Abstract
Along with serum phosphate and parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and Klotho play key roles in disordered mineral metabolism in chronic kidney disease (CKD). Through independent and combined effects on renal phosphorus and vitamin D metabolism, alterations in FGF23 and Klotho expression are essential for maintaining mineral homeostasis in kidney disease. However, increasing evidence showing that disturbances in FGF23 and Klotho expression have direct cardiovascular and renal toxicity have accelerated interest in therapies that directly target these hormones in the interest of improving outcomes in CKD. Importantly, recent studies showing that reversing CKD-induced elevations in FGF23 may have adverse cardiovascular effects have shed new light on the potential unintended consequences linked with developing therapeutic targets in this arena, ultimately supporting the notion that addressing root causes of disturbances of FGF23 and Klotho such as phosphorus excess should remain the main focus in managing mineral metabolism in CKD.
INTRODUCTION
The relatively recent emergence of fibroblast growth factor 23 (FGF23) and Klotho has illuminated much of our understanding of phosphorus and vitamin D metabolism in chronic kidney disease (CKD). Prior to the discovery and characterization of these two hormones, parathyroid hormone (PTH) was considered the primary hormonal regulator of phosphorus and vitamin D metabolism and thus, the main target of intervention in the management of bone and mineral metabolism in CKD patients. However, with the recognition that FGF23 and Klotho regulate renal phosphorus handling and vitamin D metabolism and are among the earliest (if not the earliest) detectable biochemical manifestations of disturbed phosphorus homeostasis in CKD, the focus in treating disturbances in phosphorus and vitamin D homeostasis in CKD has extended beyond serum phosphate and PTH to encompass FGF23 and Klotho in a rapidly unfolding tapestry of endocrine feedback pathways. These developments have been accelerated by the growing body of evidence suggesting that altered expression of these hormones may have direct cardiovascular and renal toxicity, supporting the concept that FGF23 and Klotho may be ideal therapeutic targets for improving outcomes in CKD. However, more recent experimental data showing that reversing alterations in FGF23 and Klotho expression in CKD may also have adverse effects provide a measure of caution in thinking about how to design therapies that target these hormones. Collectively, these data underscore the central struggle of the classical “trade-off” hypothesis—namely, how to reverse the long-term harmful effects of physiological adaptions to kidney injury in response to disturbances in mineral homeostasis without mitigating their shorter-term beneficial effects in terms of maintaining phosphorus balance.
Overview of the role of FGF23 and Klotho in mineral homeostasis in CKD
FGF23
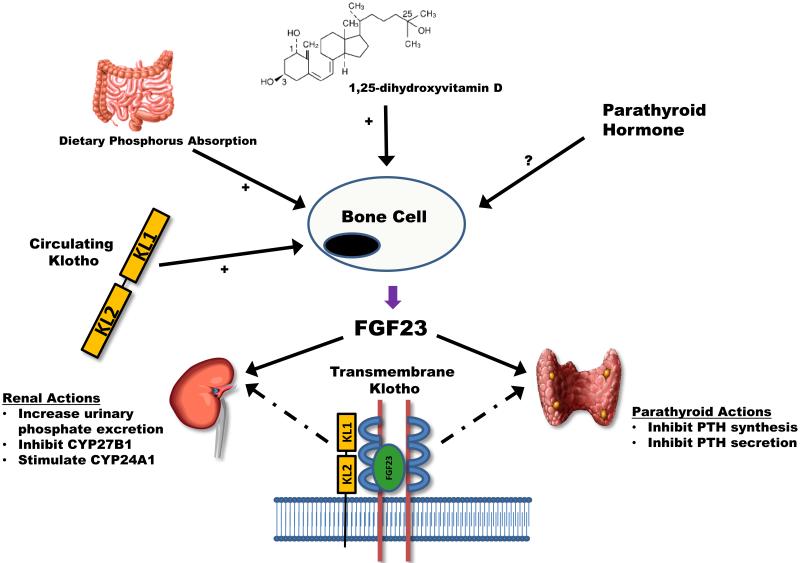
FGF23 is a 251 amino acid protein that is most highly expressed in osteocytes and osteoblasts.1 FGF23 belongs to a subfamily of FGFs (known as the FGF19 subfamily) that lack several amino acid residues in their C-terminal domain allowing them to be more soluble in circulation due to their low binding affinity to heparin.1 Thus, unlike most other FGFs, FGF23 is able to exert systemic actions with its two main sites of action being the kidneys and parathyroid glands (Figure 1).
Figure 1. Regulation and action of fibroblast growth factor 23 (FGF23).
Bone cells are the primary cells that synthesize and secrete FGF23. There are a number of systemic stimuli of FGF23 secretion—increased dietary phosphorus absorption, increased 1,25-dihydroxyvitamin D concentrations, and circulating Klotho. The effects of parathyroid hormone on FGF23 are more controversial, with some studies suggesting that parathyroid hormone directly stimulates FGF23 while others do not. FGF23 acts primarily in the kidneys and parathyroid glands. In the kidneys, FGF23 augments urinary phosphate excretion by down-regulating sodium-phosphate co-transporters in renal proximal tubular cells. In addition, FGF23 inhibits the synthesis of CYP27B1 and up-regulates CYP24A1, both of which serve to decrease circulating 1,25-dihydroxyvitamin D concentrations. In the parathyroid glands, FGF23 inhibits both the synthesis and secretion of parathyroid hormone. Transmembrane Klotho is needed for FGF23 to bind to its receptor in the kidney and parathyroid glands with high-enough affinity to effect signal transduction (dashed lines in the figure). The actions of FGF23 on the kidney and parathyroid glands appear to serve the primary purpose of maintaining phosphorus homeostasis in kidney disease by enhancing urinary phosphate excretion and decreasing intestinal phosphorus absorption via lower 1,25-dihydroxyvitamin D concentrations.
In the kidneys, FGF23 stimulates urinary phosphate excretion by inducing the endocytosis of sodium-phosphate co-transporters (NPT2a and NPT2c) from the apical membrane of renal proximal tubular cells.2 In addition, FGF23 regulates vitamin D synthesis via two pathways.3,4 First, FGF23 inhibits the synthesis of CYP27B1, the enzyme required to convert 25-hydroxyvitamin D (25(OH)D) to its more active metabolite, 1,25-dihydroxyvitamin D (1,25 (OH)2D). Second, FGF23 up-regulates the activity of CYP24A1, the enzyme that represents the major catabolic pathway for both 25(OH)D and 1,25(OH)2D. In the parathyroid glands, FGF23 directly inhibits the synthesis and secretion of PTH, perhaps representing a secondary mechanism for inhibiting 1,25(OH)2D synthesis.5 Collectively, these actions appear to be critical for maintaining phosphorus homeostasis in CKD by enhancing urinary phosphate excretion and diminishing intestinal phosphorus absorption by reducing the stimulatory effect of 1,25(OH)2D on gut phosphorus absorption.
In support of FGF23’s key role in maintaining phosphorus homeostasis in CKD are two studies which examined the impact of lowering FGF23 activity in animal models of CKD. In the first study, Hasegawa et al. lowered circulating FGF23 concentrations by injecting anti-FGF23 antibody in rats with experimentally-induced kidney disease.6 Importantly, the fall in circulating FGF23 in treated animals resulted in a decrease in urinary fractional excretion of phosphate, an increase in 1,25(OH)2D concentrations and as a consequence, a significant rise in serum phosphate concentrations despite concurrently elevated PTH concentrations. Similarly, Shalhoub and colleagues injected FGF23-neutralizing antibodies or control antibodies in 5/6th nephrectomized rats placed on a high phosphorus diet for three weeks.7 While mean PTH concentrations were lower in animals injected with FGF23-neutralizing antibody than in those injected with control antibody—most likely due to higher 1,25(OH)2D concentrations in treated as compared to control animals—mean serum phosphate concentrations were higher in treated as compared to control animals, in large part due to diminished urinary phosphate excretion. Together, these data indicate that increased FGF23 concentrations are essential for maintaining phosphorus balance in CKD. Consistent with this, studies have shown that restriction of dietary phosphorus absorption (whether through lower dietary intake or therapy with oral phosphorus binders) decreased and in some cases normalized circulating FGF23 concentrations in individuals with CKD_ENREF_30.8-10
Klotho
Klotho is a 1,012 amino acid long peptide with a single transmembrane domain and an extracellular domain with two internal repeats (KL1 and KL2) that exhibit β-glucosidase activity.11 The transmembrane form serves as the critical co-factor required for FGF23 to bind to its cognate receptor with high enough affinity to effect signal transduction. Klotho also has a circulating form that is derived either from alternative RNA splicing or cleavage of the extra-cellular domain.11 Secreted forms of Klotho have a variety of systemic effects, including regulation of phosphorus and calcium metabolism.12,13
Like FGF23, disturbances in Klotho expression play an important role in mineral homeostasis in CKD. Renal klotho expression has been shown to be down-regulated in animals with experimentally induced kidney injury.14-16 Further, Klotho expression was shown to be lower in nephrectomy specimens and arteries from individuals with CKD as compared to individuals without CKD.17,18 Since transmembrane Klotho is needed for FGF23 to bind to FGFR with high affinity, the decrease in Klotho expression in CKD observed in these studies may contribute to lower FGF23 binding to FGFR, with attendant consequences such as lower urinary phosphate excretion and/or lower inhibition of PTH secretion. In support of this possibility is a study showing that over-expression of Klotho increased urinary phosphate excretion, lowered serum phosphate concentrations and lowered circulating PTH concentrations in animals with moderate CKD.19 Unfortunately, it was unclear whether these findings were due to an improved ability of FGF23 to bind to its receptor, a direct effect of circulating Klotho, or a combination of both. If future studies more definitively show that these findings were even partly due to enhanced FGF23 signal transduction via increased Klotho expression, it would support the concept that higher FGF23 concentrations in CKD may be due, in part, to end-organ resistance at the level of the kidney and parathyroid glands.
FGF23, Klotho and Adverse Cardiovascular Outcomes in CKD
Beyond their central roles in regulating mineral metabolism, FGF23 and Klotho have also been implicated in cardiovascular disease and mortality in CKD. Higher FGF23 concentrations were independently associated with higher risk of kidney disease progression, cardiovascular disease events, and death in patients across the spectrum of CKD.20-25 Though the mechanisms for these associations remain unclear, higher FGF23 concentrations have been independently associated with higher left ventricular mass,26-28 and higher prevalence of left ventricular hypertrophy, endothelial dysfunction and vascular calcification.26-31 In addition, FGF23 has been shown to directly induce hypertrophy of cardiomyocytes in vitro and in vivo.26 Interestingly, a recent study showed that FGF23 has anti-calcific effects on human aortic smooth muscle cells,18 suggesting that excess FGF23 concentrations may also have beneficial effects on vascular calcification. Importantly, however, this latter study showed that the anti-calcific effects of FGF23 were abrogated in the setting of decreased Klotho expression in vascular smooth muscle cells, suggesting that in states of diminished Klotho expression such as CKD, the adverse effects of FGF23 on myocardial hypertrophy may outweigh any beneficial effects related to diminished vascular calcification.
Klotho also has an important role in cardiovascular health. Circulating Klotho increases nitric oxide (NO) synthesis in endothelial cells.32,33 The importance of this was shown in Klotho-deficient mice which had impaired vasodilation in response to acetylcholine challenge as compared to wild-type controls,32 a finding that was reversed following parabiosis between wild-type and Klotho-deficient animals.32 In addition, over-expression of Klotho in an animal model of accelerated atherogenesis improved vascular endothelial dysfunction, increased nitric oxide production, and reduced elevated blood pressure.34 Further, Klotho knockdown accelerated vascular calcification whereas over-expression of Klotho inhibited vascular calcification in an animal model of CKD,18 in part by inhibiting phosphate-induced osteogenic changes in vascular smooth muscle cells. In the aggregate, these data suggest that diminished Klotho expression contributes to excess cardiovascular disease in CKD.
“Treating” disturbances in FGF23 and Klotho expression in CKD: assessing the landscape
As emphasized by the above discussion, disturbances in FGF23 and Klotho expression are early and nearly universal manifestations of disordered mineral metabolism in CKD which contribute to excess skeletal fragility, cardiovascular disease, and death in individuals with CKD. A natural conclusion of these findings is that reversing alterations in FGF23 and Klotho in CKD would have a wide spectrum of beneficial effects in CKD. However, the findings of several recent studies suggest that the development of any such therapeutic intervention should proceed with due consideration of potential unintended consequences.
In a particularly illuminating study referenced above, Shaloub and colleagues showed that injection of anti-FGF23 antibodies in rats with experimentally-induced CKD consuming a high phosphorus diet partially reversed CKD-induced decreases in 1,25(OH)2D and calcium concentrations and associated secondary hyperparathyroidism, and improved histomorphometric parameters of bone health.7 However, this came at the cost of higher serum phosphate concentrations, accelerated aortic calcification, and most importantly, higher mortality in animals treated with anti-FGF23 antibodies as compared to animals treated with control antibody. While the accelerated aortic calcification observed in animals treated with anti-FGF23 antibodies may have been due to higher serum phosphate concentrations, it is interesting to speculate whether the anti-calcific effects of FGF23 on vascular smooth muscle cells may also have played a role. In another interesting study, over-expression of Klotho in mice with normal kidney function was shown to stimulate the secretion of FGF23 through an FGF receptor-dependent mechanism.35 When coupled with a recent study showing that FGF23 may inhibit Klotho expression,36 this suggests a novel endocrine regulatory pathway in which Klotho stimulates FGF23 which then negatively feeds back to inhibit Klotho expression. Though entirely speculative, this may partly explain the finding of decreased Klotho expression in CKD—namely, lower Klotho expression may occur via direct FGF23 inhibition as an adaptive mechanism to avoid further Klotho-induced increases in FGF23.
Together, these studies have important implications of therapies aimed at reversing alterations in FGF23 and Klotho that occur in the course of kidney disease. As demonstrated in animal models of CKD, directly targeting FGF23 with biologic interventions such as anti-FGF23 antibodies in CKD patients would likely improve some biochemical indices of mineral metabolism (1,25(OH)2D, PTH, calcium) while worsening others (phosphate, vascular calcification), without any clear net benefit and the possibility of real harm.6,7 In addition, while investigators have shown that restoring Klotho expression has beneficial effects on vascular calcification and kidney disease progression in animal models of CKD,18,19 the finding that Klotho stimulates FGF23 suggests that any such therapy may also exacerbate FGF23 excess with attendant potential side effects such as myocardial hypertrophy. Future studies will need to study the effects of Klotho over-expression on FGF23 concentrations in CKD.
Given the potential for unintended consequences in therapies that directly target FGF23 or Klotho, a better strategy may be to focus on interventions that treat the root causes of disturbances in FGF23 and Klotho in CKD (i.e., phosphorus overload) such as oral phosphorus binders, which have already shown some efficacy in reducing FGF23 concentrations. While avoidance of activated vitamin D analogs may also be important in this vein, a recent study showing a beneficial effect of activated vitamin D on vascular calcification in part through restoration of Klotho expression in endothelial cells18 suggests that further study is needed to determine the risk/benefit ratio of vitamin D therapy vis-à-vis its effect on phosphorus balance, Klotho expression and FGF23 in CKD. As the design of studies that directly target FGF23 and Klotho lurch forward, these considerations will need to be addressed carefully to ensure that targeting mineral metabolism can become a viable strategy in the long-term management of CKD patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol. 2012 doi: 10.1038/nrendo.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–11. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 3.Bowe AE, Finnegan R, Jan de Beur SM, et al. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–81. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–80. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 7.Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–53. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant. 2011;26:2567–71. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 9.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–91. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 12.Alexander RT, Woudenberg-Vrenken TE, Buurman J, et al. Klotho prevents renal calcium loss. J Am Soc Nephrol. 2009;20:2371–9. doi: 10.1681/ASN.2008121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–50. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aizawa H, Saito Y, Nakamura T, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–71. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–51. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiura H, Yoshida T, Shiohira S, et al. Reduced Klotho Expression Level in Kidney Aggravates Renal Interstitial Fibrosis. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00294.2011. [DOI] [PubMed] [Google Scholar]
- 17.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–20. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 18.Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 19.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq309. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–6. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 25.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–22. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Kanbay M, Nicoleta M, Selcoki Y, et al. Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1780–6. doi: 10.2215/CJN.02560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirza MA, Hansen T, Johansson L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–31. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 31.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–90. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–9. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 33.Nagai R, Saito Y, Ohyama Y, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738–46. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito Y, Nakamura T, Ohyama Y, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–72. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 35.Smith RC, O’Bryan LM, Farrow EG, et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122:4710–5. doi: 10.1172/JCI64986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsell R, Krajisnik T, Goransson H, et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant. 2008;23:827–33. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]