Abstract
We report synthesis and characterization of a novel PEG2000-conjugated hexadecylcarbamoylmethyl hexadecanoate (HDAS-PEG) as a PEG-phospholipid substitute for enhancing circulation persistence of liposomes. HDAS-PEG showed critical micelle concentration of 4.25 μM. We used post-insertion technique to introduce HDAS-PEG in outer lipid layer of the preformed liposomes. The presence of surface HDAS-PEG was confirmed by altered electrophoretic mobility, confocal microscopy and PEG estimation by ELISA. The post-inserted HDAS-PEG desorbed at approximately half the rate at which post-inserted DSPE-PEG desorbed from the liposome surface. HDAS-PEG significantly reduced liposome-induced complement activation (C4d, Bb and SC5b); HDAS-PEG was more effective than more commonly used DSPE-PEG in this capacity. For studying circulation persistence, the liposomes were labeled with 99mTc radionuclide and administered in rats. 99mTc-HDAS-PEG-liposomes showed prolonged persistence in blood as compared to that shown by 99mTc-plain liposomes. After 24 h of administration, < 1% of 99mTc-plain liposomes remained in blood, whereas approximately 28% of injected 99mTc-HDAS-PEG-liposomes were present in blood. In comparison, only 4.8% of 99mTc-DSPE-PEG-liposomes was measured in blood after 24 h. As expected, the clearance route of the liposomes was through liver and spleen. These results demonstrate the potential of a novel non-phosphoryl HDAS-PEG for surface modification of preformed liposomes with a goal of prolonging their circulation persistence and more effective inhibition of complement activation.
Keywords: Liposomes, Poly(ethylene glycol), Circulation persistence, Post-insertion, Complement
Introduction
Phospholipids are major constituents of biomembranes and have been the classic ingredients of liposomes. Being present naturally, they are assumed to be not toxic. However, molecular entities containing phosphoryl moiety are reactive in biological milieu. Oxidative modification and/or fragmentation of phosphatidylcholines generate potent inflammatory mediators that mimic the biologic action of platelet-activating factor (Zimmerman et al., 1995). We have also reported that negatively charged dimyristoylphosphatidylcholine (DMPG)-liposomes have a tendency to activate platelets (Awasthi et al., 2007). The net anionic charge on the phosphate moiety of polyethylene glycol (PEG)-phospholipids has been reported to play a key role in complement activation and anaphylotoxin production (Moghimi et al., 2006). On the other hand, Gamma irradiation, a potential technique for sterilization of liposome preparations, has been shown to increase hemolytic behavior of liposomes containing phospholipids (Stensrud et al., 1999). Moreover, phospholipids are susceptible to phospholipase-mediated breakdown and their extraction and synthesis is associated with significant costs. Considering these characteristics of phospholipids, the development of non-phospholipid amphiphiles as liposome constituents has considerable merit (Bastiat et al., 2007). As compared to the phospholipids, non-phospholipid substituents offer significant advantages related to economics, safety, stability and versatility.
Previously, we reported synthesis of an anionic non-phospholipid as a replacement of DMPG in liposomes, and its use in the enhancement of hemoglobin encapsulation (Agashe et al., 2010). The resultant liposome-encapsulated hemoglobin (LEH) was structurally similar to the liposomes composed only of phospholipids, and improved cerebral energy metabolism in a rat model of hemorrhagic shock (Awasthi et al., 2010). With a long-term goal of complete replacement of phospholipids in liposomes without compromising structural characteristics of liposomes, here we report a novel PEG-lipid, PEG2000-conjugated hexadecylcarbamoylmethyl hexadecanoic acid (HDAS-PEG). PEG-phospholipids have been successfully used for the preparation of long-acting and more stable and efficacious formulations, such as liposomal amphotericin B, doxorubicin, daunorubicin, etc (Cattel et al., 2003). Surface modification of liposomes with PEG delays their rapid clearance by the mononuclear phagocyte system (MPS) and increases the mean residence time in circulation (Awasthi et al., 2004). It is believed that the surface PEG forms a highly hydrated film of water which sterically hinders opsonization of liposomes and their subsequent uptake by the MPS (Ahl et al., 1997; Bradley et al., 1998; Szebeni et al., 2000; Torchilin et al., 1994). This stealth property of PEG-modified liposomes is governed by the thickness of the PEG layer, which in turn is a function of the length of PEG chain.
The reported HDAS-PEG could be synthesized from readily available and inexpensive chemicals. It prolongs circulation persistence of liposomes while remaining non-reactive towards complement proteins. We introduced HDAS-PEG into preformed liposomes by post-insertion technique. While the physicochemical mechanism of post-insertion has been explained (Sou et al., 2000), a direct evidence of the presence of PEG on the post-inserted liposome surface is lacking. We provide for the first time the verification of PEG-lipid existence on the liposome surface and its time- and dilution-dependent desorption.
Materials and methods
Unless otherwise mentioned, all the chemicals were obtained from Sigma–Aldrich (St. Louis, MO) and/or various suppliers through VWR Scientific (West Chester, PA) and were used without further purification. Tetradecenyl succinic anhydride was a gift from Vertellus Specialties Inc. (Indianapolis, IN). Methoxy PEG Amine, HCl Salt (MW 2000) was purchased from Jenkem Technology (Allen, TX). For liposome preparations, the phospholipids were purchased from Lipoid (Ludwigshafen, Germany), Avanti Polar Lipids (Alabaster, AL) or NOF Corporation (Tokyo, Japan). High purity cholesterol was obtained from Calbiochem (Gibbstown, NJ). 1H NMR spectra and 13C NMR spectra were recorded at 300 MHz, and 75 MHz on Mercury-VX 300 (Varian Inc., CA).
Synthesis of HDAS-PEG
A novel amphiphilic lipid (HDAS-PEG) containing hydrophilic polyethylene glycol (PEG2000) on one end and hydrophobic ethylene chains on the other end was synthesized (Figure 1a) by amidification between PEG amine and NHS-ester of 2-Hexadecylcarbamoylmethyl-hexadecanoic acid (HDAS). The precursor lipid HDAS was obtained by reacting tetradecenyl succinic anhydride and hexadecylamine, followed by catalytic hydrogenation. The final product was purified by dialysis and characterized by 1H NMR and DSC (Figure 1). Differential scanning calorimetry (DSC) was outsourced to Photometrics, Inc. (Huntington Beach, CA). Briefly, the DSC thermograms were obtained on a 2920 Modulated DSC machine (TA Instruments New Castle, DA) with a refrigerated cooling System. The calibration of the equipment for temperature and for enthalpy was performed using indium. Samples (5–10 mg ) were placed in aluminum pans and nitrogen gas purged at 40 mL/min. An empty aluminum pan was used as a reference. Samples were heated from −20 to 150°C, with a rate of 2°C/min. The data was analyzed using the “TA Universal Analysis” software.
Figure 1.
(a) Synthetic scheme for HDAS-PEG: (i) Pyridine, 80oC for 3 h, (ii) Palladium/charcoal (5%, 40 mg), Toluene, 40oC, under H2 flow for 24 h, (iii) N-hydroxysuccinimide, dicyclohexylcarbodiimide (DCC), DMF, room temperature for 28 h, and (iv) CH3O(CH2CH2O)44CH2CH2NH2.HCl, Triethylamine, DMSO, room temperature for 24 h. (b) 1H NMR spectrum of HDAS-PEG. (c) DSC thermogram of HDAS-PEG, showing values of peak maxima.
2-Hexadecylcarbamoylmethyl-hexadec-3-enoic acid (1, HDA)
Tetradecenyl succinic anhydride (8.60 g, 29.21 mmol) and hexadecylamine (5.86 g, 24.27 mmol) were allowed to react in presence of pyridine (10.0 mL) at 80°C for 3 h. The reaction mixture was extracted into dichloromethane (DCM, 200 mL) and washed three times with 10% HCl (100 mL). The organic phase was separated, dried with sodium sulfate and concentrated to obtain white solid of unsaturated HDA (11.50 g, yield 88%). 1H NMR (300 MHz, CDCl3): 5.77 (br, 1H, NH, exchanged with D2O), 5.51 (1H, CH) 5.43 (1H, CH),3.25 (m, 2H, CH2), 2.88 (m,1H), 2.94–2.12(m, 4H), 2.05–1.92 (m, 2H), 1.48 (br, 4H) 1.35–1.15 (m, 42H, CH2), 0.87 (t, 6H, CH3).
2-Hexadecylcarbamoylmethyl-hexadecanoic acid (2, HDAS)
HDA (5.8 g, 10.82 mmol) was dissolved in 200 mL of toluene at 60°C and reduced by passing hydrogen gas in the presence of catalyst palladium/charcoal (5%, 50 mg) at atmospheric pressure for 48 h. The reaction mixture was filtered on a Buchner funnel and passed through a short silica gel column to remove the catalyst. The product (5.6 g, yield 96%) was obtained as white solid by evaporating toluene under vacuum. 1H NMR (300 MHz, CDCl3): 5.74 (br, 1H, NH, exchanged with D2O), 3.26 (t, 2H), 2.87–2.31 (m, 3H), 1.78-1.59 (m, 2H), 1.60–1.40 (br, 4H), 1.35–1.15 (br, 48H, CH2), 0.87 (t, 6H, CH3). 13C NMR (75 MHz, CDCl3): 176.81, 171.31, 41.75, 37.21, 31.90, 31.58, 29.68, 29.64, 29.58, 29.54, 22.67, 14.09. ESI HRMS calculated for C34H68NO3 538.42, found 538.40 [M+H]+.
2-Hexadecylcarbamoylmethyl hexadecanoic acid 2,5-dioxo-pyrrolidin-1-yl ester (3, HDAS-NHS)
HDAS (5.0 g, 9.29 mmol ), N-hydroxysuccinimide (1.07 g, 9.29 mmol), N,N’-dicyclohexylcarbodiimide (1.92 g, 9.32 mmol) were allowed to react in anhydrous dimethylformamide (100 mL) with vigorous stirring for 24 h. The solid precipitate appeared after the completion of reaction was removed by filtration. The solution was concentrated by evaporating the solvent under reduced pressure. The crude product was purified with silica gel column chromatography using dichloromethane and ethyl acetate (90:10) to obtain (3.8 g, yield 64%) white powder. 1H NMR (300 MHz, CDCl3): 5.76 (br, 1H, NH, exchanged with D2O), 3.49 (t, 2H), 2.88-2.30 (m, 7H), 1.96-163 (br, 4H), 1.60-1.39 (br, 4H), 1.35–1.15 (m, 46H, CH2), 0.87 (t, 6H, CH3). 13C NMR (75 MHz, CDCl3): 180.16, 176.84, 172.92, 40.37, 39.85, 31.90, 31.39, 29.67, 29.60, 29.55, 29.28, 25.58, 22.66, 14.08. ESI HRMS calculated for C38H70N2O5 (M+) 634.53, found 635.40 [M+H]+.
Poly(ethylene glycol-conjugated-HDAS (4, HDAS-PEG)
HDAS-NHS (0.10 g, 0.16 mmol ), methoxy PEG2000 amine, HCl salt (0.325 g, 0.15 mmol), triethlyamine (0.040 mL) and dimethylsulfoxide (DMSO, 7 mL) were stirred vigorously at 45°C for 24 h. The product was purified by dialysis using benzoylated cellulose tubing (MWCO 2000) against DMSO and water for 24 and 48 hour, respectively. Finally, the dry product (0.24 g, 63%) was obtained by removing water under freeze drying conditions (Triad Lyophilizer, Labconco, Kansas City, MO). 1H NMR (300 MHz, CDCl3): 3.87 (t, 2H, CH3O–(CH2–CH2–O)44–CH2–CH2–NH–CO–), 3.63 ( br, 176H, CH3O–(CH2–CH2–O)44–CH2–CH2–NH–CO–), 3.54 (m, 2H,CH3O–(CH2–CH2–O)44– CH2–CH2–NH–CO–), 3.54 (m, 2H), 3.37 (s, 3H, CH3O–(CH2–CH2–O)44– CH2–CH2–NH–CO–), 2.71-2.54 (br, 2H), 2.35-2.26 (m,1H) 1.91-1.56 (br, 4H), 1.55–1.38 (br, 4H), 1.35-1.19 (br, 46H, CH2), 0.87 (t, 6H, CH3).
Critical Micellar Concentration (CMC) of HDAS-PEG
In order to examine the hydrophobic domain formation of HDAS-PEG in aqueous solution, we measured fluorescence spectral changes of N-phenylnaphthylamine (NPN) as a function of [HDAS-PEG]. Briefly, the samples were prepared by adding increasing concentrations of HDAS-PEG (0.5 to 8 μM) into a saturated aqueous solution of NPN. The solutions were kept undisturbed overnight at room temperature before measuring fluorescence spectra. The fluorescence spectra were recorded at 90° detection angle on Shimadzu 5000U-DR15 spectrofluorophotometer (Columbia, MD) equipped with a Xenon excitation source.
Cytotoxicity of HDAS-PEG
Human Umbilical Vein Endothelial Cells (HUVEC) and RAW 264.7 cells (ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium supplemented with FBS (20% and 10%, respectively), penicillin G (100 U/mL) and streptomycin (100 μg/mL) at 37°C in 5% CO2 atmosphere. The cells were plated in 96-well plates at a density of 4,000 cells/well. After 24 h of culture, the cells were treated with 100 μL of medium containing 1, 2 and 5 mg/mL of phospholipid in HDAS-PEG-containing liposomes. Separately, cytotoxicity of HDAS-PEG solution in DMSO was also evaluated (1-5 mg/mL, 100 μL). After 48 h of exposure to the treatments at 37°C, the cell viability was measured by colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay at 570 nm. The results were presented as percent viability as compared to the untreated control.
Preparation of Liposomes
Lipid hydration method was used for the preparation of liposomes. 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-ditetradecanoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (DMPG), cholesterol (CHO) and vitamin E in 45:10:44.8:0.2 mol% were dissolved in a mixture of chloroform : methanol (2:1), filtered through 0. 2 μm nylon filter, and transferred into a round bottom flask. The solvent mixture was evaporated at 45 °C on a rotavapor (Buchi R-210) to form a thin film of lipid mixture. Any trace of organic solvents was removed by keeping the film under vacuum for additional 12 h. The lipid film was rehydrated with water, vortexed and the suspension was lyophilized overnight (Triad Lyophilizer, Labconco, Kansas City, MO). The dried mixture was again hydrated with 100 mM aqueous solution of glutathione (pH 6.8-7.0, adjusted with dilute NaOH). The lipid suspension was sequentially extruded (Lipex Biomembranes Inc., Vancouver, Canada) through polycarbonate membranes of 1, 0.6, 0.4 and 0.2 μm pore size. The extrusion was performed at 55°C and repeated at least 5 times for each pore size. The final extrudate was divided into two equal halves. One-half of the preparation was subjected to PEGylation with HDAS-PEG and the other was stored at 4°C as plain liposomes. For comparison purposes, an additional liposome preparation was also manufactured in order to post-insert DSPE-PEG2000.
Insertion of HDAS-PEG in Preformed Liposomes
External insertion of PEG-lipid into the liposome bilayer was performed essentially by the method reported previously (Awasthi et al., 2004). Briefly, 0.05 mmol (equivalent to approximately 10% of the total lipid in the liposomes) of HDAS-PEG was dissolved in 50 mL of water and filtered through 0.2 μm. The solution was gradually added to a diluted suspension of liposomes, such that the concentration of HDAS-PEG remained below its CMC as determined above. The mixture was stirred overnight at 37°C in inert (N2) atmosphere during the addition of HDAS-PEG solution using a programmable syringe pump (Chemyx Inc., Satfford, TX). The preparation was washed free of any unincorporated HDAS-PEG, by tangential flow filtration using 10 volumes of PBS (pH 7.4) in a 2 sq ft 0.05 μm hollow fiber filter (Minntech Filtration Technology Group, Minneapolis, MN). The PEGylated preparation was concentrated by centrifugation at 45,000 rpm for 45 min at 5°C (Optima L-100 XP ultracentrifuge, Beckman Coulter, Fullerton, CA). The supernatant was removed, the pellet was resuspended in PBS, and the centrifugation cycle was repeated two times to completely eliminate extravesicular glutathione and unincorporated HDAS-PEG. The second half of liposome preparation (plain liposomes) was identically handled except that no PEGylation was performed in these liposomes. The final pellets of PEG-liposomes and plain liposomes were resuspended in PBS (pH 7.4) containing 300 mM sucrose and stored at 4°C until further use. Identical methods were used for the post-insertion of DSPE-PEG2000.
The phospholipid concentration in the liposome preparations was determined by colorimetric method of Stewart (Stewart, 1980) using Perkin-Elmer Lambda 4B UV/Vis spectrophotometer (Waltham, MA). Particle size, size distribution and zeta potential of the liposomes were studied with dynamic light scattering (DLS) using a Zeta PLUS analyzer (Brookhaven Instruments Corp, Holtsville, NY) equipped with 532 nm laser.
Desorption of PEG-lipid from liposomes
The rates of PEG-lipid desorption from post-inserted liposomes post-inserted with HDAS-PEG and DSPE-PEG was investigated in vitro. Approximately 1:50 diluted PEG-liposomes were dialyzed against 150 mL of PBS (pH 7.4) at 37°C with constant shaking. A similar dilution of plain liposomes served as a negative control. The dialysis chamber consisted of Pierce’s 7,000 Da MWCO Slide-A-Lyzer dialysis cassettes (Thermo Fisher Scientific, Rockford, IL). The external buffer was sampled at various times for PEG estimation by ELISA as described below. The slope of percent PEG released versus time plot afforded the apparent rate of PEG-lipid desorption (k’dsorp). In an alternate set up, the PEG-liposomes were diluted with PBS to various levels. The diluted samples were continuously and vigorously vortexed at room temperature. After 24 h, the samples were centrifuged at 14,000 rpm for 1 h at 4°C to sediment the PEG-liposomes. The percent of PEG released was plotted against the dilution factor. The concentration of PEG in the supernatant was determined by ELISA.
Transmission electron microscopy (TEM)
To visually document the formation of liposomes using non-phospholipid HDAS-PEG, we performed electron microscopy at the Oklahoma Medical Research Foundation’s Imaging Core Facility, Oklahoma City (OK). Transmission electron micrographs were obtained using a Hitachi H-7600 Transmission Electron Microscope at 30,000 X operating at 120 kV. Briefly, the TEM samples were prepared by placing dilute suspension of liposomes on a formvar-coated copper grid (400 mesh) and allowing it to stand for 3 min in air. The excess sample was removed, followed by negative staining with 1% aqueous uranyl acetate solution. The stained sample grid was allowed to air dry for 5 min before the measurements.
Laser Scanning Microscopy (LSM)
To confirm HDAS-PEG insertion into the liposome, we performed LSM of PEGylated-liposomes immunolabeled with Alexa Fluor® 488 Dye. Briefly, an 8-chambered tissue culture glass slide was coated with liposome samples for 16 h at 4°C. The wells were washed three times with 0.05% Tween 80, followed by washing twice with deionized water. The nonspecific sites were blocked with 0.25% bovine serum albumin for 30 min. The wells were washed and incubated with 2,000 X dilution of goat anti-PEG antibody (Epitomics, Burlingame, CA) for 2 h. The excess antibody was removed by washing with PBS, followed by another 2 h-incubation with Alexa Fluor® 488 Dye-conjugated donkey anti-goat IgG antibody (Invitrogen, Carlsbad, CA) at a dilution of 1:1,000 in blocking buffer. Finally the samples were imaged using a Zeiss LSM-710 Multiphoton Laser Microscope (Oklahoma Medical Research Foundation, Oklahoma City).
PEG Enzyme-Linked Immunosorbent Assay (ELISA)
For the estimation of inserted PEG-lipid within the liposomes, we employed indirect PEG-ELISA. Briefly, the wells of Immulon 4 strips (Thermo, Milford, MA) were coated overnight with pure standard PEG-lipid diluted with 0.1 M NaHCO3/Na2CO3, pH 8.0 (1:1,000,000). The wells were washed three times with 0.05% Tween 80, followed by washing twice with deionized water. The nonspecific sites were blocked with 0.25% bovine serum albumin for 30 min. The wells were washed and incubated for 2 h with 2,000 X dilution of rabbit anti-PEG antibody. After washing the wells, horseradish peroxidase-conjugated anti-rabbit IgG antibody (Cell signaling, Danvers, MA) was added at a dilution of 1:1,000. After a 2 h incubation, the wells were washed again and incubated with tetramethylbenzidine substrate (Amresco, Solon, Ohio). The reaction was stopped by adding 50 μL of 2 N H2SO4 and the plate was read at 450 nm. The regression coefficient for a least-square linear fit to the standard curve of PEG was 0.99. The limit of detection for PEG-lipid was 5 ng/well.
Complement Activation
We examined both classical and alternative complement pathways by determining C4d (classical marker), Bb (alternate marker) and SC5b (S protein-bound terminal complex) using Quidel’s MicroVue complement EIA kits (Santa Clara, CA). Plain liposomes without HDAS-PEG, but of identical composition were used for comparison. The test preparations were incubated with serum from healthy donors (1:5 v/v) for 1 h at 37°C. The mixtures were diluted with PBS and centrifuged to remove any liposome particles (14 krpm for 30 min at 4°C). The supernatants were diluted 1:30 (Bb), 1:40 (SC5b) and 1:70 (C4d).
For comparison of HDAS-PEG and DSPE-PEG, preformed liposomes (DSPC: DMPG: CHO, 50:10:40 mol%) were post-inserted with either HDAS-PEG or DSPE-PEG at a concentration of 5 mole percent. Serum was collected from blood obtained from three volunteers and complement C3a plus and complex SC5B-9 was measured.
Radiolabeling of Liposomes
The presence of encapsulated glutathione enables the labeling of liposomes with 99mTc-labeled hexamethylpropyleneamine oxime (99mTc-HMPAO) using a procedure described previously (Phillips et al., 1992). Freshly prepared 99mTc-HMPAO was obtained from OUHSC-Nuclear Pharmacy (Oklahoma City, OK). 99mTc-HMPAO (0.15 mL, approximately 5 mCi) was added to 0.50 mL of liposome preparations, and incubated at room temperature with occasional swirling. After 30 min, the labeled liposomes were passed through a PD-10 column (GE Healthcare Life Sci, Pittsburg, PA) to separate any radioactivity not associated with the liposomes. The labeling efficiencies of 99mTc-liposomes were determined by performing paper chromatography in saline.
Imaging and Tissue Distribution Studies in Rats
The animal experiments were performed according to the National Institutes of Health Animal Use and Care Guidelines and were approved by the Institutional Animal Care Committee of the University of Oklahoma Health Science Center. Sprague Dawley rats were divided in two groups of five each. The first group received 99mTc-plain liposomes, whereas the second group received 99mTc-PEG-liposomes. All injections (0.4 - 0.6 mCi, 0.5 mL and approximately 4.3 mg phospholipid dose) were performed in the tail vein of rats under 2% isoflurane anesthesia (Butler Schein Animal Health, Dublin, OH) in a stream of oxygen. After radioactivity administration, the anesthetized rats were imaged by a NanoSPECT machine (Bioscan Inc., Washington, DC) at various times before euthanasia with an overdose of Euthasol (Virbac Corporation, Fort Worth, TX) at 24 h post-injection. Various organs were excised, washed with saline, weighed, and appropriate tissue samples were counted in a gamma counter (PerkinElmer Life and Analytical Sciences, Boston, MA). Femur with bone marrow was taken as representative of bone. Total blood volume and bone and muscle mass were estimated at 5.4%, 10% and 40% of body weight, respectively. A diluted sample of injected liposomes served as a standard for comparison.
Data Analysis
The data were statistically analyzed by the univariate analysis of variance using GraphPad Prism software for Windows (La Jolla, CA). For multiple comparisons, Bonferroni’s post hoc test was applied. All average values were given ± standard error of mean. The acceptable probability for significance was p < 0.05. In order to obtain profiles of liposome distribution in blood and MPS, the images were analyzed by drawing regions of interest (ROI) around heart and liver voxels. The radioactive counts were normalized by the counts associated with the whole body and plotted with respect to time. All calculations pertaining to 99mTc radioactivity involved correction for decay of 99mTc (t1/2 6.0 h).
Results and Discussion
In recent years considerable resources have been directed toward the development of lipid bilayer vesicle delivery systems using lipid amphiphiles other than phospholipids (Mathur and Capasso, 1997). The reasons for this interest in non-phospholipids are the biological reactivity, relative instability and the costs associated with extraction and synthesis of phospholipids (Bastiat et al., 2007; Gupta et al., 1996). Phosphoryl moiety of phospholipids carries a negative charge which has been linked to many hypersensitivity reactions observed with the usage of intravenous liposome products (Bonte and Juliano, 1986; Chonn et al., 1991). Such biological reactions are frequently seen even with highly purified phospholipids, and are believed to be associated with complement activation (Funato et al., 1992; Harashima et al., 1994; Ishida et al., 2002; Liu et al., 1995; Matsuo et al., 1994). Therefore, we hypothesized that a synthetic PEG-lipid devoid of phosphoryl moiety is biologically more acceptable. We synthesized a novel PEG-conjugated neutral lipid and post-inserted it into a liposome preparation for prolonging their circulation persistence in a rat model. PEGylation results in an increase in particle diameter and change in electrophoretic mobility secondary to a reduction in the surface charge of liposomes upon PEG binding (Sugiyama and Sadzuka, 2011). Being able to accurately detect and quantify the extent of liposome surface modification is required for achieving the necessary precision when developing and testing their biological properties. Here, we provide conclusive evidences that the technique of PEG post-insertion in preformed liposomes achieves the intended physical stealthiness of liposome surface for prolonging circulation persistence.
HDAS-PEG Synthesis and characterization
HDAS-PEG was synthesized by following the scheme provided in Figure 1a. In a previous report, we published the synthesis of an intermediate anionic lipid 2 (Agashe et al., 2010). In order to conjugate PEG, PEG2000-amine was reacted with NHS-activated 2. The entire synthesis is relatively easy and provides excellent yields. The structure of HDAS-PEG was confirmed by NMR (Figure 1b). In DSC, HDAS-PEG showed a major endothermic transition at 52.5°C followed by another endothermic reaction at 56.6°C (Figure 1c). The peak temperatures on the graph are printed by the instrument software and represent onset of the peak; the major peak maximum occurred at 51.1°C (-117.6 J/g). These may not be melting points, but are the temperatures corresponding to the maximum release of heat.
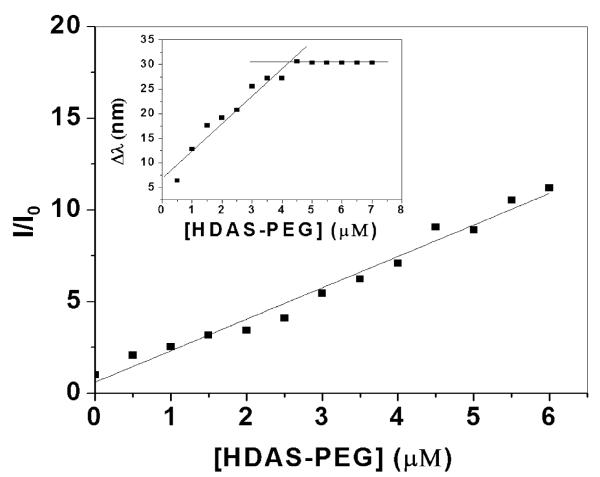
Amphiphilic molecules are characterized by a characteristic concentration, known as critical micellar concentration, at which the individual molecules aggregate into micellar structures. We determined CMC of HDAS-PEG by a fluorescence-based method using NPN as a probe. Though NPN is weakly fluorescent in aqueous solution, its fluorescence spectrum in the presence of HDAS-PEG shows about 10 times increase in intensity accompanied by a 30 nm blue-shift. The change of relative fluorescence intensity (I/I0, where I and I0 are the fluorescence intensities of NPN in the presence and absence of HDAS-PEG, respectively) with increasing polymer concentration is increased (Figure 2). This suggests that the probe molecules are encapsulated in non-polar environments within the lipids aggregates (Saitoh et al., 2002). The corresponding shift of the emission maxima of NPN (Δλ = λin water - λin HDAS-PEG solution) with increasing [HDAS-PEG] shows an inflection point at 4.25 μM which can be referred to as CMC of HDAS-PEG (Figure 2, inset). Widely used PEG-phosphatidylethanolamines (PEG-PE) show CMC in the concentration range of 8-20 μM (Priev et al., 2002).
Figure 2.
Relative fluorescence intensity (I/I0) of NPN and change in emission maxima wavelength (Δλ, inset) of NPN as a function of [HDAS-PEG]. The spectra were obtained by exciting the samples at 340 nm and the emission was recorded from 467 to 437 nm.
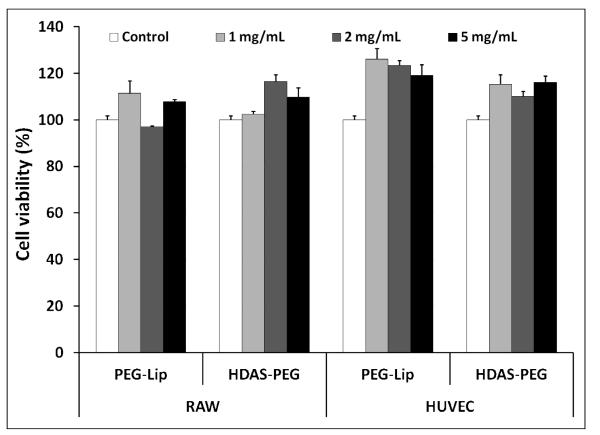
As a particulate and long-circulating drug delivery vehicle, PEG-liposomes are expected to mostly interact with two cell types-vascular endothelial cells and macrophages responsible for clearing the particulate material. Therefore, we tested potential toxicity of HDAS-PEG in HUVEC and RAW 264.7 cells. HDAS-PEG was tested both in liposome form as well as alone in solution (DMSO) form. As shown in Figure 3, HDAS-PEG as well as liposomes containing HDAS-PEG did not show any effect on the viability of these two cell lines. In general, cationic lipids and phospholipids with low phase transition temperature are toxic to cells (Fraga et al., 2011; Senior et al., 1991). Liposomes bearing rigid lipid chains provide more stable and less toxic interface between biological system and the formulation. Earlier, Miller, et al showed that HeLa (epithelial) cells endocytose positively charged liposomes to a greater extent than either neutral or negatively charged liposomes, whereas J774 macrophages interacted more with both cationic and anionic liposomes as compared to neutral liposomes; PEGylation eliminated the endocytic uptake of negatively charged liposomes (Miller et al., 1998). Like its phospholipid analog DSPE-PEG, HDAS-PEG is also a high melting lipid.
Figure 3.
Cytotoxicity of HDAS-PEG and HDAS-PEG liposomes (PEG-Lip) in RAW 264.7 and HUVEC cells evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The viable cells are represented as percent of control.
Liposome preparation
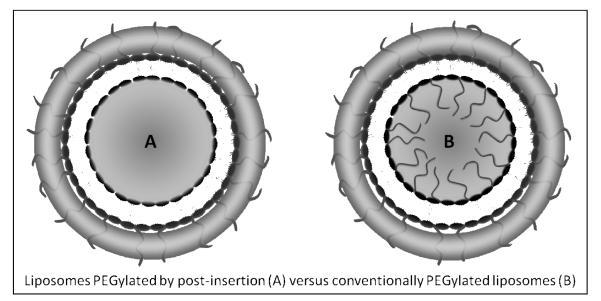
After characterizing the new HDAS-PEG lipid, we used it to modify the surface of pre-formed liposomes (DPPC:DMPG:CHOL:Vit-E = 45:10:44.8:0.2 mol%). We used approximately 10 mole % of HDAS-PEG solubilized in warm water. Conventionally, the PEG modification of liposome surface is accomplished by incorporating PEG-PEs in the lipid phase just prior to its hydration with an aqueous phase (Phillips et al., 1999). However, this technique also results in the PEG brush or mushroom occupying the limited encapsulation-worthy space inside the liposomes. The same steric hindrance that makes PEG useful for prolonged circulation may also exclude the drugs and biomolecules from getting encapsulated (Nicholas et al., 2000). In addition to undesirably restricting the real estate available for drugs, the internal PEG-phospholipids is also amenable to acid/base-catalyzed hydrolytic degradation in pH gradient liposomes. The hydrolysate has the potential of compromising the active loading and retention of drugs inside such liposomes (Nakamura et al., 2012). Equally important is the economic consideration of having expensive, but essentially ineffective (if not detrimental) internal presence of PEG-lipid which is not contributing to the intended enhancement of stability and circulation persistence of liposomes (Figure 4). The smaller the liposome size, the greater is the impact of PEG on each of the above outcomes. In the case of multi-lamellar liposomes, the magnitude of this wastage is even greater (Awasthi et al., 2004).
Figure 4.
A schematic comparison between post-inserted (A) and conventionally PEGylated liposomes (B). Post-inserted PEG-liposomes offer as clear advantage over conventionally PEGylated liposomes B where the internal space is limited by the presence PEG chains from PEG-conjugated lipid in the internal layer of liposomes.
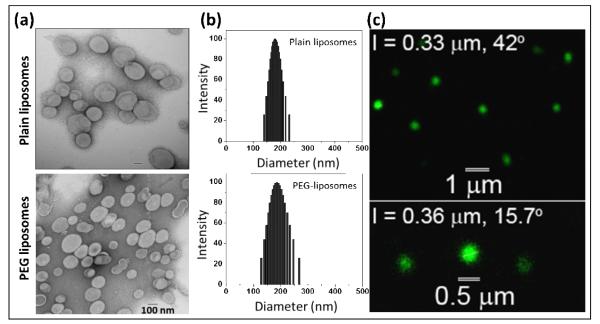
Realization of the problems associated with conventional PEG-phospholipid incorporation led to the technique inserting PEG-PE in the outer layer of pre-formed liposomes (Uster et al., 1996). This useful technique is known as post-insertion or post-modification of liposomes, and is beginning to attract applications in liposome-based drug and biologic delivery (Awasthi et al., 2004; Nakamura et al., 2012; Puri et al., 2009; Sou et al., 2000). We also chose post-insertion technique to introduce HDAS-PEG into the preformed liposomes. To prevent self-assembly of HDAS-PEG during post-insertion, its concentration was maintained at a level lower than CMC. The thermodynamic barrier to insertion into liposome bilayers is lower in case of monomeric PEG-lipids than that in case of self-assembled micellar PEG-lipids. Estimation of PEG concentration by ELISA indicated that the molar ratio of DPPC to HDAS-PEG was approximately 0.25. The physicochemical characteristics of the resultant liposomes are listed in Table 1. The images from negative-staining TEM revealed the spherical nanostructure formations with average diameter below 200 nm which was expected after extrusion through polycarbonate filter with a well-defined pore size 200 nm filter (Figure 5a and 5b). Furthermore, the mean diameters of the liposomes were in good agreement with the determination made using DLS. The narrow polydispersity of both of the preparations suggest that liposomes had homogeneous size distributions. On the other hand, PEGylation significantly altered the zeta potential of liposomes (Table 1). The relatively high negative zeta potential of plain liposomes was reduced by approximately 26 and 22 mV after PEGylation with HDAS-PEG and DSPE-PEG, respectively. The shift in electrical mobility provided us with the first proof that post-inserted PEG was occupying the surface of the liposomes. The presence of PEG on the surface moves the slipping plane further away from the liposome surface (Boada et al, 2001). At the same time, the drag or brushing caused by the presence of PEG chains on the liposome surface reduces the mobility of the liposomes (http://www.azonano.com, 2005). It is notable that the negative zeta potential value of liposomes PEGylated with HDAS-PEG is even lower than the similarly manufactured liposomes of alike composition but PEGylated with commercial PEG-phospholipid 1,2-distearoyl-sn-glycero-3-phosphoethanolamine [methoxy (polyethyleneglycol)] (DSPE-PEG) (Hinrichs et al., 2006). This is probably due to the net neutral charge of synthetic HDAS-PEG compared to the net negative charge of PEG-phospholipids (Yang et al., 2007). To further confirm the presence of HDAS-PEG on the liposome surface, the liposomes were labeled with anti-PEG antibody and confocally micrographed using Alexa Fluor® 488 Dye-conjugated anti-IgG antibody. The green fluorescence (Alexa Fluor® 488 emission) emanating from the liposome surface clearly shows the presence of surface PEG (Figure 5c). Plain liposomes without HDAS-PEG did not show any emission (not shown).
Table 1.
Characteristics of plain and PEGylated liposomes
| Average diameter (nm) |
[Phospholipid] (mg/mL) |
[HDAS- PEG] mM |
99mTc-labeling efficiency (%) |
Zeta potential (mV) |
||
|---|---|---|---|---|---|---|
| TEMa | DLSb | |||||
| Plain liposomes |
179 | 179.6 (0.02c) |
8.34 | - | >89 | −30.9 |
|
| ||||||
| HDAS- PEG- liposomes |
138 | 185.9 (0.05c) |
10.82 | 3.4 | >85 | −4.6 |
|
| ||||||
| DSPE- PEG- liposomes |
ND | 186.4 (0.06c) |
11.67 | - | 83 | −8.9 |
Transmission electron microscopy,
Dynamic light scattering,
Polydispersity , ND= not determined
Figure 5.
Physicochemical characterization of liposomes: (a) Transmission electron micrographs, (b) Dynamic light scattering for particle size distribution, and (c) One-photon Laser Scanning microscopic images of HDAS-PEG liposomes. The sample was stained with Alexa Fluor® 488 conjugated donkey anti-goat IgG antibody. The emission was collected at 500-700 nm. The wavelength for the excitation was 495 nm.
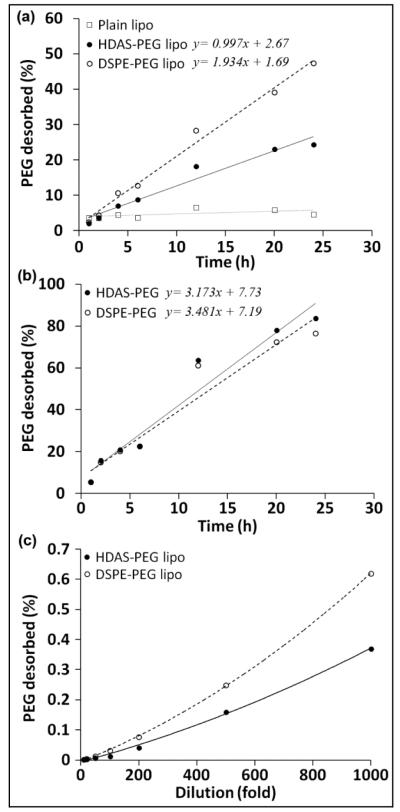
Regardless of the presence of surface PEG, PEGylated liposomes are eventually cleared by MPS in vivo. Why PEGylation does not provide more extended circulation persistence than is normally observed? We hypothesized that the PEG-lipid molecules desorb from the liposome surface as the biological fluids dilute the administered PEG-liposomes, resulting in gradual loss of PEG-conferred stealth property. In order to test this hypothesis, we estimated the desorbed PEG in a buffer separated from PEG-liposomes by a 7 KDa MWCO dialysis membrane. Figure 6a shows the profile obtained of PEG in the dialysate of HDAS-PEG-liposomes during 24 h incubation at 37°C. For comparison, an identically prepared DSPE-PEG-liposome preparation was also subjected to the same treatment. We were able to detect PEG in the dialysate starting at 1 h, and the [PEG]dialysate increased with time. PEG desorption from both PEG-liposome preparations demonstrated linearity. The k’dsorp of DSPE-PEG (1.9% h−1) appears to be almost double the k’dsorp of HDAS-PEG (0.99% h−1). In case of micellar HDAS-PEG or DSPE-PEG also, the amount of PEG released in the dialysate increased linearly, and there was not much difference between the release profiles of the two (Figure 6b). Over 80% of micellar HDAS-PEG or DSPE-PEG equilibrated with dialysis medium over the course of 24 h. The amount of PEG desorbed in relationship with dilution is shown in Figure 6c. Because the dilution experiment was carried out at room temperature (25°C) which was substantially below the melting temperature of the major lipid DPPC (Tm= 41°C), the quantity of PEG desorbed from the liposome surface was small. However, there was a difference in the desorption profile between HDAS-PEG and DSPE-PEG, with the former appearing more resistant to desorption than the latter. These results also suggest that PEG desorption is a time- and dilution-dependent phenomenon and might also be occurring in vivo after administration. The rate of desorption in vivo could be expected to be higher because physiological milieu is characterized by the better maintenance of sink conditions and the presence of plasma as the bathing fluid. Earlier, Parr et al, studied the retention of PEG coating on the liposome surface and found that removal of the hydrophilic coating in vivo is dependent on the nature of the lipid portion and the nature of the linkage between the lipid and PEG (Parr et al., 1994).
Figure 6.
(a) HDAS-PEG and DSPE-PEG desorption (with respect to time) after its insertion into the preformed liposomes. A 7 KDa MWCO dialysis cassette was used to separate desorbed PEG-lipids (MW ~2,500) from the PEGylated liposomes. No PEG was detected in the dialysates from plain liposomes (open squares). (b) The micellar solutions of HDAS-PEG and DSPE-PEG (1 mM) served as positive controls in the assay. A linear release of the majority of PEG-lipids confirms the ability of dialysis
Complement activation
Complement proteins play an important role in liposome clearance from circulation (Bradley and Devine, 1998; Szebeni et al., 2003; Szebeni et al., 1997c). A complement-dependent rapid, but transient, decrease in circulating platelets immediately following infusion of liposomes has also been noted (Phillips et al., 1997). Studies in animal models have revealed that the presence of anionic phospholipids in liposomes causes complement activation (Moghimi et al., 2006; Szebeni et al., 1997a, b), resulting in platelet micro-aggregates that are sequestered by the MPS (Loughrey et al., 1990). Recently, we reported the effect of liposome charge on platelet activation by LEH in a rabbit model (Awasthi et al., 2007). This platelet activation is partially prevented by PEGylation and reduction in anionic phospholipid content (Awasthi et al., 2007). Since, HDAS-PEG is devoid of anionic phosphoryl group, we hypothesized that liposomes prepared from HDAS-PEG will not activate complement proteins.
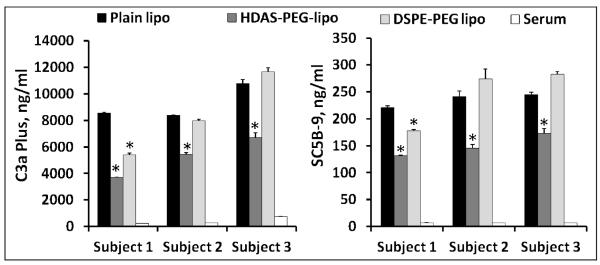
We examined both classical and alternative complement activation pathways by determining anaphylotoxins C4d (classical marker), Bb (alternate marker) and SC5b (S protein-bound terminal complex). Plain liposomes without PEG2000, but of composition identical to that of PEG-liposomes, were used for comparison. The levels of C4d, Bb and SC5b-9 in serum treated with various preparations are shown in Figure 7. The results clearly suggest that post-insertion of HDAS-PEG eliminated the liposome-induced complement activation. The results confirm the ability of post-inserted HDAS-PEG in preventing reactions which have been classified as complement-activation-related pseudoallergy or CARPA (Szebeni, 2005; Szebeni et al., 2000). Others have also reported attenuation of complement activating tendency of liposomes after PEGylation (Ahl et al., 1997; Bradley and Devine, 1998; Bradley et al., 1998). In a separate set of experiments we also compared the relative abilities of HDAS-PEG liposomes and DSPE-PEG liposomes to inhibit complement activation. Both DSPE-PEG and HDAS-PEG were post-inserted in the preformed liposomes as described previously. We found that HDAS-PEG post-insertion was more effective in reducing the liposome-induced complement activation (Fig. 8). While HDAS-PEG was effective in inhibiting complement activation in all three subjects, DSPE-PEG was significantly effective in only one subject. The reasons for this variation are unknown at present, but could be ascribed to inherent physiology of the respective subjects or timing of blood collection.
Figure 7.
PEGylation by post-insertion of HDAS-PEG attenuates liposome-induced complement activation. The complement proteins in human serum were determined by enzyme-immunoassay after treatment with plain liposomes or HDAS-PEG liposomes for 1 h. C4d and Bb are the markers for classical and alternate pathways, respectively, whereas SC5b is S protein-bound terminal complex. Average titers and standard error of mean were determined for six individual reading (* p < 0.05).
Figure 8.
Post-inserted HDAS-PEG is more effective than post-inserted DSPE-PEG in inhibiting liposome-induced complement activation. Preformed liposomes (DSPC: DMPG: CHO, 50:10:40 mol%) were post-inserted with either HDAS-PEG or DSPE-PEG at a concentration of 5 mole percent. The post-insertion technique was same as described in the methods section. Serum was collected from three volunteers and complement C3a plus and complex SC5B-9 was measured. The results are average of three replicates (±sem).
Imaging and Biodistribution of PEG-liposomes
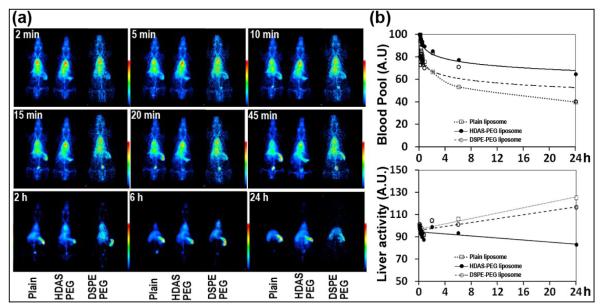
The ultimate test of success of PEGylation strategy is its performance after administration in a live animal model. The intended in vivo goal of liposome PEGylation is to prolong its circulation persistence and reduce MPS uptake secondary to their opsonization with complement proteins. In order to test the in vivo performance of liposomes post-inserted with HDAS-PEG, the liposome preparations were labeled with gamma ray-emitting radionuclide (99mTc) and injected in rats. The labeling of the liposomes with 99mTc was reproducible and the efficiency of labeling was always ≥ 85% (Table 1). Further purification by gel exclusion chromatography yielded over 95% 99mTc-liposomes. The circulation of liposomes was visually monitored over a period of 24 h after administration. For the best comparison, we simultaneously imaged two animals receiving the labeled HDAS-PEG liposomes and plain liposomes. Separately, an additional group of animals was investigated for circulation and biodistribution of 99mTc-labeled DSPE-PEG liposomes. The dynamic images (Figure 9a) taken immediately after injection show that the difference in blood pool signal in a pair of rats injected with plain liposome and HDAS PEGylated liposomes starts to appear within 5 min of injection. The heart-bound radioactivity is taken as 99mTc-liposomes circulating in blood pool. By 6 h post-injection (and more so by 24 h), the majority of injected plain liposomes was cleared from circulation, whereas the HDAS-PEG and DSPE-PEG liposomes were still in circulation. Liver and spleen were the major organs of plain liposome accumulation. The early (45 min) accumulation of plain liposome-associated 99mTc radioactivity in urinary bladder indicates the release of 99mTc radioactivity after metabolic processing of liposomes in the MPS. The difference in circulation persistence between HDAS-PEG and DSPE-PEG liposomes was only evident at 24 h. While blood pool in heart still carried substantial signal from HDAS-PEG liposomes at 24 h, DSPE-PEG liposomes were more or less eliminated. Further analysis of images was performed by drawing ROI around heart pixels and comparing the decrease in signal emanating from the ROI with respect to time. Figure 9b provides a profile of blood circulation and liver accumulation for plain, HDAS-PEG and DSPE-PEG liposomes. It is clear that simultaneous to the enhanced circulation, the liver-associated liposomes are lower in the liposomes post-inserted with HDAS-PEG-lipid than either of the other preparations. It must be noted however that even if this image-based circulation kinetics provides clear differences in relative performances of liposome preparations, it is semiquantitative and is limited by the lack of more image data points for appropriate curve fitting. A more elaborate intermittent blood sampling-based approach is best suited for absolute quantification of circulation half-lives.
Figure 9.
Demonstration of enhanced circulation persistence of liposomes PEGylated by post-insertion of HDAS-PEG or DSPE-PEG: (a) Representative scintiimages of rats injected with 99mTc-labeled plain, HDAS-PEG and DSPE-PEG liposomes, and (b) the profiles of heart- and liver-associated 99mTc-liposomes with respect to time.
To quantify the accumulation of the injected liposomes in various organs, the rats were euthanized after imaging at 24 h (Table 2). The major organs of radioactivity accumulation were blood, spleen, liver, bone and kidney; other organs accumulated negligible amount of activity. In the case of HDAS-PEG liposomes, significantly higher radioactivity (40 times) was observed in the blood as compared to that in case of plain liposomes. Although the levels of plain and PEG liposomes accumulation in the MPS (liver and spleen) appear similar, much of the MPS-associated plain liposomes are cleared from the body by 24 h. The higher muscle-associated PEG-liposomes are indicative of their presence in muscular vascular space. Both the images and the biodistribution data is consistent with previously published reports on plain and PEG-liposomes (Awasthi et al., 2004; Nakamura et al., 2012; Puri et al., 2009; Sou et al., 2000). Compared to 28% of HDAS-PEG liposomes in blood, only ~5% of DSPE-PEG liposomes were measured in circulation after 24 h. These images and the biodistribution results provide clear differences in circulation persistence between the two preparations, and offer an in vivo evidence of HDAS-PEG performing to enhance circulation of liposomes. The difference between circulation persistence of HDAS-PEG and DSPE-PEG liposomes could be partially attributed to potentially lower opsonization of former by complement proteins. Another factor that was not investigated in this study might be the resistance of HDAS-PEG to the metabolic processing.
Table 2.
Biodistribution of plain liposomes and PEG-liposomes in rats (n ≥ 4 per group) at 24 h.
| Percentage of injected dose |
||||||
|---|---|---|---|---|---|---|
| Plain liposomes | HDAS-PEG liposomes | DSPE-PEG-liposomes | ||||
|
|
||||||
| Organ | Per organ | Per gram | Per organ | Per gram | Per organ | Per gram |
|
|
||||||
| Blood | 0.70 ± 0.25 | 0.04 ± 0.01 | 27.92 ± 3.94 | 1.57 ± 0.23 | 4.78 ± 2.30 | 0.20 ± 0.09 |
| Heart | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.19 ± 0.03 | 0.18 ± 0.03 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| Lung | 0.40 ± 0.14 | 0.23 ± 0.08 | 0.64 ± 0.10 | 0.42 ± 0.06 | 0.21 ± 0.04 | 0.11 ± 0.02 |
| Liver | 10.28 ± 2.67 | 0.86 ± 0.21 | 11.10 ± 1.51 | 0.94 ± 0.13 | 9.66 ± 0.17 | 0.83 ± 0.07 |
| Spleen | 19.36 ± 6.29 | 27.41 ± 8.63 | 19.27 ± 3.53 | 27.24 ± 4.91 | 9.74 ± 1.62 | 15.67 ± 2.01 |
| Kidney | 7.17 ± 1.41 | 3.27 ± 0.55 | 9.01 ± 1.27 | 4.26 ± 0.63 | 6.74 ± 0.37 | 2.78 ± 0.29 |
| Muscle | 1.05 ± 0.36 | 0.01 ± 0.00 | 3.70 ± 0.91 | 0.03 ± 0.01 | 0.88 ± 0.26 | 0.01 ± 0.00 |
| Bone | 10.08 ± 1.99 | 0.31 ± 0.04 | 9.34 ± 1.27 | 0.30 ± 0.04 | 8.77 ± 0.78 | 0.21 ± 0.02 |
Conclusions
We report synthesis of a novel PEG-conjugated lipid that could be used to PEGylate liposomes for enhancement of circulation persistence. HDAS-PEG is as effective in enhancing circulation persistence of liposomes as the conventional PEG-phospholipids, such as DSPE-PEG, but with more inhibitory capacity of complement activation. We believe that as a substituent for PEG-phospholipids, HDAS-PEG could be easily scaled up because the precursor chemicals are readily available and inexpensive. We provided structural, biochemical and in vivo evidence of successful HDAS-PEG application in enhancing liposome characteristics. While the long-term toxicity of such non-phospholipid analogs remains to be evaluated, the new lipid appears to be nontoxic to cells in vitro, attenuates complement activation and reduces uptake and clearance of liposomes in MPS. Moreover, the potential of liposome destabilization and accompanying leakage of encapsulated material should be considered during post-insertion and appropriate measures should be taken to eliminate this possibility.
Acknowledgements
The funding for the work reported in this article was provided by the National Heart, Lung and Blood Institute (R01HL104286). Both OKN and VRY contributed equally to the experimental work. VA provided conceptual framework and manuscript writing. AH assisted in imaging and biodistribution studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids Surf B Biointerfaces. 2010;75:573–583. doi: 10.1016/j.colsurfb.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahl PL, Bhatia SK, Meers P, Roberts P, Stevens R, Dause R, Perkins WR, Janoff AS. Enhancement of the in vivo circulation lifetime of L-alpha-distearoylphosphatidylcholine liposomes: importance of liposomal aggregation versus complement opsonization. Biochim Biophys Acta. 1997;1329:370–382. doi: 10.1016/s0005-2736(97)00129-6. [DOI] [PubMed] [Google Scholar]
- Awasthi V, Agashe H, Doblas S, Towner R. Magnetic resonance spectroscopy for evaluation of liposome-encapsulated hemoglobin as a resuscitation fluid. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:69–78. doi: 10.3109/10731191003634638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: Effect of post-inserted poly (ethylene glycol)-distearoylphosphatidylethanolamine on distribution and circulation Kinetics. J. Pharmacol. Exp. Ther. 2004;309:241–248. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- Awasthi VD, Goins B, Phillips WT. Insertion of poly(ethylene glycol)-lipid reduces the liposome-encapsulated hemoglobin-induced thrombocytopenic reaction. Am J Pharmacol Toxicol. 2007;2:98–105. [Google Scholar]
- Bastiat G, Oliger P, Karlsson G, Edwards K, Lafleur M. Development of non-phospholipid liposomes containing a high cholesterol concentration. Langmuir. 2007;23:7695–7699. doi: 10.1021/la700824m. [DOI] [PubMed] [Google Scholar]
- Boada J, Gallardo M, Alsina MA, Estelrich J. Adsorption of cyanuric chloride-activated polyethylene glycol on liposomes. Colloids and Surfaces A-Physicochemical and Engineering Aspects. 2001;182:191–198. [Google Scholar]
- Bonte F, Juliano RL. Interactions of liposomes with serum proteins. Chem Phys Lipids. 1986;40:359–372. doi: 10.1016/0009-3084(86)90079-4. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Devine DV. The complement system in liposome clearance: Can complement deposition be inhibited? Adv Drug Deliv Rev. 1998;32:19–29. doi: 10.1016/s0169-409x(97)00129-4. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Devine DV, Ansell SM, Janzen J, Brooks DE. Inhibition of liposome-induced complement activation by incorporated poly(ethylene glycol)-lipids. Arch Biochem Biophys. 1998;357:185–194. doi: 10.1006/abbi.1998.0798. [DOI] [PubMed] [Google Scholar]
- Cattel L, Ceruti M, Dosio F. From conventional to stealth liposomes: a new frontier in cancer chemotherapy. Tumori. 2003;89:237–249. doi: 10.1177/030089160308900302. [DOI] [PubMed] [Google Scholar]
- Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–4241. [PubMed] [Google Scholar]
- Fraga M, Bruxel F, Lagranha VL, Teixeira HF, Matte U. Influence of phospholipid composition on cationic emulsions/DNA complexes: physicochemical properties, cytotoxicity, and transfection on Hep G2 cells. Int J Nanomedicine. 2011;6:2213–2220. doi: 10.2147/IJN.S22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato K, Yoda R, Kiwada H. Contribution of complement system on destabilization of liposomes composed of hydrogenated egg phosphatidylcholine in rat fresh plasma. Biochim Biophys Acta. 1992;1103:198–204. doi: 10.1016/0005-2736(92)90087-3. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Varanelli CL, Griffin P, Wallach DF, Siber GR. Adjuvant properties of non-phospholipid liposomes (Novasomes) in experimental animals for human vaccine antigens. Vaccine. 1996;14:219–225. doi: 10.1016/0264-410x(95)00182-z. [DOI] [PubMed] [Google Scholar]
- Harashima H, Sakata K, Funato K, Kiwada H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm Res. 1994;11:402–406. doi: 10.1023/a:1018965121222. [DOI] [PubMed] [Google Scholar]
- Hinrichs WL, Mancenido FA, Sanders NN, Braeckmans K, De Smedt SC, Demeester J, Frijlink HW. The choice of a suitable oligosaccharide to prevent aggregation of PEGylated nanoparticles during freeze thawing and freeze drying. Int J Pharm. 2006;311:237–244. doi: 10.1016/j.ijpharm.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Liposomes and the use of zeta potential measurements to study sterically stabilized liposomes. 2005 http://www.azonano.com. 2012.
- Ishida T, Harashima H, Kiwada H. Liposome clearance. Biosci Rep. 2002;22:197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- Liu D, Song YK, Liu F. Antibody dependent, complement mediated liver uptake of liposomes containing GM1. Pharm Res. 1995;12:1775–1780. doi: 10.1023/a:1016286310475. [DOI] [PubMed] [Google Scholar]
- Loughrey HC, Bally MB, Reinish LW, Cullis PR. The binding of phosphatidylglycerol liposomes to rat platelets is mediated by complement. Thromb Haemost. 1990;64:172–176. [PubMed] [Google Scholar]
- Mathur R, Capasso P. Nonphospholipid liposomes: Properties and potential use in flavor encapsulation (Chapter 17) In: Ho C-T, Tan C-T, Tong C-H, editors. Flavor Technology. American Chemical Society; 1997. pp. 219–230. [Google Scholar]
- Matsuo H, Funato K, Harashima H, Kiwada H. The complement-but not mannose receptor-mediated phagocytosis is involved in the hepatic uptake of cetylmannoside-modified liposomes in situ. J Drug Target. 1994;2:141–146. doi: 10.3109/10611869409015902. [DOI] [PubMed] [Google Scholar]
- Miller CR, Bondurant B, McLean SD, McGovern KA, O’Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamashita K, Itoh Y, Yoshino K, Nozawa S, Kasukawa H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochim Biophys Acta. 2012;1818:2801–2807. doi: 10.1016/j.bbamem.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Nicholas AR, Scott MJ, Kennedy NI, Jones MN. Effect of grafted polyethylene glycol (PEG) on the size, encapsulation efficiency and permeability of vesicles. Biochim Biophys Acta. 2000;1463:167–178. doi: 10.1016/s0005-2736(99)00192-3. [DOI] [PubMed] [Google Scholar]
- Parr MJ, Ansell SM, Choi LS, Cullis PR. Factors influencing the retention and chemical stability of poly(ethylene glycol)-lipid conjugates incorporated into large unilamellar vesicles. Biochim Biophys Acta. 1994;1195:21–30. doi: 10.1016/0005-2736(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Phillips W, Klipper R, Awasthi V, Rudolph A, Cliff R, Kwasiborski V, Goins B. Polyethylene glycol-modified liposome-encapsulated hemoglobin: A long circulating red cell substitute. J. Pharmacol. Exp. Ther. 1999;288:665–670. [PubMed] [Google Scholar]
- Phillips WT, Klipper R, Fresne D, Rudolph AS, Javors M, Goins B. Platelet reactivity with liposome-encapsulated hemoglobin in the rat. Exp Hematol. 1997;25:1347–1356. [PubMed] [Google Scholar]
- Phillips WT, Rudolph AS, Goins B, Timmons JH, Klipper R, Blumhardt R. A simple method for producing a technetium-99m-labeled liposome which is stable in vivo. Int J Rad Appl Instrum B. 1992;19:539–547. doi: 10.1016/0883-2897(92)90149-s. [DOI] [PubMed] [Google Scholar]
- Priev A, Zalipsky S, Cohen R, Barenholz Y. Determination of critical micelle concentration of lipopolymers and other amphiphiles: Comparison of sound velocity and fluorescent measurements. Langmuir. 2002;18:612–617. [Google Scholar]
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Taguchi K, Hiraide M. Evaluation of hydrophobic properties of sodium dodecyl sulfate/γ-alumina admicellesbased on fluorescence spectra of N-phenyl-1-naphthylamine. Analytica Chimica Acta. 2002;454:203–208. [Google Scholar]
- Senior JH, Trimble KR, Maskiewicz R. Interaction of positively-charged liposomes with blood: implications for their application in vivo. Biochim Biophys Acta. 1991;1070:173–179. doi: 10.1016/0005-2736(91)90160-a. [DOI] [PubMed] [Google Scholar]
- Sou K, Endo T, Takeoka S, Tsuchida E. Poly(ethylene glycol)-modification of the phospholipid vesicles by using the spontaneous incorporation of poly(ethylene glycol)-lipid into the vesicles. Bioconjug Chem. 2000;11:372–379. doi: 10.1021/bc990135y. [DOI] [PubMed] [Google Scholar]
- Stensrud G, Passi S, Larsen T, Sandset PM, Smistad G, Monkkonen J, Karlsen J. Toxicity of gamma irradiated liposomes. 1. In vitro interactions with blood components. Int J Pharm. 1999;178:33–46. doi: 10.1016/s0378-5173(98)00356-1. [DOI] [PubMed] [Google Scholar]
- Stewart JCM. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama I, Sadzuka Y. Correlation of fixed aqueous layer thickness around PEG-modified liposomes with in vivo efficacy of antitumor agent-containing liposomes. Curr Drug Discov Technol. 2011;8:357–366. doi: 10.2174/157016311798109344. [DOI] [PubMed] [Google Scholar]
- Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, Stahl GL, Bunger R, Alving CR. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol. 2000;279:H1319–1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Savay S, Milosevits J, Bodo M, Bunger R, Alving CR. The interaction of liposomes with the complement system: in vitro and in vivo assays. Methods Enzymol. 2003;373:136–154. doi: 10.1016/S0076-6879(03)73010-9. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Spielberg H, Cliff RO, Wassef NM, Rudolph AS, Alving CR. Complement activation and thromboxane secretion by liposome-encapsulated hemoglobin in rats in vivo: inhibition by soluble complement receptor type 1. Artificial Cells Blood Substitutes & Immobilization Biotechnology. 1997a;25:347–355. doi: 10.3109/10731199709118925. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Spielberg H, Cliff RO, Wassef NM, Rudolph AS, Alving CR. Complement activation and thromboxane secretion by liposome-encapsulated hemoglobin in rats in vivo: inhibition by soluble complement receptor type 1. Artif Cells Blood Substit Immobil Biotechnol. 1997b;25:347–355. doi: 10.3109/10731199709118925. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Wassef NM, Hartman KR, Rudolph AS, Alving CR. Complement activation in vitro by the red cell substitute, liposome-encapsulated hemoglobin: mechanism of activation and inhibition by soluble complement receptor type 1. Transfusion. 1997c;37:150–159. doi: 10.1046/j.1537-2995.1997.37297203517.x. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA, Jr., Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994;1195:11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Uster PS, Allen TM, Daniel BE, Mendez CJ, Newman MS, Zhu GZ. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 1996;386:243–246. doi: 10.1016/0014-5793(96)00452-8. [DOI] [PubMed] [Google Scholar]
- Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, Kim DD. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338:317–326. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Zimmerman GA, Prescott SM, McIntyre TM. Oxidatively fragmented phospholipids as inflammatory mediators: the dark side of polyunsaturated lipids. J Nutr. 1995;125:1661S–1665S. doi: 10.1093/jn/125.suppl_6.1661S. [DOI] [PubMed] [Google Scholar]