Abstract
High serum follicle-stimulating hormone (FSH) levels have been associated with diminished ovarian reserve; however, the association between high urinary FSH and reduced natural fertility has yet to be established. We sought to characterize the relationship between a single or multiple measurements of early follicular phase urinary FSH and fertility. Women (n = 209), 30 to 44 years old with no history of infertility, who had been trying to conceive for less than 3 months, provided early follicular phase urine. Participants subsequently kept a diary to record bleeding and intercourse and conducted standardized pregnancy testing for up to 6 months. A subset of women (N = 95) collected urine on cycle day 3 for up to 6 cycles. Urine was analyzed for FSH and creatinine (cr) corrected. Proportional hazard models were used to calculate fecundability ratios (FRs). Urinary FSH levels across cycles from the same woman were highly correlated (adjusted intraclass correlation = .77); within-woman variance was 3-fold lower than variance among women. Women with an initial urinary FSH level <7 mIU/mg cr exhibited a nonsignificant reduction in the probability of pregnancy (adjusted FR 0.71, 95% confidence interval [CI]: 0.45-1.13), as did women with elevated urinary FSH (≥12 mIU/mg cr; adjusted FR 0.78, 95% CI: 0.46-1.32). Using the most recent or maximum urinary FSH value did not strengthen the association. In the general population, urinary FSH levels appear to be nonlinearly associated with fertility; however, broad CIs indicate a lack of statistical significance. Repetitive testing appears to be of little benefit.
Keywords: fecundability, fertility, follicle-stimulating hormone, ovarian reserve, urine
Introduction
Women are delaying their attempts to conceive to later ages, increasing the prevalence of age-related infertility.1 The American Society for Reproductive Medicine launched a campaign in 2002 to increase awareness of age-related infertility. While chronologic age is a strong predictor of fertility, women of similar ages differ in their reproductive potential. Fertility tests could potentially identify those women at risk for infertility due to reproductive aging irrespective of their chronologic age.
Reproductive aging is thought to be due to the decline in the quality and quantity (ovarian reserve) of oocytes and the follicular pool.2–5 The decline in the follicular pool leads to less production of inhibin, a granulosa cell product.6 Lower serum inhibin levels lead to elevated early follicular phase serum follicle-stimulating hormone (FSH) levels7 and a shortened follicular phase.8 Accordingly, early follicular phase serum FSH levels are inversely correlated with the number of follicles in the ovary as determined histologically.9
Because urinary and serum FSH levels are strongly correlated with each other,10,11 urinary FSH has been used as a tool for field studies in epidemiology or for commercial testing in at-home kits. A number of commercial urinary FSH kits are FDA approved for this purpose and marketed to consumers. However, the validity of these tests in predicting natural fertility has yet to be determined.
In a preliminary analysis of 100 women, we noted a nonsignificant, 40% reduction in day-specific fecundability in women with elevated urinary FSH values.12 We concluded that a larger study was needed to refine estimates and to explore cutoff values. In addition, the potential benefit of repetitive testing for predicting fertility needs further study, as previous studies have shown significant intercycle variability, especially among older women.13 Thus the objective of this study was to explore the potential value of urinary FSH in predicting natural fertility, as measured by cycle-specific and day-specific probabilities of conception.
Materials and Methods
Time to conceive, a time-to-pregnancy study, was approved by the institutional review board of the University of North Carolina. English-speaking women between 30 and 44 years of age, who had been attempting to conceive for 3 months or less or were about to start trying to conceive, were eligible for participation in the study. Women with a history of infertility, polycystic ovarian disease, pelvic inflammatory disease, endometriosis, pelvic radiation, or with a partner with a history of infertility were excluded from participation. Women were recruited through community flyers, mass e-mails, and print advertising. Eligible women were enrolled and provided informed consent at their study visit, which was scheduled for the second, third, or fourth menstrual cycle day (first day defined as the first day of menses) following determination of eligibility. Women, who were determined to be eligible while contracepting, were enrolled in the menstrual cycle immediately following cessation of birth control.
At the study visit, women provided a baseline urine sample. A subset of this cohort agreed to collect first morning urine on cycle days 2 and 3 in each subsequent cycle for up to 5 additional cycles or until pregnancy was detected. For each cycle, urine samples were stored in the participant’s refrigerator for up to 3 days and then shipped via overnight delivery in a cooler with ice packs to the study center.
In the study center, all urine samples (5 mL) were transferred to polypropylene vials. To prevent degradation of FSH that otherwise occurs in frozen samples, glycerol was added to a final 7% dilution.14 The urine samples were stored frozen at −80°C until analysis. While trying to conceive, women completed a study diary, which was designed to collect daily information on vaginal bleeding, intercourse, pregnancy test results, and medication use. Participants were asked to complete the diary daily until pregnancy was detected or 3 menstrual cycles had passed, whichever occurred first. Women were given free home pregnancy tests (sensitivity = 20 mIU hCG/mL) and instructed to use them at the time of missed menses.
Women were instructed to inform study staff of a positive pregnancy test and were provided a free pregnancy ultrasound to encourage notification of pregnancy results. Women who did not report a positive pregnancy test were contacted at 3 and 6 months after the study visit. Women were followed until a positive pregnancy test or until 6 months of attempt following the study visit, whichever occurred first.
Urine samples were shipped frozen to the National Institute for Occupational Safety and Health (NIOSH) Reproductive Endocrinology Laboratory for endocrine analyses. Urinary FSH concentrations were assayed in duplicate using a noncompetitive, 2-site time-resolved immunofluorometric assay.15 Urinary creatinine (cr) concentrations were measured using a Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics, Raritan, New Jersey). Urinary FSH values were divided by the respective sample’s creatinine concentration to adjust for urine flow rate. All samples were measured in 1 assay per analyte. Intra-assay coefficients of variation were 3.5% for FSH and 1.1% for cr. When possible, the sample from cycle day 3 was selected for analysis. If a sample from cycle day 3 was not available, a sample from cycle day 2 or day 4 was analyzed. We previously showed high correlation between serum FSH and cr-corrected urinary FSH within our cohort (r = .85, P < .01).12
Statistical Analysis
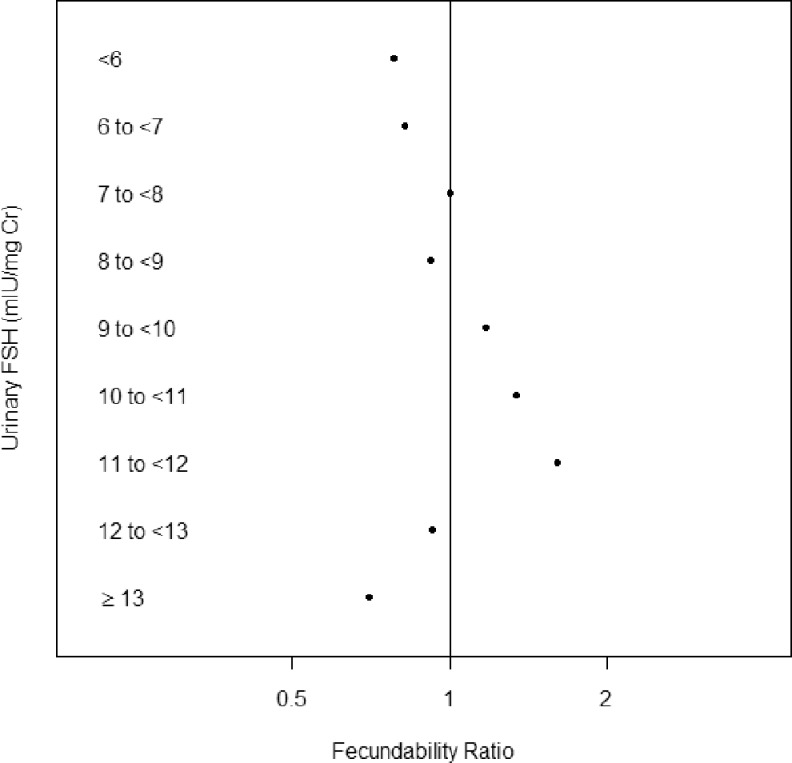
Statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, North Carolina), R 2.10.116, and WinBUGS 1.4.17 Initially, we evaluated the associations between the baseline urinary FSH hormone levels and fecundability using the full cohort (N = 209) using discrete Cox proportional hazard models to calculate a cycle-specific fecundability ratio (FR).18 These models accounted for both the right censoring and left truncation (due to women enrolling in the first, second, third, or fourth cycle of pregnancy attempt) present in the data. An FR less than 1.0 suggests reduced fecundability. Pregnancy was defined by the report of a positive home pregnancy test. Maternal age was classified into 3 categories for modeling (30-34, 35-37, and 38-44 years of age). To determine the most appropriate method to model cr-corrected urinary FSH, Kaplan-Meier plots were used to examine time-to-pregnancy by baseline FSH level (categorized <6.0, 6.0-6.9, 7.0-7.9, 8.0-8.9, 9.0-9.9, 10.0-10.9,11.0-11.9, 12.0-12.9, and ≥13.0 mIU/mg cr). Visual inspection of the 9 curves was used to categorize FSH into 3 groups (<7, 7-11.9 and ≥12 mIU/mg cr). The associated unadjusted FRs for the 9 categories illustrate the nonlinear pattern (Figure 1).
Figure 1.
Unadjusted fecundability ratios by level of baseline urinary follicle-stimulating hormone ([FSH] mIU/mg cr). Women with values from 7 to 7.9 mIU/mg cr served as the reference group.
Bivariate analyses were conducted to compare demographic characteristics between women with low (<7 mIU/mg cr), middle (7-11.9 mIU/mg cr), and high (≥12 mIU/mg cr) urinary FSH values. For categorical variables, unadjusted Cochran-Mantel-Haenszel tests for association were used in order to take advantage of the ordinal nature of some of these variables.19 For continuous variables, the nonparametric Kruskal-Wallis test was employed.
To accommodate for differences in timing and frequency of intercourse, the day-specific probabilities model by Scarpa and Dunson20 was used to generate day-specific FRs. The model accommodates multiple acts of intercourse during the fertile window, assumes independence between acts of intercourse, and allows for both the right censoring and left truncation present in the data. Day-specific FRs were calculated using the subcohort of women who provided urine and daily diary data (“baseline urine and diary” subcohort, N = 172). Information from the diary on days of menstrual bleeding, days of intercourse, and results of pregnancy tests were used to estimate day-specific probabilities of pregnancy (probability of pregnancy given an act of intercourse on a fertile day). Ovulation was assumed to have occurred 14 days prior to first day of menses or first positive home pregnancy test, with the fertile window designated as extending from 5 days before to 3 days after day of ovulation based on the standard days method.21 Prospectively observed cycles were included in the model for up to 3 observed cycles or until pregnancy was detected.
To determine the intercycle variability of urinary FSH, data from the full cohort were subsequently analyzed, first using a mixed model with the cycle-specific urinary FSH value, a random intercept for each woman, and covariates (the pregnancy attempt cycle, body mass index, age, and menstrual cycle day of collection). Women with higher fecundability contributed fewer cycles to the analysis of variance, which we define as informative conception. To account for potential bias due to informative conception, a mixed model and time-to-conception model were jointly fit with a common random intercept (Guo and Carlin model),22 also adjusting for pregnancy attempt cycle, BMI, age, timing of void, and menstrual cycle day of collection. The 2 models led to similar results, implying that informative conception had little effect on estimates. Estimates from the mixed model are presented.
To determine the value of repetitive urinary FSH testing in the assessment of fecundability, the smaller “repetitive urine” subcohort (N = 95), made up of those women who provided multiple urine samples, was used to determine the associations between (1) the baseline urinary FSH level, (2) the most recent urinary FSH level, or (3) the maximum urinary FSH level and fecundability using 3 separate, discrete Cox proportional hazard models that allowed FSH to vary over time. Time-varying FSH levels were included in the latter 2 models.
Results
Participant Characteristics
A total of 209 women provided a baseline urine sample and completed follow-up; 95 of these women were enrolled in the repetitive urine collection protocol with 71 women providing more than 1 urine sample. Of the 209 women, 172 women completed the study diary for at least 1 cycle while trying to conceive. The full cohort and subcohorts did not differ significantly by age distribution, parity, race, male partner age, intercourse frequency, or distribution of baseline FSH levels (Supplemental Table 1). However, time-to-pregnancy was significantly longer in the repetitive urine cohort (N = 95) compared to those not in the repetitive urine cohort (N = 114; P = .03). Time-to-pregnancy for the entire cohort is shown in Supplemental Figure 1. Enrollment baseline urinary FSH levels ranged from 2.37 to 41.42 mIU/mg cr with a mean ± standard deviation of 9.63 ± 4.81 mIU/mg cr (n = 209).
Nonlinear Association Between Urinary FSH and Fecundability
Given that elevated serum FSH is a marker for ovarian aging23 and is associated with decreased egg production in response to ovarian stimulation,24 we anticipated that early follicular phase urinary FSH would be inversely associated with fecundability in our population. This was not the case. Both low and high urinary FSH levels had lower point estimates of fecundability (Figure 1).
The nonlinear association does not appear to be due to chance selection that resulted in women with low FSH being significantly different from the other participants. Characteristics of the women varied little by urinary FSH level at enrollment. Women with low (<7 mIU/mg cr), middle (7-11.9 mIU/mg cr), and high (≥12 mIU/mg cr) FSH levels are compared in Table 1. Across the levels of FSH, women did not significantly differ in current use of tobacco, history of sexually transmitted diseases, body mass index, cycle day of urine collection, or self-reported menstrual cycle length. As expected, women in the high FSH group were older (P = .002). A larger proportion of women in the low and high FSH group tended to have used hormonal birth control at some point during the preceding year (P = .07). Women in the low FSH group were most likely to report regular menstrual cycles (P = .04). Using data from the daily diaries, it was noted that women in the lowest FSH group had the longest observed menstrual cycles and greatest intercycle variance in menstrual cycle length, although these findings were not statistically significant.
Table 1.
Characteristics of Study Cohort by Baseline Level of Urinary FSH (Low, Middle, and High; N = 209).a
| Low (N = 59), <7 mIU/mg cr | Middle (N = 108), 7-11.9 mIU/mg cr | High (N = 42), ≥12 mIU/mg cr | P Valueb | |
|---|---|---|---|---|
| Age, years | .002 | |||
| 30-34 | 45 (76) | 78 (72) | 21 (50) | |
| 35-37 | 9 (15) | 19 (18) | 9 (21) | |
| 38-44 | 5 (9) | 11 (10) | 12 (29) | |
| Body mass index, kg/m2 | .50 | |||
| <18.5 | 2 (3) | 3 (3) | 1 (2) | |
| 18.5-24 | 37 (64) | 70 (65) | 24 (58) | |
| 25-29 | 11 (19) | 24 (22) | 8 (20) | |
| ≥30 | 8 (14) | 11 (10) | 8 (20) | |
| History of gonorrhea or chlamydia | 4 (7) | 3 (3) | 1 (2) | .38 |
| Current smoker | 1 (2) | 2 (2) | 1 (2) | .97 |
| Hormonal contraceptivec use in past year | 33 (57) | 42 (39) | 21 (50) | .07 |
| Menstrual cycle day of urine collection | 3 (2-4) | 3 (2-4) | 3 (2-4) | .80 |
| Questionnaire: regular menstrual cycles | 57 (97) | 89 (83) | 37 (88) | .04 |
| Diaryd: intercycle menstrual length variancee | 7.0 (2.3-21.3) | 4.5 (1.3-10.3) | 4.3 (1.3-13.0) | .24 |
| Menstrual cycle length, days | ||||
| Questionnaire | 28.0 (28.0-30.0) | 28.5 (28.0-30.0) | 28.0 (27.0-29.5) | .29 |
| Diary datad | 29.4 (27.0-32.6) | 28.5 (27.0-31.4) | 28.2 (26.3-31.5) | .36 |
Abbreviations: cr, creatinine; FSH, follicle-stimulating hormone.
a Data presented as number (%) or median (interquartile range).
b For categorical variables, the Cochran-Mantel-Haenszel tests for association were used. For the continuous variables, the nonparametric Kruskal-Wallis test was employed. P values were not adjusted for other variables in the table.
c Oral contraceptive pills, contraceptive patch, or contraceptive vaginal ring.
d For women that have diary data (n = 174).
e For women with diary data for more than 1 cycle (N = 131).
Association Between Baseline Urinary FSH and Fecundability
Cycle-specific FRs for each category of baseline urinary FSH were determined using the full cohort of women (N = 209). Women with urinary FSH values between 7 and 11.9 mIU/mg cr served as the reference group. The association between urinary FSH and fecundability appeared U shaped; women with low urinary FSH levels and women with high urinary FSH levels exhibited a nonsignificant 30% to 33% reduction in fecundability (Table 2). Adjusting for age alone did not significantly alter the association for women with low urinary FSH but did slightly weaken the association for those with high urinary FSH values. Further adjustment for hormonal contraceptive use in the past year did not substantially alter the association between low or high urinary FSH and fecundability.
Table 2.
Cycle-Specific and Day-Specific FRs With 95% CIs by Level of Baseline Urinary FSH Value in the Full Cohort.
| Urinary FSH, mIU/mg cr | Cycle-Specific Fecundability Ratio | Day-Specific Fecundability Ratio | ||
|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | |
| Unadjusted | ||||
| <7 | 0.70 | 0.44-1.10 | 0.58 | 0.29-1.01 |
| 7-11 | Reference | Reference | ||
| ≥12 | 0.67 | 0.41-1.12 | 0.58 | 0.25-1.08 |
| Adjusted for age | ||||
| <7 | 0.69 | 0.43-1.08 | 0.62 | 0.31-1.08 |
| 7-11 | Reference | Reference | ||
| ≥12 | 0.75 | 0.45-1.27 | 0.63 | 0.27-1.18 |
| Adjusted for age and hormonal contraceptive use | ||||
| <7 | 0.71 | 0.45-1.13 | 0.68 | 0.31-1.25 |
| 7-11 | Reference | Reference | ||
| ≥12 | 0.78 | 0.46-1.32 | 0.79 | 0.31-1.59 |
Abbreviation: CI, confidence interval; FR, fecundability ratios; FSH, follicle-stimulating hormone.
As estimated with the unadjusted day-specific probabilities models, women (N = 172) with baseline urinary values of FSH less than 7 mIU/mg cr exhibited a nonsignificant 42% reduction in the probability of pregnancy, given an act of intercourse on a fertile day compared to women with normal urinary values. Women with elevated urinary FSH values (≥12 mIU/mg cr) also exhibited a nonsignificant 42% reduction in probability of pregnancy (Table 2). After adjustment for age and hormonal contraceptive use in the preceding year, the associated reductions were weakened for women with low urinary FSH and high urinary FSH.
Intercycle Variability of Urinary FSH
A woman’s urinary FSH values over multiple cycles were highly correlated (adjusted intraclass correlation coefficient = 0.77). Variance in urinary FSH between cycles within a woman was significantly less than variance in urinary FSH between women (variance components of 4.3 and 14.8, respectively). Variability in FSH between cycles within a woman varied by age of the woman (likelihood ratio test, P = .001), varying most in women ≥38 years of age (9.06) and least in women <35 (3.3) as measured by mixed models. Guo and Carlin joint models, which account for informative dropout, showed similar results. Of the 71 women who provided multiple urine samples, 25 (35%) had no change in FSH categories over time, 32 (45%) had changes between normal and low, 6 (9%) had changes between normal and high, and 8 (11%) had a mixture of low, normal, and high FSH levels.
Repetitive Urinary Testing and Fecundability
The association between low and high urinary FSH and fecundability was stronger in the smaller “repetitive urine” cohort (N = 95) that provided early follicular phase urine samples in each pregnancy attempt cycle for up to 6 cycles. These 95 women contributed a total of 363 cycles to the analysis. Cycle-specific FRs by level of FSH (baseline FSH, most recent FSH value, or maximum FSH value) are presented in Table 3. Using a woman’s maximum or most recent urinary FSH value resulted in little substantive difference from analyses based on the baseline urinary FSH value obtained during the first study cycle. Adjusting for frequency and timing of intercourse using day-specific fecundability analyses (N = 71) revealed similar results (data not presented).
Table 3.
Cycle-Specific FRs With 95% CIs by Level of Baseline Urinary FSH Value, Most Recent Urinary FSH Value, and Maximum Urinary FSH Value Among Women in the “Repetitive Urine” Subcohort.
| Urinary FSH, mIU/mg cr | Fecundability Ratio, Unadjusted | Fecundability Ratio, Adjusteda | ||
|---|---|---|---|---|
| Baseline FSH | FR | 95% CI | FR | 95% CI |
| <7 | 0.43 | 0.21-0.89 | 0.47 | 0.23-0.96 |
| 7-11 | Reference | |||
| ≥12 | 0.42 | 0.17-1.00 | 0.47 | 0.18-1.20 |
| Most recent FSH | ||||
| <7 | 0.44 | 0.22-0.84 | 0.41 | 0.21-0.81 |
| 7-11 | Reference | |||
| ≥12 | 0.60 | 0.24-1.49 | 0.75 | 0.28-2.01 |
| Maximum FSH | ||||
| <7 | 0.32 | 0.14-0.72 | 0.32 | 0.14-0.74 |
| 7-11 | Reference | |||
| ≥12 | 0.44 | 0.19-1.01 | 0.54 | 0.22-1.33 |
Abbreviations: CI, confidence interval; FR, fecundability ratios; FSH, follicle-stimulating hormone.
a Adjusted for age and hormonal contraceptive use in the previous year.
Discussion
Findings from this study suggest that the relationship between urinary FSH and fecundability may be hyperbolic, not linear, with women with low or high FSH values both exhibiting reduced fecundability. The findings were strongest in the “repetitive urine” group, but this may be due to chance associated with the small sample size or composition of this group. While statistically significant in the “repetitive urine” subcohort, neither high nor low urinary FSH values were significantly associated with fecundability in the full cohort. Most studies of the relationship between FSH levels (urinary or serum) have not described reduced fecundability in women with low FSH25,26; however, these studies are generally conducted in women receiving gonadotropin therapy for assisted reproduction. It is possible that negative effects of low early follicular phase FSH can be corrected with gonadotropin therapy.
There are a few studies of early follicular phase FSH levels in women trying to conceive without gonadotropin therapy. The largest study examined the relationship between early follicular phase serum FSH levels and the probability of a spontaneous pregnancy within 12 months of infertility workup among 3519 couples with unexplained infertility.27 The authors found a lower probability of pregnancy among women with serum FSH values over 8 mIU/mL. The relationship between low serum FSH and fecundability was not discussed; however, graphically the data suggested a small, nonsignficant decline in fecundability at lower FSH values. Li et al noted higher serum and urinary peak FSH levels in conception cycles compared to nonconception cycles among 14 women who conceived with donor insemination.28 However, others have not noted a relationship between FSH levels and conception following intrauterine insemination without ovarian stimulation.29
It is possible that the women in our study with low urinary FSH values (<7 mIU/mg cr, equivalent to <4.5 mIU/mL in serum) exhibited hypothalamic–pituitary–ovarian (HPO) axis dysfunction. Data from the North Carolina Early Pregnancy Study suggest that low follicular phase luteinizing hormone levels are also associated with reduced fecundability among ovulatory women.30 The HPO dysfunction may lead to menstrual cycle irregularities. Although participants with low urinary FSH values were less likely to report menstrual cycle irregularity than others, their menstrual cycle lengths as calculated from their daily diaries tended to be longer and exhibit greater variability between cycles. A long menstrual cycle length and menstrual cycle irregularity have been associated with reduced fecundability.31,32
Low FSH levels could result in anovulatory cycles or ovulatory dysfunction with lower oocyte quality. In a randomized controlled trial, a large dose of recombinant FSH at the time of human chorionic gonadotropin trigger (mimicking the LH surge) was associated with improved oocyte recovery rates and fertilization following in vitro fertilization.33 Also, low FSH levels could be indicative of luteal phase deficiency. Regularly menstruating, exercising women with luteal phase defects have been shown to have lower urinary FSH levels during the luteal–follicular transition.34
Alternatively, women with lower early follicular phase urinary FSH levels may have advanced follicular development resulting in elevated estradiol levels and correspondingly suppressed FSH. Advanced follicular development has been attributed to lower inhibin levels due to reproductive aging. Recent hormone use could also suppress FSH levels and recent birth control pill use has been associated with reduced fecundability35; however, we adjusted for recent hormonal contraceptive use and the relationship between low urinary FSH and reduced fecundability persisted.
A general trend of reduced fecundability was observed among women with elevated urinary FSH levels. High FSH levels have been associated with diminished ovarian reserve in clinical studies. However, the association between high urinary FSH and reduced fecundability has yet to be established. A number of home urinary FSH kits are marketed to consumers as a fertility test. A positive test, and presumed reduced fertility, is observed with elevated urinary FSH levels. A positive test is noted when urinary values exceed the cutoff value equivalent to a serum value of 10 mIU/mL. Using our assay, the equivalent cutoff value would be 11.5 mIU/mg. Thus, all the women in our high urinary FSH group would have tested positive. While our point estimates would suggest that women with a positive test (high FSH) would exhibit reduced fertility (1) the confidence intervals (CIs) were wide and crossed one and (2) a woman with a negative test cannot be reassured about her fertility, diminishing its value as a “fertility test.” Given our study sample, our findings should not be generalized to women younger than age 30. Fertility tests would likely have poor predictive value in a group at low risk for reproductive aging.
As seen in previous studies, within a woman intercycle variability of FSH is low.36 Previous studies have suggested that variability of FSH levels increases with age.13 However, younger women conceive in fewer cycles than older women, which could lead to bias due to informative conception (women with higher fecundability contribute fewer cycles to the analysis of variance). Our analysis, which accounted for informative conception, confirmed that variability of FSH increases with age.
Information from repetitive testing of early follicular phase urine for FSH does not appear to strengthen the association between urinary FSH and fecundability; FRs using the time-varying FSH models with the most recent urinary FSH value were not significantly different from those models using only the baseline FSH value. Sampling FSH values over multiple cycles and choosing the maximum FSH also did not strengthen the association. There appears to be little benefit to obtaining urinary FSH values over multiple cycles to predict fecundability.
In this study all baseline urine samples were non–first morning void urine samples, while 90% of the urine samples from subsequent cycles were first morning void urines. This could be viewed as a limitation in our study of the value of repetitive urinary FSH testing. Most protocols measure urinary FSH in first morning urine. However, we did adjust for cr in the samples, which should adjust for differing rates of urine production (and subsequent concentrations) between samples. In general, cr levels tend to be higher in first morning samples due to decreased urine production overnight. However, early follicular phase serum FSH levels appear to have some diurnal variation, with lower levels observed during sleep.37 Thus within a woman, one can presume that a first void urine sample would have lower cr-corrected FSH levels compared to a urine sample collected later in the day. To accommodate for differences in timing of collection in the urine samples, time of urine collection (first morning void versus later) was added to the model created to measure intercycle variability in urinary FSH. This did not alter our conclusions that multiple measures are of little benefit.
After adjusting for factors (such as age, contraceptive use, etc) which are correlated with urinary FSH levels, the CIs for the adjusted FRs from the full cohort crossed one, so that the observed effects of FSH may be driven by its relationship with these factors, particularly age. If this were to be true, our concerns about the validity of commercial urinary FSH kits would remain. Women with partners who had known fertility problems were excluded from the study, but no semen analyses were performed. We assumed that the prevalence of male subfertility will be equally distributed across urinary FSH levels. This study used models to measure the association between urinary FSH and natural fertility: Cox models and day-specific models, which adjust for frequency and timing of intercourse. Results from the 2 models were similar.
In summary, early follicular phase urinary FSH values appear to have a nonlinear association with fecundability in women between the ages of 30 and 44; both low and high values suggest reduced fecundability. However, the findings of reduced fecundability in both groups were not statistically significant, suggesting the need for larger studies. Due to the generally low intercycle variability in urinary FSH, repetitive urinary testing appears to be of little benefit. Future studies are needed to confirm and further explore the potential mechanism by which low early follicular phase FSH could lead to reduced fertility. In the meantime, a woman with a negative fertility test based on urinary FSH should not presume normal fertility.
Supplementary Material
Supplementary Material
Acknowledgments
Drs Allen Wilcox and Carmen Williams provided helpful comments on an earlier draft of this manuscript. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ie, support for coauthor D.B.).
Footnotes
Authors’ Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of company names and/or products does not constitute endorsement by the National Institute for Occupational Safety and Health/Centers for Disease Control and Prevention. The online data supplement is available at http://rsx.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.S. has previously consulted for Roche Diagnostics. For the remaining authors no possible conflicts of interests were declared.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the NICHD/NIH (grant number R21HD060229 and K12HD050113 [UNC WRHR]) and NIEHS/NIH (grant number T32ES007018).
References
- 1.Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital Health Stat 23 1997;(19):1–114. [PubMed] [Google Scholar]
- 2.Hansen KR, Thyer AC, Sluss PM, Bremner WJ, Soules MR, Klein NA. Reproductive ageing and ovarian function: is the early follicular phase FSH rise necessary to maintain adequate secretory function in older ovulatory women? Hum Reprod. 2005;20(1):89–95. [DOI] [PubMed] [Google Scholar]
- 3.Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81(2):433–442. [DOI] [PubMed] [Google Scholar]
- 4.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346. [DOI] [PubMed] [Google Scholar]
- 5.Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218(5136):22–28. [DOI] [PubMed] [Google Scholar]
- 6.Roberts VJ, Barth S, el Roeiy A, Yen SS. Expression of inhibin/activin subunits and follistatin messenger ribonucleic acids and proteins in ovarian follicles and the corpus luteum during the human menstrual cycle. J Clin Endocrinol Metab. 1993;77(5):1402–1410. [DOI] [PubMed] [Google Scholar]
- 7.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–5509. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Fukuda K, Andersen CY, Byskov AG. Characteristics of human ovulation in natural cycles correlated with age and achievement of pregnancy. Hum Reprod. 2001;16(12):2501–2507. [DOI] [PubMed] [Google Scholar]
- 9.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. [DOI] [PubMed] [Google Scholar]
- 10.Oosterhuis GJ, Lambalk CB, Michgelsen HW, de Koning CH, Vermes I, Schoemaker J. Follicle-stimulating hormone measured in unextracted urine: a reliable tool for easy assessment of ovarian capacity. Fertil Steril. 1998;70(3):544–548. [DOI] [PubMed] [Google Scholar]
- 11.Liu JH, Kao L, Rebar RW, Muse K. Urinary beta-FSH subunit concentrations in perimenopausal and postmenopausal women: a biomarker for ovarian reserve. Menopause. 2003;10(6):526–533. [DOI] [PubMed] [Google Scholar]
- 12.Steiner AZ, Herring AH, Kesner JS, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol. 2011;117(4):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain T, Klein NA, Lee DM, Sluss PM, Soules MR. Endocrine assessment of relative reproductive age in normal eumenorrheic younger and older women across multiple cycles. Am J Obstet Gynecol. 2003;189(4):1080–1084. [DOI] [PubMed] [Google Scholar]
- 14.Kesner JS, Knecht EA, Krieg EF., Jr Stability of urinary female reproductive hormones stored under various conditions. Reprod Toxicol. 1995;9(3):239–244. [DOI] [PubMed] [Google Scholar]
- 15.Kesner JS, Knecht EA, Krieg EF., Jr Time-resolved immunofluorometric assays for urinary luteinizing hormone and follicle stimulating hormone. Anal Chim Acta. 1994;285:13–22. [Google Scholar]
- 16.R: A language and environment for statistical computing. R Foundation for Statistical Computing 2009. http://www.R-project.org. Accessed December 6, 2011.
- 17.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Stokes ME, Davis CS, Koch GG. The s x r Table. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 20.Scarpa B, Dunson DB. Bayesian selection of predictors of conception probabilities across the menstrual cycle. Paediatr Perinat Epidemiol. 2006;20(suppl 1):30–37. [DOI] [PubMed] [Google Scholar]
- 21.Arevalo M, Sinai I, Jennings V. A fixed formula to define the fertile window of the menstrual cycle as the basis of a simple method of natural family planning. Contraception. 1999;60(6):357–360. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Carlin BP. Separate and joint modeling of longitudinal and event time data using standard computer packages. The American Statistician. 2004;58:16–24. [Google Scholar]
- 23.Backer LC, Rubin CS, Marcus M, Kieszak SM, Schober SE. Serum follicle-stimulating hormone and luteinizing hormone levels in women aged 35-60 in the U.S. population: the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). Menopause. 1999;6(1):29–35. [PubMed] [Google Scholar]
- 24.Muasher SJ, Oehninger S, Simonetti S, et al. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50(2):298–307. [DOI] [PubMed] [Google Scholar]
- 25.Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82(1):180–185. [DOI] [PubMed] [Google Scholar]
- 26.Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79(5):1091–1100. [DOI] [PubMed] [Google Scholar]
- 27.van der Steeg JW, Steures P, Eijkemans MJ, et al. Predictive value and clinical impact of basal follicle-stimulating hormone in subfertile, ovulatory women. J Clin Endocrinol Metab. 2007;92(6):2163–2168. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Nakajima ST, Chen J, Todd HE, Overstreet JW, Lasley BL. Differences in hormonal characteristics of conceptive versus nonconceptive menstrual cycles. Fertil Steril. 2001;75(3):549–553. [DOI] [PubMed] [Google Scholar]
- 29.Witt BR, Barad DH, Barg P, et al. Basal serum follicle stimulating hormone (FSH) and estradiol levels as predictors of pregnancy in unstimulated donor insemination cycles. J Assist Reprod Genet. 1995;12(3):157–160. [DOI] [PubMed] [Google Scholar]
- 30.Baird DD, Weinberg CR, Zhou H, et al. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71(1):40–49. [DOI] [PubMed] [Google Scholar]
- 31.Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006;21(5):1279–1284. [DOI] [PubMed] [Google Scholar]
- 32.Small CM, Manatunga AK, Klein M, et al. Menstrual cycle variability and the likelihood of achieving pregnancy. Rev Environ Health. 2010;25(4):369–378. [DOI] [PubMed] [Google Scholar]
- 33.Lamb JD, Shen S, McCulloch C, Jalalian L, Cedars MI, Rosen MP. Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2011;95(5):1655–1660. [DOI] [PubMed] [Google Scholar]
- 34.De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83(12):4220–4232. [DOI] [PubMed] [Google Scholar]
- 35.Linn S, Schoenbaum SC, Monson RR, Rosner B, Ryan KJ. Delay in conception for former ‘pill’ users. JAMA. 1982;247(5):629–632. [PubMed] [Google Scholar]
- 36.Penarrubia J, Fabregues F, Manau D, et al. Initial analysis of variability among basal hormone biomarkers of ovarian reserve. Reprod Biomed Online. 2004;8(2):191–195. [DOI] [PubMed] [Google Scholar]
- 37.Mortola JF, Laughlin GA, Yen SS. A circadian rhythm of serum follicle-stimulating hormone in women. J Clin Endocrinol Metab. 1992;75(3):861–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials