Abstract
Objective
Following menopause, women are at increased risk for cardiovascular disease. The present study assessed cardiovascular hemodynamics in pre- versus postmenopausal women, with a focus on systemic vascular resistance (SVR) at rest and during stress. Sympathetic nervous system (SNS) activity, and cardiovascular adrenergic receptor (AR) function were also examined.
Methods
A total of 90 women (N = 45 premenopausal; N=45 postmenopausal) completed a laboratory protocol comprised of a resting baseline and 4 mental stress tasks. Measurements included blood pressure (BP), cardiac output (CO), SVR, and plasma catecholamines. In addition, α and β AR responsiveness to the infusion of selective pharmacological agonists were assessed.
Results
Compared to premenopausal women, postmenopausal women were characterized by similar BP, but lower CO and higher SVR, both at rest and during stress (p’s < 0.05). Postmenopausal women also had higher baseline plasma norepinephrine levels (p=.007) and reduced β AR responsiveness (p=.02), although β AR differences may have been confounded by aging effects.
Conclusions
After menopause, women exhibit altered SNS activity and a sustained increase in hemodynamic load that may contribute to pathological structural and functional changes in the heart and blood vessels.
Descriptors: cardiovascular, menopause, hemodynamics, catecholamines, adrenergic receptors
INTRODUCTION
CHD risk in women increases dramatically after menopause (1–4), ultimately accounting for about one third of all deaths in women (5). Epidemiological evidence suggests that changes in female reproductive hormones, particularly the decline in estrogen, are primary factors contributing to this increased risk of CHD among postmenopausal women (2, 3, 6, 7). In addition to beneficial effects on the lipid profile, estrogen causes vasodilation through both direct and indirect effects on the vasculature (8–13). Therefore, when plasma estrogen levels fall dramatically following menopause, similar levels of blood pressure may be maintained with a lower cardiac output and higher systemic vascular resistance (SVR). The purpose of this study was to examine cardiovascular hemodynamics at rest and during stress in premenopausal and postmenopausal women. We hypothesized that postmenopausal women would show increased SVR at rest and during stress, as well as larger SVR responses during stress, compared to premenopausal women. Plasma catecholamines and adrenergic receptor (AR) responsiveness were examined as potential contributors to the hemodynamic effects of menopause.
METHODS
Participants
Women were recruited through advertisements in local newspapers. Recruitment was designed to obtain samples of pre- and postmenopausal women that were matched by age, weight, ethnicity, and blood pressure. Telephone screening was used to establish whether potential participants met the study’s inclusion/exclusion criteria. Over 90% of both pre- and postmenopausal women presenting for screening physical examination were eligible to participate in the study and were consented and enrolled.
A total of 90 women (N=45 premenopausal; N=45 postmenopausal) women aged 47–56 years comprised the study sample. The study protocol was reviewed and approved by Duke University Medical Center’s Institutional Review Board, and all participants provided verbal and written consent prior to participation. Exclusion criteria included use of exogenous reproductive hormones (e.g., hormone replacement therapy, oral contraceptives), hysterectomy, history of cardiovascular disease or systemic disease affecting the cardiovascular system; hypertension, defined as blood pressure > 160/100 mmHg on blood pressure screening examination; chronic use of drugs which alter systemic hemodynamics (including antihypertensives, antidepressants, sympathomimetic agents); and current tobacco or illegal drug use. Women who reported regular menstruation were considered premenopausal and women who had not menstruated in at least 9-months were considered postmenopausal. Reproductive hormone assays were used to further document menopausal status. Sample characteristics are reported in Table 1.
Table 1.
Descriptive Characteristics of Study Sample and Baseline Values
| Variable | Premenopausal (N=45) Mean ± SD |
Postmenopausal (N=45) Mean ± SD |
P |
|---|---|---|---|
| Ethnic (No. Black/White) | 15/30 | 15/30 | |
| Age (yrs) | 49.2 ± 1.3 | 51.3 ± 2.1 | <.01 |
| Weight (kg) | 68.4 ± 10.8 | 69.6 ± 11.0 | 0.9 |
| BMI (kg/m2) | 25.8 ± 3.3 | 26.4 ± 4.3 | 0.7 |
| Glucose (mg/dl) | 92.5 ± 6.4 | 92.0 ± 7.0 | 0.9 |
| Creatinine (mg/dl) | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.5 |
| Total Cholesterol (mg/dl) | 189.5 ± 31.9 | 215.8 ± 43.8 | <.05 |
| Triglyceride (mg/dl) | 84.3 ± 42.5 | 97.1 ± 74.5 | 0.2 |
| Estrogen (pg/mL) | 94.0 ± 79.1 | 11.5 ± 12.7 | <.01 |
| Progesterone (ng/mL) | 4.5 ± 6.1 | 0.2 ± 0.3 | <.01 |
| LH (mIU/mL) | 11.9 ± 13.0 | 51.9 36.1 | <.01 |
| FSH (mIU/mL) | 11.1 ± 11.9 | 72.3 ± 29.5 | <.01 |
Note: SBP=systolic blood pressure, DBP=diastolic blood pressure, BMI=body mass index, LH=luteinizing hormone, FSH=follicle stimulating hormone
Hormone Assessment
Blood (2 ml) was drawn from an antecubital vein and collected into a serum-separator tube and refrigerated. The sample was analyzed within 12-hours of collection using immunochemoluminometric assay (Labcorp Inc., Burlington, NC) to determine concentrations of estrogen, progesterone, follicle stimulating hormone (FSH), and luteinizing hormone (LH). Performance characteristics for these assays were as follows: estrogen range 10–1000 pg/ml; progesterone range 0.1–40 ng/ml; FSH range 0.3–200 mIU/ml; LH range 0.07–200 mIU/ml.
Catecholamine Measurement
To obtain resting plasma epinephrine and norepinephrine values, blood was sampled from a cannula inserted into a forearm vein and connected by heparin-treated polyethylene tubing to a blood withdrawal pump (Cormed ML6 continuous blood withdrawal system: Dakmed, Inc. Buffalo, NY). Blood was drawn at a rate of 3 ml/min into chilled ethylenediaminetetraacetic acid (EDTA)-treated tubes. Samples were placed on ice, cold-centrifuged, and plasma was pipetted and frozen at −80°C until assay. Assays were performed using high-performance liquid chromatography procedures (HPLC). The lower limits of quantification using the HPLC method is 25pg/ml, and the intra- and inter-day coefficients of variation are <10%.
Cardiovascular Measures
Blood pressure (BP) was measured utilizing a Suntech 4240 automatic auscultatory BP monitor. A minimum of 3 stethoscopic auscultatory readings were made concurrently with the automatic ausculatory readings by a trained staff member to ensure optimal placement of the electrocardiogram (ECG) electrodes and the Korotkoff sound microphone.
Impedance cardiography signals were recorded via a Minnesota Impedance Cardiograph (Model HIC-1, Bio-Impedance Technology, Chapel Hill, NC) using a tetrapolar band-electrode configuration. The recording electrode bands were positioned around the base of the neck and around the thorax over the xiphisternal junction. The current electrode bands were positioned to encompass the neck and thorax, at least 3 cm away from each of the recording electrodes. The ECG was recorded from the Minnesota Impedance Cardiograph using disposable ECG electrodes. The basal thoracic impedance (Zo), the first derivative of the pulsatile impedance (dZ/dt) and the ECG waveforms were processed using specialized ensemble-averaging software (COP, BIT Inc., Chapel Hill, NC), which was used to derive stroke volume using the Kubicek equation (14).
Cardiac Output (CO)
Following instrumentation, cardiac output obtained from the impedance cardiography system was calibrated against a pulsed-Doppler system. The Doppler system was used to measure ascending aortic blood flow velocity during cardiac cycles occurring during the first 30-sec. of five consecutive minutes. Impedance cardiographic signals were recorded over the exact same five 30-sec. periods. The cardiac output values derived from the Doppler flow measures were used to scale all impedance volume-based measures CO, stroke volume, and SVR to absolute values. For example, if resting calibration Doppler stroke volume was 10% lower than for impedance, a scale factor of 0.9 was applied to impedance stroke volume measures. This strategy provided a means of estimating absolute CO and extends the widely accepted validity of impedance cardiography as a reliable method for measuring changes in cardiac output over time (15). Finally, all CO measurements were divided by body surface area (BSA), to provide an index of CO adjusted for body size (liter/min/m2).
Systemic Vascular Resistance (SVR)
The following equation was used to derive SVR:
SVR (dyne-sec.cm−5.m2) = ((MAP/CO) × 80) * BSA
where MAP = mean arterial pressure (mmHg) and CO = cardiac output (liter/min); with MAP = DBP+(SBP-DBP)/3. SVR was adjusted for body size by multiplying by BSA.
Laboratory Stress Testing Protocol
Participants completed the laboratory protocol at least six hours following the most recent consumption of caffeine. All test procedures were conducted while participants were seated in an electrically shielded, sound attenuated, temperature-controlled (24°C) room. Following instrumentation with the blood pressure monitor and the impedance cardiograph, participants were seated in a comfortable chair and asked to relax as much as possible for 20 minutes. Resting baseline cardiovascular measures were taken once each minute during the last 3 minutes of this relaxation period. Blood drawn over this same 3-minute period was used to determine resting baseline plasma catecholamines. Participants then completed four laboratory stress tasks, separated by 3-minute rest periods; balance for order of task presentation was achieved using a Williams’ square design. Cardiovascular measures were recorded each minute during these tasks.
Anger Interview (AI)
The AI required participants to recall an incident from their lives that made them feel angry, and that also involved active interpersonal interaction. Participants were given three minutes to prepare and three minutes to describe the incident to the interviewer. Participants first described the scenario, then their emotions and interpersonal interactions, followed by a summary of the outcome and their level of satisfaction with their behavior in the situation. The AI is considered ecologically valid, and has been found to produce large increases in blood pressure, similar to public speaking tasks, but with a greater hemodynamic contribution from increased vascular resistance (16).
Reaction-Time Shock-Avoidance (RT)
The RT consisted of a three-minute task during which a loud audible tone presented at varying, unpredictable intervals (average = 23 sec). Participants were told to press a key as fast as possible upon hearing each tone. They were further instructed that a "painful, but harmless" electric shock would be immediately delivered by electrodes previously applied to the leg if, on any given trial, a reaction time was considered too slow. In fact, no shocks were delivered. The RT is considered a potent means of eliciting blood pressure increases mediated predominantly by β-adrenergic mechanisms (17).
Mirror Trace (MT)
In this three-minute task, the participants were required to trace the shape of a star using a metal stylus guided only by its reflection in a mirror. Participants were told to trace the shape as many times as possible, without making any errors associated with deviating beyond the narrow boundaries of the star shape. Errors caused an aversive buzzing sound and were recorded with a digital counter. The MT is considered an alpha-adrenergic stimulus and has been associated with perceived frustration and increased SVR (18).
Foot Cold Pressor (CP)
The CP required the participants to immerse their foot, up to the ankle, in a mixture of ice and water (0–4 C) for 100 seconds. The CP results primarily in an increase in SVR due to alpha-adrenergic stimulation (19).
Cardiovascular Measurements During Receptor Responsiveness Testing
All receptor responsiveness testing was conducted on a separate day from the laboratory stress testing protocol. Participants were completely reclined and had not consumed caffeine for at least 6 hr. Blood pressure was measured continuously using the Finapres (Ohmeda, Madison, WI) noninvasive blood pressure monitor, which uses the vascular unloading technique to measure systolic, diastolic, and mean blood pressure on a beat-by-beat basis. This instrument has been validated against intra-arterial measures under various conditions including pressor responses to phenylephrine (20). Heart rate (HR) was derived from an ECG as the interval between successive R-waves.
β-Adrenergic Receptor Responsiveness
Beta-adrenergic receptor responsiveness was assessed using an isoproterenol sensitivity test. Cardiac β-receptor responsiveness was indexed as the chronotropic dose of isoproterenol required to increase HR by 25 bpm (CD25)(21). Progressively increasing bolus-doses of isoproterenol (0.125, 0.25, 0.5, 1.0, 2.0, 4.0 µg) were injected into an antecubital vein until an increase in HR of at least 25 bpm was observed. HR responses following each dose were computed as the shortest three successive ECG R-R intervals following drug injection, compared to the shortest three R-R intervals at rest (pre-injection). Following each dose, the next higher dose was not injected for at least 5-minutes, or until cardiovascular activity had returned to resting levels, usually within 5–10 minutes. The linear regression model of log-dose/HR response for each subject was used to determine CD25 exactly by interpolation. The CD25 measure provides an index of cardiovascular β1 and β2 receptor responsiveness, and is inversely related to receptor responsiveness.
Alpha1-adrenergic Receptor Responsiveness
A procedure analogous to the β-responsiveness test described above was used for assessing alpha1 receptor responsiveness, using the alpha1 agonist phenylephrine to stimulate vascular alpha1 receptors (22). In this test, the criterion response is defined as the dose required to increase mean arterial pressure by 25 mmHg (PD25). An initial dose of 25 µg phenylephrine was used, with successive doses doubled until the 25 mmHg response was exceeded, or until a maximum dose of 800 µg. Again, at least 5-minutes, or longer if required for recovery of cardiovascular activity to resting levels, preceded administration of successive doses. The linear log-dose/MAP response curve was used to determine the exact PD25 dose. The PD25 index is inversely related to vascular alpha1 receptor responsiveness.
Data Analytic Strategy
Two-factor (menopause × ethnicity) analysis of variance tests were employed to evaluate the effects of menopause, ethnicity and menopause-by-ethnicity interactions on clinical characteristics (Table 1). Similar models were used to evaluate pre- versus postmenopausal differences in the hemodynamic and SNS characteristics (Table 2). Analyses involving the CD25 index of cardiac β-AR responsiveness included baseline HR as a covariate, and analyses involving the PD25 index of vascular α-AR responsiveness included baseline SBP as a covariate. In order to assess whether observed effects were independent of age, BMI and cholesterol levels, additional ANCOVAs were performed that included these characteristics as covariables.
Table 2.
Baseline Values of Hemodynamic Measures, Catecholamine Levels, and α- and β-AR responsiveness Indices.
| Variable | Premenopausal (N=45) Mean ± SD |
Postmenopausal (N=45) Mean ± SD |
P |
|---|---|---|---|
| SBP (mmHg) | 107.5 ± 14.3 | 107.7 ± 13.0 | 0.9 |
| DBP (mmHg) | 71.0 ± 8.3 | 71.4 ± 7.1 | 0.9 |
| Heart Rate (bpm) | 68.0 ± 9.9 | 67.6 ± 9.6 | 0.6 |
| CO (liter/min/m2) | 3.2 ± 0.7 | 2.6 ± 0.7 | <0.001 |
| SVR (dyne-sec.cm−5.m2) | 2152.5 ± 532.4 | 2722.5 ± 726.0 | <0.001 |
| Norepinephrine* (pg/ml) | 337.5 ± 105.7 | 458.2 ± 248.3 | 0.007 |
| Epinephrine** (pg/ml) | 30.2 ± 29.8 | 33.1 ± 50.5 | 0.9 |
| CD25*** (µg isoproterenol) | 2.43 ± 1.21 | 3.03 ± 1.89 | 0.02 |
| PD25**** (µg phenylephrine) | 265.3 ± 128.1 | 303.0 ± 165.8 | 0.9 |
Note: SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, CO = cardiac output index, SVR = systemic vascular resistance, CD25 = β-adrenergic receptor responsiveness, PD25 = α-adrenergic receptor responsiveness.
N = 73;
N = 64;
N=80;
N=74.
Baseline hemodynamic measurements (SBP, DBP, HR, stroke volume, CO, and SVR) were computed as the mean of the three measurements taken during the last three minutes of the 20-minute initial relaxation period. Cardiovascular measurements were taken every minute during each of the stress tasks, and response to the stressor was defined as the mean of the minute-by-minute readings taken during the task. Hemodynamic responses to the stress tasks were examined using three factor (menopause×ethnicity×task) repeated measures ANCOVAs, in which all two-way and the three-way interaction were evaluated. Primary analyses included baseline as a repeated measures condition, in order to evaluate the persistence of any menopausal effects across resting and stress conditions; confirmatory analyses examined moderating effects of menopausal status on stress responses, with corresponding baseline measurement included as a covariate. These analyses were also repeated, controlling for age, BMI and cholesterol levels. A p value of <.05 was considered significant.
RESULTS
Demographic and Clinical Characteristics
Demographic and clinical characteristics are presented in Table 1. Post-menopausal women were an average of 2 years older than pre-menopausal women (p < .01), and total cholesterol was higher in postmenopausal than premenopausal women (p<.01) Sample characteristics of reproductive hormone assays are also reported in Table 1with expected pre- versus postmenopausal differences in estrogen, progesterone, FSH, and LH (p’s<.001). There were no ethnic differences, nor ethnicity by menopause interactions, for the characteristics shown in Table 1.
Baseline Cardiovascular Measures
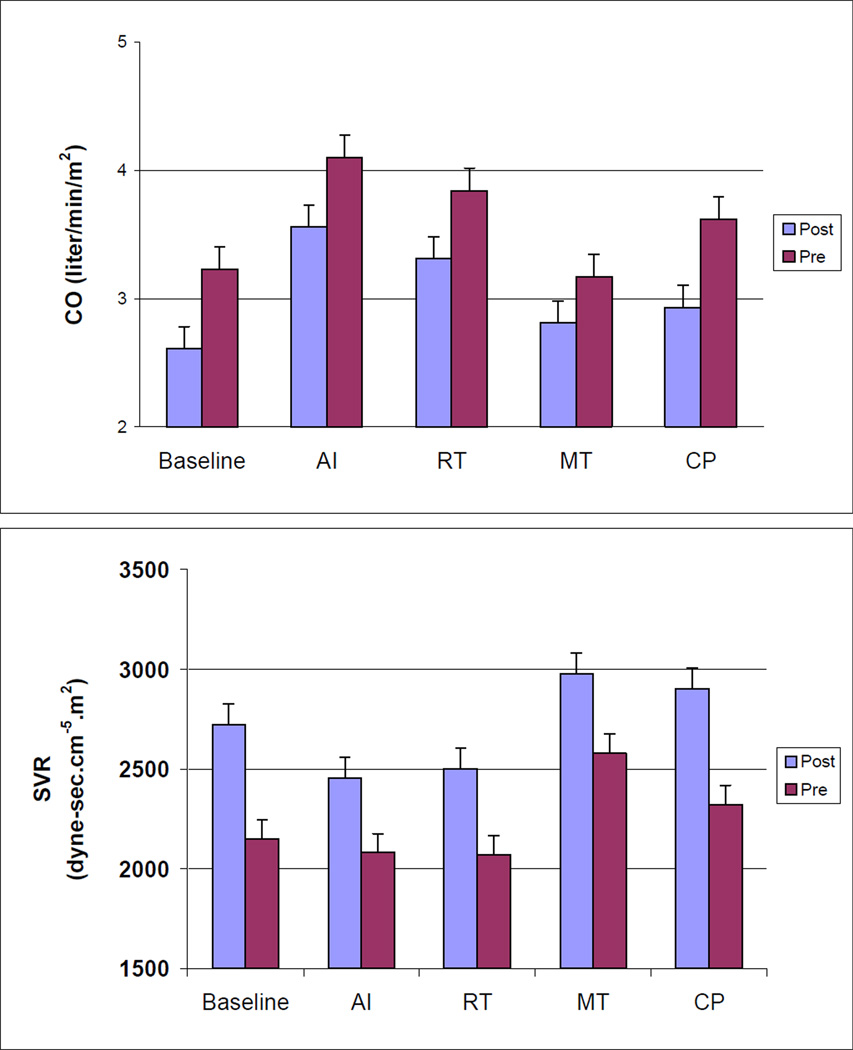
Baseline values of cardiovascular measures are presented in Table 2. ANOVAs comparing menopause and ethnic groups with respect to baseline hemodynamic measures revealed that premenopausal women exhibited higher baseline CO than postmenopausal women (p < .001), as well as lower baseline SVR (p < .001). No other main effects or interactions involving ethnic and menopause status were observed for heart rate, blood pressure, CO and SVR. Due to problems establishing a venous line and sample contamination, plasma norepinephrine assays were available only for a subset of 73 subjects and epinephrine assays for 64 subjects. Postmenopausal women had higher resting plasma norepinephrine levels than premenopausal women (p = .007), but did not differ in resting epinephrine. Baseline CO, SVR, and norepinephrine differences between pre- and postmenopausal women remained significant after controlling for age and BMI.
Adrenergic Receptor (AR) Responsiveness
Complete α- and β-AR responsiveness testing data were available for 74 women, due to difficulties establishing a venous infusion-line in the remaining study participants. ANOVAs comparing menopause and ethnic groups with respect to baseline catecholamine values revealed that no menopausal differences were observed for the PD25 index of α-AR responsiveness. However, in postmenopausal women, significantly higher values for the CD25 index of β-AR responsiveness were found compared with premenopausal women, p = .02. However, this menopausal difference in CD25 was no longer evident after controlling for age and BMI. There were no ethnic differences or menopausal status by ethnicity interactions involving these measures.
Hemodynamic Responses to stressors
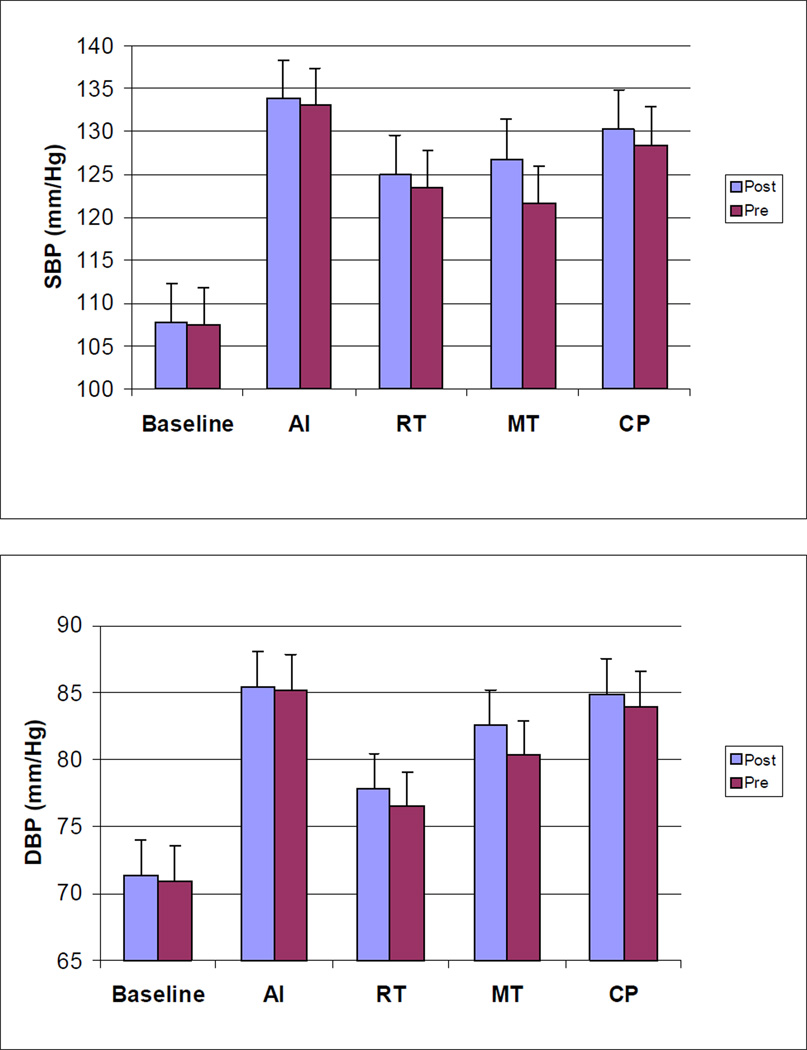
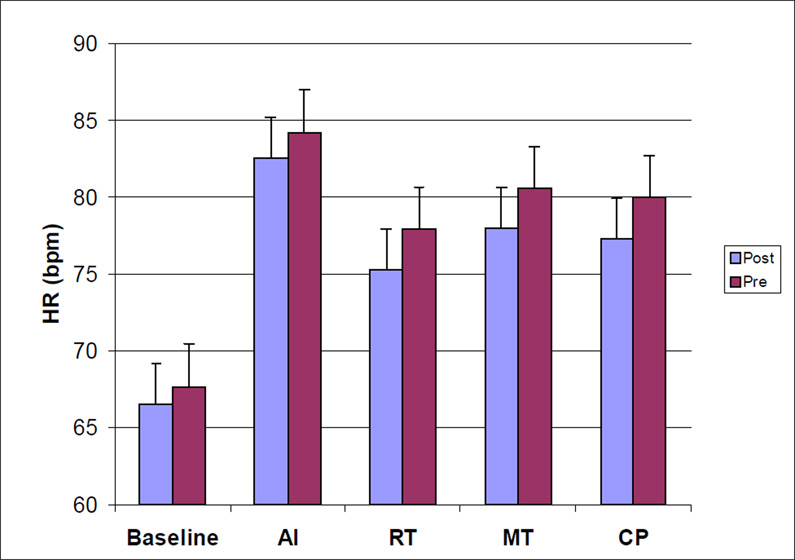
Results of ANCOVAs comparing menopause and ethnic groups with respect to cardiovascular measures at rest and during each of the laboratory stress tasks revealed that the tasks altered cardiovascular activity for all response measures (p’s < .001). Planned contrasts revealed that HR, SBP, and DBP were higher during each task than during baseline (p’s < .0001). CO increased during AI, RT, and CP (p’s < .05), but was not significantly different from baseline during MT (p = .08). Relative to baseline, SVR values decreased during AI and RT (p’s < .05) and increased during MT (p=.056) and CP (p<.001).
A significant main effect of menopause status revealed that postmenopausal women had lower CO values throughout the laboratory protocol (p = .004). Postmenopausal women also exhibited higher SVR values throughout the protocol (p = .002). These menopausal effects for CO and SVR remained unaltered after age and BMI were included as covariates. No main effects were observed for ethnicity, and no interactions involving menopause status, ethnicity, or the repeated measures factor (task) were observed. Repeated measures ANCOVAs focusing on task responses, incorporating corresponding baseline values as covariates, confirmed that menopausal status did not moderate task responses (menopause effects and menopause by task interactions all non-significant), supporting the interpretation that menopausal differences in CO and SVR at resting baseline simply persisted, rather than be enhanced or attenuated, during exposure to the stress tests. Values of CO and SVR for premenopausal and postmenopausal women at each phase of the laboratory protocol are presented in Figure 1.
Figure 1.
Values of SBP, DBP, HR, CO, and SVR for Premenopausal and Postmenopausal women at Each Phase of the Laboratory Stress Testing Protocol
Note: SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate CO = cardiac output, SVR = systemic vascular resistance, AI = anger interview, RT = reaction time, MT = mirror trace, CP = foot cold pressor.
DISCUSSION
Our observations that postmenopausal women evidenced greater SVR and lower CO suggest that menopause is associated with increased hemodynamic load that is sustained during mental stress. These findings are consistent with evidence from two recent trials that hormone replacement therapy reduced SVR and increased CO among postmenopausal women (23) and perimenopausal women (24). Increased vascular resistance may be involved in the higher levels of ambulatory blood pressure observed among postmenopausal women (25, 26). Furthermore, increased hemodynamic load associated with menopause may have pathophysiological consequences, as adverse left ventricular remodeling has been reported as an early sequel of menopause (27).
There are several possible mechanisms for menopause-related increases in SVR. Elevated sympathetic nervous system activity following menopause may explain increased SVR among postmenopausal women, which is suggested by evidence that hormone replacement therapy has been shown to reduce SNS activity (23, 28, 29) and NE mediated vasoconstriction (29). Postmenopausal women in the present study also exhibited higher plasma NE than premenopausal women. In addition, increased SVR among postmenopausal women may reflect altered adrenergic receptor functioning. Reduced β–AR and/or increased α–AR would favor an elevated SVR. In the present study we found β-adrenergic responsiveness estimated by isoproterenol infusion (CD25) was significantly lower in postmenopausal women than in premenopausal women. However, this difference was not robust as it was no longer evident after controlling for age. Nonetheless, blunted β–AR responsiveness may be one mechanism contributing to elevated SVR observed in postmenopausal women. Our observations also showed that stress did not moderate menopausal differences in cardiovascular hemodynamics is consistent with an interpretation that menopausal status was unrelated to stress response, but instead represented an altered physiological foundation supporting hemodynamic differences that persisted across a range of stimulus conditions.
Postmenopausal alterations in several other regulatory systems may also contribute to elevated SVR. Impaired vascular endothelial function, which is well documented to occur in postmenopausal women, may increase vascular resistance (30–34). Postmenopausal impairment of endothelial function may be due to the decline in estrogen-potentiated vasodilation (35–37). Estrogen appears to increase blood flow by enhancing nitric oxide bioavailability, thus improving endothelium-dependent vasodilation (38–40). A recent study reported that menopause exacerbates the age-related increase in arterial stiffness during the early postmenopausal phase and that this augmentation is probably related, at least in part, to estrogen deficiency (41) Another study showed that surgically-induced menopause causes an increase in peripheral vascular resistance and blood pressure, suggesting a role of ovarian hormones in the homeostatic pressure modulation (41,42). Estrogen also modulates calcium influx into smooth muscle cells, reducing contractile activity (40).
There are several limitations to the present study. First, our sample size is relatively small. Comparatively, however, it did exceed the size of most other study populations characterizing cardiovascular and endothelial responses to mental stress (43,44). We also made many statistical comparisons, which in a study of our sample size may have given rise to findings by chance. Another limitation is that premenopausal women participants were not assessed during a controlled menstrual cycle phase. There is some evidence suggesting that natural hormonal fluctuations may alter sympathetic outflow and the regulation of vascular resistance (45, 46).
In summary, after menopause, women may be characterized by increased hemodynamic load that is sustained during mental stress. Altered SNS activity may be one mechanism leading to elevated vascular resistance in postmenopausal women. Increased hemodynamic load following menopause may contribute to pathological structural and functional changes in the heart and blood vessels that are characteristic of postmenopausal women.
Acknowledgements
Supported by NIH grants HL53724, M01-RR-30, National Center for Research Resources, General Clinical Research Centers Program
References
- 1.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. New England Journal of Medicine. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Annals of Internal Medicine. 1978;89:157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Annals of Internal Medicine. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. American Journal of Obstetrics & Gynecology. 1981;139:47–51. doi: 10.1016/0002-9378(81)90410-5. [DOI] [PubMed] [Google Scholar]
- 5.Wold Health Organization Vital Statistics and causes of death. World Health Statistics Annual. 1980
- 6.Utian WH. Biosynthesis and physiologic effects of estrogen and pathophysiologic effects of estrogen deficiency: a review. [Review] [8 refs] American Journal of Obstetrics & Gynecology. 1989;161:1828–1831. doi: 10.1016/s0002-9378(89)80002-x. [DOI] [PubMed] [Google Scholar]
- 7.La Vecchia C, Decarli A, Franceschi S, Gentile A, Negri E, Parazzini F. Menstrual and reproductive factors and the risk of myocardial infarction in women under fifty-five years of age. American Journal of Obstetrics & Gynecology. 1987;157:1108–1112. doi: 10.1016/s0002-9378(87)80271-5. [DOI] [PubMed] [Google Scholar]
- 8.Mercuro G, Longu G, Zoncu S, Cherchi A. Impaired forearm blood flow and vasodilator reserve in healthy postmenopausal women. American Heart Journal. 1999;137(4 Pt 1):692–697. doi: 10.1016/s0002-8703(99)70225-5. [DOI] [PubMed] [Google Scholar]
- 9.Mercuro G, Pitzalis L, Podda A, et al. A. Effects of acute administration of natural progesterone on peripheral vascular responsiveness in healthy postmenopausal women. American Journal of Cardiology. 1999;84:214–218. doi: 10.1016/s0002-9149(99)00237-4. [DOI] [PubMed] [Google Scholar]
- 10.Mercuro G, Podda A, Pitzalis L, et al. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. American Journal of Cardiology. 2000;85:787–789. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- 11.Mercuro G, Zoncu S, Cherchi A, Rosano GM. Can menopause be considered an independent risk factor for cardiovascular disease?. [Review] [88 refs] Italian Heart Journal: Official Journal of the Italian Federation of Cardiology. 2001;2:719–727. [PubMed] [Google Scholar]
- 12.Lin AL, McGill HC, Jr, Shain SA. Hormone receptors of the baboon cardiovascular system. Biochemical characterization of aortic and myocardial cytoplasmic progesterone receptors. Circulation Research. 1982;50:610–616. doi: 10.1161/01.res.50.5.610. [DOI] [PubMed] [Google Scholar]
- 13.Lin AL, Gonzalez R, Jr, Carey KD, Shain SA. Estradiol-17 beta affects estrogen receptor distribution and elevates progesterone receptor content in baboon aorta. Arteriosclerosis. 1986;6:495–504. doi: 10.1161/01.atv.6.5.495. [DOI] [PubMed] [Google Scholar]
- 14.Kubicek WG, Karnegis JN, Patterson RP, Witsoe DA, Mattson RH. Development and evaluation of an impedance cardiac output system. Aerospace Medicine. 1966;37:1208–1212. [PubMed] [Google Scholar]
- 15.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJP. Methodological Guidelines for Impedance Cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 16.Ironson G, Taylor CB, Boltwood M, et al. Effects of anger on left ventricular ejection fraction in coronary artery disease. American Journal of Cardiology. 1992;70(3):281–285. doi: 10.1016/0002-9149(92)90605-x. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Savage DD, Sachs I, Laragh JH. Relation of hemodynamic load to left ventricular hypertrophy and performance in hypertension. American Journal of Cardiology. 1983;51:171–176. doi: 10.1016/s0002-9149(83)80031-9. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92:2102–2108. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 19.Girdler SS, Hinderliter AL, Light KC. Peripheral adrenergic receptor contributions to cardiovascular reactivity: influence of race and gender. Journal of Psychosomatic Research. 1993;37:177–193. doi: 10.1016/0022-3999(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 20.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–655. doi: 10.1161/01.hyp.13.6.647. [DOI] [PubMed] [Google Scholar]
- 21.Cleaveland CR, Rangno RE, Shand DG. A standardized isoproterenol sensitivity test. The effects of sinus arrhythmia, atropine, and propranolol. Archives of Internal Medicine. 1972;130:47–52. doi: 10.1001/archinte.130.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Kotchen TA, Guthrie GP, McKean H, Kotchen JM. Adrenergic responsiveness in prehypertensive subjects. Circulation. 1982;65:285–290. doi: 10.1161/01.cir.65.2.285. [DOI] [PubMed] [Google Scholar]
- 23.Light KC, Hinderliter AL, West SG, et al. Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. Journal of Hypertension. 2001;19:269–278. doi: 10.1097/00004872-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Kamali P, Muller T, Lang U, Clapp JF., III Cardiovascular responses of perimenopausal women to hormonal replacement therapy. American Journal of Obstetrics & Gynecology. 2000;182:17–22. doi: 10.1016/s0002-9378(00)70485-6. [DOI] [PubMed] [Google Scholar]
- 25.Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. Journal of Human Hypertension. 1997;11:507–514. doi: 10.1038/sj.jhh.1000476. [DOI] [PubMed] [Google Scholar]
- 26.Owens JF, Stoney CM, Matthews KA. Menopausal status influences ambulatory blood pressure levels and blood pressure changes during mental stress. [see comments.] Circulation. 1993;88:2794–2802. doi: 10.1161/01.cir.88.6.2794. [DOI] [PubMed] [Google Scholar]
- 27.Hinderliter AL, Sherwood A, Blumenthal JA, et al. Changes in hemodynamics and left ventricular structure after menopause. American Journal of Cardiology. 2002;89:830–833. doi: 10.1016/s0002-9149(02)02193-8. [DOI] [PubMed] [Google Scholar]
- 28.Blum I, Vered Y, Lifshitz A, et al. The effect of estrogen replacement therapy on plasma serotonin and catecholamines of postmenopausal women. Israel Journal of Medical Sciences. 1996;32:1158–1162. [PubMed] [Google Scholar]
- 29.Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- 30.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 31.Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–582. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 32.Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine inprimary and secondary forms of human hypertension. Hypertension. 1993;21:929–933. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- 33.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. Journal of Clinical Investigation. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeiher AM, Drexter H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans: Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. Journal of Clinical Investigation. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal RS, Brinker JA, Resar JR, et al. Long-term estrogen therapy abolishes acute estrogen-induced coronary augmentation in postmenopausal women. American Heart Journal. 1997;133:323–328. doi: 10.1016/s0002-8703(97)70227-8. [DOI] [PubMed] [Google Scholar]
- 36.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO. Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 37.Collins P, Rosano GMC, Sarrel PM, et al. 17β-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- 38.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women.[comment] Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- 39.Guetta V, Quyyumi AA, Prasad A, Panza JA, Waclawiw M, Cannon RO., III The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation. 1997;96:2795–2801. doi: 10.1161/01.cir.96.9.2795. [DOI] [PubMed] [Google Scholar]
- 40.Lang U, Baker RS, Clark KE. Estrogen-induced increases in coronary blood flow are antagonized by inhibitors of nitric oxide synthesis. European Journal of Obstetrics, Gynecology, & Reproductive Biology. 1997;74:229–235. doi: 10.1016/s0301-2115(97)00104-8. [DOI] [PubMed] [Google Scholar]
- 41.Zaydun G, Tomiyama H, Hashimoto H, et al. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis. 2006;184(1):137–142. doi: 10.1016/j.atherosclerosis.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Mercuro G, Zoncu S, Saiu F, Mascia M, Melis GB, Rosano GM. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas. 2004;47(2):131–138. doi: 10.1016/s0378-5122(03)00252-4. [DOI] [PubMed] [Google Scholar]
- 43.Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 44.Sherwood A, Johnson K, Blumenthal JA, Hinderliter AL. Endothelial function and hemodynamic responses during mental stress. Psychosomatic Medicine. 1999;61(3):365–370. doi: 10.1097/00006842-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 46.McFetridge JA, Sherwood A. Hemodynamic and sympathetic nervous system responses to stress during the menstrual cycle. AACN Clinical Issues. 2000;11:158–167. doi: 10.1097/00044067-200005000-00002. [DOI] [PubMed] [Google Scholar]