Abstract
Fear conditioning and experimental extinction have been presented as models of anxiety disorders and exposure therapy, respectively. Moreover, the return of fear serves as a model of relapse after exposure therapy. Here we present two experiments, with rats as subjects in a lick suppression preparation, in which we assessed the additive effects of two different treatments to attenuate the return of fear. First, we evaluated whether two phenomena known to generate return of fear (i.e., spontaneous recovery and renewal) summate to produce a stronger reappearance of extinguished fear. At test, rats evaluated outside the extinction context following a long delay after extinction (i.e., a delayed context shift) exhibited greater return of extinguished fear than rats evaluated outside the extinction context alone, but return of extinguished fear following a delayed context shift did not significantly differ from the return of fear elicited in rats tested following a long delay after extinction alone. Additionally, extinction in multiple contexts and a massive extinction treatment each attenuated the strong return of fear produced by a delayed context shift. Moreover, the conjoint action of these treatments was significantly more successful in preventing the reappearance of extinguished fear, suggesting that extensive cue exposure administered in several different therapeutic settings has the potential to reduce relapse after therapy for anxiety disorders, more than either manipulation alone.
Keywords: extinction, return of fear, massive extinction, extinction in multiple contexts, exposure therapy, relapse
If fears and phobias are acquired through stimuli pairings as associative accounts suggest (e.g., Bouton, Mineka, & Barlow, 2001; Field, 2006; Laborda & Miller, 2011; Mineka & Oehlberg, 2008), then the study of manipulations that reduce the expression of this type of association could be informative for those interested in developing new techniques to treat these behavioral disorders (e.g., Craske et al., 2008; Laborda, Miguez, Polack, & Miller, 2012). After a conditioned stimulus (CS) has acquired behavioral control through pairings with an unconditioned stimulus (US), presenting it without the US reduces its potential to elicit conditioned responding. This phenomenon has been labeled extinction (Pavlov, 1927) and has been presented as a model of exposure therapy (e.g., Bouton, 2000; Bouton & Nelson, 1998), which is currently considered the most successful therapeutic approach for treating anxiety and related disorders (e.g., Chambless & Ollendick, 2001).
Pavlov (1927) proposed that during extinction treatment a new learning experience takes place and, after a theoretical detour in which extinction was thought to erase memories (e.g., Rescorla & Wagner, 1972), contemporary researchers have reached a consensus consistent with his approach. Evidence suggests that what is learned through extinction treatment is an inhibitory-like association that is most readily expressed in the spatiotemporal context in which extinction took place (for reviews, see Bouton, 1993, 2000; Craske, Liao, Brown, & Vervliet, 2012; Craske et al., 2009). Among the evidence demonstrating that extinction learning involves acquisition of a context-specific inhibitory-like association are signature phenomena of extinction such as renewal (Bouton & Bolles, 1979) and spontaneous recovery (Pavlov, 1927), both of which indicate that original associations remain largely intact after extinction. In the case of renewal, testing outside the context of extinction usually elicits more conditioned responding than when testing occurs in the extinction context (e.g., Bouton & Bolles, 1979; Bouton & King, 1983; Laborda, Witnauer, & Miller, 2011). In the case of spontaneous recovery, a long delay imposed between extinction and testing encourages the expression of the extinguished excitatory information rather than the inhibitory-like information learned during extinction training (e.g., Pavlov, 1927).
Evidence of the reappearance of extinguished responding in humans has also been reported (e.g., Effting & Kindt, 2007; Milad, Orr, Pitman, & Rauch, 2005; Neumann & Kitlertsirivatana, 2010; Vansteenwegen et al., 2005). For example, using a differential fear conditioning preparation in which one CS predicted the occurrence of an aversive shock and a second CS predicted no shock, Effting and Kindt found stronger return of fear in participants who were tested back in the acquisition context after extinction in a second context (i.e., ABA renewal; here we use the common notation for different types of renewal of three successive letters in which the first letter denotes the context of acquisition, the second the context of extinction, and the third the context of testing) than in participants who received acquisition, extinction, and testing in the same context (i.e., AAA control). For a recent review on the return of fear in human fear conditioning experiments see Vervliet, Baeyens, Van den Bergh, and Hermans (in press). Reappearance of extinguished responding can also be found in experimental studies with non-clinical human samples. For example, Rodriguez, Craske, Mineka, and Hladek (1999) provided support for the context-specificity of exposure treatment. Spider fearful participants who were tested in the same context in which they previously received exposure sessions exhibited less fear response (i.e., lower heart rate level) than those participants who were treated and tested in two different contexts. In a different experimental preparation, Collins and Brandon (2002) found a return of urges to drink in social drinkers when they were tested outside of the context in which they received exposure sessions to alcohol-related cues.
Many theoretical accounts for renewal and spontaneous recovery have been proposed, but presenting them is beyond of the scope of the present research report (see McConnell & Miller, 2012). Here we adopt a general theoretical framework in which extinction treatment is viewed as an example of retroactive outcome interference. From this perspective, extinction treatment forms a CS-noUS association that interferes with the CS-US association formed during acquisition training. Whether and to what degree behavior indicative of acquisition or extinction is expressed at test depends of the comparison between the reactivated US and noUS memories from acquisition and extinction. If the reactivated US memory from acquisition, which is a product of the strength of the CS-US association and the facilitatory cues from acquisition present at test, is stronger than the reactivated noUS memory from extinction, which is a product of the strength of the CS-noUS association and the facilitatory cues from extinction present at test, then we expect to see strong conditioned responding at test. Conversely, if the reactivated noUS memory from extinction is stronger than the reactivated US memory from acquisition, we would expect to see weak conditioned responding at test (Laborda & Miller, 2012; Miller & Laborda, 2011).
One consequence of this logic is that the dissimilarities between the contexts of extinction and testing should positively correlate with the degree of return of fear evidenced at test because fewer facilitatory cues from extinction treatment are present. In terms of the present series, testing in a different spatiotemporal context should produce more reappearance of extinguished responding than testing in a different spatial or different temporal context alone. Using a conditioned taste aversion preparation with rats, Rosas and Bouton (1998) provided initial evidence of summation of these two extinction-to-test contextual manipulations, each of which alone produces some reappearance of extinguished responding. This would be expected if changes in the physical and temporal contexts summate to produce a stronger form of return of extinguished responding. Rosas, Vila, Lugo, and López (2001) successfully extended Rosas and Bouton's results to a human causal learning preparation.
In Experiment 1 we evaluated whether renewal and spontaneous recovery would also summate in eliciting a stronger return of fear. The potential additivity of these phenomena is of interest in practical terms because it gives us a higher level of recovered fear that should be more sensitive to the evaluation of the effects of different manipulations aimed at reducing the return of fear. In addition, a situation in which renewal and spontaneous recovery summate may be considered more naturalistic, in the sense that relapse after exposure therapy usually occurs when patients confront disorder-related stimuli outside of the treatment context and after a relatively long time after the end of treatment (e.g., Bouton, 1988; Craske, 1999; Rachman, 1979, 1989).
A second consequence of an associative interference view of extinction (e.g., Laborda & Miller, 2012; Miller & Laborda, 2011) is that the return of conditioned fear following extinction (and likely, relapse from exposure therapy) can be attenuated by manipulations that enhance either the strength of the extinction association (i.e., CS-noUS) or the presence at test of cues from extinction that facilitate retrieval of memories from extinction treatment. Here we focus on two of these manipulations: the use of extinction in multiple contexts, which arguably promotes generalization of facilitatory cues from the extinction to the test context, and the use of a massive number of extinction trials, which arguably enhances the extinction learning.
Gunther, Denniston, and Miller (1998) assessed whether using multiple extinction contexts would have an effect on the level of return of fear expressed at test. In their study, rats in a fear conditioning preparation were trained to fear an auditory CS by pairing it with a mild footshock (i.e., US) in Context A. After this training, rats in the experimental groups received a moderate amount of extinction treatment, either in a single new context (Context B) or in three different new contexts (Context B, C, and D). At test in the associatively neutral Context E, rats that received extinction treatment in multiple contexts were less fearful of the CS (i.e., they showed less renewal) than rats that received the same extinction treatment but in a single context. The attenuation of the return of extinguished responding by training extinction in multiple contexts has been replicated and extended in many different situations (e.g., Bandarian Balooch & Neumann, 2011; Chelonis, Calton, Hart, & Schachtman, 1999; Glautier & Elgueta, 2009; Neumann, 2006; Pineño & Miller, 2004; Thomas, Vurbic, & Novak, 2009; Vansteenwegen et al., 2007; for negative results see Betancourt et al., 2008; Bouton, García-Gutiérrez, Zilski, & Moody, 2006; Neumann, Lipp, & Cory, 2007), and today is a well-documented result. Successful applications of this technique have also been reported in preclinical studies with humans. Neumann (2006) found reduced ABC and ABA renewal after extinction in multiple contexts in a conditioned suppression task, and Vansteenwegen et al. (2007) found that exposing spider fearful participants to a spider videotaped in three different rooms of a house attenuated recovery of extinguished fear when the test occurred in a neutral context (i.e., an ABC-like design).
Denniston, Chang, and Miller (2003) assessed whether using a massive number of extinction trials would have an effect in the level of return of fear expressed at test. In their study, rats were trained to fear an auditory CS by pairing it with a mild footshock (i.e., US) in Context A. After this training, rats received either a moderate (160) or a massive (800) number of extinction trials in Context B. The rats were then tested either in a neutral context or back in the acquisition context. Rats that received massive extinction treatment were less fearful to the CS (i.e., they showed less renewal) than rats that received a moderate amount of extinction. As discussed elsewhere (Laborda, McConnell, & Miller, 2011), those studies that have failed to find decreased recovery of fear with massive extinction trials used far fewer extinction trials (e.g., Thomas et al. [2009] used 144 trials, Rauhut, Thomas, & Ayres [2001] used 100 trials, and Tamai & Nakajima [2000] used 112 trials) than Denniston et al. (800 trials), which explains the different results. Of interest, the effect of massive exposure has also been found in clinical settings. For example, some researchers (Foa et al., 2005; Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010) have observed that prolonged exposure reduces relapse in patients treated for posttraumatic stress disorder. Given that prolonged exposure and massive extinction both increase the time spent in the presence of the CS, it is possible that these two manipulations share the same underlying mechanisms (e.g., enhanced CS-noUS learning).
Critically, Thomas et al. (2009) found that extinction in multiple contexts was only effective in reducing ABA renewal when a moderate number of extinction trials were used (144) in comparison with few extinction trials (36). Therefore, at least under certain parameters, the amount of extinction treatment seems to interact (instead of summate) with the number of contexts in which the extinction treatment takes place, which could explain why some researchers have failed to find an effect of extinction in multiple contexts alone (e.g., Bouton, García-Gutiérrez, et al., 2006). In Thomas et al.'s study, each manipulation alone was ineffective in reducing the return of fear when subjects were tested back in the acquisition context, but they had a multiplicative (i.e., interactive) effect when used together.
In summary, in the present series of experiments we evaluated a) whether spontaneous recovery and ABC renewal summate provoking an especially strong return of fear (Experiment 1), and b) whether two effective behavioral techniques for reducing return of conditioned fear (i.e., extinction in multiple contexts and massive extinction) summate to further prevent the return of fear after a spatiotemporal context shift.
Experiment 1: Renewal and Spontaneous Recovery
Experiment 1 was designed to evaluate whether the return of fear produced by a mismatch between the context of extinction and testing (i.e., renewal) summates with the return of fear produced by inserting a long retention interval between extinction and testing (i.e., spontaneous recovery) in a fear conditioning preparation (see Table 1 for details). We hypothesized that if these manipulations summate, then subjects tested after a long delay and outside the context of extinction should exhibit stronger recovery of extinguished fear responses than subjects that are tested with either restorative manipulation alone. The additivity of these phenomena would be evident if in a 2 (Time of testing: Short delay v. Long delay) × 2 (Context of testing: Context of extinction v. Neutral context) factorial ANOVA we find both a main effect of time of testing and of context of testing, in the absence of an interaction between these factors. In fact, an interaction between them would suggest that the effects of our manipulations are multiplicative (i.e., interactive) rather than additive (i.e., summative, see Myers & Well, 2003). An interaction of these two manipulations seemed unlikely because space and time seemingly constitute independent dimensions.
Table 1.
Design Summary of Experiment 1
| Groups | Acquisition | Retention Interval 1 | Extinction | Retention Interval 2 | Test | |
|---|---|---|---|---|---|---|
|
Acq
|
8 X + (A) | – | (B) / (C) | – | X (C) | |
| Ext |
Ext1
|
8 X + (A) | – | 162 X- (B) / (C) | – | X (B) |
| Ext2 | 8 X + (A) | 21 Days | 162 X- (B) / (C) | – | X (B) | |
|
|
||||||
| Ren |
Ren1
|
8 X + (A) | – | 162 X- (B) / (C) | – | X (C) |
| Ren2 | 8 X + (A) | 21 Days | 162 X- (B) / (C) | – | X (C) | |
|
|
||||||
|
Sp-Rec
|
8 X + (A) | – | 162 X- (B) / (C) | 21 Days | X (B) | |
| Ren+Sp-Rec | 8 X + (A) | – | 162 X- (B) / (C) | 21 Days | X (C) | |
Note: CS X was a 10-s click train. “+” denotes reinforcement with a brief footshock. “−” denotes no reinforcement. “−" denotes no treatment. A, B, and C, are different contexts. Numbers preceding letter X indicate total number of trials in that phase. See text for details.
To evaluate the effect of a delay between extinction and testing (i.e., spontaneous recovery), two controls were necessary. Groups Ext1 and Ren1 controlled for the time between acquisition and extinction, but confound the total interval between acquisition and testing, and Groups Ext2 and Ren2 controlled for the total interval between acquisition and testing, but confound the interval between acquisition and testing. Collectively Groups Ext1 and Ext2 permit assessment of spontaneous recovery. Groups Ext1 and Ext2, Like Groups Ren1 and Ren2, were expected to behave similarly, but that is an empirical question.
Methods
Subjects
The subjects were 42 male (292–430 g) and 42 female (213–288 g), experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Subjects were randomly assigned to one of seven groups (ns = 12; Acq, Ext1, Ext2, Ren1, Ren2, Sp-Rec, and Ren+Sp-Rec, where Acq = Acquisition, Ext = Extinction, Ren = Renewal, and Sp-Rec = Spontaneous recovery), counterbalanced within groups for sex. The animals were individually housed in standard hanging stainless-steel wire-mesh cages in a vivarium maintained on a 16/8-hr light/dark cycle. Experimental manipulations occurred during the light phase. The animals received free access to Purina Lab Chow, whereas water availability was limited to 20 min per day following a progressive deprivation schedule initiated one week prior to the start of the study. From the time of weaning until the start of the study, all animals were handled for 30 s, three times per week.
Apparatus
Twenty-four experimental chambers, of three different types, were used. Chamber type 1 (Ch1) was 30-cm long, 30-cm wide, and 27-cm high. The sidewalls of the chamber were made of stainless steel sheet metal, and the front wall, back wall, and ceiling of the chamber were made of clear Plexiglas. The floor was constructed of 0.3 cm diameter rods, spaced 1.3 cm center-to-center, and connected by NE-2 neon bulbs that allowed a 1.0-mA, 0.5-s, constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each of twelve copies of Ch1 was housed in an environmental isolation chest that was dimly illuminated by a houselight (1.12-Watt, #1820 incandescent bulb) mounted high on one wall of the experimental chamber.
Chamber type 2 (Ch2) was rectangular, measuring 24.0 × 9.0 × 12.5-cm (l × w × h). The walls and ceiling of Ch2 were clear Plexiglas, and the floor was comprised of stainless steel rods measuring 0.5-cm diameter, spaced 1.3-cm apart (center to center). The floor of Ch2 could provide footshock identical to that of Ch1. Each of six copies of Ch2 was housed in a separate light- and sound-attenuating environmental isolation chest. Each chamber was dimly illuminated by a 2-W (nominal at 120 VAC, but driven at 50 VAC) incandescent house light mounted on an inside wall of the environmental chest located approximately 30-cm from the center of the animal enclosure.
Chamber type 3 (Ch3) was 27-cm long, 29.5-cm high, 21.5-cm wide at the top, and 5.5-cm wide at the bottom. The floor was comprised of two 27-cm long plates, 2-cm wide, with a 1.5-cm gap between the two plates. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the side walls were stainless steel. The floor and side walls of Ch3 could provide footshock identical to that of Ch1 and Ch2. Each of six copies of Ch3 was housed in a separate sound- and light-attenuating environmental isolation chest. The chamber was illuminated by a 7-W (nominal at 120 VAC, but driven at 60 VAC) light bulb, which was mounted on the inside wall of the environmental chest, approximately 30-cm from the center of the experimental chamber. Light entered the chamber primarily by reflection from the ceiling of the environmental chest.
Each instance of Ch2 and Ch3 could be equipped with a water-filled lick tube that extended 1-cm into a cylindrical niche, which was 4.5-cm in diameter, left right centered, with its bottom 1.75-cm above the floor of the apparatus and 5.0-cm deep. In the same manner, Ch1 could be equipped with a water-filled lick tube that extended about 1 cm from the rear of a niche (4 × 4 × 5.5 cm, l × w × h) placed on the front wall. The lick tube entered the center of this niche 3.3 cm above the niche floor, which was at the level of the grid floor. In all chambers there was a photobeam detector 1-cm in front of the lick tube that was broken whenever the subject licked the tube. A 45-Ω speaker on an inside walls of each isolation chest could deliver a click train (6 Hz, 6 dB on the C scale) above background). Ventilation fans in each enclosure provided a constant 76-dB background noise. The light intensities inside the three chambers were approximately equal due to the difference in opaqueness of the walls.
A 10-s click train served as CS X and a 0.5-s footshock served as US. Context A consisted of an instance of Ch1 with the house light (HL) off, and a block of wood with two drops of banana essence located inside the isolation chest. Contexts B and C were Ch2 and Ch3, counterbalanced in these roles within groups. No nominal odor cue was added to these contexts.
Procedure
Acclimation
On Days 1 and 2, all subjects were acclimated to Contexts A, B, and C for 30 min. All subjects were exposed to Context B and C (in that order) on Day 1 and to Context A on Day 2. During the acclimation phase subjects did not have access to the water-filled lick tubes. There were no presentations of the CS or US during this phase.
Acquisition
On Days 3 and 4, all subjects received 60-min conditioning training sessions in Context A. Subjects received 4 daily presentations of X co-terminating with the US with a mean intertrial interval (ITI) of 15 min (from CS onset to CS onset). The reinforced trials occurred at 5, 16, 36, and 52 min into the session.
Retention interval 1
Between Days 5 and 25, subjects of Groups Ext2 and Ren2 stayed in their home cages and no further training took place. During this period, subjects were handled for 30 sec, three times per week.
Extinction
All groups, except Group Acq, received 162 extinction trials in Context B and equal amount of exposure to Context C. Group Acq simply received equal exposure to both contexts. All sessions were 2.25 hr in duration. The long duration of these sessions was unnecessary in Experiment 1, but was used to maintain similarity of parameters with Experiment 2. For all groups receiving extinction treatment, trials occurred with a mean ITI of 30 s (CS onset to CS onset; range = 15–45 s). The extinction trials were delivered in a block at the beginning or at the end of the extinction session, counterbalanced within groups. For Groups Ext1, Ren1, Sp-Rec, and Ren+Sp-Rec, the extinction sessions consisted of non-reinforced presentations of X in Context B on Days 6, 8, and 10, and context exposure sessions consisted of exposure to Context C without presentations of X on Days 5, 7, and 9. Group Acq was exposed to Context B and C on the same schedule. For Groups Ext2 and Ren2 the extinction sessions consisted of non-reinforced presentations of X in Context B on Days 27, 29, and 31, and context exposure sessions consisted of exposure to Context C without presentations of X on Days 26, 28, and 30.
Retention interval 2
Between Days 11 and 31, subjects of Groups Sp-Rec and Ren+Sp-Rec stayed in their home cages and no further treatment took place. Subjects were handled for 30 sec, three times per week.
Reacclimation
All subjects were reacclimated to Contexts B and C in two daily 30-min sessions (with the order of exposure to the contexts counterbalanced within groups). In these sessions subjects had free access to the water-filled lick tubes, and no nominal stimuli were programmed to occur. The purpose of these sessions was to establish a stable rate of drinking behavior, thereby providing similar baseline behavior across groups upon which conditioned lick suppression could be assessed. For Groups Ext1, Ren1, and Acq, the reacclimation sessions occurred on Days 11 and 12. For Groups Ext2, Ren2, Sp-Rec, and Ren+Sp-Rec, these sessions occurred on Days 32 and 33.
Testing
All subjects were tested for conditioned lick suppression to the target cue X on the day after reacclimation was completed. Upon placement in the test chamber, time spent drinking by each subject was recorded. Immediately after completion of an initial 5 cumulative seconds of licking in the absence of any nominal stimulus, subjects were presented with CS X (i.e., the click train) for 15 min. Thus, all subjects were drinking at the time of CS onset. Time to complete an additional 5 cumulative seconds of licking in the presence of X was recorded. The times recorded during the presentation of X were interpreted as reflecting subjects' expectancy of the US following onset of X. The test session was 16 min in duration, and a ceiling score of 15 min was imposed on the time to complete 5 cumulative seconds of drinking in the presence of X. In practice, only one rat (from Group Acq) reached the 15 min ceiling score. Following the convention of our laboratory, all animals that took more than 60 s to complete their first 5 cumulative seconds of licking (i.e., prior to CS onset) during the test session were scheduled to be eliminated from the study because such long latencies may be considered indicative of unusually great fear of the test context. In practice, one subject from Group Sp-Rec met this elimination criterion and was excluded from all analyses.
Consistent with the experimental design, some groups were tested in a test session on Day 13 and others in a test session on Day 34. During the Day 13 test session, subjects from Groups Ren1 and Acq were tested in Context C, while subjects from Group Ext1 were tested in Context B. During the Day 34 test session, subjects from Groups Ext2 and Sp-Rec were tested two at the time in Context B, while subjects from Groups Ren2 and Ren+Sp-Rec were tested in the same manner but in Context C.
Data analysis
Latencies to drink for five cumulative seconds before the onset of the test stimulus (i.e., pre-CS measure) and after the onset of the test stimulus (i.e., CS measure) were transformed to log10 to better approximate the normal distributions assumed by parametric statistical analyses. Analyses of variance (ANOVA) were used to evaluate potential baseline differences and to determine whether our manipulations affected subjects' log latencies to drink in the presence of the test stimulus. The error term from the ANOVA served as an estimate of within-group variance in planned comparisons. Effect size was estimated using Cohen's f (Myers & Wells, 2003). Alpha was set at .05.
Results and Discussion
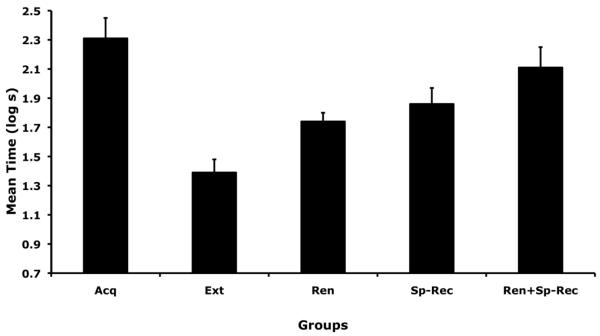
As can be seen in Figure 1, in the present study we found acquisition, extinction, ABC renewal, spontaneous recovery, and more important, we found that ABC renewal and spontaneous recovery summated producing significantly more return of fear than renewal, but only numerically more return of fear than spontaneous recovery. The following statistical analysis supported these conclusions.
Figure 1.
Results of Experiment 1. Mean log10 time to complete 5 cumulative seconds of licking in the presence of target CS (X). Brackets represent the standard error of the means. Higher scores indicate more conditioned fear. Acq = group that received no extinction trials and was tested in a neutral but familiar context (C); Ext = group that received a moderate number of extinction trials in only one context (B) and was tested in the extinction context following a short delay after extinction; Ren = group that received a moderate number of extinction trials in only one context (B) and was tested in a neutral but familiar context (C) after a short delay following extinction; Sp-Rec = group that received a moderate number of extinction trials in only one context (B) and was tested in the extinction context after a long delay following extinction; Ren+Sp-Rec = group that received a moderate number of extinction trials in only one context (B) and was tested in a neutral but familiar context (C) after a long delay following extinction. See text and Table 1 for further details.
A one-way ANOVA applied to the log pre-CS test data showed no differences among groups in baseline drinking behavior, F(6, 76) = 1.76, p = 0.12, MSE = 0.04, indicating that the experimental groups did not appreciably differ in their baseline behavior. However, the same analysis proved to be significant when the log CS data were examined, F(6, 76) = 8.31, p < .001, MSE = 0.17, Cohen's f = 0.73. Four planned comparisons were then performed. A significant difference was found when Group Acq was compared with Groups Ext1, F(1, 76) = 29.58, p < .001, and Ext2, F(1, 76) = 30.67, p < .001, indicating that the extinction treatment used was successful in reducing fear responding in our preparation. The level of conditioned responding after extinction treatment did not differ between Groups Ext1 and Ext2, F(1, 76) = 0.01, p = 0.92, indicating that a delay between acquisition and extinction had negligible impact on the consequences of the extinction treatment. The similar level of responding between Groups Ext1 and Ext2 was confirmed by a Bayesian analysis with odds of 16.68 in favor of the null hypothesis (Rouder et al., 2009). Considering these results, Groups Ext1 and Ext2 were pooled as Group Ext for all further statistical analyses. A similar situation occurred with Groups Ren1 and Ren2. The level of responding did not differ between Groups Ren1 and Ren2, F(1, 76) = 0.01, p = 0.94, also indicating that a delay between acquisition and extinction had negligible impact on the consequences of the extinction treatment. The similar level of responding between Groups Ren1 and Ren2 was confirmed by a Bayesian analysis with odds of 16.78 in favor of the null hypothesis (Rouder et al., 2009). Considering these results, Groups Ren1 and Ren2 were pooled as Group Ren for all further statistical analyses.
The additivity of ABC renewal and spontaneous recovery was tested with a 2 (Time of testing: Short delay vs. Long delay) × 2 (Context of testing: B vs. C) factorial ANOVA. This analysis showed a main effects of the time of testing, F(1, 67) = 17.75, p < .001, MSE = 0.15, Cohen's f = 0.49, indicating that more return of fear occurred when subjects were tested after a long delay following extinction than when they were tested after a short delay following extinction (i.e., spontaneous recovery was observed). The main effect of the context of testing was also significant, F(1, 67) = 9.29, p < .001, Cohen's f = 0.34, indicating that more return of fear occurred when subjects were tested outside the context of extinction than when subjects were tested in the context of extinction (i.e., ABC renewal was observed). Of importance, the interaction between these factors did not prove significant, F(1, 67) = 0.27, p = .60, suggesting that these effects summated instead of interacted to produce more return of fear. Two planned comparisons were then performed. A significant difference was found when Group Ren was compared with Group Ren+Sp-Rec, F(1, 67) = 7.04, p < .001, confirming that the return of fear was significantly stronger following a spatiotemporal context shift than following a spatial context shift alone. A similar analysis showed that Group Sp-Rec did not significantly differ from Group Ren+Sp-Rec, F(1, 67) = 2.37, p = 0.13, although the numerical difference was in the expected direction (i.e., Sp-Rec < Ren+Sp-Rec).
In summary, the present results showed return of fear when testing occurred in a neutral but familiar context and when a long delay was imposed between extinction treatment and testing. Importantly, these manipulations summated to produce a stronger return of fear than did either of them used separately; the combined effect was significantly stronger than renewal alone, but only numerically stronger than spontaneous recovery alone. This result is not surprising, but neither it is obvious, as the two manipulations could have been exploiting a common source that might have been exhausted by either manipulation alone. The extremely effective return of fear observed with combined ABC renewal and spontaneous recovery provided a high baseline to investigate potential means of preventing the return of fear in Experiment 2 (also see, Laborda, Miguez, & Miller, 2012).
Experiment 2: Massive Extinction in Multiple Contexts
Experiment 2 was designed to evaluate the conjoint effects of extinction in multiple contexts (Gunther et al., 1998) and massive extinction (Denniston et al., 2003) in a situation in which renewal and spontaneous recovery summate. Thomas et al. (2009) recently assessed the conjoint effect of these manipulations in an ABA renewal situation, finding reduced return of fear only when both manipulations were used together, with each manipulation alone not been more effective than a control group that received a few extinction trials in only one context. No summative effect was detected; instead, two underpowered manipulations were found to have a multiplicative effect making their collective effects significant.
There are several differences between Thomas et al.'s (2009) and the present study, but most importantly: a) we used parameters that have proven to be effective in reducing the return of fear with extinction in multiple context alone (Gunther et al., 1998), b) we used parameters that have proven to be effective in reducing the return of fear with massive extinction treatment alone (Denniston et al., 1998), c) and we evaluated the summative effect of these manipulation in reducing a stronger return of fear provoked by a delayed context shift. Critically, Thomas et al. confounded the increase in extinction trials with an increase in the number of extinction sessions, which by itself may have reduced the return of fear as extinguishing in multiple sequential sessions can be considered extinction in multiple temporal contexts (for a similar analysis of the role of sessions in the return of fear, see Laborda, Miguez, & Miller, 2012). Here we controlled for this confounding by keeping the number and length of the extinction sessions constant while manipulating only the number of extinction trials.
We hypothesized that extinction treatment in multiple contexts and massive amounts of extinction treatment would reduce the strong return of fear seen in Experiment 1 (i.e., Group Ren+Sp-Rec). Moreover, we hypothesized that both of these manipulations applied together would be more effective in attenuating the return of fear than each manipulation alone (see Table 2 for details). Massive extinction was expected to strengthen the memory of extinction treatment, whereas extinction in multiple contexts was expected to encourage the generalization of facilitatory cues from the extinction context(s) to the test context. As these two mechanisms appear to be independent (e.g., Miller & Laborda, 2011), summation of these two manipulations was expected rather than an interaction. The additivity of these techniques would be evident if in a 2 (Extinction contexts: Single v. Multiple) × 2 (Extinction trials: Moderate v. Massive) factorial ANOVA we found significant main effects of these factors in the absence of an interaction between them. In fact, an interaction between them would suggest that the effects of our manipulations are multiplicative (or interactive) rather than additive (or summative) (see Myers & Well, 2003).
Table 2.
Design Summary of Experiment 2
| Groups | Acquisition | Extinction | Retention Interval | Test | |||
|---|---|---|---|---|---|---|---|
|
Single/Moderate
|
8 X+ (A) | 162 X- (B) | (C) | (D) | (E) | 21 Days | X (E) |
|
Single/Massive
|
8 X+ (A) | 810 X- (B) | (C) | (D) | (E) | 21 Days | X (E) |
|
Multiple/Moderate
|
8 X+ (A) | 54 X- (B) | 54 X- (C) | 54 X- (D) | (E) | 21 Days | X (E) |
| Multiple/Moderate | 8 X+ (A) | 270 X- (B) | 270 X- (C) | 270 X- (D) | (E) | 21 Days | X (E) |
Note: CS X was a 10-s click train. “+” denotes reinforcement with a brief footshock. “-” denotes no reinforcement. A, B, C, D, and E are different contexts. Numbers preceding letter X indicate total number of trials in that phase. See text for details.
Method
Subjects
Subjects were 24 male (167 – 272 g) and 24 female (166 – 221 g), experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Subjects were randomly assigned to one of four groups (ns = 12; Single/Moderate, Single/Massive, Multiple/Moderate, and Multiple/Massive, where Single = single extinction context, Multiple = multiple extinction contexts, Moderate = moderate number of extinction trials, and Massive = massive number of extinction trials), counterbalanced within groups for sex. The maintenance and housing of subjects were the same as in Experiment 1.
Apparatus
The chambers and stimuli used in Experiment 2 were the same as those used in Experiment 1 except where otherwise described. Six additional Ch2 were used. Five physical contexts were used in this study, one for conditioning (Context A), three for extinction (Contexts B, C, and D), and one for testing (Context E). Context A consisted of an instance of Ch1 with the house light (HL) off, and a block of wood with two drops of banana essence located inside the isolation chest. No Plexiglas floor was used in this context. The physical contexts used as Contexts B, C, and D were counterbalanced within groups. The three extinction contexts were: (1) an instance of Ch2 with HL off, a block of wood with two drops of 98% methyl salicylate, and Plexiglas floor; (2) an instance of Ch2 (different from the one used as [1]) with HL on (no Plexiglas floor or odor cue was used in this context); (3) an instance of Ch3 with the HL on. Finally, Context E consisted of an instance of Ch1 (different from the one used as Context A) with HL on and a Plexiglas floor. No odor cue was used in this context.
Procedure
Acclimation
On Day 1, all subjects were acclimated to their versions of Context E for 30 min. Subjects were acclimated only to Context E because that is where all animals were tested. During the acclimation phase, subjects had access to water-filled lick tubes. As in Experiment 1, there were no presentations of the CS or US during this phase.
Acquisition
On Days 2 and 3, all subjects received 60-min conditioning training sessions in Context A, exactly as described in Experiment 1.
Extinction
During this phase of the experiment, all subjects received an equal amount of exposure to Contexts B, C, D, and E in accordance with the experimental design (see Table 3 which summarizes the order of sessions, the amount of extinction, and the context of extinction for all groups). During these sessions, subjects in Condition Single received extinction trials in Context B and equal exposure to Contexts C, D, and E. Subjects in Condition Multiple received extinction trials in Contexts B, C, and D and equal exposure to Context E. Condition Moderate received moderate extinction treatment (162 trials), whereas Condition Massive received massive amount of extinction treatment (810 trials). Extinction sessions consisted of nonreinforced presentations of X in the appropriated context (Days 7, 11, and 15). Context exposure sessions consisted of exposure to the appropriated context without presentations of X to equally expose subject to Context B, C, D, and E (Days 4–6, 8–10, and 12–14). Lick tubes were present only during exposure to Context E, which served later as the test context for all groups. All sessions were 2.25 hr in duration. For all groups, extinction trials had a mean ITI of 30 s (CS onset to CS onset; range = 15–45 s). To accomplish this, while avoiding confounding session duration and trial spacing, groups that received 54 extinction trials per session received them in a block at the middle of the extinction session.
Table 3.
Order of extinction and context exposure sessions in Experiment 2
| Days 4, 5, 6 | Day 7 | Days 8, 9, 10 | Day 11 | Days 12, 13, 14 | Day 15 | |
|---|---|---|---|---|---|---|
|
Single/Moderate
|
(E), (D), (C) | 54 X- (B) | (E), (D), (C) | 54 X- (B) | (E), (D), (C) | 54 X- (B) |
|
Single/Massive
|
(E), (D), (C) | 270 X- (B) | (E), (D), (C) | 270 X- (B) | (E), (D), (C) | 270 X- (B) |
|
Multiple/Moderate
|
(E), (D), (C) | 54 X- (B) | (E), (D), (B) | 54 X- (C) | (E), (B), (C) | 54 X- (D) |
| Multiple/Massive | (E), (D), (C) | 270 X- (B) | (E), (D), (B) | 270 X- (C) | (E), (B), (C) | 270 X- (D) |
Note: Following the experimental design each group received a number of extinction trials in the indicated context on Days 7, 11, and 15. During the rest of the days (Days 4–6, 8–10, and 12–14), exposure to Contexts B, C, D, and E was equated for each group over the entire extinction session. See text for details.
Retention interval
Between Days 16 and 36, all subjects stayed in their home cages and no treatment took place. Subjects were handled for 30 s, three times per week.
Reacclimation
On Days 37 and 38, all subjects were reacclimated in two daily 30-min sessions to Contexts E. The rest of the procedure was identical to that of Experiment 1.
Testing
On Day 39, all subjects were tested one at a time for conditioned lick suppression to X in Context E. The rest of the testing procedures were the same as in Experiment 1. In the present experiment no subjects met the exclusion criteria (i.e., more than 60 s to complete their first 5 cumulative seconds of licking prior to CS onset) and no subjects reached the ceiling of 15 min during testing.
Data analysis
As in Experiment 1, latencies to drink for five cumulative seconds before the onset of the test stimulus (i.e., pre-CS measure) and after the onset of the test stimulus (i.e., CS measure) were transformed to log10 to better approximate the normal distributions assumed by parametric statistical analyses. Analyses of variance (ANOVA) were used to evaluate potential baseline differences and to determine whether our manipulations affected subjects' log latencies to drink in the presence of the test stimulus. The error term from the ANOVA served as an estimate of within-group variance in planned comparisons. Effect size was estimated using Cohen's f (Myers & Wells, 2003). Alpha was set at .05.
Results and Discussion
The results of Experiment 2 are depicted in Figure 2. In this experiment strong return of fear was expected in all subjects (i.e., they were trained in a design that allows ABC renewal and spontaneous recovery to summate, see Experiment 1); however, in three of the four groups we observed some attenuation of this reappearance of fear. As predicted, Group Single/Moderate exhibited the strongest return of fear at test (highly similar in degree to the recovery of fear observed in the similarly treated Group Ren+Sp-Rec of Experiment 1, but this assessment should be taken with caution given it relies on a cross-experiment comparison). This return of fear was attenuated when extinction took place in multiple contexts (Group Multiple/Moderate) and when massive extinction treatment was administered (Group Single/Massive). Importantly, when both behavioral manipulations were used together, return of fear was weaker than when each manipulation was used alone. Moreover, it seems that the joint effect of our manipulations totally abolished the return of fear, as is suggested by rats in Group Massive/Multiple exhibiting even less suppression than the Group Ext in Experiment 1 (however, as a cross-experiment comparison, this claim should be taken with caution). The following statistical analysis supported these conclusions.
Figure 2.
Results of Experiment 2. Mean log10 time to complete 5 cumulative seconds of licking in the presence of the target CS (X) after a long delay following extinction in a neutral but familiar context (E) for all groups. Brackets represent the standard error of the means. Higher scores indicate more conditioned fear. Single = condition that received extinction trials in only one context (B); Multiple = condition that received extinction trials in three different contexts (B, C, and D); Moderate = condition that received a moderate number of extinction trials (162); Massive = condition that received a massive number of extinction trials (810). See text and Tables 2 and 3 for further details.
A 2 (Contexts of extinction: Single vs. Multiple) × 2 (Number of extinction trials: Moderate vs. Massive) ANOVA applied to the log pre-CS on the test data showed a nonsignificant main effect of number of extinction contexts, F(1, 44) = 0.04, p = .85, MSE = 0.06, a nonsignificant main effect of number of extinction trials, F(1, 44) = 0.05, p = .83, and a nonsignificant interaction of these factors, F(1, 44) = 1.77, p = .19, indicating that the experimental groups did not significantly differ in baseline drinking behavior. A similar analysis was performed to evaluate the additivity of massive extinction and extinction in multiple contexts. This analysis on conditioned suppression during the CS (i.e., log CS data) revealed a significant main effects of number of extinction contexts, F(1, 44) = 17.23, p < .001, MSE = 0.19, Cohen's f = 0.58, indicating that extinction in multiple context decreased the return of fear. The main effect of number of extinction trials was also significant, F(1, 44) = 15.04, p < .001, Cohen's f = 0.54, indicating that massive extinction trials reduced the return of fear. Of central importance, the interaction between these factors did not prove significant, F(1, 44) = 2.04, p = .16, suggesting that these effects primarily summated instead of interacted to reduce the return of fear. Two planned comparisons were then performed. A significant difference was found when Group Single/Massive was compared with Group Multiple/Massive, F(1, 44) = 15.60, p < .001, indicating that the return of fear was weaker following massive extinction in multiple contexts than following massive extinction alone. A significant difference was also found when Group Multiple/Moderate was compared with Group Multiple/Massive, F(1, 44) = 14.07, p < .001, indicating that the return of fear was weaker following massive extinction in multiple contexts than following extinction in multiple contexts alone.
In summary, the present results showed that massive extinction treatment and extinction in multiple contexts were effective in reducing the return fear produced by a spatiotemporal context shift. Moreover, both techniques together further reduced this strong return of fear. Analogous to the summation effect seen in Experiment 1, the opposing summation effect observed in Experiment 2 is not surprising; however, it is not foreordained, as one of the manipulations to prevent the return of fear could have rendered the other ineffectual.
General Discussion
In Experiment 1, subjects that were tested after a spatiotemporal context shift displayed more return of fear than subjects that were tested after a physical or temporal context shift alone. In other words, ABC renewal and spontaneous recovery summated, producing a stronger return of fear. However, the stronger return of fear evidenced with conjoint renewal and spontaneous recovery was only numerical when compared to spontaneous recovery alone. These results extend Rosas and Bouton's (1998) findings to a fear conditioning preparation. For research purposes, a preparation that produces strong return of fear is especially useful because it should be highly sensitive to techniques designed to reduce fear reappearance. Also, a preparation that encourages return of fear through spatiotemporal changes seems to be more naturalistic than other situations, given that relapse from exposure therapies is most common when patients confront disorder-related stimuli outside the psychotherapeutic clinic and after an interval following the end of treatment (e.g., Craske, 1999; Rachman, 1989).
In Experiment 2, subjects that received massive extinction treatment in multiple contexts displayed less return of fear than subjects that received massive extinction treatment alone or extinction treatment in multiple contexts alone. Each of these techniques alone was effective in reducing the return of fear after a spatiotemporal context shift (extending Gunther et al.'s [1998] and Denniston et al.'s [2003] findings to a different preparation); moreover, both techniques together were highly effective in reducing this strong return of fear.
In previous research, Thomas et al. (2009) found an interactive (instead of a summative) effect between extinction in multiple contexts and massive extinction training. The difference with the present results is likely due to two factors. First, each manipulation in Thomas et al.'s study was by itself ineffective in reducing the return of fear. It may be true that the implementation of underpowered versions of these techniques together interact and reach a threshold to affect behavior. In our study, each technique by itself was effective in reducing the return of fear, which seemed to encourage a summation of their individual effects. Second, in Thomas et al.'s study more extinction trials were confounded with more extinction sessions. Given that each successive extinction session may enhance the generalization of extinction learning to different temporal test contexts (for an analysis of time as context, see Bouton, 2010; also see Laborda, Miguez, & Miller, 2012), their results should be considered with caution.
It is worth noting that an interference account of extinction (e.g., Laborda & Miller, 2012; Miller & Laborda, 2011) predicts all of the results presented here. In the case of the present study, the amount of contextual change between the context of extinction and the context of testing was expected to be positively correlated with the amount of return of fear at test, as was observed in Experiment 1. Also, according to this account, techniques that enhance extinction learning (such as administering a very large amount of extinction treatment) and techniques that augment the potential facilitatory cues from the context of extinction that are present at test (such as extinguishing in multiple contexts) are expected to summate in reducing the return of fear, as was observed in Experiment 2.
An important constraint for the translation of the present results to clinical situations comes from the results of Gunther et al.'s (1998) Experiment 2. The benefit of extinguishing in multiple contexts disappeared when acquisition was also conducted in multiple contexts. An unfortunate consequence of their results is that patients who have experienced multiple traumas (e.g., war combatants) or patients who have used drugs in many situations (e.g., smokers) may not benefit from this technique alone. However, whether such constrain is eliminated when using massive extinction in multiple contexts is still an experimental question.
In future research some limitations of the present studies should be considered. First, in Experiment 1 the summative effects of ABC renewal and spontaneous recovery was documented based on the finding of both effects being significant in the absence of a significant interaction between them. Then, a planned comparison confirmed that both manipulations used together evoked more return of fear than only a physical context shift. However, a second planned comparison failed to confirm that both manipulations used together evoked more return of fear than only a long delay between extinction and testing (p = .13), although the difference in responding was in the expected direction. Future research interested in the relationships between different sources of return of fear should use longer delays between extinction and testing toward inducing more spontaneous recovery of fear so that there will be more return of fear to potentially be countered. Second, our results of Experiment 2 are mute with respect to the effect of our manipulations on extinction itself, as we did not include ABB control groups. Future research should include such groups to evaluate whether our manipulations have an effect in behavior during extinction (which might well reflect both associative and nonassociative processes; Rescorla, 2001), or they are just techniques that enhance generalization to contexts different from the context of extinction.
Future efforts should also: a) evaluate whether these techniques can reduce other sources of return of fear (e.g., reinstatement and rapid reacquisition), b) evaluate whether these techniques can reduce the return of extinguished responses in other preparations (e.g., alcohol tolerance), and importantly, c) investigate ways to implement these findings in more clinical situations. Exposure sessions using many fear-eliciting cue exposures in multiple settings are likely to reduce the return of fear after therapy, but they are apt to require more time and effort on the part of the therapist.
The implementation of the techniques studied here must be done considering some specific constrains. The implementation of the multiple contexts manipulations in clinical settings may seem easy in principle, but clinicians should bear in mind that a “context” is created by a multitude of features of different dimensions. For example, a context can be influenced by the physical background, drug states, mood states, hormonal states, and even by the passage of time (Bouton, 2010). To put this manipulation into therapeutic practice, we would suggest planning therapeutic sessions in different rooms and buildings, with different settings, odors, luminescence, at different times of the day and in different days of the week, maybe even with different therapists. Implementation of the massive exposure can take different forms. The problem here is what is considered a “trial” in a therapeutic setting. Does an exposure trial consist of every time a patient thinks about the feared stimulus or only direct exposure to the feared stimulus? Depending on the conceptualization of what is an exposure trial, different applications of massive exposure are possible. Also, other factors should be taken in count such as the spacing between exposure trials (e.g., Laborda, Miguez, & Miller, 2012; Urcelay, Wheeler, & Miller, 2009).
As a final remark, we think that more effort should be directed toward identifying and evaluating techniques to reduce the return of fear in highly controlled analogue animal models (such as in the present report) and in translational studies (such as reported by Collins & Brandon [2002] and Rodriguez et al. [1999]) because these are the types of studies which could give us tools to reduce relapse in clinical settings. Importantly, the summation of techniques to reduce the return of fear, such as the one presented in Experiment 2 and in other reports (e.g., Laborda, Miguez, & Miller, 2012), is a promising result that may lead the way to the development of enhanced extinction procedures. Grounding procedures on solid laboratory research could greatly increase the efficacy of exposure therapy.
Highlights
-
>
We used fear conditioning in rats to model anxiety disorders, extinction to model exposure therapy, and the return of fear to model relapse.
-
>
Return of fear was facilitated by both a change of context from extinction to testing (i.e., renewal) and a delay in testing (i.e., spontaneous recovery).
-
>
These two effects summated to produce more robust return of fear than renewal alone.
-
>
Massive extinction and extinction in multiple contexts each attenuated this robust reappearance of fear.
-
>
These two manipulations in compound summated to reduce return of fear more than either treatment alone.
Acknowledgments
National Institute of Mental Health Grant 33881 supported this research. Mario A. Laborda was supported by the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT-Chile). The authors would like to thank Henry Cham, Lisa Mash, Gonzalo Miguez, and Cody Polack for their comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandarian Balooch S, Neumann DL. Effect of multiple contexts and context similarity on the renewal of extinguished conditioned behaviour in an ABA design with humans. Learning and Motivation. 2011;42:53–63. [Google Scholar]
- Betancourt R, Corada L, Dominichetti J, Laborda MA, Martínez G, Miguez G. Efecto de la extinción en múltiples contextos sobre la renovación de la tolerancia a las drogas. Psicothema. 2008;20:279–283. [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigm of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: Implications for exposure therapy. Behaviour Research and Therapy. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychology. 2000;19:57–63. doi: 10.1037/0278-6133.19.suppl1.57. [DOI] [PubMed] [Google Scholar]
- Bouton ME. The multiple forms of context in associative learning theory. In: Mesquita B, Feldman Barrett L, Smith E, editors. The mind in context. The Guilford Press; New York, NY: 2010. pp. 233–258. [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Bouton ME, García-Gutiérrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behaviour Research and Therapy. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Nelson JB. The role of context in classical conditioning: Some implications for cognitive behavior therapy. In: O'Donohue W, editor. Learning and behavior therapy. Allyn and Bacon; Boston: 1998. pp. 59–84. [Google Scholar]
- Chambless DL, Ollendick T. Empirically supported psychological interventions: Controversies and evidence. Annual Review of Psychology. 2001;52:685–716. doi: 10.1146/annurev.psych.52.1.685. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Calton JL, Hart JA, Schachtman TR. Attenuation of the renewal effect by extinction in multiple contexts. Learning and Motivation. 1999;30:1–14. [Google Scholar]
- Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. Journal of Consulting and Clinical Psychology. 2002;70:390–397. [PubMed] [Google Scholar]
- Craske MG. Anxiety disorders: Psychological approaches to theory and treatment. Westview Press; Boulder, CO: 1999. [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Craske MG, Liao B, Brown L, Vervliet B. Role of inhibition in exposure therapy. Journal of Experimental Psychopathology. 2012;3:322–345. [Google Scholar]
- Denniston JC, Chang RC, Miller RR. Massive extinction attenuates the renewal effect. Learning and Motivation. 2003;34:68–86. [Google Scholar]
- Effting M, Kindt M. Contextual control of human fear associations in a renewal paradigm. Behaviour Research and Therapy. 2007;45:2002–2018. doi: 10.1016/j.brat.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Field AP. Is conditioning a useful framework for understanding the development and treatment of phobias? Clinical Psychology Review. 2006;26:857–875. doi: 10.1016/j.cpr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Cahill SP, Rauch SAM, Riggs DS, Feeny NC, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. Journal of Consulting and Clinical Psychology. 2005;73:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Glautier S, Elgueta T. Multiple cue extinction effect on recovery of responding in causal judgments. International Journal of Comparative Psychology. 2009;22:254–270. [Google Scholar]
- Gunther LM, Denniston JC, Miller RR. Conducting exposure treatment in multiple contexts can prevent relapse. Behaviour Research and Therapy. 1998;36:75–91. doi: 10.1016/s0005-7967(97)10019-5. [DOI] [PubMed] [Google Scholar]
- Laborda MA, McConnell BL, Miller RR. Behavioral techniques to reduce relapse after exposure therapy: Applications of studies of experimental extinction. In: Schachtman TR, Reilly S, editors. Associative learning and conditioning theory: Human and non-human applications. Oxford University Press; New York, NY: 2011. pp. 79–103. [Google Scholar]
- Laborda MA, Miguez G, Miller RR. Preventing return of fear in an animal model of anxiety: Additive effect of spacing extinction trials and sessions. Manuscript submitted for publication; 2012. [Google Scholar]
- Laborda MA, Miguez G, Polack CW, Miller RR. Animal models of psychopathology: Historical models and the Pavlovian contribution. Terapia Psicológica. 2012;40:45–59. [Google Scholar]
- Laborda MA, Miller RR. S-R associations, their extinction, and recovery: A new associative account of phobias without recall of original trauma. Behavior Therapy. 2011;42:153–169. doi: 10.1016/j.beth.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda MA, Miller RR. Reactivated outcome memories compete for expression after Pavlovian extinction. Behavioural Processes. 2012;90:20–27. doi: 10.1016/j.beproc.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda MA, Witnauer JE, Miller RR. Contrasting AAC and ABC renewal: The role of contexts associations. Learning & Behavior. 2011;39:46–56. doi: 10.3758/s13420-010-0007-1. [DOI] [PubMed] [Google Scholar]
- McConnell BL, Miller RR. Associative theories of extinction and recovery-from-extinction effects. Manuscript submitted for publication; 2012. [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Miller RR, Laborda MA. Preventing recovery from extinction and relapse: A product of current retrieval cues and memory strengths. Current Directions in Psychological Science. 2011;20:325–329. [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Myers JM, Wells AD. Research design and statistical analysis. 2nd ed Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- Neumann DL. The effects of physical context changes and multiple extinction contexts on two forms of renewal in a conditioned suppression task with humans. Learning and Motivation. 2006;37:149–175. [Google Scholar]
- Neumann DL, Kitlertsirivatana E. Exposure to a novel context after extinction causes a renewal of extinguished conditioned responses: Implications for the treatment of fear. Behaviour Research and Therapy. 2010;48:565–570. doi: 10.1016/j.brat.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Lipp OV, Cory SE. Conducting extinction in multiple contexts does not necessarily attenuate the renewal of shock expectancy in a fear-conditioning procedure with humans. Behaviour Research and Therapy. 2007;45:385–394. doi: 10.1016/j.brat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, editor. Oxford University Press; London: 1927. [Google Scholar]
- Pineño O, Miller RR. Signaling a change in cue-outcome relations in human associative learning. Learning & Behavior. 2004;32:360–375. doi: 10.3758/bf03196034. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rachman S. The return of fear. Behaviour Research and Therapy. 1979;17:164–166. doi: 10.1016/0005-7967(79)90028-7. [DOI] [PubMed] [Google Scholar]
- Rachman S. The return of fear: Review and prospect. Clinical Psychology Review. 1989;9:147–168. [Google Scholar]
- Rauhut AS, Thomas BL, Ayres JJB. Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: Implications for treating human phobias. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27:99–114. [PubMed] [Google Scholar]
- Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Erlbaum; Hillsdale, NJ: 2001. pp. 119–154. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current theory and research. Appleton-Century Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Rodriguez BI, Craske MG, Mineka S, Hladek D. Context-specific of relapse: Effects of therapist and environmental context on return of fear. Behaviour Research and Therapy. 1999;37:845–862. doi: 10.1016/s0005-7967(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Rosas JM, Bouton ME. Context change and retention interval can have additive, rather than interactive, effects after taste aversion extinction. Psychonomic Bulletin and Review. 1998;5:79–83. [Google Scholar]
- Rosas JM, Vila NJ, Lugo M, López L. Combined effect of context change and retention interval on interference in causality judgment. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27:153–164. [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD. Bayesian t-test for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Tamai N, Nakajima S. Renewal of formerly conditioned fear in rats after extensive extinction training. International Journal of Comparative Psychology. 2000;13:137–147. [Google Scholar]
- Thomas BL, Vurbic D, Novak C. Extensive extinction in multiple contexts eliminates the renewal of conditioned fear in rats. Learning and Motivation. 2009;40:147–159. [Google Scholar]
- Urcelay GP, Wheeler DS, Miller RR. Spacing extinction trials alleviates renewal and spontaneous recovery. Learning & Behavior. 2009;37:60–73. doi: 10.3758/LB.37.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay GP, Witnauer JE, Miller RR. The dual role of the context in postpeak performance decrements resulting from extended training. Learning & Behavior. doi: 10.3758/s13420-012-0068-4. in press. doi:10.3758/s13420-012-0068-4. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P. Return of fear in a human differential conditioning paradigm caused by a return to the original acquisition context. Behaviour Research and Therapy. 2005;43:323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Vervliet B, Iberico C, Baeyens F, Van den Bergh O, Hermans D. The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behaviour Research and Therapy. 2007;45:1169–1179. doi: 10.1016/j.brat.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: A critical review of renewal research in humans. Biological Psychology. doi: 10.1016/j.biopsycho.2012.01.006. in press. doi:10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]