Abstract
Women are using estrogens for many purposes, such as to prevent pregnancy or miscarriage, or to treat menopausal symptoms. Estrogens also have been used to treat breast cancer which seems puzzling, since there is convincing evidence to support a link between high lifetime estrogen exposure and increased breast cancer risk. In this review, we discuss the findings that maternal exposure to the synthetic estrogen diethylstilbestrol during pregnancy increases breast cancer risk in both exposed mothers and their daughters. In addition, we review data regarding the use of estrogens in oral contraceptives and as postmenopausal hormone therapy and discuss the opposing effects on breast cancer risk based upon timing of exposure. We place particular emphasis on studies investigating how maternal estrogenic exposures during pregnancy increase breast cancer risk among daughters. New data suggest that these exposures induce epigenetic modifications in the mammary gland and germ cells, thereby causing an inheritable increase in breast cancer risk for multiple generations.
Keywords: Synthetic estrogens, Pregnancy, In utero, Oral contraceptives, Hormone therapy, Epigenetics
Estrogens are needed for many normal physiological functions in the woman’s body, including in the brain, heart, liver, bone, adipose tissue, breast and uterus. Although estrogens act through the estrogen receptors (ERα and ERβ) in these tissues, the specific tasks of estrogens differ from tissue to tissue. For example, in the central nervous system estrogens protect against neurodegenerative diseases, and in adipose tissue, estrogens regulate adipogenesis, adipose deposition and adipocyte differentiation [1,2]. Consequently, loss of estrogens at menopause increases a woman’s risk of developing osteoporosis, neuroregenerative diseases, including Altzheimer’s disease and Parkinson’s disease, and cardiovascular diseases [2]. Menopause also is associated with weight gain [3, 4].
Interestingly, results obtained in the Women’s Health Initiative (WHI) study indicate that treatment with estrogen alone, or estrogen and progestin in combination, does not protect against stroke or cardiovascular and neurodegenerative diseases, but in fact may increase them [5, 6]. In the bone and adipose tissue, estrogen therapy has beneficial effects [5–7]. Mechanisms driving these opposing effects of hormone therapy (HT) on different estrogen target tissues are poorly understood.
Breast cancer risk also increases after menopause: over 80 % of breast cancers in the Western world are diagnosed in women aged 50 and over. [8]. The drop in estrogens and increased breast cancer risk seem to be in conflict with the findings showing that high total lifetime exposure to estrogens, including estradiol, estrone and estriol, increases breast cancer risk [9], and blocking estrogen receptor activation with antiestrogens reduces the risk [10,11]. To complicate the issue further, estrogens can prevent the development of some breast cancers [12] and can be effective in treating this disease [13]. Thus, the complex associations between estrogens and breast cancer remain to be elucidated.
In this review, the evidence that estrogens not only promote the growth of existing breast cancer cells [14], but also can be used to prevent their growth is first briefly reviewed. Then we discuss findings regarding the effects of estrogenic exposures on breast cancer risk when women take them during pregnancy, premenopausally and postmenopausally. Our focus will be on the exposures that occur during pregnancy and their effect on breast cancer risk in daughters. We also will discuss our recent findings showing that maternal exposure during pregnancy to synthetic estradiol or a high fat (HF) diet that elevates pregnancy estradiol levels [15, 16] increases breast cancer risk in multiple generations of female offspring [17].
Estrogens and Breast Cancer: General Observations
Estrogens Increase Breast Cancer Risk
Endogenous factors linked to increased breast cancer risk include those that lead the breast tissue being exposed to high levels of hormones for extended periods of time, such as early age of menarche, and late age of first pregnancy and menopause [18]. Other factors that alter the hormonal environment and, thus, affect breast cancer risk include obesity [19] and antiestrogens [20].
We highlight here three examples of the complex association between estrogenic environment and breast cancer risk. First, women who started menstruating early (before age 12) and/or went through menopause later (after age 55), and thus have a higher number of menstrual cycles and longer lifetime exposure to hormones, are at a slightly elevated risk of breast cancer [21, 22]. However, while a short menstrual cycle (the time from menses to menses) and therefore a higher lifetime exposure to estrogen in some studies is associated with an increased risk of breast cancer [23–26], in some studies no link has been found [27, 28]. Results of a recent meta-analysis, involving 117 epidemiological studies, suggest that the effects of early menarche and late menopause on breast cancer risk are not caused simply by the lengthening of a woman’s total number of reproductive years [22].
Second, since adipose tissue contains high levels of aromatase that converts androgens to estrogens, obesity is linked to increased estrogen levels [29, 30]. However, the increase is seen mostly in postmenopausal women [19], and not in premenopausal women [31]. This difference may be because an increase in adipose-derived estrogens suppresses ovarian estrogen production in premenopausal women. Interestingly, premenopausal obesity provides a protective effect, while postmenopausal obesity increases breast cancer risk. A pooled analysis of seven prospective cohort studies clearly shows a direct association of obesity with risk of postmenopausal breast cancer (RR=1.26; CI=1.09–1.46) and the inverse association of obesity with risk of premenopausal breast cancer risk (RR=0.54; CI=0.34–0.85) [32]. A subsequent prospective study of 12,159 postmenopausal women reported an increased risk in women with a BMI >28.5 (RR= 1.54; CI=1.01–2.35) [33]. In this study, postmenopausal breast cancer risk was increased in women who were obese either pre- and/or postmenopausally.
Postmenopausal HT may mask the effects of obesity on breast cancer. In the European Prospective Investigation into Cancer and Nutrition (EPIC) study, a significant association between obesity and breast cancer was found only in women who were not using HT (n=78,541 women; RR=1.31; CI= 1.08–1.59) [34]. In the same EPIC study, 24,314 postmenopausal women reported using HT (either estrogen alone or in combination with progestin) and their risk of developing breast cancer tended to be inversely linked to obesity (RR= 0.71; CI=0.50–1.01). Other studies have confirmed that breast cancer risk is not increased in obese HT users, compared with lean HT users [35, 36]. Both lean and obese women using HT have a higher risk of breast cancer than lean non-users [34].
Estrogens perhaps not only mask, but may shield against the adverse effects of obesity, especially in premenopausal women. Among healthy premenopausal women at high risk of developing breast cancer and therefore taking antiestrogens (tamoxifen or raloxifen), obesity is associated with an increased risk of invasive breast cancer, compared with normal weight women (HR=1.70, p=0.01) [20]. As already mentioned, obese premenopausal women who are not using endocrine therapy are at lower risk of developing breast cancer than lean premenopausal women. Data for this study were obtained from two recent antiestrogen-based breast cancer prevention trials: (i) the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial that involved 300 centers in the USA and Canada, and (ii) the STAR (The Study of Tamoxifen and Raloxifene) trial that was conducted by the NSABP and involved 500 centers across USA, Canada and Puerto Rico.
Obese pre- and postmenopausal women differ from each other not only regarding the effects of adipose tissue on their circulating estrogen levels, but also where the fat is stored. Among men and postmenopausal women, excess fat tends to accumulate as visceral fat in the waist area, while in premenopausal women fat is stored in the hips and thighs [37]. Excess visceral fat is linked to an increased risk of insulin resistance and type 2 diabetes, conditions which are associated with elevated breast cancer risk [38]. The mechanism mediating the effects of insulin resistance and type 2 diabetes on breast cancer risk remain to be identified.
Third, high mammographic density is one of the strongest risk factors for breast cancer [39, 40], and among postmenopausal women with high breast density, this risk further increases by 1.7-fold with the use of HT [41]. Nevertheless, the interactions between breast density and changes in the hormonal environment seem complex [42, 43]. For example, findings regarding the effect of antiestrogens on mammographic density are conflicting, with some studies reporting a reduction in density [44, 45] and some reporting no effect with antiestrogen use [46].
Estrogens and Prevention of Breast Cancer
Findings obtained in animal studies suggest that estrogens can reduce breast cancer risk. In the early 1960’s, Huggins et al. [47] reported that administration of high levels of 17-β estradiol (E2) and progesterone (P) in rats prior to carcinogen (9,12-dimethylbenz[a]anthracene; DMBA) exposure inhibited mammary tumorigenesis in rats. We have obtained similar findings in rats treated prepubertally with E2 alone. [48]. These data indicate that E2 or E2+P exposure of a developing pre-pubertal mammary gland alters its sensitivity to carcinogens that initiate mammary tumorigenesis. The protective effects of estrogens appear not to be limited to exposure during mammary gland development (e.g., prepubertal exposure), as short-term exposure to high levels of E2 and P after treatment with the carcinogen methylnitrosourea (MNU) is as effective as ovariectomy in preventing mammary carcinogenesis [49]. Thus, this hormone combination can inhibit both the susceptibility of epithelial cells to carcinogens and the carcinogenic process at its initial stages. Perhaps the most unexpected discovery regarding estrogens and breast cancer is that in postmenopausal women estrogen only based HT reduces the risk of developing breast cancer by almost one quarter [50, 51].
Estrogen levels are high during pregnancy, and since first pregnancy before age 20 reduces breast cancer risk by 50 % [52], the protective effects of estrogens reported by Huggins et al., [47] and Grubbs et al., [49] may reflect physiological changes that occur in the mammary gland during pregnancy. Therefore, many subsequent studies utilized doses of E2 and P that mimic the levels seen during pregnancy. Using this approach, both Grubb’s [53] and Sivaraman’s groups [54] found that rats exposed to pregnancy-mimicking doses of E2 and P for 3 or 5 weeks had at least 4-fold lower incidence of mammary tumors than the control rats. Guzman and collaborators [12, 55] subsequently showed that E2 alone was as effective as E2 combined with P in reducing mammary tumorigenesis. Treatment with P alone enhanced mammary carcinogenesis [12]. These findings provide convincing evidence that estrogen exposure during pregnancy reduces later mammary cancer risk.
Several explanations have been offered as to why early pregnancy and high pregnancy estrogen levels reduce breast cancer risk (reviewed by Britt et al. [56] and Russo & Russo [57]). The proposed mechanisms include (i) altered sensitivity of the mammary gland to later hormonal exposures [56]; (ii) reduction in the number of stem/progenitor cells [56, 58] and consequently, elimination of targets for malignant transformation; and (iii) changes in gene expression patterns resulting in reduced proliferation and increased differentiation [59–61]. Most likely, all three are involved in protecting the breast against cancer.
Estrogens and Treatment of Breast Cancer
Over half a century ago the synthetic estrogen diethylstilbestrol (DES) was used to treat metastatic breast cancer, and was replaced by tamoxifen (TAM) in the early 1980’s as TAM has fewer side effects [62]. Since then TAM and other antiestrogens have been successfully used to treat ER positive breast cancers. However, about 30 % of these cancers recur [63]. It was recently discovered that 46 % of women with metastatic breast cancer who developed resistance to multiple antiestrogen treatments exhibit clinical response to high-dose estrogens, including DES [64]. Further, even a low dose of E2 is reported to cause growth arrest or shrinkage of the tumor in one third of women with metastatic breast cancer who stop responding to antiestrogens [65]. This effect may be caused by estrogens inducing apoptosis and killing antiestrogen resistant cancer cells [13]. Thus, estrogens can inhibit the growth of existing breast tumors.
Maternal Synthetic Estrogen Use and Breast Cancer
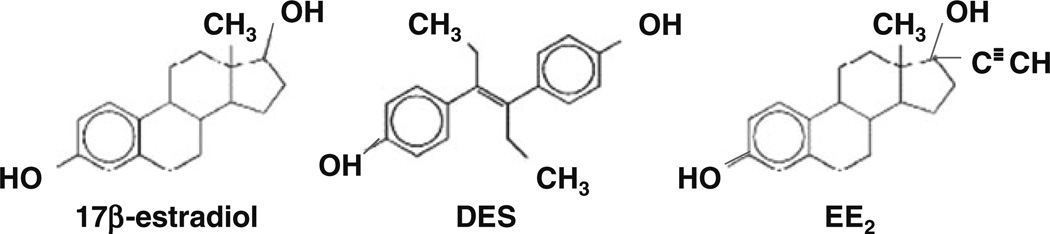
Synthetic estrogens are compounds obtained by chemical synthesis that possess estrogenic activity. One such compound is ethinyl estradiol (EE2); it is used alone or in combination with progestins as an oral contraceptive and some forms of menopausal HT, and also to treat advanced breast and prostate cancers. DES is another type of synthetic estrogen, and because it contains diethyl substitution at the ethylenic bond of stilbestrol, it is a highly potent estrogen (Fig. 1). From the early 1940’s until 1970’s, DES was given to pregnant women to prevent miscarriage, which is often proceeded by a decline in estrogen levels. It later became apparent that DES treatment was mostly ineffective in preventing miscarriage [66], but nevertheless physicians continued prescribing DES to pregnant women. A recent article summarizes the effects of maternal exposure to DES during pregnancy and its adverse effects on pregnancy and fetal development in women [67], and show that this exposure increased 2nd trimester miscarriage by 3.8 -fold.
Fig. 1.
Structures of natural estradiol (E2) and synthetic estrogens diethylstilbestrol (DES) and ethinyl estradiol (EE2)
The recommended DES regimen in women started at 5 mg per day in the 7th and 8th week of pregnancy, increasing every other week by 5 mg per day through the 14th week, then increasing every week by 5 mg per day from 25 mg per day in the 15th week to 125 mg per day in the 35th week of pregnancy [68]. The FDA banned DES in 1971 when physicians reported several cases of a rare type of cancer, clear cell adenocarcinoma (CCA) of the vagina and cervix in young women whose mothers took DES. It soon was discovered that women whose mothers were prescribed DES during pregnancy had a 40 times greater risk of developing this type of cancer than unexposed women [69].
Maternal Exposure to DES During Pregnancy and Breast Cancer Risk Among Daughters
Findings in Human Studies
Increased incidence of CCA in young daughters of DES exposed mothers was not the only long-term adverse outcome. These daughters also develop more breast cancers [67, 70, 71]. In addition, breast cancer incidence is increased in the mothers themselves [72].
Several studies have been published that investigate breast cancer risk in the daughters of DES mothers, the majority of which are cohort studies done in the US. The same cohort of daughters has been followed in many of these studies, and as the women in the cohort grew older, their breast cancer risk grew higher, compared with matched non-exposed controls [67, 70, 71, 73, 74]. These studies clearly indicate that when both groups of women are old enough to develop breast cancer, the incidence is at least 2-fold higher in the daughters of DES-exposed mothers.
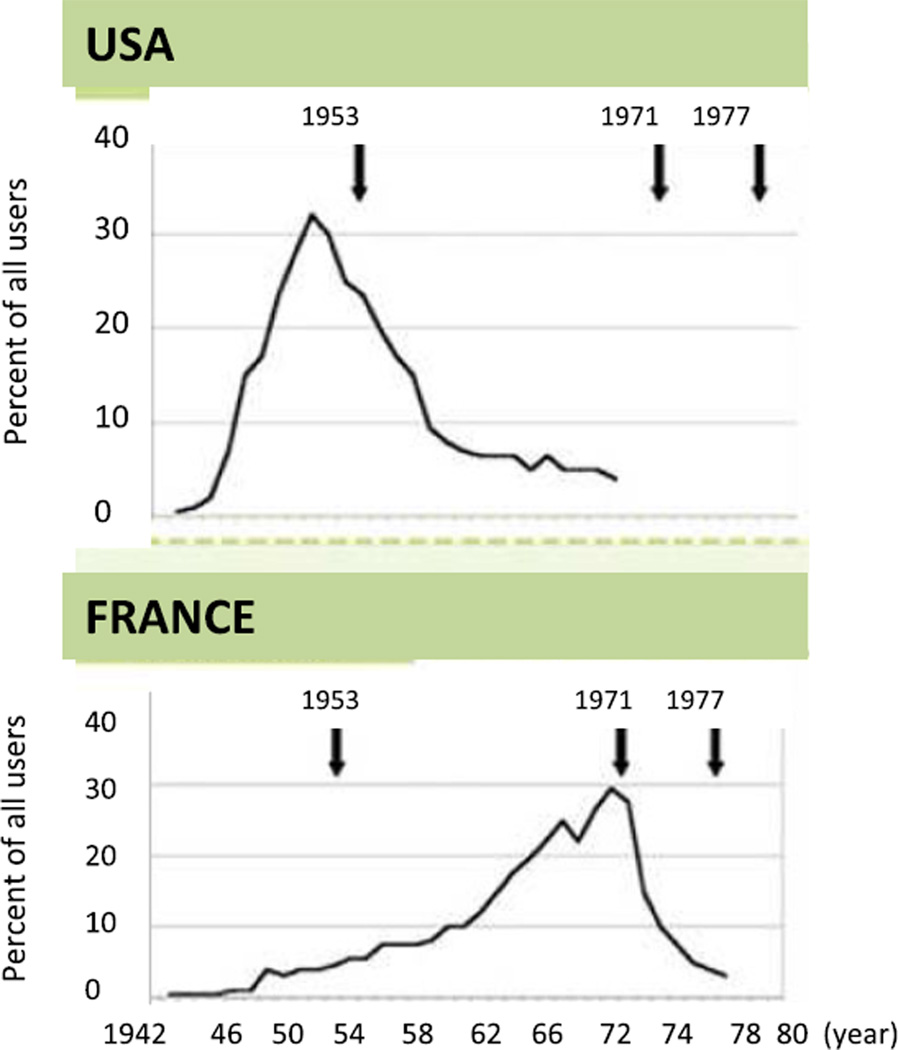
Many pregnant women in Europe and Australia also used DES, but the peak exposure occurred later than in the USA. Fig. 2 shows that the peak exposure in the USA was in the early 50’s, while in France it was in the late 1960’s and early 70’s. The fact that the daughters of DES-exposed mothers in Europe are younger than the daughters in the USA probably explains why a recent study done in Europe found a trend but not a significant increase in breast cancer risk among them [75]. Once the European daughters reach the age when breast cancer is more commonly detected, they too are likely to exhibit increased breast cancer risk. In summary, the findings in humans provide strong evidence that maternal DES exposure during pregnancy increases their daughters’ breast cancer risk. In the UK and France, half a million pregnant women may have taken it, and 740,000 in Australia. The exact number of pregnant women who used DES during pregnancy is not known, but it is estimated to have been approximately 5 to 10 million women worldwide. These numbers may be an underestimation, as DES was marketed by several drug companies and under several different trade names.
Fig. 2.
Difference in the peak intake of DES during pregnancy in the USA and France (from http://diethylstilbestrol.co.uk)
Findings in Animal Studies
Many animal studies have investigated the effect of in utero DES exposure on later mammary tumorigenesis [76–81]. The amount of DES given to pregnant mice or rat dams varied from study to study (0.2–12,000 µg/day, which translates in rats to approximately 1 µg/kg – 60 mg/kg DES per day; in pregnant women the DES dose ranged from 100 µg/kg to 2 mg/kg), as did the route of administration (subcutaneous injection or via feed) and mammary tumor model used (spontaneous, carcinogen-induced, or ACI rats which develop mammary tumors upon estrogen exposure). The animal studies show that the doses of DES relevant to pregnant women increased later risk of developing mammary tumors. The increase did not occur with the highest doses and exposures which started early in pregnancy [81, 82], probably because they prevented implantation or caused miscarriage in most of the exposed dams.
Other Estrogenic Exposures in Utero and Breast Cancer Risk
The increase in breast cancer risk due to maternal exposure to DES is not limited to this synthetic estrogen. We have been studying the effects of maternal exposure to excess natural (E2) or synthetic (EE2) estrogens during pregnancy on mammary tumorigenesis in rat models of human breast cancer. Both natural and synthetic estrogens can lead to an increase in mammary tumorigenesis in the offspring [16, 17]. In addition, we have found that maternal exposure to a HF diet elevates pregnancy E2 levels [15] and leads to elevated mammary cancer risk among offspring [16, 17]. Similar results have been seen in another rat model of mammary tumorigenesis [83]. Further, findings in mice show that a maternal HF diet during pregnancy increases the offsprings’spontaneous (out-bred CD-1 mice) [84] and genetically-induced (ErbB2/c-neu mice) mammary tumorigenesis [85].
These findings indicate that dietary factors which elevate maternal estrogen exposure during pregnancy may increase daughter’s later risk of developing breast cancer. These estrogens can originate from various sources, such as from livestock treated with estrogens to promote their growth: cattle was exposed to DES until it was banned in 1973, and thereafter cattle has been given other estrogenic growth stimulating products [86, 87]. In addition, humans are daily exposed to many industrial chemicals with estrogenic properties that may end up in the food and drinking water consumed by pregnant women.
Mechanisms Involved in Mediating the Effects of Elevated in Utero Estrogenic Environment on Later Increase in Breast Cancer Risk
Fetal Mammary Gland Development
The length of gestation in mice and rats is 21 days. Development of the mammary gland starts with the formation of the mammary lines on gestation day 10 in the mouse [88]. Within 24–36 h, five pairs of placodes are formed on the ectoderm surface, from where they invaginate into the underlying mesenchyme and form a mammary bud. This process is completed on gestational day 14 [88]. Ductal branching morphogenesis begins on gestational day 16 when epithelial cells from the mammary bud grow down into the mesenchyme and the mammary fat pad, which consists of preadipocytes. At this point, mammary epithelial cells start to form the ductal tree which contains 10–15 branches until the mouse reaches puberty during postnatal week 4 [89, 90].
Development of the mammary lines, placodes and mammary buds, and outgrowth of the mammary ducts are driven by several signaling molecules, including members of the Wnt/β-catenin pathway, fibroblast growth factors (FGF) and their receptors, T-box-containing transcription factor 3 (TBX3), parathyroid hormone-related protein (PTHrP) and parathyroid hormone 1 receptor (PTH1R), bone morphogenic protein 4 (BMP4), members of the insulin-like growth factor (IGF) family, and the homeodomain-containing transcription factors MSX1 and MSX2 [90]. These genes are expressed in the epithelial cells and/or mesenchyme [90, 91], and interactions between these two types of tissue are critical for mammary gland development [91].
The estrogen receptors are first present in the mesenchyme [91, 92], and only sparse ER staining in the mouse mammary epithelial cells has been detected during the fetal period [92]. These findings are consistent with the data from human studies showing “punctate” expression of ER in some, but not all fetal mammary glands [93, 94]. After birth, the expression of ER is clearly seen in the prepubertal mammary epithelium in humans and rodents, and is markedly higher than during fetal development [94]. Findings obtained in mice without functional ER-α (ERKO [95] or ENERKI [96]) show that estrogens are not needed for fetal mammary gland development but they are necessary for ductal and alveolar development from puberty onwards. This seems puzzling, since in utero or neonatal exposure to estrogens, including DES, alters fetal and neonatal mammary gland development in humans [97] and mice [98]. As discussed in more detail below, maternal hormonal exposures during pregnancy also lead to changes in postnatal mammary gland development among the offspring.
A likely explanation for these findings is that estrogen is not required for fetal mammary gland development but can modify the process. All the genes that are essential for fetal mammary gland development are either regulated or they interact with estrogens and ER: Wnt/β-catenin family [99], FGF [100, 101] and IGF [102] families, TBX3 [103], PTHrP and PTH1R [104–106], BMP4 [107], and MSX1/2 [101]. Expression of ER itself is affected by the fetal estrogenic environment. We have observed that ER protein levels are reduced in the mammary glands [108] and hypothalamus [109] of 2-month-old female offspring of dams fed a HF diet that elevates pregnancy E2 levels. Neonatal exposure to high, but not low or moderate doses of DES, down-regulates ER in the uterus and vagina [110]. A similar reduction in ER-α expression is seen in the offspring of E2 exposed dams, but only before the onset of puberty [111]. After puberty onset, this receptor is up-regulated in in utero E2 exposed rats [111]. Up-regulated ER-α expression also is seen in the postpubertal mammary glands of rats exposed to alcohol in utero; alcohol consumption increases E2 levels in pregnant dams and elevates mammary cancer risk in their offspring [112]. Mechanisms explaining persistent changes in ER expression in in utero estrogen exposed individuals are likely to be epigenetically induced (see below).
In summary, several signaling pathways in the fetal mammary mesenchyme and epithelium are required to ensure normal development. Although rudimentary mammary gland can form in the absence of estrogens and ER, this hormone and its receptors nevertheless are important regulators of the process. Mammary gland seems to develop differently in the presence of excess estrogens (such as an exposure to DES) than normally (see below), and these differences can be manifested at different times during postnatal life, particularly after the onset of puberty.
Changes in Postnatal Mammary Gland Morphology
In utero estrogenic exposures have many different effects on mammary gland development. Most frequently reported changes include dilated ducts and cyctic alveolar structures [113–115]. In addition, these exposures lead to an increase in the number of terminal end buds (TEBs) [116]. TEBs are located at the tips of growing epithelial ducts and consist of a mass of body cells and a top layer of cap cells. They lead the growth of the mammary epithelial tree, and give rise to malignant mammary tumors in carcinogen-treated animals [117]. Similar structures in the human breast, terminal ductal lobular units 1 (TDLU1), appear to be the sites of breast cancer initiation in most women [118]. The reason why tumors arise from TEB/TDLU1 is not entirely clear, but might be related to increased cell proliferation in this structure [119] that, in turn, is associated with increased levels of DNA adducts and reduced capacity to repair DNA damage [120]. Bifurcation of TEBs gives rise to ducts and alveolar buds, which further differentiate to lobules, and when TEBs reach the edges of the fat pad in adult mice and rats, they regress to terminal buds [117].
The number of TEBs in the mammary gland has been proposed to directly correlate with breast cancer susceptibility [116, 117]. Importantly, in utero estrogenic exposures that increase susceptibility to mammary tumorigenesis often lead to an increase in TEB numbers, either before the onset of puberty or at 50 days of age when the gland is most susceptible to malignant transformation [116]. Neonatal exposure to DES also increases the number of TEBs in the mammary gland [82, 121].
Changes in the Expression of Estrogen-Regulated Genes
Relatively little is known about long-term changes in the transcriptome of the mammary glands in animals exposed to estrogen in utero. We have found that maternal exposure to estrogenic compounds during pregnancy induces persistent changes in estrogen-regulated genes in the female offspring [111, 116, 122]. These genes include caveolin1, pAkt, BCL-2, EGFR and NFkB [111, 122]. Further, our on-going studies indicate that many polycomb target genes (PgTGs) are significantly down-regulated in the mammary glands of adult rats exposed to E2 in utero, consistent with increased methylation of these genes [17]. As these genes are all linked to breast cancer, they might be associated with the observed increase in mammary cancer risk among female offspring.
The effect of neonatal exposure to DES (1 µg/kg) on gene expression in the TEBs has been investigated by Umekita et al. [121] using a dose of DES known to increase the number of TEBs in the mammary gland [82]. The most significant change in gene expression seen in this study involved the NFkB signaling pathway, which is linked to breast cancer progression [123, 124] and anti-estrogen resistance [125].
Epigenetic Processes
A potential mechanism of developmental programming of cancer susceptibility by in utero estrogenic exposures may be related to alterations in the epigenome. Embryonic and primordial germ cells in the developing fetus undergo extensive epigenetic programming during fetal life, and the epigenome then interprets the information in the genetic code by means that do not involve a change in DNA sequence [126]. It has been reported by us [17] and others [127, 128] that in utero estrogenic exposures modify the epigenome by altering DNA methylation, enzymes which regulate histones, polycombs, and miRNA expression, and thus leave a permanent epigenetic mark on estrogen-sensitive cells.
Changes in DNA Methylation
Alterations in the methylation of CpG islands within the promoter region of genes are the most frequently studied epigenetic modifications in cancer. DNA methyltransferases (DNMTs) catalyze the methylation of genomic DNA by adding a methyl group (CH3) onto the 5-carbon of the cytosine ring within CpG dinucleotides. These enzymes include DNMT1 and DNMT3a and 3b [129, 130]. Studies in vitro show that overexpression of DNMT1 induces genomic hypermethylation and loss of imprinting [131], while RNAi-induced depletion of DNMT1 leads to demethylation of CpG islands in breast cancer cells [132]. In utero exposure to DES is reported to increase the expression of DNMT1 in the epididymis [128] and uterus [127]. In the uterus of DES exposed mice, DNMT1 was significantly reduced immediately after birth, but at puberty, DNMT1 levels were higher in the DES exposed mice than in the controls [127]. Our findings indicate that expression of DNMT1 is increased in the mammary glands of rats exposed to EE2 in utero when determined approximately three weeks after puberty onset [17]. Several other maternal exposures also modify the offspring’s epigenome, such as dietary intake of folic acid [133], genistein [134, 135] and Bisphenol A [136].
Maternal exposure to DES during pregnancy has been reported to alter methylation patterns of Hox genes [137, 138], c-fox [139], and Nsbp1 [134] in estrogen-regulated tissues in the offspring. We identified several genes which exhibited either increased or reduced DNA methylation in the mammary glands of offspring exposed to synthetic EE2 during pregnancy [17].
Histone Modifications
Histone modifications are complex, as they involve not just methylation but also acetylation and deacetylation and other changes. These modifications occur in the N-terminal tails of histones and affect the “openness” of the chromatin, which determines whether a gene is expressed or silenced (e.g., acetylation allows for transcription, while deacetylation represses transcription) [140, 141]. Histone H3 trimethylation at lysine K4, and acetylation of histones H3 and H4 are all associated with gene activation, whereas trimethylation of H3 at lysine K27 induces gene silencing [142, 143]. During development, those genes that induce differentiation of stem cells, including PcTGs, are repressed by the polycomb and H3K27me3 complex [144].
Methylation of H3K27 is catalyzed by the Polycomb Enhancer of Zeste-2 (EZH2), the catalytic subunit of Polycomb repressive complex PCR2. This complex also contains polycombs SUZ12 and EED, and it is important in the establishment and maintenance of silencing of PcGTs and tumor suppressor genes (TSGs) [145]. For example, PCR1 contains Bmi-1 and Ring1 with YY1 binding protein, and it recognizes chromatin marked with methylated H3K27, and consequently mediates transcriptional repression. As an increase in EZH2 expression in the mammary glands of mice exposed to DES in utero has been reported [146], this process seems to be influenced by maternal exposure to synthetic estrogens during pregnancy.
Together, the PCR1/PCR2/H3K27me3 complex recruits DNMTs, which then methylate PcGTs. PcGTs are defined as genes to which either EZH2, SUZ12, EED or H3K27me3 bind in human embryonic stem cells (hESC) [147] or embryonic fibroblasts [144]. Over 2,500 PcGTs have been identified [144, 148], many of which are known TSGs. For example, of the nine TSGs methylated in peripheral blood and Random Periareolar Fine-Needle Aspiration (RPFNA) in women at high risk for breast cancer (RARB, ER-α, INK4a/ARF, BRCA1, PRA, PRB, RASSF1A, HIN-1, and CRBP1) [149], four (44 %) are PcGTs (PcGTs are underlined). We have found that maternal exposure to the synthetic estrogen EE2 during pregnancy causes a permanent increase in the methylation of several PcTGs [17]. These genes control stem cell differentiation [150–153] and are known to be methylated in cancer cells [154–156].
Although most studies thus far have focused on investigating long-lasting changes in DNA methylation in estrogen’s target organs in in utero estrogen exposed animals, histone modifications also have been found. For example, maternal HF diet during pregnancy induces alterations in fetal hepatic H3 acetylation [157], and in postnatal hepatic H4 acetylation [158]. In the mammary gland, maternal HF intake during pregnancy is reported to reduce acetylation of histone H4 and increase recruitment of HDAC3 within p16 in the offspring, silencing the expression of this tumor suppressor [159]. There is some evidence that in utero exposure to DES alters histone methylation in the uterus [160]. Since DNA methylation and histone modification are closely associated and cross-regulated processes, it is possible that a change in either DNMT or HDAC activity is sufficient to alter the epigenome and cancer risk.
MicroRNAs (miRNAs)
miRNAs are short non-coding single stranded RNAs composed of approximately 21–22 nucleotides that regulate gene expression by sequence-specific basepairing with the 3’-untranslated region (3’UTR) of target mRNAs. miRNA binding induces the post-transcriptional repression of target genes [161], either by inducing inhibition of protein translation or mRNA degradation. miRNA target genes can be identified by searching for conserved sequences complementary to the seed region of miRNAs. It has been estimated that each miRNA binds to as many as 200 targets [162], and thus they regulate the expression of at least one third of human mRNAs [163], and likely more [164]. A single miRNA can target, and potentially silence, several hundred genes. Futher, any given gene can be targeted by several miRNAs [162].
Expression of many miRNAs is suppressed by estrogens [165, 166]. The mechanisms involved in this suppression are currently unknown but may include the inhibition of miRNA maturation from pri-miRNAs via Drosha and Dicer RNases [167–169]. In our on-going study [170], we noted that in utero exposure to EE2 lowers the expression of many of the same miRNAs in the adult mammary gland as have been reported to be down-regulated by E2 in MCF-7 human breast cancer cells [165]. Since miRNAs can be silenced by methylation [171–173], the increase in DNA methylation by in utero estrogenic exposures might explain these findings. Further, miRNAs also can suppress polycombs [174, 175] and polycombs in turn can regulate miRNAs [176], suggesting that some histone modifications interact with miRNAs. Therefore, the increase in mammary cancer risk caused by maternal exposure to excess estrogens may also involve persistent changes in the expression of miRNAs, especially those that target oncogenes.
Epigenetics and Mammary Gland Morphology
Changes in the mammary gland morphology in in utero exposed animals may be driven by changes in the epigenome [177]. For example, mammary stem cell differentiation and lineage determination is regulated epigenetically [178–181] Further, similar to DNA methylation, miRNAs are key regulators of normal development [182, 183], including the development of the mammary gland [178, 184, 185]. Therefore, epigenetic modifications induced by an exposure to estrogenic compounds in utero might have caused persistent alterations in the mammary gland morphology.
Maternal Exposure to Excess Estrogens During Pregnancy Induces a Transgenerational Increase in Mammary Cancer Risk
If epigenetic changes occur in germ cells after fetal estrogen exposure, they may be inherited across multiple generations. To study this possibility we fed pregnant rat dams diets containing synthetic estradiol EE2 or a HF diet that elevates pregnancy estradiol levels [15, 16]. Maternal exposure to a HF diet during pregnancy increased mammary cancer risk in daughters and granddaughters, whilst maternal exposure to EE2 increased mammary cancer risk in daughters, granddaughters and great granddaughters [17]. In addition, the increase in risk was associated with a higher number of TEBs in the mammary glands of all three generations.
The increase in mammary cancer risk in the F1-F3 generations of female offspring of dams exposed to EE2 during pregnancy was associated with an increased expression of DNMTs (in the F1: Dnmt1, and in the F2-F3 generation offspring: Dnmt1, Dnmt3a and Dnmt3b) in the offspring’s mammary glands [17]. In addition, methylation patterns were altered in the same 375 gene promoter regions in the mammary glands of daughters, granddaughters and great granddaughters. Methylated genes included PgTGs. This epigenetic signature is likely induced and maintained by the high levels of DNMTs, but it remains to be seen whether the altered DNA methylation explains the multigenerational increase in mammary cancer risk observed in in utero estrogen exposed offspring.
In summary, a high maternal estrogenic environment during pregnancy, especially the increase induced by synthetic estrogens such as DES, increase later breast cancer risk in the exposed daughters. While the precise pathways mediating this increase in breast cancer risk have not been conclusively established, the key change likely involves epigenetic modifications in genes which regulate (i) proliferation and differentiation of progenitor cells, (ii) apoptosis and autophagy, (iii) DNA repair, (iv) metabolism, and (v) immune functions [111, 116, 122, 186]. Changes in any of these five functions may increase susceptibility of the mammary gland to malignant transformation upon exposure to a breast cancer initiating factor, such as carcinogens or radiation.
Estrogens During Pregnancy and Mother’s Breast Cancer Risk
There is both direct and indirect evidence that an elevated estrogenic environment during pregnancy increases a mother’s risk of developing breast cancer. Direct evidence comes from observations that women who exhibited the highest estrogen levels, either in the first [187] or third trimesters of pregnancy [188], subsequently had elevated breast cancer risk. In the case of high early pregnancy E2 levels, an increase in breast cancer risk was seen only before age 40 [187]. Mothers who used the synthetic estrogen DES during pregnancy also are at increased breast cancer risk [72]. Indirect evidence comes from studies showing that breast cancer risk is elevated in women who suffered from severe pregnancy-related nausea [189] or gave birth to heavy newborns [190]. These women exhibit elevated pregnancy estrogen levels [191, 192].
Pregnancy permanently alters gene expression in the mammary gland, both in animal models [59, 60] and humans [193], and these are thought to explain the protective effects of an early first pregnancy. These changes include suppression of several growth factors or their key down-stream targets, such as IGF-1, growth hormone receptor and amphiregulin, and suppression of genes which induce an epithelial-mesenchymal transition, including fibronectin 1, lumican, and collagens Col5a2 and Col1a1. Importantly, the changes in the transcriptome do not reflect distinct morphological changes that take place in the mammary gland during pregnancy. Breasts of nulliparous women contain mainly TDLU1 and 2, which during pregnancy mature to TDLU3 and 4 [57]. The differentiated TDLU3 and 4 lobules persist until menopause, but are then converted back to TDLU1/2, making the breast tissue of postmenopausal parous and nulliparous women morphologically indistinguishable from each other [57, 194].
Using a preclinical rodent model we have investigated whether an exposure to E2 during pregnancy alters gene signaling in the dam’s mammary gland [195]. The analysis of gene expression patterns by microarrays and verification by PCR indicate that pregnancy E2 exposure reverses the reported protective changes in several genes induced by pregnancy, which have been observed in both rodent models and humans, including transforming growth factor β3 (TGFβ3). Most of the genes that were down-regulated in the mammary glands of rats treated with E2 during pregnancy are those that are markers of differentiation (casein alpha s1, ceruloplasmin, lactalbumin) or immune functions (lipocalin 2, lipopolysaccharide binding protein), while up-regulated genes were associated with increased angiogenesis (VegfA), growth (growth hormone receptor, pleitrophin), or epithelial to mesenchymal transition (collagen type 1 alpha 1). Since cell proliferation also remained elevated in the mammary gland of parous rats exposed to E2 during pregnancy, it is possible that excess estrogens during pregnancy prevent differentiation of mammary stem and progenitor cells, an explanation that was proposed to explain the protective effects of pregnancy against breast cancer [58, 196, 197].
Estrogen Use in Adult Life
There are two main categories of estrogenic compounds that are used by adult women: (a) oral contraceptives (OC), which are used during the reproductive years, and (b) HTs, which are used during and after menopause.
Premenopausal Oral Contraceptive Use
Hormonal contraceptives came to the market in the 1960’s, and since then three generations of oral contraceptives have been available. The first generation OCs contained more than 35 µg of estrogens and more than 2.5 mg of progestin. The second generation contained less of both, and the progestin source was norethindrone or levonorgestel. In the third generation OCs, progestin is either norgestimate or desogestrel. Further, the latest generation of OC drugs contain approximately five times less estrogen and four time less progestin than the first pills.
OCs work primarily by preventing ovulation, as a consequence of inhibiting pituitary gonadotropin secretion [198]. In addition to OC, other routes of administration are currently available, including transdermal and transvaginal routes, allowing for the use of lower doses of estrogen and progesterone than in OC. Contraceptives also are available via intramuscular injection or through an intrauterine device using progesterone only [199]. Consistent with their ability to inhibit ovulation, contraceptives reduce circulating levels of E2 and progesterone [200], and depending on the level of these hormones in the contraceptives, the overall estrogen exposure is the same or lower in women using contraceptives as it is in those who do not use them.
Effects on Breast Cancer Risk
A number of older epidemiological studies reported that current or recent OC use slightly increased the risk of developing breast cancer, compared with women who had never used OC. A meta-analysis of 54 studies, published over 15 years ago, found an increased risk (relative risk [RR], 1.24, 95 % CI, 1.15–1.33) of breast cancer in women using OCs [201]. The risk was highest for women who had started using OC as teenagers [201]. However, the risk returned to normal 10 years after a woman stopped using OC [201, 202]. Results from another meta-analyses [202], and a study involving slightly over 100,000 women [203], reported an increased risk for breast cancer among current/recent users of OCs versus woman who had never used hormonal contraception. Results were similar for combined and progestin-only OCs [203]. In the Nurses’ Health Study [204], lifetime OC use and breast cancer incidence were assessed among 116,608 young women (age<42 years). Past use of any oral contraceptive was not linked to breast cancer risk, while current use was (multivariate RR, 1.33; 95 % CI, 1.03–1.73). This increase may be explained almost solely with current use of triphasic preparations with levonorgestrel as the progestin (RR, 3.05, 95 % CI, 2.00–4.66). Interestingly, in women diagnosed with breast cancer who had stopped using OC over 10 years the cancer was less likely to be advanced than in women who had never used OC [201, 202], implying a persistent protective influence on breast biology.
The most recent meta-analysis, containing data published between 1996 and 2011, and obtained from women who likely had taken 3rd generation OC, found no indication of an increase in premenopausal breast cancer risk in OC users [205]. This analysis included 12 studies that compared whether women had ever used vs. never used OC. Eight of these studies contained information on past use. Data obtained in a recent multicenter, population-based, case–control investigation confirm the results obtained over the past few years. In a multivariable analysis containing 10 different contraceptive formulations (n=50 women per formulation), breast cancer risk was not affected by any of the OC formulations [206].
In conclusion, several studies over a number of years investigating whether OC use increases breast cancer risk suggested that current, but not past use may slightly increase risk. However, the most recent studies provide no evidence that current use of any of the contraceptives available, increase breast cancer risk. This is likely due to the fact that OCs used by women today are less estrogenic and contain less progestin than those available decades ago.
Postmenopausal Hormone Therapy
Menopausal HT with estrogen alone or estrogen combined with progesterone has been used for many years to help relieve symptoms of menopause and prevent osteoporosis [207, 208]. The estrogens in HT originate from a variety of animal (conjugated equine estrogens) and plant (bioidentical HT) sources, or are synthetic estrogens (ethinyl estradiol). The conjugated equine estrogens are derived from the urine of pregnant mares, The chemical structure of all these hormones is similar to natural hormones produced by women’s bodies.
Initially, findings obtained in observational studies were interpreted to indicate that HT might have other health benefits, such as protection from cardiovascular disease (Nurses’ Health Study 1981; Barrett-Connor et al. 1998; PEPI trial 1995) [208–210]. However, the WHI study, which was the first large, randomized, placebo-controlled clinical trial of postmenopausal hormone treatment, provided surprising results. These results dramatically challenged the previous view, and raised questions about the short-term risks and long-term benefits of HT [211]. The HERS study (Heart and Estrogen/progestin Replacement Study), which is a randomized, double-blind, placebo-controlled trial of combination HT for secondary prevention of coronary heart disease in postmenopausal women, also demonstrated that there was no benefit of initiation of HT in women with established coronary heart disease [212].
Combination HT
The WHI trial, launched by the NIH in 1991, included a postmenopausal Hormone Therapy trial with two separate treatment arms: the estrogen-plus-progestin arm (conjugated equine estrogens, CEE, 0.625 mg daily and medroxyprogesterone acetate, MPA, 2.5 mg daily) involving women with a uterus, and the estrogen-alone arm involving women who had undergone hysterectomy [6, 211, 213]. Progestin was given to prevent estrogen’s stimulatory effect on the endometrium. A total of 16,608 women, aged 50 through 79 years, participated in the study. In both study arms, women were randomly assigned to receive either the hormone medication or placebo. Primary end points of the study were invasive breast cancer and coronary heart disease. The unexpected results indicated more harm than benefits and caused the trial to be terminated 3 years early in 2002 after 5.6 years of follow-up. Analysis of the WHI data for the first 3 years of HT intervention indicated a greater risk of fatal and non-fatal malignancies, the global risk index being 12 % higher in women on CEE plus MPA compared with placebo [211]. No increase in cardiovascular diseases was observed 3 years after the termination of the intervention [211].
In the Nurses’ Health Study, which is observational and thus did not include any trial or intervention, no increase in coronary heart disease risk was seen, but breast cancer risk was increased with HT use [214]. The discrepancy in cardiovascular disease risk between these two studies might be largely explained by differences in the distribution of time between the onset of menopause and the start of HT [214]. This is supported by findings obtained in the follow-up of the WHI trial [50], performed 10 years after the termination of the study. A detailed re-analysis of the data indicated that most of the adverse effects (increased risk of breast cancer, heart disease and stroke) that led to the early termination of the study, occurred in women who had started HT an average of 12 years past menopause and were in their 60s and 70s. In contrast, among women aged 50 to 59, those on HT had less heart disease and were less likely to die for any reason than women taking the placebo. Regardless of age when HT was initiated, an increase in breast cancer risk from combined HT was seen as soon as 2 years after the start of use [50, 211].
The most recent large scale intervention study concerning the potential benefits/risks of HT in postmenopausal women [41] followed almost 13,000 women between the ages of 50 and 79 (average age 64 at the start of the study) for 11 years. The results indicate that women on combination HT (CEE plus MPA, which was used by 95 % of U.S. women at the time the intervention study began) had a greater risk of more aggressive cancers and death compared to women not taking HT. Thus, estrogen plus progestin could have direct effects on the biology of the cancer, making them grow more aggressively. This conclusion is in contrast to earlier studies which suggested that HT increased the risk of less malignant breast tumors [215, 216].
Estrogen-Alone HT
This treatment has been an option only for women who have had a hysterectomy, as progesterone protects against estrogen’s stimulatory effect on the endometrium. Some studies have suggested that estrogen actually lowers the risk of breast cancer. Although the NIH discontinued the estrogen-alone arm of the WHI study due to an increased risk of stroke and no reduction in risk of coronary heart disease in these women [217], the initial data found that treatment with CEE alone for 7.1 years yielded a 20 % lower risk of invasive breast cancer compared with those in the placebo group [6]. However, due to the presence of abnormalities in the previous mammograms, treatment with CEE increased the frequency of mammography screening. When used long term (for more than 10 years), estrogen alone has been found to increase the risk of ovarian cancer in some studies [213].
As a result of the findings regarding HT, the use of combination HT by menopausal and postmenopausal women dropped by almost 70 % from 2002 to 2003 [218]. Consequently, between 2001 and 2004, the overall incidence of postmenopausal breast cancer went down by 8.6 % [219]. The rates of estrogen receptor-positive breast cancer dropped by 14.7 % in women between the ages of 50 and 69, which further suggests that stopping HT played a role in the decline.
Based upon these findings, the current consensus is that combination HTs increase breast cancer risk, but OCs do not. The biological basis for these opposing findings remains to be discovered, but may be related to the presence versus absence of ovarian estrogens in pre- and postmenopausal women, respectively.
HT and Mammographic Density
Mammographic density drops at menopause, and this improves the ability of imaging techniques to detect abnormalities in the mammary epithelium. Combined estrogen plus progestin therapy adversely affects breast cancer detection by mammograms and breast biopsies due to maintaining higher mammographic density (cumulative frequency of mammograms with abnormalities in HT vs placebo: 35.0 % vs 23.0 %; P<0.001; cumulative breast biopsy frequency: 10.0 % vs 6.1 %; P<001) [220]. Even 12 months after discontinuation of combined HT, the adverse effect of breast density on mammographic detection was still seen (P <0.001) [220].
Separate studies by the Breast Cancer Surveillance Consortium [41] and Gierich et al. [221] (the Breast Imaging Reporting and Data System, BIRADS) using data from seven registries, representing more than 580,000 women with normal BMI and nearly 1,350,000 screening mammograms, focused on women with dense breast tissue as determined by radiologists’ mammogram scores. The results confirmed several previous studies which show that women with dense breasts are at an increased risk of developing breast cancer compared with women whose breasts are of average density, the association being strongest for premenopausal women. Combination HT increased the risk of postmenopausal breast cancer by up to twofold [41]. For postmenopausal women who used HT and had low or average breast density, their 5-year risk ranged from 0.3 to 2.5 %, whereas it ranged from 1.1 to 4.4 % for women with dense or very-dense mammographic density [41]. The risk was slightly higher for those who used combination HT versus estrogen alone. It is not known whether HT slows the natural atrophy of the mammary gland after menopause, or whether it stimulates the growth of existing premalignant or malignant cells, or both.
The conclusions of the WHI post-trial analysis and newer studies have led to current guidelines suggesting that HT should only be used for the treatment of severe hot flashes and for no longer than 5 years in women who have recently entered menopause. Estrogen plus progestin and estrogen alone decreased the risk for fractures, but increased the risk for stroke, thromboembolic events, gallbladder disease, and urinary incontinence [51]. Estrogen plus progestin increased the risk for breast cancer and dementia, whereas estrogen alone decreased the risk of breast cancer [41, 51, 222].
Conclusion
Women use estrogens for many purposes. During pregnancy, synthetic estrogen DES was used to prevent miscarriage and promote healthy pregnancy, although it turned out to cause the opposite. During the reproductive years when a woman’s own estrogen levels are high, women use synthetic estrogens as contraceptives. Since estrogens play an important role in normal physiological functions in women, some menopausal and postmenopausal women use estrogen supplementation to regain the benefits of natural estrogens.
The effects of estrogens on breast cancer risk differ depending upon when during a woman’s life time they are used. Maternal exposure to DES during pregnancy increases breast cancer risk in mothers and their daughters. The adverse effects of synthetic estrogen exposure during pregnancy may not be limited to mothers and their daughters. Our preclinical study in rodents showed that maternal exposure to EE2 increases breast cancer risk in daughters, granddaughters, and great granddaughters. The first generation of OCs increased breast cancer risk at the time women were taking them, but the increase in risk was not permanent. The current, third generation contraceptives do not increase breast cancer risk. Menopausal and postmenopausal HT, if it contains both estrogens and progestin, increases a woman’s breast cancer risk, and recent data suggest that tumors developing during therapy are more aggressive than those in women not using HT. Estrogen-only HT does not increase breast cancer risk, and might even reduce it. However, due to other adverse effects of estrogen-only HT, it is not recommended beyond using it to control the most severe menopausal symptoms.
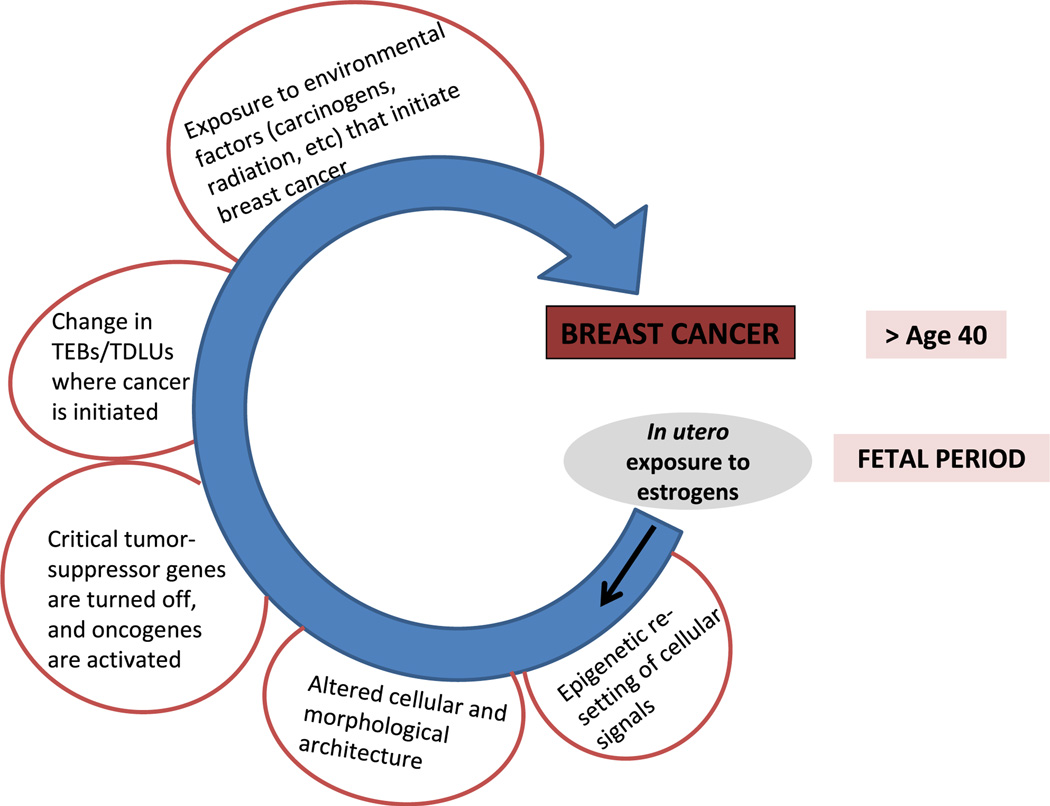
We are beginning to understand how the increase in breast cancer risk following in utero exposures to synthetic estrogens occurs. It most likely involves long-term epigenetic changes in genes that are important in determining the risk for breast cancer development, such as tumor suppressor genes, PcTGs and oncogenes. The proposed sequence of events from the fetal estrogen exposure to an increase in breast cancer risk is illustrated in Fig. 3. Briefly, an exposure to synthetic estrogens during the fetal period induces modifications in the epigenetic reprogramming of the genome, leading to changes in mammary gland morphology, and gene and protein expression. Some of these changes are transient, such as an increase in the number of TEBs in rodents, and some persist, such as an altered gene and protein expression involving tumor suppressor genes and oncogenes. Together, epigenetically induced modifications in the mammary gland morphology and gene expression increase the likelihood that environmental carcinogens and radiation induce malignant transformation, and evetually breast cancer. The next challenge is to determine whether the increase in risk can be reversed by reversing epigenetic changes that occur as a consequence of early life exposure to synthetic estrogens.
Fig. 3.
Proposed sequence of events from maternal exposure to estrogens during pregnancy to an increase in daughter’s breast cancer risk
Acknowledgments
Funding support This study was supported by the National Cancer Institute (R01 CA164384-01A1, U54 CA100970, U54CA149147, and P30 CA051668)
Abbreviations
- BIRADS
breast imaging reporting and data system
- BMP4
bone morphogenic protein 4
- CCA
clear cell adenocarcinoma
- CEE
conjugated equine estrogens
- DES
diethylstilbestrol
- DMBA
9,12-dimethylbenz[a]anthracene
- DNMT
DNA methyltransferase
- E2
17-β estradiol
- EE2
ethinyl estradiol
- EPIC
european prospective investigation into cancer and nutrition
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- EZH2
enhancer of zeste-2
- FGF
fibroblast growth factors
- HF
high fat
- HDAC
histone deacetylase
- HT
hormone therapy
- hESC
human embryonic stem cells
- IGF
insulin-like growth factor
- MNU
methylnitrosourea
- NSABP
national surgical adjuvant breast and bowel project
- MPA
medroxyprogesterone acetate
- miRNA
microRNAs
- OC
oral contraceptives
- PTHrP
parathyroid hormone-related protein
- PTH1R
parathyroid hormone 1 receptor
- PcTG
polycomb target genes
- P
progesterone
- RPFNA
random periareolar fine-needle aspiration
- STAR
study of tamoxifen and raloxifene
- TAM
tamoxifen
- TDLU
terminal ductal lobular unit
- TEB
terminal end buds
- TSG
tumor suppressor gene
- WHI
women’s health initiative
Footnotes
Disclosure L. Hilakivi-Clarke has served as an expert witness in a case concerning breast cancer risk in daughters of DES-exposed mothers on behalf of the plaintiffs
Contributor Information
Leena Hilakivi-Clarke, Email: clarkel@georgetown.edu, Department of Oncology, Georgetown University, Washington, DC, USA; Georgetown University Medical Center, Research Building, Room E407, 3970 Reservoir Road, NW, Washington, DC 20057, USA.
Sonia de Assis, Department of Oncology, Georgetown University, Washington, DC, USA.
Anni Warri, Department of Oncology, Georgetown University, Washington, DC, USA; Institute of Biomedicine, University of Turku Medical Faculty, Turku, Finland.
References
- 1.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;86:1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovejoy JC. The menopause and obesity. Prim Care. 2003;30:317–325. doi: 10.1016/s0095-4543(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 4.Davis SR, Castelo-Branco C, Chedraui P, et al. Understanding weight gain at menopause. Climacteric. 2012;15:419–429. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s health initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Samaras K, Hayward CS, Sullivan D, Kelly RP, Campbell LV. Effects of postmenopausal hormone replacement therapy on central abdominal fat, glycemic control, lipid metabolism, and vascular factors in type 2 diabetes: a prospective study. Diabetes Care. 1999;22:1401–1407. doi: 10.2337/diacare.22.9.1401. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Breast cancer: facts and figures 2011–2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 9.Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 10.Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther. 2009;9:51–60. doi: 10.1586/14737140.9.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sestak I, Cuzick J. Preventive Therapy for Breast Cancer. Curr Oncol Rep. 2012;14:568–573. doi: 10.1007/s11912-012-0273-5. [DOI] [PubMed] [Google Scholar]
- 12.Guzman RC, Yang J, Rajkumar L, Thordarson G, Chen X, Nandi S. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci U S A. 2011;108:18879–18886. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke R, Dickson RB, Lippman ME. aspects of breast cancer Growth factors, drugs and stromal interactions. Crit Rev Oncol Hematol. 1992;12:1–23. doi: 10.1016/1040-8428(92)90062-u. [DOI] [PubMed] [Google Scholar]
- 15.Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Clarke R, Lippman M. Breast cancer risk in rats fed a diet high in n-6 polyunsaturated fatty acids during pregnancy. J Natl Cancer Inst. 1996;88:1821–1827. doi: 10.1093/jnci/88.24.1821. [DOI] [PubMed] [Google Scholar]
- 16.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman ME. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci USA. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Assis S, Warri A, Cruz MI, et al. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. doi: 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiological Reviews. 1993;15:48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 19.Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res (Phila) 2012;4:583–592. doi: 10.1158/1940-6207.CAPR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritte R, Lukanova A, Tjonneland A, et al. Height, age at menarche and risk of hormone receptor positive and negative breast cancer: A cohort study. Int J Cancer. 2012 doi: 10.1002/ijc.27913. [DOI] [PubMed] [Google Scholar]
- 22.The Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson H, Landin-Olsson M, Gullberg B. Retrospective assessment of menstrual cycle length in patients with breast cancer, in patients with benign breast disease, and in women without breast disease. J Natl Cancer Inst. 1983;70:17–20. [PubMed] [Google Scholar]
- 24.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 25.Whelan EA, Sandler DP, Root JL, Smith KR, Weinberg CR. Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol. 1994;140:1081–1090. doi: 10.1093/oxfordjournals.aje.a117208. [DOI] [PubMed] [Google Scholar]
- 26.Terry KL, Willett WC, Rich-Edwards JW, Hunter DJ, Michels KB. Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1509–1513. doi: 10.1158/1055-9965.EPI-05-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler LM, Potischman NA, Newman B, et al. Menstrual risk factors and early-onset breast cancer. Cancer Causes Control. 2000;11:451–458. doi: 10.1023/a:1008956524669. [DOI] [PubMed] [Google Scholar]
- 28.Orgeas CC, Hall P, Rosenberg LU, Czene K. The influence of menstrual risk factors on tumor characteristics and survival in postmenopausal breast cancer. Breast Cancer Res. 2008;10:R107. doi: 10.1186/bcr2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 30.Diorio C, Lemieux J, Provencher L, Hogue JC, Vachon E. Aromatase inhibitors in obese breast cancer patients are not associated with increased plasma Estradiol levels. Breast Cancer Res Treat. 2012;136:573–579. doi: 10.1007/s10549-012-2278-z. [DOI] [PubMed] [Google Scholar]
- 31.Dorgan JF, Reichman ME, Judd JT, et al. The relation of body size to plasma levels of estrogens and androgens in premenopausal women (Maryland, United States) Cancer Causes Control. 1995;6:3–8. doi: 10.1007/BF00051674. [DOI] [PubMed] [Google Scholar]
- 32.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 33.Lahmann PH, Lissner L, Gullberg B, Olsson H, Berglund G. A prospective study of adiposity and postmenopausal breast cancer risk: the Malmo diet and cancer study. Int J Cancer. 2003;103:246–252. doi: 10.1002/ijc.10799. [DOI] [PubMed] [Google Scholar]
- 34.Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2004;111:762–771. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 35.Li CI, Malone KE, Daling JR. Interactions between body mass index and hormone therapy and postmenopausal breast cancer risk (United States) Cancer Causes Control. 2006;17:695–703. doi: 10.1007/s10552-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 36.Modugno F, Kip KE, Cochrane B, et al. Obesity, hormone therapy, estrogen metabolism and risk of postmenopausal breast cancer. Int J Cancer. 2006;118:1292–1301. doi: 10.1002/ijc.21487. [DOI] [PubMed] [Google Scholar]
- 37.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med. 1995;123:673–675. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 39.Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. doi: 10.1007/s11912-001-0083-7. [DOI] [PubMed] [Google Scholar]
- 40.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13:223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830–3837. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker S, Kaaks R. Exogenous and endogenous hormones, mammographic density and breast cancer risk: can mammographic density be considered an intermediate marker of risk? Recent Results Cancer Res. 2009;181:135–157. doi: 10.1007/978-3-540-69297-3_14. [DOI] [PubMed] [Google Scholar]
- 43.Martin LJ, Minkin S, Boyd NF. Hormone therapy, mammographic density, and breast cancer risk. Maturitas. 2009;64:20–26. doi: 10.1016/j.maturitas.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 45.Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2×2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749–3756. doi: 10.1200/JCO.2008.19.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearman L, Kagan R, Arsenault J, Muram D. The effects of raloxifene on mammographic breast density: a review of clinical trials. Menopause. 2010;17:654–659. doi: 10.1097/gme.0b013e3181c29e56. [DOI] [PubMed] [Google Scholar]
- 47.Huggins C, Moon RC, Morii S. Extinction of experimental mammary cancer.I Estradiol-17beta and progesterone. Proc Natl Acad Sci USA. 1962;48:379–386. doi: 10.1073/pnas.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabanes A, Wang M, Olivo S, et al. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–748. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 49.Grubbs CJ, Peckham JC, McDonough KD. Effect of ovarian hormones on the induction of l-methyl-l-nitrosurea-induced mammary cancer. Carcinogenesis. 1983;4:495–497. doi: 10.1093/carcin/4.4.495. [DOI] [PubMed] [Google Scholar]
- 50.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive services task force recommendations. Ann Intern Med. 2012;157:104–113. doi: 10.7326/0003-4819-157-2-201207170-00466. [DOI] [PubMed] [Google Scholar]
- 52.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 53.Grubbs CJ, Farneli DR, Hill DL, McDonough KC. Chemoprevention of n-nitro-n-methylurea-induced mammary cancers by pretreatment with 17beta-estradiol and progesterone. J Natl Cancer Inst. 1985;74:927–931. [PubMed] [Google Scholar]
- 54.Sivaraman L, Stephens LC, Markaverich BM, et al. Hormone-induced refractoriness to mammary carcinogenesis in wistar-furth rats. Carcinogenesis. 1998;19:1573–1581. doi: 10.1093/carcin/19.9.1573. [DOI] [PubMed] [Google Scholar]
- 55.Rajkumar L, Guzman RC, Yang J, Thordarson G, Talamantes F, Nandi S. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc Natl Acad Sci U S A. 2001;98:11755–11759. doi: 10.1073/pnas.201393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14:907–933. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 57.Russo IH, Russo J. Pregnancy-induced changes in breast cancer risk. J Mammary Gland Biol Neoplasia. 2011;16:221–233. doi: 10.1007/s10911-011-9228-y. [DOI] [PubMed] [Google Scholar]
- 58.Siwko SK, Dong J, Lewis MT, Liu H, Hilsenbeck SG, Li Y. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells-implications for pregnancy-induced protection against breast cancer. Stem Cells. 2008;26:3205–3209. doi: 10.1634/stemcells.2008-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D'Cruz CM, Moody SE, Master SR, et al. Persistent parity-induced changes in growth factors, TGF-beta3, and differentiation in the rodent mammary gland. Mol Endocrinol. 2002;16:2034–2051. doi: 10.1210/me.2002-0073. [DOI] [PubMed] [Google Scholar]
- 60.Blakely CM, Stoddard AJ, Belka GK, et al. Hormone-induced protection against mammary tumorigenesis is conserved in multiple rat strains and identifies a core gene expression signature induced by pregnancy. Cancer Res. 2006;66:6421–6431. doi: 10.1158/0008-5472.CAN-05-4235. [DOI] [PubMed] [Google Scholar]
- 61.Belitskaya-Levy I, Zeleniuch-Jacquotte A, Russo J, et al. Characterization of a genomic signature of pregnancy identified in the breast. Cancer Prev Res (Phila) 2011;4:1457–1464. doi: 10.1158/1940-6207.CAPR-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twombly R. Estrogen’s dual nature? studies highlight effects on breast cancer. J Natl Cancer Inst. 2011;103:920–921. doi: 10.1093/jnci/djr233. [DOI] [PubMed] [Google Scholar]
- 63.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 64.Mahtani RL, Stein A, Vogel CL. High-dose estrogen as salvage hormonal therapy for highly refractory metastatic breast cancer: a retrospective chart review. Clin Ther. 2009;31(Pt 2):2371–2378. doi: 10.1016/j.clinthera.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dieckmann WJ, Davis ME, Rynkiewitz LM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol. 1953;66:1062–1081. doi: 10.1016/s0002-9378(16)38617-3. [DOI] [PubMed] [Google Scholar]
- 67.Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 68.Physicians desk reference to pharmaceutical specialties and biologicals. 15th Edition. 1961. p. 625. [Google Scholar]
- 69.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 70.Palmer JR, Wise LA, Hatch EE, et al. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1509–1514. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- 71.Troisi R, Hatch EE, Titus-Ernstoff L, et al. Cancer risk in women prenatally exposed to diethylstilbestrol. Int J Cancer. 2007;121:356–360. doi: 10.1002/ijc.22631. [DOI] [PubMed] [Google Scholar]
- 72.Colton T, Greenberg R, Noller K, et al. Breast cancer in mothers prescribed diethylstilbestrol in pregnancy. JAMA. 1993;269:2096–2100. [PubMed] [Google Scholar]
- 73.Hatch EE, Palmer JR, Titus-Ernstoff L, et al. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA. 1998;280:630–634. doi: 10.1001/jama.280.7.630. [DOI] [PubMed] [Google Scholar]
- 74.Palmer JR, Hatch EE, Rosenberg CL, et al. Risk of breast cancer in women exposed to diethylstilbestrol in utero: preliminary results (United States) Cancer Causes Control. 2002;13:753–758. doi: 10.1023/a:1020254711222. [DOI] [PubMed] [Google Scholar]
- 75.Verloop J, van Leeuwen FE, Helmerhorst TJ, van Boven HH, Rookus MA. Cancer risk in DES daughters. Cancer Causes Control. 2010;21:999–1007. doi: 10.1007/s10552-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boylan ES, Calhoon RE. Mammary tumorigenesis in the rat following prenatal exposure to diethylstilbestrol and postnatal treatment with 7,12-dimethylbenz[a]anthracene. J Toxicol Environ Health. 1979;5:1059–1071. doi: 10.1080/15287397909529814. [DOI] [PubMed] [Google Scholar]
- 77.Boylan ES, Calhoon RE. Prenatal exposure to diethylstilbestrol: ovarian-independent growth of mammary tumors induced by 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1981;66:649–652. [PubMed] [Google Scholar]
- 78.Boylan ES, Calhoon RE. Transplacental action of diethylstilbestrol on mammary carcinogenesis in female rats given one or two doses of 7,12-dimethylbenz(a)anthracene. Cancer Res. 1983;43:4879–4884. [PubMed] [Google Scholar]
- 79.Rothschild TC, Boylan ES, Calhoon RE, Vonderhaar BK. Transplacental effects of diethylstilbestrol on mammary development and tumorigenesis in female ACI rats. Cancer Res. 1987;47:4508–4516. [PubMed] [Google Scholar]
- 80.Vassilacopoulou D, Boylan ES. Mammary gland morphology and responsiveness to regulatory molecules following prenatal exposure to diethylstilbestrol. Teratog Carcinog Mutagen. 1993;13:59–74. doi: 10.1002/tcm.1770130203. [DOI] [PubMed] [Google Scholar]
- 81.Kawaguchi H, Miyoshi N, Miyamoto Y, et al. Effects of exposure period and dose of diethylstilbestrol on pregnancy in rats. J Vet Med Sci. 2009;71:1309–1315. doi: 10.1292/jvms.001309. [DOI] [PubMed] [Google Scholar]
- 82.Ninomiya K, Kawaguchi H, Souda M, et al. Effects of neonatally administered diethylstilbestrol on induction of mammary carcinomas induced by 7, 12-dimethylbenz(a)anthracene in female rats. Toxicol Pathol. 2007;35:813–818. doi: 10.1080/01926230701584205. [DOI] [PubMed] [Google Scholar]
- 83.Stark AH, Kossoy G, Zusman I, Yarden G, Madar Z. Olive oil consumption during pregnancy and lactation in rats influences mammary cancer development in female offspring. Nutr Cancer. 2003;46:59–65. doi: 10.1207/S15327914NC4601_08. [DOI] [PubMed] [Google Scholar]
- 84.Walker BE. Tumors in female offspring of control and diethylstilbestrol-exposed mice fed high-fat diets. J Nat Cancer Inst. 1990;82:50–54. doi: 10.1093/jnci/82.1.50. [DOI] [PubMed] [Google Scholar]
- 85.Luijten M, Thomsen AR, van den Berg JA, et al. Effects of soy-derived isoflavones and a high-fat diet on spontaneous mammary tumor development in Tg.NK (MMTV/c-neu) mice. Nutr Cancer. 2004;50:46–54. doi: 10.1207/s15327914nc5001_7. [DOI] [PubMed] [Google Scholar]
- 86.Raun AP, Preston RL. History of diethystilbestrol use in cattle. American Society of Animal Science. 2001:1–7. [Google Scholar]
- 87.Preston RL, Byers F, Stevens KR. Estrogenic activity and growth stimulation in steers fed varying protein levels. J Anim Sci. 1978;46:541–546. doi: 10.2527/jas1978.462541x. [DOI] [PubMed] [Google Scholar]
- 88.Sakakura T. Mammary embryogenesis. In: Neville MC, Daniel CW, editors. Mammary gland: development, regulation, and function. New York: Plenum Press; 1987. p. 37. [Google Scholar]
- 89.Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4:9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- 90.Cowin P, Wysolmerski J. Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb Perspect Biol. 2010;2:a003251. doi: 10.1101/cshperspect.a003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hens JR, Wysolmerski JJ. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005;7:220–224. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-a alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keeling JW, Ozer E, King G, Walker F. Oestrogen receptor alpha in female fetal, infant, and child mammary tissue. J Pathol. 2000;191:449–451. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH661>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 94.Naccarato AG, Viacava P, Vignati S, et al. Bio-morphological events in the development of the human female mammary gland from fetal age to puberty. Virchows Arch. 2000;436:431–438. doi: 10.1007/s004280050470. [DOI] [PubMed] [Google Scholar]
- 95.Bocchinfuso WP, Lindzey JK, Hewitt SC, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- 96.Sinkevicius KW, Burdette JE, Woloszyn K, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vrettos AS, Fotiou S, Papaharalampus N. Development of the breasts of the fetus Effects of the administration of hormonal preparations during pregnancy. J Gynecol Obstet Biol Reprod (Paris) 1976;5:561–566. [PubMed] [Google Scholar]
- 98.Tomooka Y, Bern HA. Growth of mouse mammary glands after neonatal sex hormone treatment. J Natl Cancer Inst. 1982;69:1347–1352. [PubMed] [Google Scholar]
- 99.Varea O, Garrido JJ, Dopazo A, Mendez P, Garcia-Segura LM, Wandosell F. Estradiol activates beta-catenin dependent transcription in neurons. PLoS One. 2009;4:e5153. doi: 10.1371/journal.pone.0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L, Kharbanda S, Hanfelt J, Kern FG. Both autocrine and paracrine effects of transfected acidic fibroblast growth factor are involved in the estrogen-independent and antiestrogen-resistant growth of MCF-7 breast cancer cells. Cancer Res. 1998;58:352–361. [PubMed] [Google Scholar]
- 101.Nallasamy S, Li Q, Bagchi MK, Bagchi IC. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet. 2012;8:e1002500. doi: 10.1371/journal.pgen.1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:423–429. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 103.Fillmore CM, Gupta PB, Rudnick JA, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107:21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rabbani SA, Khalili P, Arakelian A, Pizzi H, Chen G, Goltzman D. Regulation of parathyroid hormone-related peptide by estradiol: effect on tumor growth and metastasis in vitro and in vivo. Endocrinology. 2005;146:2885–2894. doi: 10.1210/en.2005-0062. [DOI] [PubMed] [Google Scholar]
- 105.Funk JL, Wei H. Regulation of parathyroid hormone-related protein expression in MCF-7 breast carcinoma cells by estrogen and antiestrogens. Biochem Biophys Res Commun. 1998;251:849–854. doi: 10.1006/bbrc.1998.9568. [DOI] [PubMed] [Google Scholar]
- 106.Kajitani T, Tamamori-Adachi M, Okinaga H, Chikamori M, Iizuka M, Okazaki T. Negative regulation of parathyroid hormone-related protein expression by steroid hormones. Biochem Biophys Res Commun. 2011;407:472–478. doi: 10.1016/j.bbrc.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 107.Giacomini D, Paez-Pereda M, Stalla J, Stalla GK, Arzt E. Molecular interaction of BMP-4, TGF-beta, and estrogens in lactotrophs: impact on the PRL promoter. Mol Endocrinol. 2009;23:1102–1114. doi: 10.1210/me.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hilakivi-Clarke LA, Raygada M, Stoica A, Martin M-B. Consumption of a high-fat diet during pregnancy alters estrogen receptor content, protein kinase C activity and morphology of mammary gland in the mother and her female offspring. Cancer Res. 1998;58:654–660. [PubMed] [Google Scholar]
- 109.Cabanes A, de Assis S, Gustafsson JA, Hilakivi-Clarke L. Maternal high n-6 polyunsaturated fatty acid intake during pregnancy increases voluntary alcohol intake and hypothalamic estrogen receptor alpha and beta levels among female offspring. Dev Neurosci. 2000;22:488–493. doi: 10.1159/000017480. [DOI] [PubMed] [Google Scholar]
- 110.Bern HA, Edery M, Mills KT, Kohrman AF, Mori T, Larson L. Long-term alterations in histology and steroid receptor levels of the genital tract and mammary gland following neonatal exposure of female BALB/cCrgl mice to various doses of diethylstilbestrol. Cancer Res. 1987;47:4165–4172. [PubMed] [Google Scholar]
- 111.Shajahan A, Goel S, de Assis S, Yu B, Clarke R, Hilakivi-Clarke L. Changes in mammary caveolin-1 signaling pathways are associated with breast cancer risk in rats exposed to estradiol in utero or during prepuberty. Hormone Molecular Biology and Clinical Investigation. 2010;2:227–234. doi: 10.1515/hmbci.2010.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hilakivi-Clarke L, Cabanes A, de AS. In utero alcohol exposure increases mammary tumorigenesis in rats. Br J Cancer. 2004;90:2225–2231. doi: 10.1038/sj.bjc.6601793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hovey RC, sai-Sato M, Warri A, et al. Effects of neonatal exposure to diethylstilbestrol, tamoxifen, and toremifene on the BALB/c mouse mammary gland. Biol Reprod. 2005;72:423–435. doi: 10.1095/biolreprod.104.029769. [DOI] [PubMed] [Google Scholar]
- 114.Jones LA, Bern HA. Long-term effects of neonatal treatment with progesterone, alone and in combination with estrogen, on the mammary gland and reproductive tract of female BALB/cfC3H mice. Cancer Res. 1977;37:67–75. [PubMed] [Google Scholar]
- 115.Mori T, Nagasawa H, Bern HA. Long-term effects of perinatal exposure to hormones on normal and neoplastic mammary growth in rodents: a review. J Environ Pathol Toxicol. 1979;3:191–205. [PubMed] [Google Scholar]
- 116.Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007;7:465–474. doi: 10.2174/156800907781386641. [DOI] [PubMed] [Google Scholar]
- 117.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Investig. 1987;57:112–137. [PubMed] [Google Scholar]
- 118.Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 119.Russo J, Russo IH. Influence of differentiation and cell kinetics on the susceptibility of the rat mammary gland to carcinogenesis. Cancer Res. 1980;40:2677–2687. [PubMed] [Google Scholar]
- 120.Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992;84:634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- 121.Umekita Y, Souda M, Hatanaka K, et al. Gene expression profile of terminal end buds in rat mammary glands exposed to diethylstilbestrol in neonatal period. Toxicol Lett. 2011;205:15–25. doi: 10.1016/j.toxlet.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 122.Zhang B, Tian Y, Jin L, et al. DDN: a caBIG(R) analytical tool for differential network analysis. Bioinformatics. 2011;27:1036–1038. doi: 10.1093/bioinformatics/btr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Connelly L, Barham W, Onishko HM, et al. Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene. 2011;30:1402–1412. doi: 10.1038/onc.2010.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biswas DK, Shi Q, Baily S, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction. 2004;127:643–651. doi: 10.1530/rep.1.00221. [DOI] [PubMed] [Google Scholar]
- 127.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr J. 2009;56:131–139. doi: 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- 128.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters the expression of DNA methyltransferases and methylation of genomic DNA in the epididymis of mice. Endocr J. 2006;53:331–337. doi: 10.1507/endocrj.k06-009. [DOI] [PubMed] [Google Scholar]
- 129.Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 130.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 131.Biniszkiewicz D, Gribnau J, Ramsahoye B, et al. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22:2124–2135. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome-components and functional correlates. Genes Dev. 2006;20:3215–3231. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 133.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 134.Tang WY, Newbold R, Mardilovich K, et al. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ho SM, Tang WY, Belmonte dF, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 138.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog. 2003;38:78–84. doi: 10.1002/mc.10147. [DOI] [PubMed] [Google Scholar]
- 140.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 141.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 142.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 143.Li H, Fischle W, Wang W, et al. Structural basis for lower lysine methylation state-specific readout by MBT repeats of L3MBTL1 and an engineered PHD finger. Mol Cell. 2007;28:677–691. doi: 10.1016/j.molcel.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herranz N, Pasini D, Diaz VM, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to Diethylstilbestrol (DES) or Bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010 May 15; doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee TI, Jenner RG, Boyer LA, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meissner A, Mikkelsen TS, Gu H, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vasilatos SN, Broadwater G, Barry WT, et al. CpG island tumor suppressor promoter methylation in non-BRCA-associated early mammary carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2009;18:901–914. doi: 10.1158/1055-9965.EPI-08-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Easwaran H, Johnstone SE, Van NL, et al. A DNA hypermethylation module for the stem/progenitor cell signature of cancer. Genome Res. 2012;22:837–849. doi: 10.1101/gr.131169.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao J, Wang J, Wang Y, Dai W, Lu L. Regulation of Pax6 by CTCF during induction of mouse ES cell differentiation. PLoS One. 2011;6:e20954. doi: 10.1371/journal.pone.0020954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mohn F, Weber M, Rebhan M, et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30:755–766. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 153.Cheng AS, Culhane AC, Chan MW, et al. Epithelial progeny of estrogen-exposed breast progenitor cells display a cancer-like methylome. Cancer Res. 2008;68:1786–1796. doi: 10.1158/0008-5472.CAN-07-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moelans CB, Verschuur-Maes AH, van Diest PJ. Frequent promoter hypermethylation of BRCA2, CDH13, MSH6, PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast cancer. J Pathol. 2011;225:222–231. doi: 10.1002/path.2930. [DOI] [PubMed] [Google Scholar]
- 155.Jiang Y, Tong D, Lou G, Zhang Y, Geng J. Expression of RUNX3 gene, methylation status and clinicopathological significance in breast cancer and breast cancer cell lines. Pathobiology. 2008;75:244–251. doi: 10.1159/000132385. [DOI] [PubMed] [Google Scholar]
- 156.Park SY, Kwon HJ, Lee HE, et al. Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 2011;458:73–84. doi: 10.1007/s00428-010-1013-6. [DOI] [PubMed] [Google Scholar]
- 157.Suter MA, Chen A, Burdine MS, et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J. 2012;26:5106–5114. doi: 10.1096/fj.12-212878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang KF, Cai W, Xu JL, Shi W. Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J Mol Endocrinol. 2012;49:107–114. doi: 10.1530/JME-12-0046. [DOI] [PubMed] [Google Scholar]
- 159.Zheng S, Li Q, Zhang Y, Balluff Z, Pan YX. Histone deacetylase 3 (HDAC3) participates in the transcriptional repression of the p16 (INK4a) gene in mammary gland of the female rat offspring exposed to an early-life high-fat diet. Epigenetics. 2012;7:183–190. doi: 10.4161/epi.7.2.18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci.STKE. 2007;2007:re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 162.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Kerin MJ. MicroRNAs as Novel Biomarkers for Breast Cancer. J Oncol. 2009;2009:950201. doi: 10.1155/2010/950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 164.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maillot G, Lacroix-Triki M, Pierredon S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- 166.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 167.Yamagata K, Fujiyama S, Ito S, et al. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell. 2009;36:340–347. doi: 10.1016/j.molcel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 168.Cheng C, Fu X, Alves P, Gerstein M. mRNA expression profiles show differential regulatory effects of microRNAs between estrogen receptor-positive and estrogen receptor-negative breast cancer. Genome Biol. 2009;10:R90. doi: 10.1186/gb-2009-10-9-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Adams BD, Claffey KB, White BA. Argonaute-2 expression is regulated by epidermal growth factor receptor and mitogen-activated protein kinase signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology. 2009;150:14–23. doi: 10.1210/en.2008-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hilakivi-Clarke L, de Assis S, Clarke R, Warri A, Marian C, Zwart A, Jin L, Kim DJ, Tian Y, Zhang B, Wang Y, Xuan J. Elevated in utero estrogenic environment may increase later breast cancer risk by down-regulating miRNAs. American Association for Cancer Research. 2011;102 [Google Scholar]
- 171.Weber B, Stresemann C, Brueckner B, Lyko F. Methylation of human microRNA genes in normal and neoplastic cells. Cell Cycle. 2007;6:1001–1005. doi: 10.4161/cc.6.9.4209. [DOI] [PubMed] [Google Scholar]
- 172.Neves R, Scheel C, Weinhold S, et al. Role of DNA methylation in miR-200c/141 cluster silencing in invasive breast cancer cells. BMC Res Notes. 2010;3:219. doi: 10.1186/1756-0500-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lujambio A, Calin GA, Villanueva A, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cao Q, Mani RS, Ateeq B, et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–199. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zheng F, Liao YJ, Cai MY, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278–289. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 176.Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 177.Rijnkels M, Kabotyanski E, Montazer-Torbati MB, et al. The epigenetic landscape of mammary gland development and functional differentiation. J Mammary Gland Biol Neoplasia. 2010;15:85–100. doi: 10.1007/s10911-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang TH, Esteller M. Chromatin remodeling in mammary gland differentiation and breast tumorigenesis. Cold Spring Harb Perspect Biol. 2010;2:a004515. doi: 10.1101/cshperspect.a004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schuettengruber B, Cavalli G. Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development. 2009;136:3531–3542. doi: 10.1242/dev.033902. [DOI] [PubMed] [Google Scholar]
- 180.Maruyama R, Choudhury S, Kowalczyk A, et al. Epigenetic regulation of cell type-specific expression patterns in the human mammary epithelium. PLoS Genet. 2011;7:e1001369. doi: 10.1371/journal.pgen.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee HJ, Hinshelwood RA, Bouras T, et al. Lineage specific methylation of the Elf5 promoter in mammary epithelial cells. Stem Cells. 2011;29:1611–1619. doi: 10.1002/stem.706. [DOI] [PubMed] [Google Scholar]
- 182.Cao H, Yang CS, Rana TM. Evolutionary emergence of microRNAs in human embryonic stem cells. PLoS One. 2008;3:e2820. doi: 10.1371/journal.pone.0002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gunaratne PH. Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells? Curr Stem Cell Res Ther. 2009;4:168–177. doi: 10.2174/157488809789057400. [DOI] [PubMed] [Google Scholar]
- 184.Avril-Sassen S, Goldstein LD, Stingl J, et al. Characterisation of microRNA expression in post-natal mouse mammary gland development. BMC Genomics. 2009;10:548. doi: 10.1186/1471-2164-10-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Greene SB, Gunaratne PH, Hammond SM, Rosen JM. A putative role for microRNA-205 in mammary epithelial cell progenitors. J Cell Sci. 2010;123:606–618. doi: 10.1242/jcs.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Savarese TM, Strohsnitter WC, Low HP, et al. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lukanova A, Surcel HM, Lundin E, et al. Circulating estrogens and progesterone during primiparous pregnancies and risk of maternal breast cancer. Int J Cancer. 2012;130:910–920. doi: 10.1002/ijc.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:361–368. [PubMed] [Google Scholar]
- 189.Enger SM, Ross RK, Henderson B, Bernstein L. Breastfeeding history, pregnancy experience and risk of breast cancer. Br J Cancer. 1997;76:118–123. doi: 10.1038/bjc.1997.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wohlfahrt J, Melbye M. Maternal risk of breast cancer and birth characteristics of offspring by time since birth. Edidemiology. 1999;10:441–444. doi: 10.1097/00001648-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 191.Depue RH, Bernstein L, Ross RK, Judd HL, Henderson BE. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, amd other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987;156:1137–1141. doi: 10.1016/0002-9378(87)90126-8. [DOI] [PubMed] [Google Scholar]
- 192.Nagata C, Iwasa S, Shiraki M, Shimizu H. Estrogen and alpha-fetoprotein levels in maternal and umbilical cord blood samples in relation to birth weight. Cancer Epidemiol Biomarkers Prev. 2006;15:1469–1472. doi: 10.1158/1055-9965.EPI-06-0158. [DOI] [PubMed] [Google Scholar]
- 193.Asztalos S, Gann PH, Hayes MK, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3:301–311. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 194.Russo J, Rivera R, Russo IH. Influence of age and parity on the development of the human breast. Breast Cancer Res Treat. 1992;23:211–218. doi: 10.1007/BF01833517. [DOI] [PubMed] [Google Scholar]
- 195.Hilakivi-Clarke L, de Assis S, Warri A, Luoto R. Pregnancy hormonal environment and mother’s breast cancer risk. Horm Mol Biol Clin Investig. 2012;9:11–23. doi: 10.1515/hmbci-2012-0019. [DOI] [PubMed] [Google Scholar]
- 196.Britt KL, Kendrick H, Regan JL, et al. Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells. Breast Cancer Res. 2009;11:R20. doi: 10.1186/bcr2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tiede BJ, Owens LA, Li F, DeCoste C, Kang Y. A novel mouse model for non-invasive single marker tracking of mammary stem cells in vivo reveals stem cell dynamics throughout pregnancy. PLoS One. 2009;4:e8035. doi: 10.1371/journal.pone.0008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bronson RA. Oral contraception: mechanism of action. Clin Obstet Gynecol. 1981;24:869–877. doi: 10.1097/00003081-198109000-00014. [DOI] [PubMed] [Google Scholar]
- 199.Mishell DR., Jr State of the art in hormonal contraception: an overview. Am J Obstet Gynecol. 2004;190:S1–S4. doi: 10.1016/j.ajog.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 200.Gaspard UJ, Romus MA, Gillain D, Duvivier J, Mey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27:577–590. doi: 10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- 201.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 202.Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190:S5–S22. doi: 10.1016/j.ajog.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 203.Kumle M, Weiderpass E, Braaten T, Persson I, Adami HO, Lund E. Use of oral contraceptives and breast cancer risk: The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2002;11:1375–1381. [PubMed] [Google Scholar]
- 204.Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19:2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156:635–648. doi: 10.1059/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342–350. doi: 10.1016/j.contraception.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998;92:982–988. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 208.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 209.Bain C, Willett W, Hennekens CH, Rosner B, Belanger C, Speizer FE. Use of postmenopausal hormones and risk of myocardial infarction. Circulation. 1981;64:42–46. doi: 10.1161/01.cir.64.1.42. [DOI] [PubMed] [Google Scholar]
- 210.The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 211.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 212.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 213.Lacey JV, Jr, Mink PJ, Lubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 214.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Edidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheek J, Lacy J, Toth-Fejel S, Morris K, Calhoun K, Pommier RF. The impact of hormone replacement therapy on the detection and stage of breast cancer. Arch Surg. 2002;137:1015–1019. doi: 10.1001/archsurg.137.9.1015. [DOI] [PubMed] [Google Scholar]
- 216.Holli K, Isola J, Cuzick J. Low biologic aggressiveness in breast cancer in women using hormone replacement therapy. J Clin Oncol. 1998;16:3115–3120. doi: 10.1200/JCO.1998.16.9.3115. [DOI] [PubMed] [Google Scholar]
- 217.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 218.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 219.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 220.Chlebowski RT, Anderson G, Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–377. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 221.Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218–1227. doi: 10.1093/jnci/djs327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Davey DA. Update: estrogen and estrogen plus progestin therapy in the care of women at and after the menopause. Womens Health (Lond Engl) 2012;8:169–189. doi: 10.2217/whe.12.1. [DOI] [PubMed] [Google Scholar]