Abstract
The purpose of this study was to determine the melanoma targeting property of 177Lu-DOTA-GGNle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. 177Lu-DOTA-GGNle-CycMSHhex exhibited high receptor-mediated melanoma uptake and fast urinary clearance. The tumor uptake of 177Lu-DOTA-GGNle-CycMSHhex was 20.25 ± 4.59 and 21.63 ± 6.27% ID/g at 0.5 and 2 h post-injection, respectively. Approximately 83% of injected dose cleared out the body via urinary system at 2 h post-injection. 177Lu-DOTA-GGNle-CycMSHhex showed high tumor to normal organ uptake ratios except for the kidneys. The tumor/kidney uptake ratios of 177Lu-DOTA-GGNle-CycMSHhex were 2.76 and 1.74 at 2 and 24 h post-injection. The melanoma lesions were clearly visualized by SPECT/CT using 177Lu-DOTA-GGNle-CycMSHhex as an imaging probe at 2 h post-injection. Overall, high melanoma uptake coupled with fast urinary clearance of 177Lu-DOTA-GGNle-CycMSHhex underscored its potential for melanoma treatment in the future.
Keywords: Alpha-melanocyte stimulating hormone, 177Lu-labeled lactam bridge-cyclized peptide, melanoma targeting
Skin cancer is the most commonly diagnosed cancer in the United States, with approximately 3.5-million new cases occurring annually. Basal cell carcinoma, squamous cell carcinoma and melanoma are three major types of skin cancer. Malignant melanoma is the most lethal form of skin cancer, accounting for 75% of deaths of skin cancer despite the fact that melanoma only accounts for less than 5% of skin cancer cases.1 High mortality of melanoma is tightly associated with metastatic melanoma which is resistant to current chemotherapy and immunotherapy. Thus, it is desirable to develop receptor-targeting peptide radiopharmaceuticals for melanoma imaging and therapy.2–21 At the present time, receptor-targeting radionuclide therapy represents a promising strategy for melanoma treatment. Melanocortin-1 (MC1) receptor-targeting alpha-melanocyte stimulating hormone (α-MSH) peptides have been utilized as delivering vehicles to target therapeutic radionuclides to melanoma cells for treatment.10,12,19 This strategy takes advantage of rapid distribution through blood circulation, receptor-targeting melanoma localization, and fast urinary clearance of radiolabeled α-MSH peptides.
Over the past several years, we have identified a novel class of lactam bridge-cyclized α-MSH peptides through structure-activity-relationship studies for melanoma imaging.22–29 The MC1 receptor binding motif (His-DPhe-Arg-Trp) was cyclized by an Asp-Lys lactam bridge to yield the CycMSHhex {c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2} peptide. The radiometal chelators of DOTA (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid) and NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) were coupled to the N terminus of the CycMSHhex for SPECT (single photon emission computed tomography) and PET (positron emission tomography) imaging of melanoma.27–29 The melanoma lesions could be clearly visualized by SPECT using 111In-DOTA-GGNle-CycMSHhex or 67Ga-NOTA-GGNle-CycMSHhex as imaging probes,27,28 as well as by PET using 64Cu-NOTA-GGNle-CycMSHhex as an imaging probe. 29
Building upon the success of this novel class of lactam bridge-cyclized α-MSH peptides for melanoma imaging, we managed to extend their application to melanoma therapy using therapeutic radionuclides. We were particularly interested in 177Lu due to its attractive decay properties. 177Lu is medium-energy (0.497 MeV) β-emitter with a maximum soft tissue penetration of approximately 1.8–2 mm.30 Such short penetration in soft tissue makes 177Lu an ideal radioisotope for treating small tumors and metastases, where the therapeutic radiations stay fairly localized. Meanwhile, 177Lu is a manageable radioisotope in terms of dose preparation and waste disposal due to its half-life of 6.71 days, and is commercially available. Moreover, 177Lu emits γ-rays (113 and 208 keV) that are suitable for SPECT imaging. Thus, we prepared 177Lu-DOTA-GGNle-CycMSHhex and examined its cellular internalization and efflux properties in B16/F1 melanoma cells, and determined its melanoma targeting and pharmacokinetic properties in B16/F1 melanoma-bearing C57 mice in this study.
Firstly, DOTA-GGNle-CycMSHhex was synthesized and purified by reverse phase high pressure liquid chromatography (RP-HPLC) according to our published procedure.27 After the HPLC purification, DOTA-GGNle-CycMSHhex displayed greater than 90% purity. The identity of DOTA-GGNle-CycMSHhex was confirmed by electrospray ionization mass spectrometry. 177Lu-DOTA-GGNle-CycMSHhex (Figure 1) was readily prepared in 0.5 M ammonium acetate with greater than 95% radiolabeling yield, and was completely separated from its excess non-labeled peptide by RP-HPLC. The retention time of 177Lu-DOTA-GGNle-CycMSHhex was 17.8 min. 177Lu-DOTA-GGNle-CycMSHhex was stable in mouse serum at 37 °C for 24 h. Only 177Lu-DOTA-GGNle-CycMSHhex was detected by RP-HPLC after 24 h of incubation (Figure 2). Cellular internalization and efflux properties of 177Lu-DOTA-GGNle-CycMSHhex were examined in B16/F1 melanoma cells. Figure 3 illustrates the internalization and efflux of 177Lu-DOTA-GGNle-CycMSHhex. 177Lu-DOTA-GGNle-CycMSHhex exhibited rapid cellular internalization and prolonged cellular retention. Approximately 90% of 177Lu-DOTA-GGNle-CycMSHhex was internalized in the cells after 20 min of incubation. Cellular efflux results indicated that 40% of the 177Lu-DOTA-GGNle-CycMSHhex activity remained inside the cells at 2 h of incubation in the culture medium.
Figure 1.

Proposed schematic structure of 177Lu-DOTA-GlyGlyNle-CycMSHhex.
Figure 2.
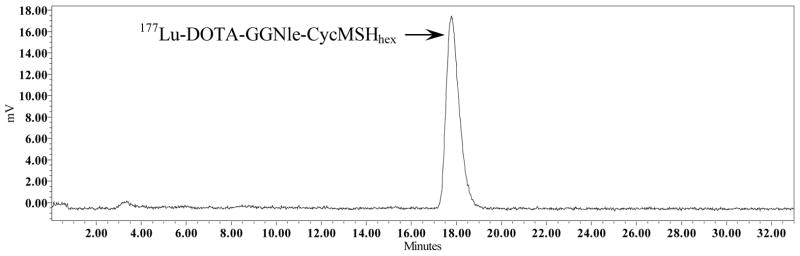
Serum stability of 177Lu-DOTA-GlyGlyNle-CycMSHhex after 24 h incubation at 37°C. The retention time of 177Lu-DOTA-GlyGlyNle-CycMSHhex was 17.8 min.
Figure 3.
Cellular internalization (A) and efflux (B) of 177Lu-DOTA-GlyGlyNle-CycMSHhex in B16/F1 melanoma cells. Total bound radioactivity (◆), internalized radioactivity (▲) and cell membrane radioactivity (■) were presented as counts per minute (cpm).
Secondly, the melanoma targeting and pharmacokinetic properties of 177Lu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing mice. The biodistribution results of 177Lu-DOTA-GGNle-CycMSHhex are presented in Table 1. 177Lu-DOTA-GGNle-CycMSHhex displayed rapid and high melanoma uptake. The tumor uptake was 20.25 ± 4.59 and 21.63 ± 6.27% ID/g at 0.5 and 2 h post-injection, respectively. 177Lu-DOTA-GGNle-CycMSHhex exhibited prolonged tumor retention, with 8.24 ± 1.51% ID/g of tumor uptake at 24 h post-injection. The co-injection of non-radioactive NDP-MSH blocked 96.3% of the tumor uptake, demonstrating that the tumor uptake was MC1 receptor-mediated. Whole-body clearance of 177Lu-DOTA-GGNle-CycMSHhex was rapid, with approximately 83% of the injected dose being washed out of the body via urinary system by 2 h post-injection. Ninety-three percent of the injected dose cleared out of the body by 24 h post-injection. Normal organ uptake of 177Lu-DOTA-GGNle-CycMSHhex was generally low (<1.37% ID/g) at 2 h post-injection except for kidneys. High tumor/blood and tumor/normal organ uptake ratios were demonstrated as early as 0.5 h post-injection. The renal uptake was 13.83 ± 2.51, 7.83 ± 1.38, and 9.68 ± 1.95% ID/g at 0.5, 2 and 4 h post-injection, respectively. At 24 h post-injection, the kidney uptake was 4.75 ± 1.03% ID/g. The co-injection of NDP-MSH didn’t reduce the renal uptake, indicating that the renal uptake of 177Lu-DOTA-GGNle-CycMSHhex was not receptor-mediated. The tumor/kidney uptake ratio was 2.76 and 1.74 at 2 and 24 h post-injection, respectively.
Table 1.
Biodistribution of 177Lu-DOTA-GlyGlyNle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. The data were presented as percent injected dose/gram or as percent injected dose (Mean ± SD, n=5)
| Tissues | 0.5 h | 2 h | 4 h | 24 h | 2-h NDP blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
| Tumor | 20.25±4.59 | 21.63±6.27 | 15.78±1.45 | 8.24±1.51 | 0.81±0.20* |
| Brain | 0.21±0.14 | 0.05±0.01 | 0.09±0.03 | 0.02±0.02 | 0.03±0.01 |
| Blood | 2.98±0.38 | 0.22±0.14 | 0.15±0.07 | 0.23±0.41 | 0.08±0.05 |
| Heart | 1.12±0.22 | 0.16±0.07 | 0.23±0.11 | 0.17±0.13 | 0.17±0.10 |
| Lung | 3.15±0.57 | 0.38±0.05 | 0.24±0.04 | 0.13±0.03 | 0.41±0.12 |
| Liver | 1.61±0.25 | 0.73±0.10 | 0.78±0.08 | 0.53±0.08 | 0.68±0.09 |
| Spleen | 1.21±0.19 | 0.27±0.14 | 0.32±0.11 | 0.24±0.10 | 0.25±0.11 |
| Stomach | 2.29±0.45 | 1.37±0.45 | 1.22±0.27 | 0.69±0.09 | 1.17±0.85 |
| Kidneys | 13.83±2.51 | 7.83±1.38 | 9.68±1.95 | 4.75±1.03 | 6.36±0.33 |
| Muscle | 1.05±0.34 | 0.17±0.09 | 0.05±0.02 | 0.14±0.05 | 0.24±0.12 |
| Pancreas | 0.88±0.32 | 0.20±0.05 | 0.24±0.09 | 0.20±0.11 | 0.21±0.06 |
| Bone | 0.95±0.33 | 0.34±0.10 | 0.64±0.33 | 0.18±0.24 | 0.57±0.32 |
| Skin | 3.28±0.50 | 0.48±0.06 | 0.50±0.09 | 0.33±0.06 | 0.29±0.20 |
|
| |||||
| Percent injected dose (%ID) | |||||
| Intestines | 1.66±0.34 | 0.90±0.18 | 1.04±0.51 | 0.47±0.13 | 0.56±0.20 |
| Urine | 62.40±3.78 | 83.33±6.63 | 83.73±7.12 | 93.31±2.34 | 93.83±2.82 |
|
| |||||
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/liver | 12.58 | 29.63 | 20.23 | 15.55 | 1.19 |
| Tumor/kidney | 1.46 | 2.76 | 1.63 | 1.74 | 0.13 |
| Tumor/lung | 6.43 | 56.92 | 65.75 | 63.38 | 1.98 |
| Tumor/muscle | 19.29 | 127.24 | 315.6 | 58.86 | 3.38 |
| Tumor/blood | 6.80 | 98.32 | 105.2 | 35.83 | 10.13 |
| Tumor/skin | 6.18 | 45.06 | 31.56 | 24.97 | 2.79 |
P<0.05 for determining significance of differences in tumor, kidney, liver, lung, muscle, skin and blood uptake between 177Lu-DOTA-GlyGlyNle-CycMSHhex with or without peptide blockade at 2 h post-injection.
Over the past several years, several MC1 receptor-targeting 177Lu-labeled metal-cyclized α-MSH peptides have been reported for melanoma therapy.14,15,19 Initially, the (Arg11)CCMSH peptide was cyclized with non-radioactive Re to retain favorable melanoma targeting properties, whereas the DOTA was conjugated to the N-terminus of the peptide for 177Lu labeling.14 177Lu-DOTA-Re(Arg11)CCMSH exhibited 14.48 ± 0.85 and 17.68 ± 3.32% ID/g of tumor uptake at 2 and 4 h post-injection in B16/F1 melanoma-bearing C57 mice. The renal uptake of 177Lu-DOTA-Re(Arg11)CCMSH was 17.99 ± 2.47% and 19.09 ± 2.38% ID/g at 2 and 4 h post-injection in B16/F1 melanoma-bearing C57 mice. Furthermore, the introduction of Glu2 between the DOTA and (Arg11)CCMSH significantly decreased the renal uptake of 177Lu-DOTA-Re(Glu2, Arg11)CCMSH by 70% as compared to 177Lu-DOTA-Re(Arg11)CCMSH at 2 and 4 h post-injection.15 Although the tumor uptake of 177Lu-DOTA-Re(Glu2, Arg11)CCMSH was lower than that of 177Lu-DOTA-Re(Arg11)CCMSH, 177Lu-DOTA-Re(Glu2, Arg11)CCMSH showed enhanced tumor/kidney uptake ratios than 177Lu-DOTA-Re(Arg11)CCMSH at 2 and 4 h post-injection.15 Remarkably, 177Lu-DOTA-GGNle-CycMSHhex exhibited higher tumor/kidney uptake ratios than 177Lu-DOTA-Re(Glu2, Arg11)CCMSH at 0.5, 2 and 24 h post-injection. The tumor/kidney uptake ratios of 177Lu-DOTA-GGNle-CycMSHhex were 1.5, 1.5 and 1.8 times the tumor/kidney uptake ratios of 177Lu-DOTA-Re(Glu2, Arg11)CCMSH at 0.5, 2 and 24 h post-injection, respectively.
The representative whole-body SPECT/CT images are presented in Figure 4. The B16/F1 melanoma lesions were clearly visualized by SPECT/CT using 177Lu-DOTA-GGNle-CycMSHhex as an imaging probe at 2 h post-injection. The imaging properties of 177Lu-DOTA-GGNle-CycMSHhex could potentially be utilized to calculate the absorbed dose of 177Lu-DOTA-GGNle-CycMSHhex treatment, as well as to monitor the therapeutic response without injecting an additional imaging probe. Furthermore, 177Lu-DOTA-GGNle-CycMSHhex exhibited high tumor to normal organ uptake ratios except for kidneys, suggesting that the kidneys would be dose-limiting normal organ for 177Lu-DOTA-GGNle-CycMSHhex treatment in the future. However, it is worthwhile to note that the renal uptake of 177Lu-DOTA-GGNle-CycMSHhex was lower than that of 177Lu-DOTA-Re(Arg11)CCMSH at all time points investigated in this study. The 177Lu-DOTA-Re(Arg11)CCMSH treatment (1×37.0 MBq or 2×18.5 MBq) significantly decreased the B16/F1 tumor growth and extended the mean survival time of B16/F1 tumor-bearing mice without any overt signs of renal toxicity.19 Accordingly, it is expected that same treatment doses of 177Lu-DOTA-GGNle-CycMSHhex would yield therapeutic effects without renal toxicity. Moreover, it is anticipated that the enhanced tumor/kidney uptake ratio of 177Lu-DOTA-GGNle-CycMSHhex would improve its therapeutic efficacy for melanoma. It would be interesting to determine the therapeutic efficacy of 177Lu-DOTA-GGNle-CycMSHhex in the future.
Figure 4.
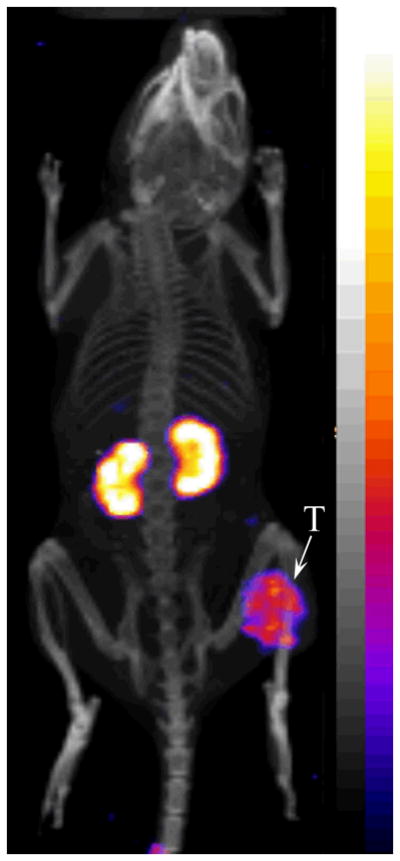
Representative whole-body SPECT/CT image of 177Lu-DOTA-GlyGlyNle-CycMSHhex in a B16/F1 melanoma-bearing C57 mouse at 2 h post-injection. The tumor lesions (T) were highlighted with an arrow on the image.
In conclusion, the tumor targeting and pharmacokinetic properties of 177Lu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 mice in this study. Overall, the properties of high melanoma uptake and fast urinary clearance of 177Lu-DOTA-GGNle-CycMSHhex underscored its potential as a therapeutic agent for metastatic melanoma detection in the future.
The experimental details are presented in References and notes.31–34
Acknowledgments
We thank Drs. Jianquan Yang and Fabio Gallazzi for their technical assistance. This work was supported in part by the NIH grant NM-INBRE P20RR016480/P20GM103451 and University of New Mexico STC Gap Fund. The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
REFERENCES AND NOTES
- 1.Siegel R, Naishadham D, Jemal A. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Giblin MF, Wang N, Hoffman TJ, Jurisson SS, Quinn TP. Proc Natl Acad Sci USA. 1998;95:12814. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Cancer Res. 2000;60:5649. [PubMed] [Google Scholar]
- 4.Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. J Nucl Med. 2001;42:1847. [PubMed] [Google Scholar]
- 5.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. Int J Cancer. 2002;101:480. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 6.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. J Nucl Med. 2002;43:1699. [PubMed] [Google Scholar]
- 7.Chen J, Cheng Z, Miao Y, Owen NK, Quinn TP, Jurisson SS. J Med Chem. 2002;45:3048. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- 8.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Bioconjug Chem. 2003;14:1177. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 9.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. J Nucl Med. 2004;45:116. [PubMed] [Google Scholar]
- 10.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. J Nucl Med. 2005;46:121. [PubMed] [Google Scholar]
- 11.Froidevaux S, Calame-Christe M, Tanner H, Eberle AN. J Nucl Med. 2005;46:887. [PubMed] [Google Scholar]
- 12.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman TJ, Quinn TP. Clin Cancer Res. 2005;11:5616. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 13.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. J Med Chem. 2005;48:2985. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 14.Miao Y, Hoffman TJ, Quinn TP. Nucl Med Biol. 2005;32:485. doi: 10.1016/j.nucmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Miao Y, Fisher DR, Quinn TP. Nucl Med Biol. 2006;33:723. doi: 10.1016/j.nucmedbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Lewis JS. J Nucl Med. 2007;48:64. [PubMed] [Google Scholar]
- 17.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. Bioconjug Chem. 2007;18:765. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao Y, Benwell K, Quinn TP. J Nucl Med. 2007;48:73. [PubMed] [Google Scholar]
- 19.Miao Y, Shelton T, Quinn TP. Cancer Biother Radiopharm. 2007;22:333. doi: 10.1089/cbr.2007.376.A. [DOI] [PubMed] [Google Scholar]
- 20.Raposinho PD, Xavier C, Correia JD, Falcao S, Gomes P, Santos I. J Biol Inorg Chem. 2008;13:449. doi: 10.1007/s00775-007-0338-3. [DOI] [PubMed] [Google Scholar]
- 21.Raposinho PD, Correia JD, Alves S, Botelho MF, Santos AC, Santos I. Nucl Med Biol. 2008;35:91. doi: 10.1016/j.nucmedbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Miao Y, Gallazzi F, Guo H, Quinn TP. Bioconjug Chem. 2008;19:539. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Nucl Med Biol. 2009;36:267. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo H, Yang J, Gallazzi F, Prossnitz ER, Sklar LA, Miao Y. Bioconjug Chem. 2009;20:2162. doi: 10.1021/bc9003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, Yang J, Shenoy N, Miao Y. Bioconjug Chem. 2009;20:2356. doi: 10.1021/bc900428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, Yang J, Gallazzi F, Miao Y. J Nucl Med. 2010;51:418. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo H, Yang J, Gallazzi F, Miao Y. J Nucl Med. 2011;52:608. doi: 10.2967/jnumed.110.086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo H, Gallazzi F, Miao Y. Bioconjug Chem. 2012;23:1341. doi: 10.1021/bc300191z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo H, Miao Y. Mol Pharmaceutics. 2012;9:2322. doi: 10.1021/mp300246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WH, Hartmann-Siantar C, Fisher D, Descalle MA, Daly T, Lehmann J, Lewis MR, Hoffman TJ, Smith CJ, Situ PD, Volkert WA. Cancer Biother Radiopharm. 2005;20:436. doi: 10.1089/cbr.2005.20.436. [DOI] [PubMed] [Google Scholar]
- 31.Peptide radiolabeling with 177Lu: Amino acid and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). DOTA-tri-t-butyl ester was purchased from Macrocyclics Inc. (Richardson, TX) for peptide synthesis. All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. DOTA-GGNle-CycMSHhex was synthesized and characterized according to our published procedure.27 177LuCl3 was purchased from Trace Life Sciences, Inc. (Dallas, TX) for peptide radiolabeling. 177Lu-DOTA-GGNle-CycMSHhex was prepared in a 0.5 M NH4OAc-buffered solution at pH 5.4 according to the published procedure.19 Briefly, 50 μL of 177LuCl3 (37–74 MBq in 0.05 M HCl aqueous solution), 10 μL of 1 mg/mL DOTA-GGNle-CycMSHhex aqueous solution and 400 μL of 0.5 M NH4OAc (pH 5.4) were added into a reaction vial and incubated at 75°C for 45 min. After the incubation, 10 μl of 0.5% ethylenediaminetetraacetic acid (EDTA) aqueous solution was added into the reaction vial to scavenge potential unbound 177Lu3+ ions. The radiolabeled complexes were purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytical column (Deerfield, IL) using a 20-minute gradient of 18–28% acetonitrile in 20 mM HCl aqueous solution with a flow rate of 1.0 mL/min. Purified peptide sample was purged with N2 gas for 20 minutes to remove the acetonitrile. The pH of final solution was adjusted to 7.4 with 0.1 N NaOH and sterile normal saline for animal studies. In vitro serum stability of 177Lu-DOTA-GGNle-CycMSHhex was determined by incubation in mouse serum at 37 °C for 24 h and monitored for degradation by RP-HPLC.
- 32.Cellular internalization and efflux of 177Lu-DOTA-GGNle-CycMSHhex: B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA). Cellular internalization and efflux of 177Lu-DOTA-GGNle-CycMSHhex were evaluated in B16/F1 melanoma cells. After being washed twice with binding medium [modified Eagle’s medium with 25 mM N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline], the B16/F1 cells seeded in cell culture plates were incubated at 25°C for 20, 40, 60, 90 and 120 min (n=3) in the presence of approximate 200,000 counts per minute (cpm) of HPLC-purified 177Lu-DOTA-GGNle-CycMSHhex. After incubation, the reaction medium was aspirated and the cells were rinsed with 2×0.5 mL of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS. Cellular internalization of 177Lu-DOTA-GGNle-CycMSHhex was assessed by washing the cells with acidic buffer [40 mM sodium acetate (pH 4.5) containing 0.9% NaCl and 0.2% BSA] to remove the membrane-bound radioactivity. The remaining internalized radioactivity was obtained by lysing the cells with 0.5 mL of 1N NaOH for 5 min. Membrane-bound and internalized 177Lu-DOTA-GGNle-CycMSHhex activities were counted in a gamma counter. Cellular efflux of 177Lu-DOTA-GGNle-CycMSHhex was determined by incubating the B16/F1 cells with 177Lu-DOTA-Nle-CycMSHhex for 2 h at 25°C, removing non-specific-bound activity with 2×0.5 mL of ice-cold PBS rinse, and monitoring radioactivity released into cell culture medium. At time points of 20, 40, 60, 90 and 120 min, the radioactivities on the cell surface and inside the cells were separately collected and counted in a gamma counter.
- 33.Biodistribution studies: All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The pharmacokinetics of 177Lu-DOTA-GGNle-CycMSHhex was determined in B16/F1 melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). Each C57 mouse was subcutaneously inoculated on the right flank with 1×106 B16/F1 cells. The weight of tumors reached approximately 0.2 g 10 days post cell inoculation. Each melanoma-bearing mouse was injected with 0.037 MBq of 177Lu-DOTA-GGNle-CycMSHhex via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. The tumor uptake specificity of 177Lu-DOTA-GGNle-CycMSHhex was determined by co-injecting 10 μg (6.07 nmol) of unlabeled NDP-MSH peptide with 177Lu-DOTA-GGNle-CycMSHhex at 2 h post-injection. Statistical analysis was performed using the Student’s t-test for unpaired data. A 95% confidence level was chosen to determine the significance of difference in tumor and renal uptake of 177Lu-DOTA-Nle-CycMSHhex with/without NDP-MSH co-injection in the biodistribution studies described above. Differences at the 95% confidence level (p<0.05) were considered significant.
- 34.Melanoma imaging with 177Lu-DOTA-GGNle-CycMSHhex: Approximately 17.4 MBq of 177Lu-DOTA-GGNle-CycMSHhex was injected in a B16/F1 melanoma-bearing C57 mouse (10 days post the cell inoculation) via the tail vein for melanoma imaging. The mouse was sacrificed for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan) imaging at 2 h post-injection. The 9-min CT imaging was immediately followed by the whole-body SPECT imaging. The SPECT scans of 24 projections were acquired. Reconstructed SPECT and CT data were visualized and co-registered using InVivoScope (Bioscan, Washington DC).



