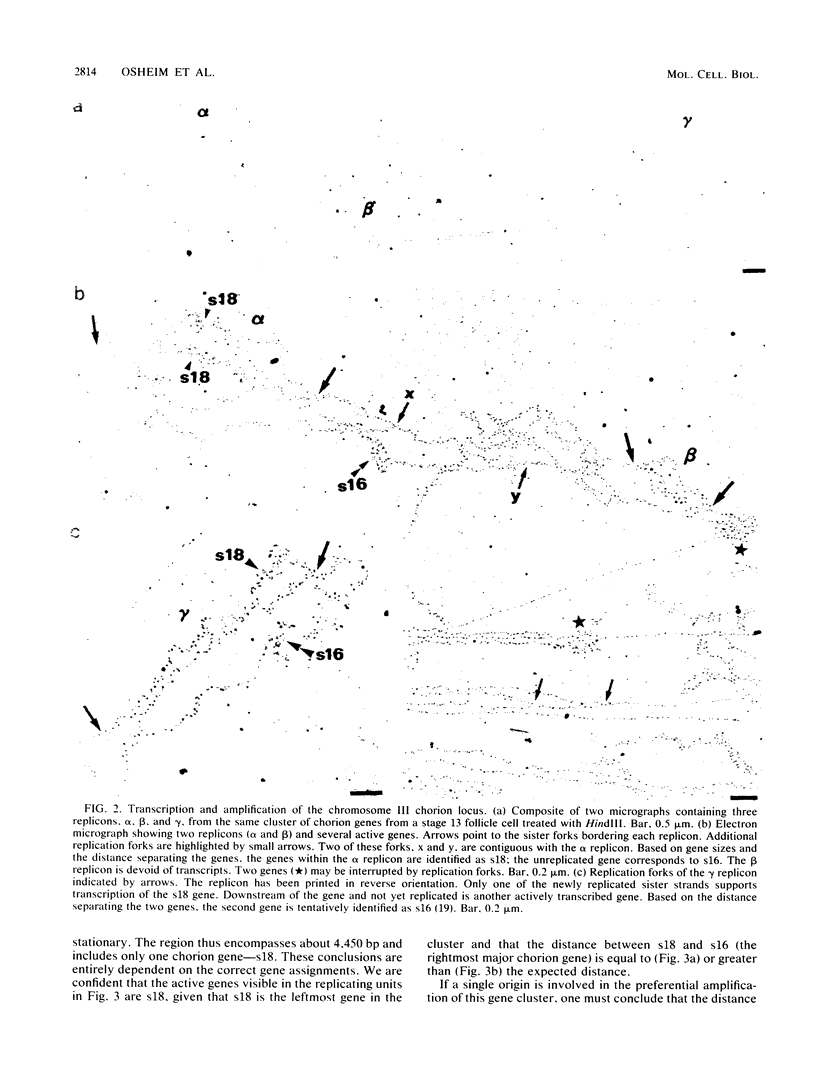
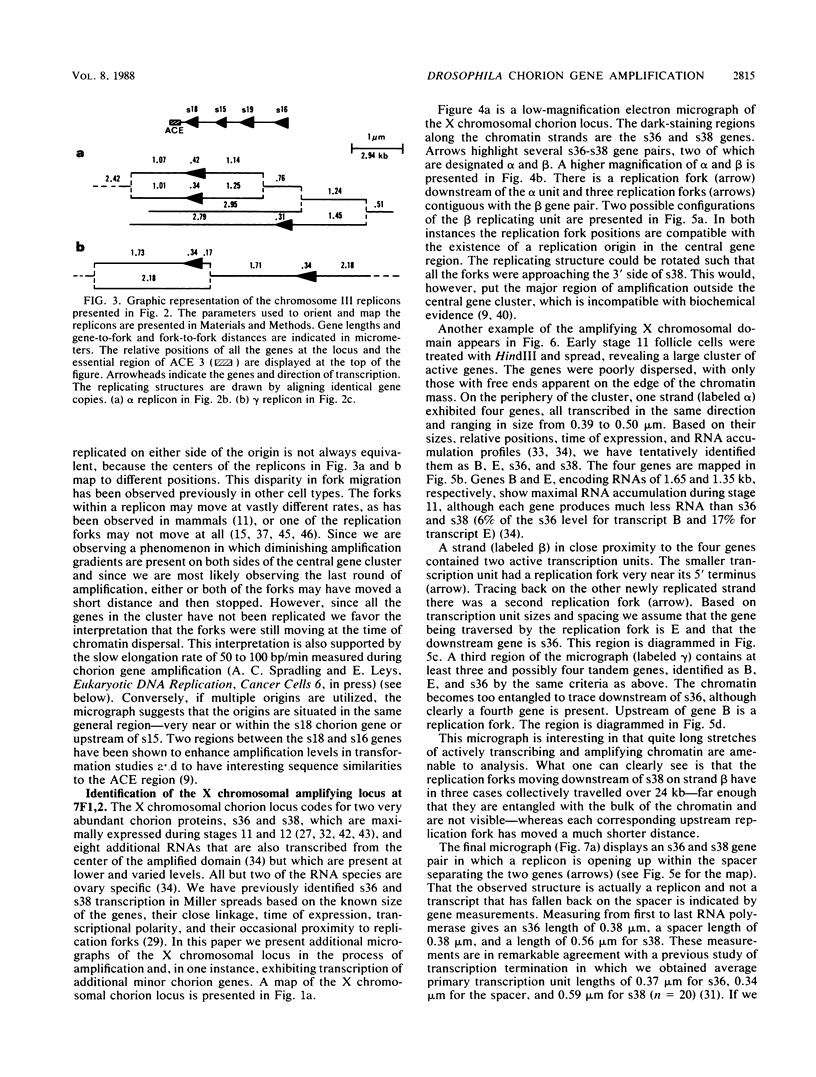
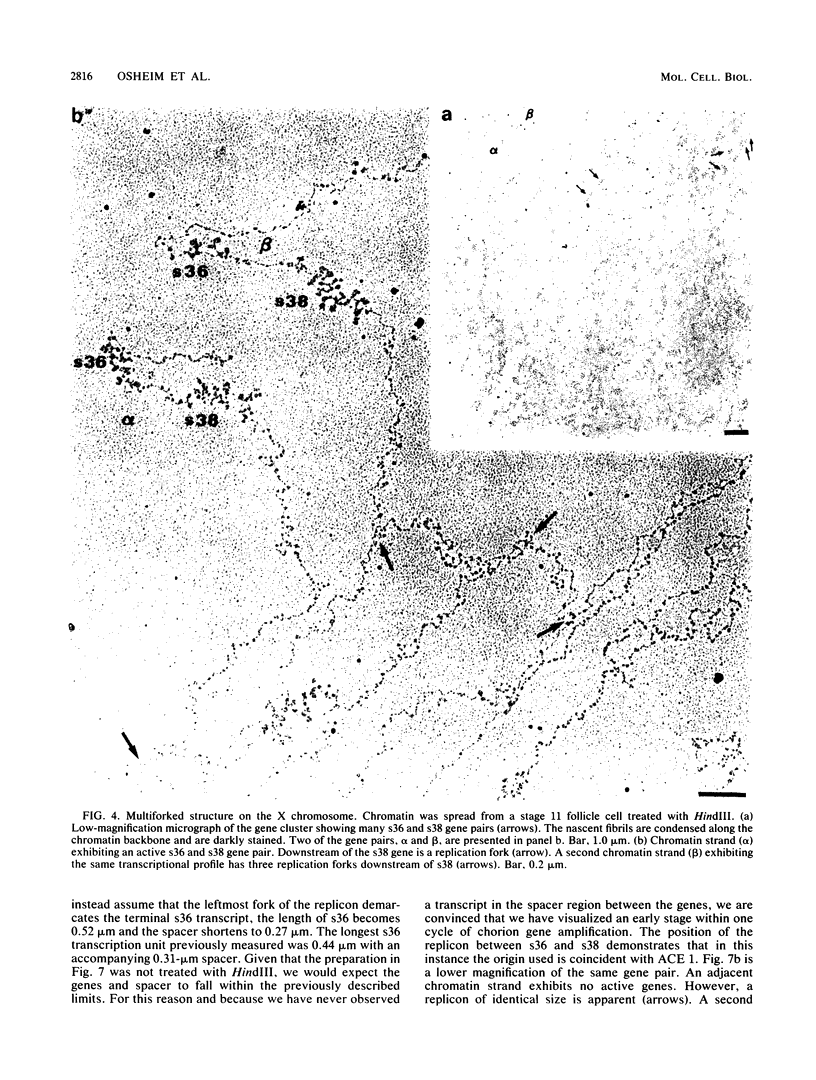
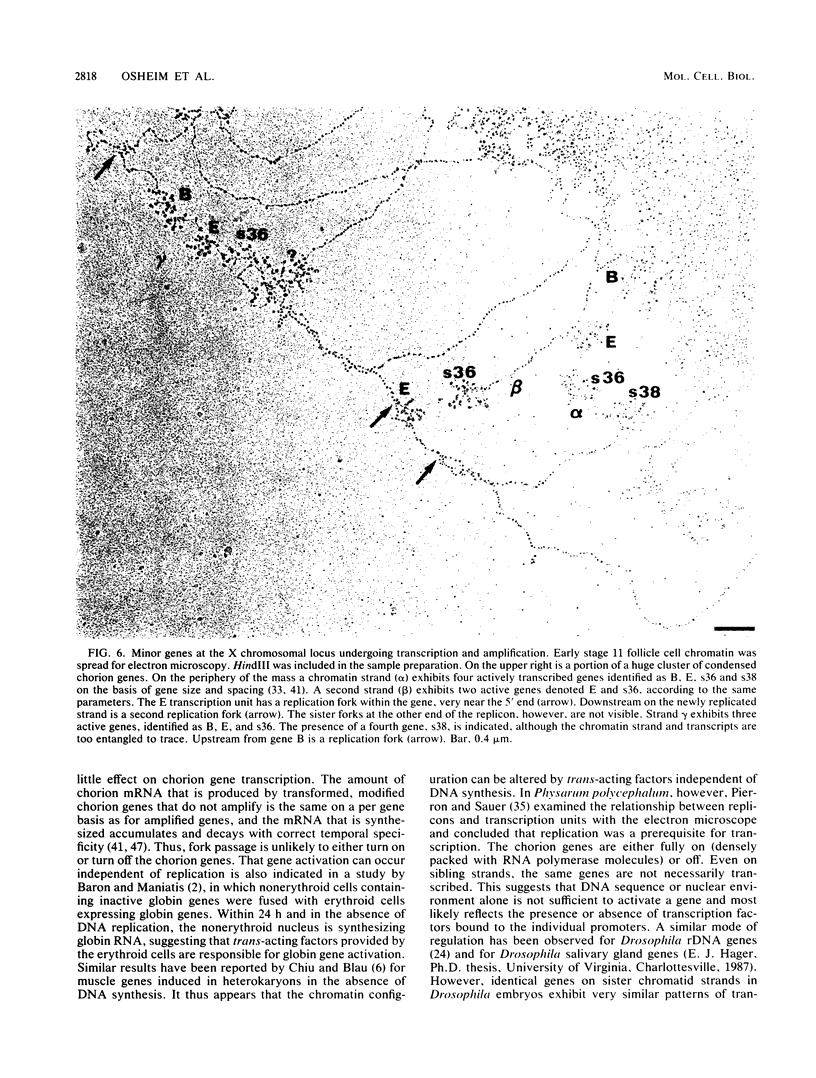
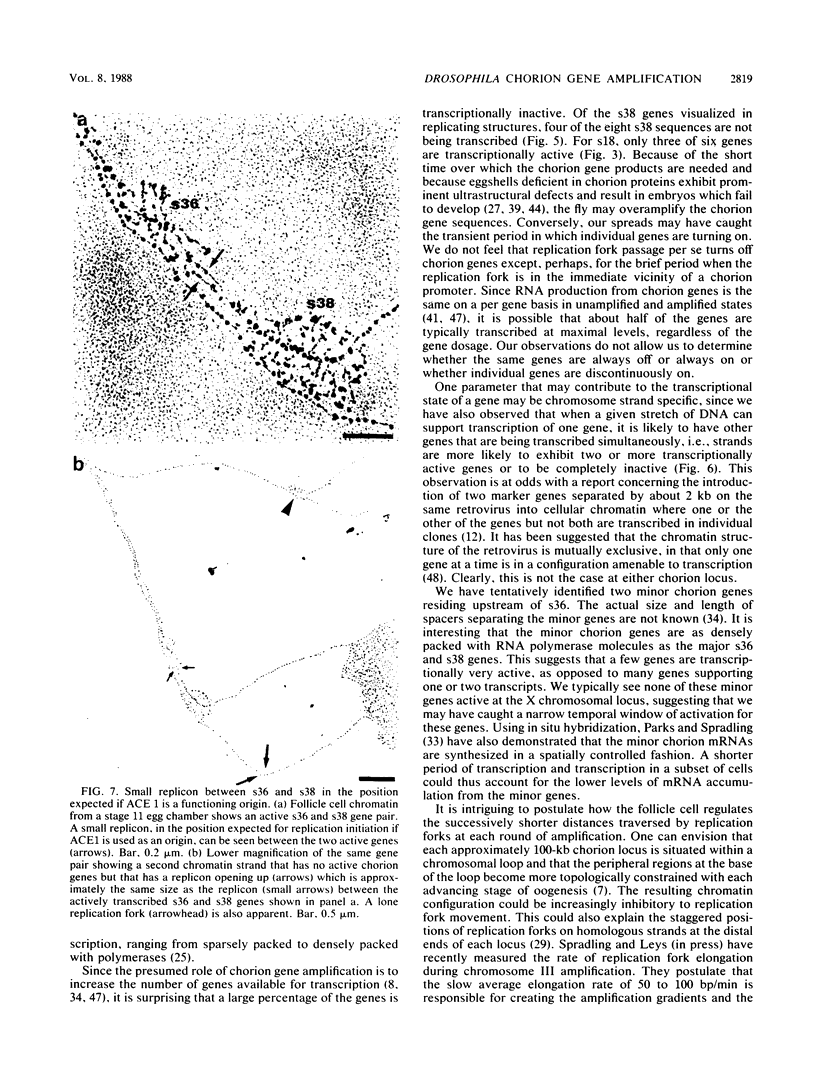
We visualized by electron microscopy the preferential amplification of Drosophila chorion genes in late-stage follicle cells. Chromatin spreads revealed large clusters of actively transcribed genes of the appropriate size, spacing, and orientation for chorion genes that were expressed with the correct temporal specificity. Occasionally the active genes were observed within or contiguous with intact replicons and replication forks. In every case, our micrographs are consistent with the hypothesis that the central region of each chorion domain contains a replication origin(s) used during the amplification event. In one case, a small replication bubble was observed precisely at the site of the essential region of the X chromosome amplification control element. The micrographs also suggest that forks at either end of a replicon frequently progress very different distances, presumably due to different times in initiation or different rates of movement. It appears that all chorion genes (even those coding for minor proteins) are transcribed in a "fully on" condition, albeit for varied durations, and that if replication fork passage does inactivate a promoter, it does so very transiently. Furthermore, a DNA segment containing one active gene is likely to have an additional active gene(s). Surprisingly, during the time frame of expected maximum activity, approximately half of the chorion sequences appear transcriptionally inactive.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre H., De Petrocellis B., Brachet J. Studies on differentiation without cleavage in Chaetopterus. Requirement for a definite number of DNA replication cycles shown by aphidicolin pulses. Differentiation. 1982;22(2):132–135. doi: 10.1111/j.1432-0436.1982.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986 Aug 15;46(4):591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. P., Blau H. M. Reprogramming cell differentiation in the absence of DNA synthesis. Cell. 1984 Jul;37(3):879–887. doi: 10.1016/0092-8674(84)90423-9. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Delidakis C., Kafatos F. C. Amplification of a chorion gene cluster in Drosophila is subject to multiple cis-regulatory elements and to long-range position effects. J Mol Biol. 1987 Sep 5;197(1):11–26. doi: 10.1016/0022-2836(87)90605-x. [DOI] [PubMed] [Google Scholar]
- Dubey D. D., Raman R. Do sister forks of bidirectionally growing replicons proceed at unequal rates? Exp Cell Res. 1987 Feb;168(2):555–560. doi: 10.1016/0014-4827(87)90028-0. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Griffin-Shea R., Thireos G., Kafatos F. C. Organization of a cluster of four chorion genes in Drosophila and its relationship to developmental expression and amplification. Dev Biol. 1982 Jun;91(2):325–336. doi: 10.1016/0012-1606(82)90039-2. [DOI] [PubMed] [Google Scholar]
- Hammond M. P., Laird C. D. Chromosome structure and DNA replication in nurse and follicle cells of Drosophila melanogaster. Chromosoma. 1985;91(3-4):267–278. doi: 10.1007/BF00328222. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. DNA replication: direction and rate of chain growth in mammalian cells. J Cell Biol. 1973 Aug;58(2):410–418. doi: 10.1083/jcb.58.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Mitsialis S. A., Spoerel N., Mariani B., Lingappa J. R., Delidakis C. Studies on the developmentally regulated expression and amplification of insect chorion genes. Cold Spring Harb Symp Quant Biol. 1985;50:537–547. doi: 10.1101/sqb.1985.050.01.066. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C. The cocoonase zymogen cells of silk moths: a model of terminal cell differentiation for specific protein synthesis. Curr Top Dev Biol. 1972;7:125–191. doi: 10.1016/s0070-2153(08)60071-x. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Levine J., Orr-Weaver T., Parks S., Wakimoto B., de Cicco D., Spradling A. Localization of sequences regulating Drosophila chorion gene amplification and expression. Cold Spring Harb Symp Quant Biol. 1985;50:527–535. doi: 10.1101/sqb.1985.050.01.065. [DOI] [PubMed] [Google Scholar]
- Levine J., Spradling A. DNA sequence of a 3.8 kilobase pair region controlling Drosophila chorion gene amplification. Chromosoma. 1985;92(2):136–142. doi: 10.1007/BF00328465. [DOI] [PubMed] [Google Scholar]
- Li J. J., Peden K. W., Dixon R. A., Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986 Apr;6(4):1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Transient replication of bovine papilloma virus type 1 plasmids: cis and trans requirements. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3609–3613. doi: 10.1073/pnas.83.11.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Post-replicative nonribosomal transcription units in D. melanogaster embryos. Cell. 1979 Jul;17(3):551–563. doi: 10.1016/0092-8674(79)90263-0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Mulligan P. K., Rasch E. M. Determination of DNA content in the nurse and follicle cells from wild type and mutant Drosophila melanogaster by DNA-Feulgen cytophotometry. Histochemistry. 1985;82(3):233–247. doi: 10.1007/BF00501400. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Spradling A. C. Drosophila chorion gene amplification requires an upstream region regulating s18 transcription. Mol Cell Biol. 1986 Dec;6(12):4624–4633. doi: 10.1128/mcb.6.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W., Komitopoulou K., Kafatos F. C. Mutants suppressing in trans chorion gene amplification in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3773–3777. doi: 10.1073/pnas.81.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. RNP particles at splice junction sequences on Drosophila chorion transcripts. Cell. 1985 Nov;43(1):143–151. doi: 10.1016/0092-8674(85)90019-4. [DOI] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. Two Drosophila chorion genes terminate transcription in discrete regions near their poly(A) sites. EMBO J. 1986 Dec 20;5(13):3591–3596. doi: 10.1002/j.1460-2075.1986.tb04687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr Novel amplification and transcriptional activity of chorion genes in Drosophila melanogaster follicle cells. Cell. 1983 Jun;33(2):543–553. doi: 10.1016/0092-8674(83)90435-x. [DOI] [PubMed] [Google Scholar]
- Parks S., Wakimoto B., Spradling A. Replication and expression of an X-linked cluster of Drosophila chorion genes. Dev Biol. 1986 Sep;117(1):294–305. doi: 10.1016/0012-1606(86)90372-6. [DOI] [PubMed] [Google Scholar]
- Pierron G., Sauer H. W., Toublan B., Jalouzot R. Physical relationship between replicons and transcription units in Physarum polycephalum. Eur J Cell Biol. 1982 Nov;29(1):104–113. [PubMed] [Google Scholar]
- Reeder R. H., Higashinakagawa T., Miller O., Jr The 5' leads to 3' polarity of the Xenopus Ribosomal RNA precursor molecule. Cell. 1976 Jul;8(3):449–454. doi: 10.1016/0092-8674(76)90158-6. [DOI] [PubMed] [Google Scholar]
- Saffer L. D., Miller O. L., Jr Electron microscopic study of Saccharomyces cerevisiae rDNA chromatin replication. Mol Cell Biol. 1986 Apr;6(4):1148–1157. doi: 10.1128/mcb.6.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh N. Timing mechanisms in early embryonic development. Differentiation. 1982;22(3):156–163. doi: 10.1111/j.1432-0436.1982.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Snyder P. B., Galanopoulos V. K., Kafatos F. C. Trans-acting amplification mutants and other eggshell mutants of the third chromosome in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 May;83(10):3341–3345. doi: 10.1073/pnas.83.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell. 1979 Mar;16(3):589–598. doi: 10.1016/0092-8674(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 1981 Nov;27(1 Pt 2):193–201. doi: 10.1016/0092-8674(81)90373-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., de Cicco D. V., Wakimoto B. T., Levine J. F., Kalfayan L. J., Cooley L. Amplification of the X-linked Drosophila chorion gene cluster requires a region upstream from the s38 chorion gene. EMBO J. 1987 Apr;6(4):1045–1053. doi: 10.1002/j.1460-2075.1987.tb04857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinemann M. Chromosomal replication in Drosophila virilis. III. Organization of active origins in the highly polytene salivary gland cells. Chromosoma. 1981;82(2):289–307. doi: 10.1007/BF00286112. [DOI] [PubMed] [Google Scholar]
- Steinemann M. Chromosomal replication of Drosophila virilis. II. Organization of active origins in diploid brain cells. Chromosoma. 1981;82(2):267–288. doi: 10.1007/BF00286111. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. DNA replication in vitro erases a Xenopus 5S RNA gene transcription complex. Cell. 1986 Oct 24;47(2):217–227. doi: 10.1016/0092-8674(86)90444-7. [DOI] [PubMed] [Google Scholar]
- Wong Y. C., Pustell J., Spoerel N., Kafatos F. C. Coding and potential regulatory sequences of a cluster of chorion genes in Drosophila melanogaster. Chromosoma. 1985;92(2):124–135. doi: 10.1007/BF00328464. [DOI] [PubMed] [Google Scholar]
- de Cicco D. V., Spradling A. C. Localization of a cis-acting element responsible for the developmentally regulated amplification of Drosophila chorion genes. Cell. 1984 Aug;38(1):45–54. doi: 10.1016/0092-8674(84)90525-7. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]