Abstract
Alterations in White Matter (WM) may be seen in young relatives at risk and may underlie vulnerability to Schizophrenia. We were interested in exploring which of the WM regions were altered in adolescent offspring at familial risk for Schizophrenia. We examined structural alterations in the offspring of subjects with Schizophrenia or Schizoaffective disorder (HR; n=65; 36 males) and healthy controls (HC; n=80: 37 males) matched for age and education. MRI images were collected using a GE 1.5T scanner at the University of Pittsburgh Medical Center. Image processing was done using FreeSurfer (MGH) by an experienced rater blind to clinical data. We used multivariate analysis of covariance, with intracranial volume (p> 0.05) and age as covariates. High Risk offspring had significant reductions in total WM, hemispheric WM and WM within left parietal and left cingulate cortices. Male offspring had more pronounced right hemisphere WM reductions than females.
Keywords: Schizophrenia, Genetic High-Risk, Offspring, FreeSurfer, MRI
1. Introduction
Schizophrenia represents a group of probably etiologically heterogeneous, severe mental disorders the neurobiological underpinnings of which are not yet fully understood (Keshavan et al, 2008a; MacDonald and Schulz, 2009). Schizophrenia has been thought to result from “disconnectivity” of white matter systems (Friston and Frith, 1995). It has been proposed that alterations in the white matter connectivity may give rise to the some of the symptoms found in schizophrenia. For example, Crow (1998) suggested that auditory hallucinations could arise from aberrant communication between language centers in the left and right temporal cortices. Hubl and colleagues reported alterations in lateral parts of the arcuate fasciculus in patients with hallucinations (Hubl et al. 2004). Aberrations in white matter connectivity between distributed systems could also lead to disorders of self-monitoring which in turn could lead to auditory hallucinations (Silbersweig and Stern, 1996, 1998).
Morphometric studies using ROI methodology have shown that in addition to the gray matter abnormalities, regional and global white matter volumes also have been compromised in schizophrenia (reviewed in Shenton et al., 2001). This is supported by several studies using different methodologies such as voxel based Morphometry [Giuliani et al, 2005; Meda et al., 2008), deformation based Morphometry (Davatzikos, 2005) and ADC (Apparent diffusion coefficient) based Morphometry (Ardekani et al., 2005). These various methods have shown, albeit with differing results, that white matter volumes in the brain are altered in schizophrenia. A recent review and meta-analysis (Olabi et al., 2011) of 928 patients and 867 controls examining 32 brain regions showed that patients’ annual WM volume reduction in several brain regions was −.32% in the frontal, −.32% in the parietal, and −.39% temporal lobes.
While there is growing evidence that schizophrenia subjects show changes in white matter (Kubicki et al., 2007), (Bloemen et al, 2010), there is very little known about whether these alterations are related to underlying liability to the illness. Family history remains one of the strongest etiologic factors in schizophrenia, with an estimated heritability of almost 80% (Mcgrath et al., 2008). The study of young relatives of patients with schizophrenia therefore offers a unique window into the premorbid liability to the illness (Keshavan et al, 2008b). The Preclinical (premorbid and prodromal) phase of the illness has been investigated in studies (Lawrie et al., 2001; Lymer et al., 2006; Lawrie et al., 2008) that have shown white matter alterations.
Although the evidence for white matter involvement is accumulating, it is unclear whether this involvement is limited to certain regions or whether they are widespread, and whether the familial susceptibility may have a role in the neurodevelopmental aspects of WM. To assess the potential role of WM in disrupted neurodevelopmental processes, we examined volumetric alterations in the white matter of familial HR subjects and Healthy controls. Few studies have looked at total WM volume, hemispheric WM volumes and regional WM volumes across both hemispheres in high risk subjects. These studies have not yielded consistent results (reviewed in Agnew-Blais & Seidman, in pressreviewed in Agnew-Blais & Seidman, 2012; Boos et al., 2007: meta-analysis).
We hypothesized that given their familial susceptibility for the illness, subjects at high risk for Schizophrenia would show volumetric WM alterations compared to age and sex matched healthy controls.
2. METHODS
2.1. Participants
The study was conducted at the Western Psychiatric Institute and Clinic, Pittsburgh. Sixty five racially diverse adolescents or young adult offspring (OS) of of schizophrenia probands and eighty healthy controls (HC) were recruited. The overall sample characteristics have been described in previous reports (Francis et al, 2011). Twenty eight OS subjects had one parent with Schizoaffective disorder (SZA) and thirty seven OS subjects has one parent with SZ. Of these, twenty three fathers had a diagnosis of SZ/SZA, while forty two mothers had a diagnosis of SZ/SZA. Offspring of parents with schizophrenia or schizoaffective disorder were recruited by approaching patients in the clinic and through advertisements. Healthy Controls were recruited through advertisements in the same community as OS subjects. Diagnostic assessments of HC and OS and parental diagnoses of schizophrenia or schizoaffective disorder used the structured clinical interviews for DSM-IV diagnoses (SCID)(First et al., 1995) and were confirmed using consensus meetings led by senior diagnosticians (M.S.K and D.M). Participants with an IQ < 80, lifetime evidence of a psychotic disorder, exposure to antipsychotic medications or anti-depressant medications, current or recent (within the previous month) substance use disorder, significant neurological or unstable medical conditions were excluded. All participants signed informed consent after the study was fully explained to them. For participants < 18 years of age, consent was provided by the parent or guardian, and the subjects provided informed assent. The study was approved by the University of Pittsburgh Institutional Review Board.
2.2. Image Acquisition
MRI scans were obtained on subjects using a GE 1.5T whole body scanner (GE Medical Systems, Milwaukee, Wisconsin). The detailed scanning protocol has been described in an earlier publication (Gilbert et al, 2001). The scans were T1 weighted images: three-dimensional spoiled gradient recalled (SPGR), acquired in a steady-state pulse sequence (124 coronal slices, 1.5 mm thickness, TE=5 msec, TR=25 msec, acquisition matrix=256×192, FOV=24 cm, flip angle 40°). Approximately 10% of the MRI scans with radio frequency inhomogeneity defects and motion artifacts were not included in the analysis.
2.3 Image Analysis
2.3.1 Semi-automated morphometric analysis using FreeSurfer
We used FreeSurfer (FS) 4.0.5 (64 bit version) (Massachusetts General Hospital, (Fischl et al, 2004; Desikan et al, 2006; Dale et al, 1999; Fischl et al, 1999; van der Kouwe et al, 2008;) running on Linux for morphometric analysis. FS, a semiautomated brain image morphometric software, has been used to study the brain morphology of several illnesses including schizophrenia (Fischl and Wald, 2007; Fischl et al, 2002;). FS has 3 automated stages (Fischl et al, 2004), each followed by manual image editing by an Image analyst (AF). Image processing included motion correction of volumetric T1-weighted images, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al., 2002, 2004b), intensity normalization, tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/CSF borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999). Detailed pictures of WM segmentation may be seen in Salat et al, (2009).
We chose FreeSurfer’s semi-automated morphometric analyses for specific reasons. Inter-subject variability and random error inherent in manual techniques is minimized by automated methods. A second reason is the economy of time for completion. By contrast, the possibility of systematic error in automated approaches can be detected and corrected by rigorous manual editing, such as that followed in this study. Each scan was carefully checked for errors of template registration, skull strip, segmentation and parcellation at each stage of the image processing. We performed Inter-Rater reliability between AF and NT (see acknowledgements) on the 33 WM regions of ten brains and obtained an average inter class correlation value = .99 (WM) and .96 (GM).
Our objective was to examine early WM volume alterations in the regions of the left and right hemispheres of non-psychotic High risk subjects and controls. Since two decades of research studies have consistently implicated regions within the frontal, temporal and parietal, and cingulate WM in the etiopathogenesis of SZ (reviewed in Shenton et al, 2001), we examined the WM volumes in these lobes. Although we did not include the other regions within the MANCOVA, we present ANCOVA analysis of the WM of all the other regions in Tables 1 and 2. The corpus callosum was not included since it was published elsewhere (Francis et al, 2011).
Table 1.
Demographic Information of Subject Populations
| Variable | Offspring (N = 65) | Control (N = 80) | |
|---|---|---|---|
| M (SD)/N (%) | M (SD)/N (%) | P b | |
| Age | 16.30 (3.40) | 16.62 (3.65) | 0.60 |
| Male | 38 (54%) | 24 (38%) | 0.08 |
| Caucasian | 34 (49%) | 41 (65%) | 0.08 |
| Education (yrs) | 9.72 (3.33) | 10.18 (3.29) | 0.43 |
Fisher’s exact test or independent t-test, two-tailed, for significant differences between OS and HC participants.
Table 2.
LEFT HEMISPHERE – ALL Regional White Matter
| LOBES | GYRI | Mean Volume in mm3 | DF | F | P | Partial Eta Squared | |||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Offspring | ||||||||
| Mean | SD | Mean | SD | ||||||
| Frontal | Lateral Orbitofrontal | 7162.1 | 1142.2 | 6652.9 | 937.6 | 145 | 7.48 | 0.007 | 0.05 |
| Medial Orbitofrontal | 3706.6 | 758.7 | 3388.2 | 676.7 | 145 | 7.16 | 0.008 | 0.05 | |
| Caudal Middle Frontal | 6486.1 | 1180.1 | 6123.6 | 1214 | 145 | 3.86 | 0.05 | 0.02 | |
| Rostral Middle Frontal | 12192.2 | 2297.2 | 11521.7 | 2044.2 | 145 | 3.18 | 0.07 | 0.02 | |
| Pars Orbitalis | 862.5 | 261.7 | 809.7 | 222.9 | 145 | 1.21 | .274 | 0.01 | |
| Pars Opercularis | 3569.3 | 572.8 | 3405.9 | 666.2 | 145 | 1.64 | .203 | 0.01 | |
| Pars Triangularis | 3491.7 | 683.9 | 3314.5 | 666.7 | 145 | 1.38 | .281 | 0.009 | |
| Superior Frontal | 17649.3 | 2588.7 | 16731.9 | 2879 | 145 | 4.19 | 0.043 | 0.02 | |
| Frontal Pole | 306.4 | 168.6 | 302.5 | 154.1 | 145 | 0.197 | 0.658 | 0.001 | |
| Temporal | Middle temporal | 5358.65 | 975.2 | 4931.02 | 1004.5 | 145 | 5.25 | 0.02 | 0.03 |
| Inferior temporal | 6138.25 | 1135.5 | 5565.35 | 1263.9 | 145 | 8.43 | 0.004 | 0.056 | |
| Planum Temporale | 1413.43 | 192.8 | 1336.97 | 191.9 | 145 | 3.94 | 0.04 | 0.026 | |
| Superior Temporal | 8026.61 | 1274 | 7599.6 | 1286 | 145 | 3.03 | 0.081 | 0.021 | |
| Entorhinal | 463.05 | 192.2 | 401.9 | 154.4 | 145 | 3.19 | 0.07 | 0.02 | |
| Fusiform | 5796.6 | 927 | 5365 | 931.6 | 145 | 6.55 | 0.012 | 0.04 | |
| Parahippocampal | 1278 | 353.3 | 1177.9 | 244.3 | 145 | 3.034 | 0.084 | 0.02 | |
| Temporal Pole | 568.2 | 247.4 | 541.9 | 173.4 | 145 | 0.498 | 0.491 | 0.003 | |
| Parietal* | Supramarginal | 8637.91 | 1357.6 | 7839.29 | 1338.2 | 145 | 13.99 | 0.001* | 0.091 |
| Superior parietal | 12723.86 | 1936.6 | 11603.38 | 2027.4 | 145 | 13.68 | 0.001* | 0.089 | |
| Angular | 9511.95 | 1662.2 | 9050.17 | 1805.9 | 145 | 2.57 | .111 | 0.018 | |
| Cuneus | 2527.4 | 530.9 | 2414.7 | 651.7 | 145 | .671 | .414 | 0.004 | |
| Pericalcarine | 3230.6 | 728.1 | 3006.3 | 767.2 | 145 | 2.146 | 0.145 | 0.015 | |
| Precuneus | 8873.9 | 1557.8 | 8409.8 | 1493.8 | 145 | 3.40 | 0.067 | 0.02 | |
| Motor Somatosensory | Post Central | 7901.84 | 1371.4 | 7252.89 | 1160.2 | 145 | 9.36 | 0.003 | 0.063 |
| Pre Central | 13247.04 | 1892.5 | 12603.02 | 1874.8 | 145 | 2.83 | 0.09 | 0.094 | |
| Paracentral | 3813 | 780 | 3697.1 | 722 | 145 | 0.28 | 0.6 | 0.001 | |
| Cingulate* | Isthmus cingulate | 2681.67 | 468.8 | 2519.37 | 422.4 | 145 | 4.27 | 0.03* | 0.032 |
| Posterior cingulate | 3980.04 | 485.1 | 3689.91 | 505.5 | 145 | 11.37 | 0.001* | 0.075 | |
| Caudal Anterior Cingulate | 2176.74 | 372.3 | 2080.2 | 352.6 | 145 | 1.84 | .177 | 0.013 | |
| Rostral Anterior Cingulate | 1787.92 | 361.1 | 1762.22 | 335.6 | 145 | .129 | .720 | 0.0009 | |
| Occipital | Lateral Occipital | 10752.3 | 1943.1 | 9726.9 | 2233.3 | 145 | 7.51 | 0.007 | 0.05 |
| Lingual | 5208.7 | 1096 | 4948.7 | 1001.5 | 145 | .867 | .353 | 0.006 | |
-survived correction for multiple comparisons
2.4. Statistical Analysis
White matter volumes were normally distributed [Shapiro–Wilk’s test (W statistic, p>0.1)]. Multivariate analyses of covariance (MANCOVA) were used with Group and Sex as categorical predictors and Age and ICV as covariates to test differences in white matter volumes between offspring and healthy controls. We first compared total, left and right hemispheric WM across groups. We next performed several MANCOVA analyses on each of the lobes (right and left Frontal, Temporal, Parietal lobes, Motor-Somatosensory cortex and Cingulate cortices) as dependent variables. Tables 2 and 3 show the regions included in the MANCOVAS. Significant MANCOVAs were followed by univariate ANCOVAs to identify specific regional volumetric deficits. Univariate ANCOVAs were carried out on other regions in secondary analysis. Bonferroni corrections were used on the MANCOVAs and ANCOVA tests to control for multiple comparisons. We derived a Bonferroni corrected p value by dividing 0.05 by the number of MANCOVAs and the univariate tests within each MANCOVA. This alpha value was then used to determine whether each p value survived the correction or not.
Table 3.
RIGHT HEMISPHERE – ALL Regional White Matter
| LOBES | GYRI | Mean Volume in mm3 | DF | F | P | Partial Eta Squared | |||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Offspring | ||||||||
| Mean | SD | Mean | SD | ||||||
| Frontal | Lateral Orbitofrontal | 6907.8 | 1055 | 6610.4 | 864.3 | 145 | 2.82 | 0.09 | 0.019 |
| Medial Orbitofrontal | 3953.2 | 677.9 | 3853.4 | 683.1 | 145 | .67 | 0.41 | 0.004 | |
| Caudal Middle Frontal | 5741.1 | 1019.5 | 5569.6 | 1060 | 145 | 0.74 | .38 | 0.005 | |
| Rostral Middle Frontal | 13049 | 2571.6 | 11893.8 | 2199.1 | 145 | 8.56 | 0.003 | 0.05 | |
| Pars Orbitalis | 1026.8 | 281 | 965.9 | 229.6 | 145 | 1.42 | 0.234 | 0.01 | |
| Pars Opercularis | 3493.7 | 644.2 | 3346.9 | 630.3 | 145 | 1.62 | 0.204 | 0.01 | |
| Pars Triangularis | 3097.3 | 663.3 | 2823.2 | 587.2 | 145 | 7.41 | 0.007 | 0.05 | |
| Superior Frontal | 18001.4 | 2894 | 17109.8 | 2787.8 | 145 | 3.6 | 0.05 | 0.025 | |
| Frontal Pole | 421.4 | 176 | 397.1 | 181.5 | 145 | .743 | .390 | 0.005 | |
| Temporal | Middle temporal | 5972.9 | 1057.6 | 5367.5 | 964.4 | 145 | 13.75 | 0.0003 | 0.09 |
| Inferior temporal | 5662.8 | 1188 | 5123.5 | 1080.8 | 145 | 7.06 | 0.008 | 0.04 | |
| Planum Temporale | 972.2 | 143.7 | 935.8 | 139.6 | 145 | 1.44 | 0.23 | 0.003 | |
| Superior Temporal | 7228.4 | 1083 | 7030.9 | 1098.7 | 145 | 0.58 | 0.44 | 0.004 | |
| Entorhinal | 551.4 | 219.6 | 532.2 | 174.1 | 145 | .449 | .504 | 0.003 | |
| Fusiform | 5601.5 | 791.2 | 5215.5 | 1077.2 | 145 | 3.68 | 0.057 | 0.02 | |
| Parahippocampal | 1347 | 232.6 | 1271.5 | 253.4 | 145 | 3.11 | 0.08 | 0.02 | |
| Temporal Pole | 647.4 | 223.2 | 611 | 166.8 | 145 | 1.30 | .256 | 0.009 | |
| Parietal | Supramarginal | 8401.2 | 1321.4 | 7778.6 | 1353.6 | 145 | 13.97 | 0.0002 | 0.06 |
| Superior parietal | 12076.1 | 1812.9 | 11444.5 | 2108.8 | 145 | 3.67 | 0.05 | 0.02 | |
| Angular | 11891.7 | 2160.6 | 10638.9 | 2207.8 | 145 | 9.85 | 0.002 | 0.09 | |
| Cuneus | 2553.8 | 630.5 | 2369.9 | 606.6 | 145 | 2.04 | .155 | 0.014 | |
| Pericalcarine | 3218 | 868.3 | 3071.8 | 865.2 | 145 | .412 | .522 | 0.002 | |
| Precuneus | 8979.6 | 1561 | 8514.6 | 1603.6 | 145 | 2.93 | 0.089 | 0.02 | |
| Motor Somatosensory | Post Central | 7435.4 | 1106.9 | 7007.7 | 1228.2 | 145 | 4.87 | 0.02 | 0.03 |
| Pre Central | 13649.1 | 2194.3 | 13119.9 | 1999.6 | 145 | 0.84 | 0.361 | 0.006 | |
| Paracentral | 4854.2 | 914.8 | 4731 | 794.5 | 145 | .35 | .554 | 0.002 | |
| Cingulate | Isthmus cingulate | 2584.3 | 405.9 | 2415.1 | 413 | 145 | 6.27 | 0.013 | 0.04 |
| Posterior cingulate | 3884.3 | 576.6 | 3762.4 | 475.8 | 145 | 1.28 | 0.259 | 0.009 | |
| Caudal Anterior Cingulate | 2310.7 | 336 | 2258 | 425.9 | 145 | .311 | .578 | 0.002 | |
| Rostral Anterior Cingulate | 1388.5 | 301.5 | 1372.2 | 344.4 | 145 | .100 | 0.753 | 0.0007 | |
| Occipital | Lateral Occipital | 10355.3 | 1964.9 | 9692.2 | 1953.7 | 145 | 2.70 | .102 | 0.01 |
| Lingual | 4938.7 | 1052.4 | 4508.4 | 956.1 | 145 | 3.93 | 0.049 | 0.02 | |
3. RESULTS
3.1. Total and Hemispheric White Matter
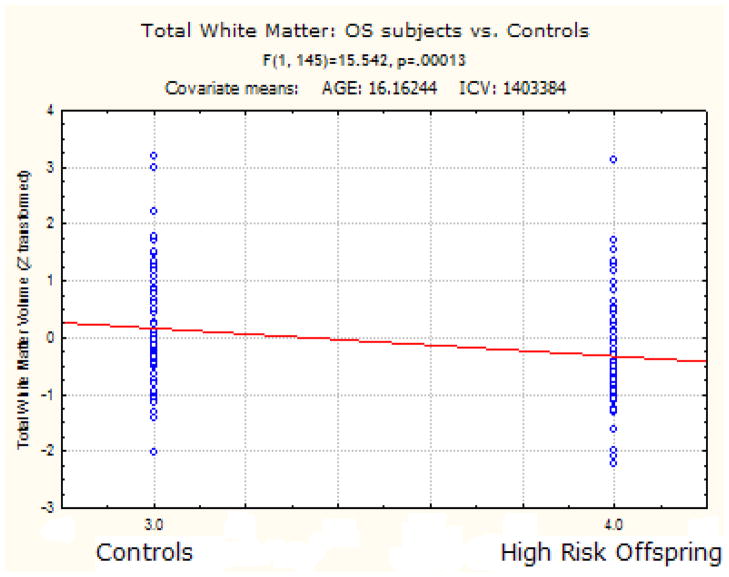
OS subjects had significantly reduced total white matter F (1, 145) = 15.54 p < 0.0001.
OS subjects had significantly less WM in the left hemisphere [HR - 204734.22 mm3 (SD) 27586] [HC - 218976.6 mm3 (SD) 27728.85] (F (1, 145) = 14.83 p < 0.0001] and right hemisphere [HR-206716.82 mm3 (SD) 28635.97] [HC - 221522.54 mm3 (SD) 27932.82] (F (1, 145) = 15.46 p < 0.0001].
3.2. Lobar effects
The MANCOVAs showed that left parietal [ F(6,142) = 3.42 p < 0.004], left motor-somatosensory [ F(3,142) = 3.3 p < 0.02] left cingulate [ F(4,141) = 3.44 p < 0.01] and right temporal[ F(8,141) = 2.5 p < 0.01], right parietal [ F(6,142) = 2.5 p< 0.02 ] had significantly reduced volume in High risk offspring compared to healthy controls. Only the left parietal cortex WM and left cingulate WM survived the correction for multiple MANCOVAs. Tables 2 and 3 shows the ANCOVA analysis for all the white matter regions in high risk subjects when compared to healthy control subjects.
3.3. Gender, Age and White Matter
There was a significant group X sex interaction in the right hemisphere WM volume F (1, 145) – 4.6408, p < 0.032. Although HR subjects had smaller RH WM volumes than their healthy counterparts, this appeared to be more pronounced in the males than females. There were no significant sex by group interaction in the left hemisphere WM. White matter correlated positively with age for all assessed regions but these correlations did not significantly differ between the groups.
4. Discussion
White matter constitutes the anatomical infrastructure for inter and intra hemispheric connectivity. In a sample of non-psychotic genetic high risk offspring and age matched healthy controls, our study showed that total WM volume in the right hemisphere was reduced by 6.68 % and the left hemisphere was reduced by 6.5 % in high risk subjects indicative of a developing vulnerability to the illness. In addition, male HR brains showed a greater reduction in the right hemisphere WM when compared to females. Our study showed that WM in the posterior regions of the brain namely left parietal cortex, and left cingulate cortex were significantly reduced in HR subjects. A study done on 22q11 deletion syndrome, which is a risk model for Schizophrenia, showed similar WM reductions in the parietal, temporal and occipital areas of the brain (da Silva Alves et al, 2011). It is possible that such a reduction of WM volume early in the preclinical phase may present as a decrease in the white matter fiber density in schizophrenia as shown in several DTI studies (Karlsgodt et al, 2009, Bloemen et al, 2010). In a separate study on the same sample, we showed that the splenium volume of the Corpus Callosum (Francis et al, 2011) was significantly reduced lending evidence that the inter-hemispheric connectivity between temporo-parietal regions could also be altered in this sample.
ROI based studies have shown that frontal WM is compromised in SZ (Brier et al., 1992; Buchanan et al,2004; Sanfilipo et al, 2000; Wible et al., 2001; Hulshoff-Pol 2002; Mathalon et al, 2003 and Buchsbaum et al, 2006). In a meta-analysis of 17 VBM studies of WM, Di et al, (2009) showed WM alterations in the right and medial frontal WM regions which was consistent with the ROI studies. In addition, they showed that the internal capsule WM is also altered. This is consistent with several DTI studies (Szeszko et al., 2005; Kubicki et al, 2005, 2007; Buchsbaum et al., 2006). In summary, WM alterations in the left hemisphere in HR subjects observed in this study lend evidence to the concept of a loss of connectivity between regions as a possible marker for susceptibility in schizophrenia. It is possible that such abnormalities may underlie a later misconnection syndrome as hypothesized by several investigators of white matter involvement in schizophrenia (Karlsgodt et al, 2009). More studies are warranted since genetic influences on WM volume and their relationship with cognitive abilities in genetic High risk subjects and healthy individuals are only being presently unraveled. While morphometric studies of WM such as the present study provide important clues to altered short and long range connectivity of WM fasciculi in the brain, Diffusion Tensor Imaging studies and fMRI connectivity studies are more likely to definitively inform these issues.
Fig 1.
FreeSurfer White Matter Segmentation:
Fig 2.
Highlights.
Our study compares WM volumes in Genetic High Risk against Control subjects.
WM volume in the R hemisphere was reduced by 6.68 % in high risk subjects.
Left hemisphere WM was also reduced by 6.5 %.
Male HR brains showed a greater reduction in the R hemisphere WM.
WM volumes of L parietal lobe, and L cingulate cortex were reduced in HR subjects.
Acknowledgments
Role of Funding Source
MH064023 (MSK)
MH045203 (MSK)
NARSAD Established Investigator award (MSK)
We are grateful to Diana Mermon MS for her assistance in clinical assessments and recruitment. Dr. Vaibhav Diwadkar helped with diagnostic ascertainments. Neeraj Tandon helped with the reliability analysis of FreeSurfer measurements.
Footnotes
Disclosure/Conflict of Interest Statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. In: Wood Steven, Yung Alison, Pantelis Christos., editors. Cognitive Neuropsychiatry. Special issue, “Cognitive Antecedents of Psychiatric Disorders”. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Bappal A, D’Angelo D, Ashtari M, Lencz T, Szeszko PR, et al. Brain morphometry using diffusion-weighted magnetic resonance imaging: application to schizophrenia. Neuroreport. 2005;16(13):1455–9. doi: 10.1097/01.wnr.0000177001.27569.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Rajarethinam R, Eack S, Kulkarni S, Prasad KM, Montrose DM, Dworakowski D, Diwadkar V, Keshavan MS. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophr Res. 2009 Dec;115(2–3):202–8. doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen OJ, de Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, Dingemans P, Linszen DH, van Amelsvoort TA. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med. 2010 Aug;40(8):1297–304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007 Mar;64(3):297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992 Dec;49(12):921–6. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, Barta PE, Pearlson GD. Morphometric assessment of the heteromodal association cortex in schizophrenia. Am J Psychiatry. 2004 Feb;161(2):322–31. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu KW, Mitelman S, Brickman AM, Shihabuddin L, Haznedar MM, Hazlett EA, Ahmed S, Tang C. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann Gen Psychiatry. 2006 Nov 28;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as a transcallosal misconnection syndrome. Schizophr Res. 1998;30(2):111–4. doi: 10.1016/s0920-9964(97)00139-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999 Feb;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- da Silva Alves F, Schmitz N, Bloemen O, van der Meer J, Meijer J, Boot E, Nederveen A, de Haan L, Linszen D, van Amelsvoort T. White matter abnormalities in adults with 22q11 deletion syndrome with and without schizophrenia. Schizophr Res. 2011 Oct;132(1):75–83. doi: 10.1016/j.schres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Voxel-based morphometric analysis using shape transformations. Int Rev Neurobiol. 2005;66:125–46. doi: 10.1016/S0074-7742(05)66004-7. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Di X, Chan RC, Gong QY. White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1390–4. doi: 10.1016/j.pnpbp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV (SCID) American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001 Jan;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004a;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004b;23( Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Wald LL. Phase maps reveal cortical architecture. Proc Natl Acad Sci U S A. 2007;104(28):11513–4. doi: 10.1073/pnas.0704515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis AN, Bhojraj TS, Kulkarni S, Prasad KM, Eack SM, Montrose DM, Keshvan MS. Abnormalities of the Corpus Callosum in Non-Psychotic high-risk offspring of Schizophrenia patients. Psychiatry Research – Neuroimaging. 2011 Jan 30;191(1):9–15. doi: 10.1016/j.pscychresns.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. Review. [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–24. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res. 2005;74(2–3):135–47. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Archives of General Psychiatry. 2004 Jul;61(7):658–68. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MG, van Haren NE, van der Tweel I, Staal WG, Baaré WF, Kahn RS. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002 Feb;159(2):244–50. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008 Fall;20(4):1297–327. doi: 10.1017/S095457940800062X. Review. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008a Part 3: neurobiology. Schizophr Res. 2008;106(2–3):89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. (2008) Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008b Aug;103(1–3):114–20. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007 Jan-Feb;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry. 2005 Mar;18(2):121–34. doi: 10.1097/00001504-200503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49(10):811–23. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008 Mar;34(2):330–40. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymer GK, Job DE, William T, Moorhead J, McIntosh AM, Owens DG, et al. Brain-behaviour relationships in people at high genetic risk of schizophrenia. Neuroimage. 2006;33(1):275–85. doi: 10.1016/j.neuroimage.2006.06.031. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Schulz SC. What we know: findings that every theory of schizophrenia should explain. Schizophr Bull. 2009;35(3):493–508. doi: 10.1093/schbul/sbp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Rapoport JL, Davis KL, Krystal JH. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry. Arch Gen Psychiatry. 2003 Aug;60(8):846–8. doi: 10.1001/archpsyc.60.8.846. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, et al. A large scale (N=400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res. 2008;101(1–3):95–105. doi: 10.1016/j.schres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011 Jul 1;70(1):88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009 Feb 15;44(4):1247–58. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000 May;57(5):471–80. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007 Apr;26(4):518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004 Jul;22(3):1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D, Stern E. Functional neuroimaging of hallucinations in schizophrenia: toward an integration of bottom-up and top-down approaches. Mol Psychiatry 1996. 1996 Nov 1;5:367–75. [PubMed] [Google Scholar]
- Silbersweig D, Stern E. Towards a functional neuroanatomy of conscious perception and its modulation by volition: implications of human auditory neuroimaging studies. Philos Trans R Soc Lond B Biol Sci. 1998 Nov 29;353(1377):1883–8. doi: 10.1098/rstb.1998.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005 Mar;162(3):602–5. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–69. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O’Donnell BF, Kikinis R, Jolesz FA, McCarley RW. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res. 2001 Nov 30;108(2):65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]