Abstract
Objective
It is routine in intensive care medicine (ICM) to measure hypothalamic-pituitary-adrenal (HPA) function, commonly utilizing the ACTH stimulation test to diagnose absolute or relative adrenal insufficiency (RAI). It is therefore important to determine whether ICM therapies and testing themselves influence HPA test results.
Design
Prospective, 96 h animal study.
Setting
Research laboratory.
Subjects
24 healthy canines.
Interventions
Animals were randomized into two groups—awake and unrestrained or treated with intensive care medicine therapies including sedation, intubation and mechanical ventilation (SIM). Animals were further randomized to receive dexamethasone (or placebo) or undergo either a total of 4 or 7 ACTH stimulation tests over 96 h.
Measurements and Main Results
SIM transiently increased both basal and post-ACTH total and free cortisol concentrations greater than two-fold as compared to baseline for the first 24 h (p ≤ 0.05 for both). Performance of 7 stimulation tests increased both basal and post-ACTH total and free cortisol concentrations from baseline by greater than 1.5 fold for the duration of the 96 h study (p ≤ 0.05). Neither SIM nor the performance of more stimulation tests affected delta cortisol measurements (total or free cortisol, p=NS). In contrast, dexamethasone suppressed basal total cortisol concentrations by greater than two-fold (p ≤ 0.005) at all time points and transiently increased delta total cortisol by approximately 35% during the first 24 h of the study (p ≤ 0.05).
Conclusions
total and free cortisol measurements—whether pre- or post-ACTH or as a calculated delta—were altered by intensive care therapies or frequent ACTH stimulation testing with one exception. Delta free cortisol was the only HPA measurement unaffected by SIM, completion of more ACTH stimulation tests or dexamethasone therapy. These findings support the need to determine normal ranges for HPA testing in subjects receiving ICM before establishing laboratory criteria for the diagnosis of RAI.
Keywords: ACTH stimulation test, canine, critical illness, glucocorticoid and relative adrenal insufficiency
Introduction
The use of the ACTH stimulation test to establish the diagnosis of absolute adrenal insufficiency in the outpatient setting is well established. By comparison, the concept of relative adrenal insufficiency (RAI) was introduced in the 1990s and has only recently gained acceptance as a clinical diagnosis (1). The premise of RAI is that a basal cortisol concentration which is elevated may, nonetheless, be inadequate in the setting of critical illness. Proponents of RAI would further argue that critically ill patients with concomitant RAI, like patients with absolute adrenal insufficiency, benefit from steroid therapy.
The interpretation of cortisol concentrations in critical illness has proven be to complex (2). As such, there is yet to be a diagnostic criterion for RAI which prospectively identifies patients who benefit from glucocorticoid therapy. To date, different total serum cortisol levels including a baseline < 18 or 25 μg/dL, an absolute post-ACTH stimulation level < 18 μg/dL, or an ACTH-induced increment (i.e., delta) in total cortisol < 7 or 9 μg/dL have all been used to define RAI in published studies (3). Uncertainty also exists as to whether free serum cortisol measurement, as opposed to total cortisol, is a superior test, especially in critically ill patients with hypoalbuminemia (2, 4). If ACTH stimulation testing is to be used to diagnose RAI, it is imperative that the reference range of results be defined in terms of both total and free serum cortisol and that treatment factors which influence test results be identified. Only then can the ACTH stimulation test be used to differentiate normal from abnormal adrenal function in critical illness.
The purpose of this study was to evaluate the impact of various intensive care medicine (ICM) therapies and interventions on tests of pituitary and adrenal function in otherwise healthy animals. First, the effect of sedation, intubation and mechanical ventilation (SIM) on pituitary and adrenal function testing was assessed. We also compared animals receiving a dexamethasone infusion with controls as this glucocorticoid is used in ICUs because it is believed to neither affect performance of assays that measure cortisol concentrations nor, in the short-term, alter the absolute post-ACTH cortisol concentration (5). Finally, we randomized animals to receive either 4 or 7 ACTH stimulation tests with low- and high-dose ACTH over the 96 h time period in order to evaluate the effect of performing more than one test in a day—what can occur clinically when practitioners either accidentally repeat the test or decide to confirm the results. Besides comparing the low- and high-dose ACTH stimulation tests, this design allowed us to evaluate the precision of the ACTH stimulation test (i.e., the reproducibility of results) and determine whether the administration of ACTH itself affected test results. The impact of all of these interventions on serum cortisol concentrations (both total and free) measured at baseline, peak response or the calculated increment of change from baseline was assessed, as each of these measurements has been used to diagnose RAI in critically ill patients.
Materials and Methods
Study Design
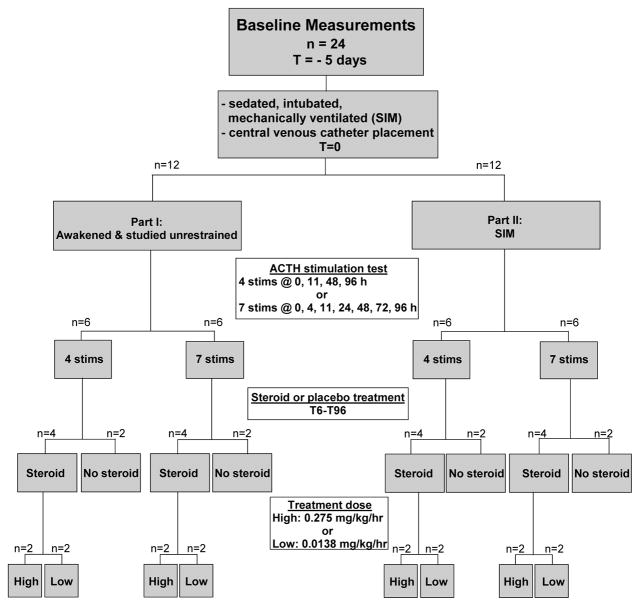
The experiments described below were performed as part of a protocol approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health. At baseline, all animals (n=24, 12–18 month old, 10–12 kg, male, purpose-bred beagles) had a peripheral venous catheter placed aseptically (Fig. 1). Blood was drawn from each animal for assessment of basal pituitary and adrenal function via measurement of serum total and free cortisol, aldosterone, and anti-diuretic hormone (ADH) concentrations and plasma endogenous adrenocorticotropic hormone (eACTH) concentration. Low- and high-dose synthetic ACTH (cosyntropin [Cortrosyn, Amphastar Pharmaceuticals, Inc., Rancho Cucamonga, CA]) stimulation tests were then performed sequentially. One hour after administration of low-dose ACTH (1.0 μg IV), a blood sample was drawn, and immediately thereafter, high-dose ACTH (5.0 μg/kg IV) was administered; blood was again drawn 1 h later. Serum free and total cortisol concentration was determined in both post-ACTH samples. The peripheral catheter was then removed, and the animals returned to standard holding.
Figure 1.
Study Design
Five days after baseline evaluation, all animals were taken to a surgical suite and placed under general anesthesia (see below for description). A central venous catheter was inserted in all animals; completion of this procedure was designated as t=0. In Part I, animals were then awakened. In Part II, animals further had femoral arterial and urinary catheters placed and a tracheostomy performed (see below for description of surgical procedures). After these procedures were completed (approximately 20 min) general anesthesia was converted to continuous sedation (fentanyl, midazolam, and medetomidine), and mechanical ventilation was initiated and continued for the duration of the study (96 h).
All animals in Parts I and II had blood drawn for measurement of plasma eACTH concentration and serum aldosterone, ADH, and basal free cortisol and total cortisol concentrations at t = 4, 11, 24, 48, 72 and 96 h after catheter placement. Animals in both parts were further randomly assigned to undergo a total of either 4 (t = baseline, 11, 48, or 96 h; n=6) or 7 (t = baseline, 4, 11, 24, 48, 72, 96; n=6) sequential low- and high-dose ACTH stimulation tests as described above. Animals in Parts I and II were also randomly assigned to receive continuous intravenous infusion (1.7 ml/hr) of low-dose dexamethasone (0.0138 mg/kg/hr; n=4), high-dose dexamethasone (0.275 mg/kg/hr; n=4) or saline (n=4). The dose chosen are 10X and 200X as potent as the 24 h cortisol dose used to treat adrenal insufficiency in canines, and are thus comparable to the higher dose cortisol therapy tested in early human RTCs and the more recent, lower dose cortisol therapy (300 mg/day) used more commonly today (6, 7). Infusions were initiated at t = 6 h and continued until the end of the study (t=96 h). Oxacillin (20 mg/kg IV every 8h) was administered beginning at t=0 h and continued until the end of the study for prophylaxis.
Surgical Procedures
Animals were fasted for 18 h prior to anesthesia induction, and all procedures were performed using aseptic technique. After intravenous induction with propofol (4–6 mg/kg), tracheal intubation (Rusch, 6 Fr, Duluth, GA) and initiation of mechanical ventilation (Fabius Trio, Drager Medical, Telford, PA), anesthesia was maintained with isoflurane (0.5–1.5 %). A catheter (Maxxim Medical, Athens, TX, 20-gauge) was then placed in the external jugular vein and covered with a self-adhesive bandage (Co-Flex, Andover Coated Products, Andover, MA,1.5 inch × 2 inch elastic bandage) to prevent catheter access by the animal. In Part I, animals were then awakened, extubated and placed in individual cages with free access to food and water. Animals in Part II remained anesthetized and underwent femoral artery catheter (Maxxim Medical, Athens, TX, 20-gauge) and urinary catheter (Cook, Foley 8 F, 55 cm) placement. A tracheostomy was also performed on each animal as previously described (8).
Mechanical Ventilation
In Part II, the ventilator (Servovent 300, Siemens Medical, Sweden) was initially set with a fraction of inspired oxygen (FiO2) = 25%, positive end expiratory pressure (PEEP) = 5 cm H2O, tidal volume = 20 ml/kg, and respiratory rate = 15 breaths/min. Tidal volumes of 15–20 ml/kg are standard in intensive care units treating critically ill septic canines (8, 9). An O2 saturation below 92% was treated by alternately increasing FiO2 in increments of 25% or the PEEP, initially by 5 cm H20 followed by 2 cm H20. The maximum settings were FiO2 =100% and PEEP = 12 cm H2O. Both the FiO2 and PEEP were reduced by the same decrements if the O2 saturation was above 93% for 6 h. Blood gas determinations (every 2 h until t = 8 h and then every 8 h thereafter) were used to set respiratory rates on the mechanical ventilator. Respiratory rates were increased in increments of 5 breaths/minute to maintain pCO2 under 35 mm Hg; alternatively, the respiratory rate was decreased by 5 breaths/minute if both the pH < 7.3 and pCO2 ≤ 30 mmHg. The minimum and maximum settings were 15 and 35 breaths per minute, respectively.
Other ICU Therapies
In addition to human therapies, veterinary critical care specifically for animals was instituted in Part II of the study based on the standard of care for critically ill large animals requiring sustained mechanical ventilation in the clinical setting (9, 10). Animal mouth and endotracheal tube care along with body positioning and intravenous catheter site dressings were attended to at scheduled intervals as previously described (8). Humidity in the ventilator tubing was maintained using a humidifier (Conchatherm III, Hudson RCI-AB, Temecula, CA) attached to the airway system. Throughout the study, a heated water blanket and other heavy blankets were used to maintain a core body temperature between 36.5°C and 37.5°C. Famotidine (1 mg/kg IV q12 h) was given to prevent stress-induced stomach ulcers and unfractionated heparin (3000 IU IM, q8 h) was administered for venous thrombosis prophylaxis.
Sedation Management
In Part II, adequacy of the level of sedation was evaluated and adjusted continuously by a clinician or trained technician constantly present in the animal laboratory or 96 h after initiation of administration of midazolam (0.2 mg/kg loading dose, 50 μg/kg/min infusion IV) and fentanyl (5 μg/kg loading dose, 0.7 μg/kg/min infusion IV). Both the fentanyl and midazolam doses were increased in 25% increments every 5 min until adequate sedation was obtained. Medetomidine infusion (2–5 μg/kg/min) was used to supplement sedation as needed according to set criteria. Criteria for adequacy of sedation were continuously monitored as previously described (8).
Laboratory Data
Blood samples used for measurement of serum aldosterone and total and free cortisol concentrations were allowed to clot and were centrifuged, and the serum was separated and frozen in plastic tubes at −20°C until assayed in duplicate. For measurement of serum aldosterone concentration, an assay was performed using a previously validated (11) radioimmunoassay kit (Coat-A-Count Aldosterone Assay, DPC, Los Angeles, CA). The sensitivity of the assay was 20 pg/mL; all measurements below the standard curve were recorded as 10 pg/mL. Measurement of ADH as arginine vasopressin was determined using a radioimmunoassay kit (Nichols Institute, Diagnostics BV) as previously described (12, 13). For measurement of total cortisol concentration, assay was performed using a previously validated (14) radioimmunoassay kit (Coat-A-Count Cortisol Assay, DPC, Los Angeles, CA). The sensitivity of the assay was 14 nmol/L; all measurements below the standard curve were recorded as 7 nmol/L.
For measurement of serum free cortisol concentration, the samples were assayed by a modified ultrafiltration technique. A sample volume of 500 ml serum was placed in a micropartition device (Centrifree Micropartition Device, Millipore Corporation, Billerica, MA). The device was covered to prevent evaporation, warmed to 37°C and centrifuged in a swinging bucket rotor (37°C, 1200×g, 30 min). The sample remaining in the top of the device (“top”) and the ultrafiltrate were harvested and stored at −80°C until analysis. All samples were analyzed in duplicate as above. The percent free cortisol in each sample was determined by the formula:
The free cortisol concentration in each sample was determined by multiplying the % free cortisol by the total cortisol concentration measured in the non-centrifuged sample.
To validate the procedure, free cortisol percent and concentration were determined in 53 samples obtained from healthy animals before and after administration of either 1 μg ACTH per animal or 5 μg/kg body weight. A significant (p<0.0001) linear relationship between total plasma cortisol and percentage free cortisol was found by linear regression. The regression equation (% free cortisol = 6.5 + [0.04 × total cortisol concentration]) was similar to that found by Kemppainen et al. when free cortisol was measured in canine plasma by a centrifugal ultrafiltration-dialysis technique (% free cortisol = 8.7 + [0.05 × total cortisol concentration]) (15). In addition, a single basal sample from an animal was divided into 6 equal aliquots and processed as described. The cortisol concentration was measured in all 6 ultrafiltrate portions obtained. The coefficient of variation was 3.2% showing repeatability of the technique.
Samples for measurement of plasma eACTH concentration were collected at the time of the pre-ACTH sample. Blood was collected into EDTA tubes containing the proteinase inhibitor aprotinin (final concentration 1000 kallikrein inhibitor U/mL blood) (16). Samples were centrifuged immediately; plasma was separated and placed in plastic tubes and stored at −20°C until assayed in duplicate using a previously validated (17) immunoradiometric kit (ACTH assay, Nichols Institute, San Clemente, CA). The sensitivity of the assay was 4.4 pg/mL. All measurements below the standard curve were recorded as 2.2 pg/mL.
Statistical Methods
Treatment effects found at various contiguous time points (4, 11 and 24 h; 72 and 96 h) were qualitatively similar for all interventions studied and therefore combined for ease of presentation and to enhance our ability to find significant effects. Pituitary-adrenal function parameters were analyzed using an analysis of variance (ANOVA) procedure (18). Serum bound cortisol concentration was calculated as the difference between serum total and free cortisol concentrations. A delta cortisol concentration was calculated by subtracting a pre-ACTH serum total or free cortisol concentration from its respective post-ACTH serum total or free cortisol concentration. To analyze the effect of dexamethasone and ACTH stimulation, the ANOVA had two main effects, time and dose of dexamethasone (low and high). When two-way interaction indicated results were qualitatively similar, data from the animals receiving the two doses of dexamethasone were pooled to increase the power of the study to find time effects. In addition, single degree-of-freedom tests were used to compare subsequent time points to the baseline data. To analyze the effect of SIM, a three-way ANOVA was used; the three factors in the ANOVA model included time, dose of dexamethasone (low and high), and each intervention analyzed separately (e.g. sedation, steroids, and number of ACTH stimulation tests), as well as all interactions. As in the model above, when interactions associated with the dose of dexamethasone showed qualitatively similar results, data from the animals receiving the two doses of dexamethasone were pooled to increase study power. Correlations between pre- and post-ACTH serum cortisol concentration were assessed by using a general linear model, treating the baseline values as a continuous variable and controlling for animal-to-animal variation within the linear model (19).
Results
The effect of dexamethasone on pituitary-adrenal function
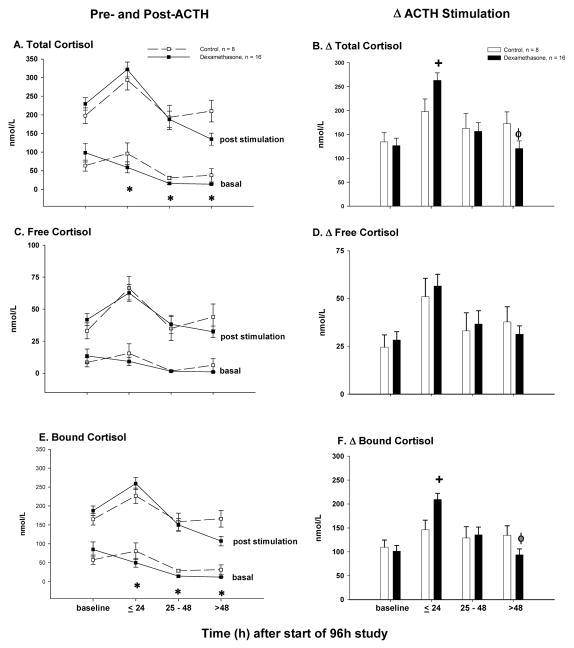
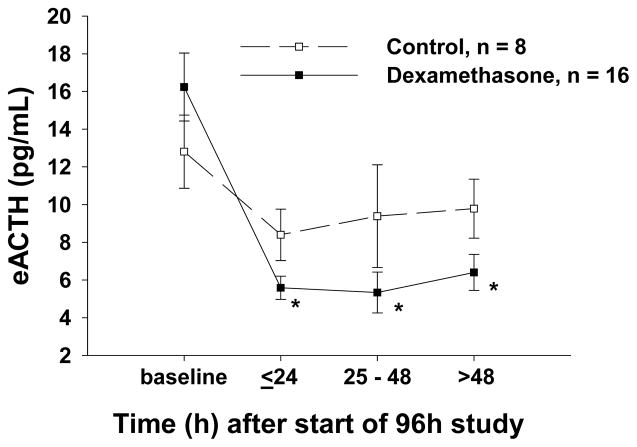
The effects of low- and high-dose dexamethasone infusion on pituitary-adrenal function were qualitatively similar and, therefore, data were combined to increase our ability to find significant effects (Table 1, Supplement). Animals assigned to the treatment and control groups had similar basal cortisol (total, free and bound) (Fig. 2 A, C and E), eACTH (Fig. 3), aldosterone and ADH concentrations (data not shown). Throughout the dexamethasone infusion, basal cortisol (total and bound) (Fig. 2A and E) and eACTH concentrations (Fig 3) significantly declined compared to controls. In contrast, dexamethasone infusion had no significant effect on basal free cortisol (Fig. 2C), aldosterone or ADH concentrations throughout the study (data not shown).
Figure 2.
For dexamethasone-treated animals (closed boxes connected by a solid line) and controls (open squares connected by a dashed line), the mean concentrations (nmol/L) ± SEM pre- and post-ACTH stimulation (results of low and high dose ACTH challenge combined) at serial time points are shown for total cortisol (panel A), free cortisol (panel C) and protein-bound cortisol (panel E). For dexamethasone-treated animals (closed bars) and controls (open bars), the mean ± SEM change from pre- to post-ACTH stimulation (i.e., delta cortisol; results of low and high dose cosyntropin challenge combined) is shown for total cortisol (panel B), free cortisol (panel D) and protein bound cortisol (panel F).
Figure 3.
The effect of a 90 h dexamethasone infusion (from T=6 to 96h; results of low and high dose dexamethasone combined) on endogenous adrenocorticotropic hormone (eACTH). The mean ± SEM concentrations (pg/mL) at serial time points are shown for dexamethasone-treated animals (closed squares connected by a solid line) and control animals (open squares connected by a dashed line).
The effect of low- and high-dose ACTH stimulation testing (Fig. 2A, C and E) on cortisol concentrations (total, free and bound) were qualitatively similar; therefore, data were combined (Table 1, Supplement). Dexamethasone significantly altered the ACTH-stimulated total and bound delta cortisol concentrations in a time-dependent manner. Delta total and bound cortisol concentrations were augmented by dexamethasone treatment through the first 24 h compared to controls (Fig. 2B and F). After 24 h, the effect of dexamethasone on ACTH stimulation testing was reversed, and by the end of the study (>48 h), dexamethasone significantly blunted the ACTH response. In contrast, dexamethasone had no significant effect on the delta free cortisol concentration throughout the study (Figure 2D).
The effect of sedation, intubation and mechanical ventilation (SIM) or increasing the number of ACTH stimulation tests (7 vs. 4) on pituitary-adrenal function
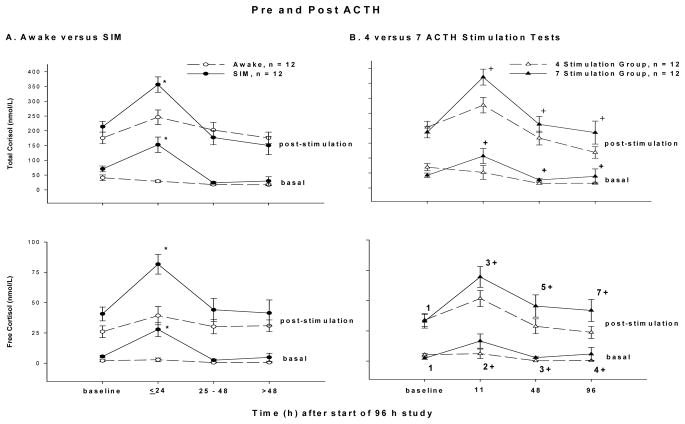
Both the addition of SIM and the performance of more ACTH stimulation tests (i.e. 7 vs. 4) raised the basal and ACTH-stimulated serum cortisol concentrations (total and free). Basal and ACTH-stimulated cortisol (total and free) concentrations were significantly increased transiently by the SIM in the early stages, i.e. ≤ 24h, but were similar to controls thereafter (Fig. 4A). In comparison, the animals receiving 7 vs. 4 stimulation tests had significantly increased basal and ACTH-stimulated serum cortisol concentrations (total, and free) throughout the study after t=0 (Fig. 4B). Since both SIM and the performance of 7 stimulation tests each raised both basal and ACTH-stimulated cortisol concentrations concomitantly and to similar degrees, the delta cortisol concentrations (total and free) were unchanged by these interventions at all time points studied (data not shown). Thus, more than one stimulation test per 24 h (7 vs. 4 over 96 h), on average, elevated basal total and free cortisol greater than two-fold and likewise elevated post-ACTH concentrations by a similar amount over the course of the 96 h study.
Figure 4.
Panel A: The mean concentrations (nmol/L) ± SEM of pre- and post-ACTH total and free cortisol for sedated, intubated and mechanically ventilated (SIM) animals (closed circles connected by a solid line) and awake animals (open circles connected by a dashed line). Panel B shows the mean concentrations ± SEM of pre- and post-ACTH total and free cortisol for animals receiving a total of 7 (closed triangles connected by a solid line) and 4 (open triangles connected by dashed lines) stimulation tests.
The addition of SIM and the performance of 7 vs. 4 stimulation tests also induced significant increases in serum aldosterone concentration from baseline (Fig. 5; p<0.001 and p <0.05, respectively). The effect of SIM was again transient, occurring up to 48h after initiation of SIM. However, the significant increase from baseline in serum aldosterone induced by the performance of 7 ACTH stimulation tests persisted throughout the study.
Figure 5.
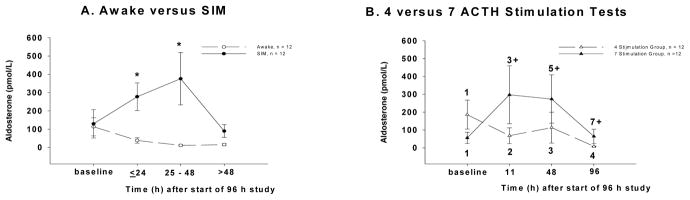
Panel A: Mean basal aldosterone concentration (pmol/L ± SEM) in sedated, intubated and mechanically ventilated (SIM) animals (closed circles connected by a solid line) and awake animals (open circles connected by a dashed line). Panel B: Mean basal aldosterone concentration (pmol/L ± SEM) in animals undergoing 7 (closed triangles connected by a solid line) versus 4 (open triangles connected by a dashed line) ACTH stimulation tests.
The decline in eACTH concentrations over the course of the study seen in both control and dexamethasone-treated animals (Fig. 3) was blunted with SIM (p = 0.06) and the performance of 7 vs. 4 ACTH stimulation tests (p= 0.04) (data not shown). Lastly, these two therapies had no effect on serum ADH concentrations throughout the study (data not shown).
Discussion
We examined the combined effect of sedation, intubation and mechanical ventilation (SIM) on low (1 μg/animal) and high dose (5 μg/kg) ACTH stimulation tests in 24 healthy canines. In addition, using a factorial design, we investigated if testing frequency or administration of a continuous infusion of low dose (0.0138 mg/kg/hr) or high dose (0.275 mg/kg/hr) dexamethasone for 90h altered test results. At baseline, the mean (±SEM) basal total cortisol concentrations were 56 ± 14 nmol/mL (n = 24 animals). Administration of low- and high-dose ACTH resulted in doubling (111 ± 17) and quadrupling (242 ± 27), respectively, of the total cortisol concentrations after 60 min. We ultimately combined the data obtained from the low- and high-dose ACTH stimulation tests because the results were qualitatively similar regardless of the variable tested (i.e., SIM, number of stimulations or dexamethasone therapy).
Over the first 24 h, SIM equally increased both the basal and post-ACTH total and free cortisol concentrations greater than two-fold such that there was no alteration in the calculated delta total cortisol concentration. After 24 h and until the end of the experiment at 96 h, SIM had no effect on ACTH stimulation testing.
Increasing the frequency of ACTH stimulation testing to more than 1 per 24 hours (i.e., 2 per day) also increased both the basal and post-ACTH total and free cortisol concentrations greater than 1.5-fold and had no effect on the incremental cortisol response to ACTH. As opposed to SIM, this effect was sustained throughout the 96 h experiment.
Low- and high-dose dexamethasone therapy altered pituitary-adrenal function similarly, so we combined the data to increase our ability to find a significant effect. Beginning before 24 h and continuing until the end of the experiment at 96 h, dexamethasone therapy persistently lowered basal total cortisol concentrations approximately seven-fold compared to controls. In contrast to SIM and performance of more ACTH stimulation tests, dexamethasone therapy affected the delta total cortisol concentration; from 4 to 24 h, the delta total cortisol concentration increased approximately 33%. Over the remainder of the study, dexamethasone therapy caused a greater decline in the delta total cortisol as compared to control.
We also measured free cortisol levels under the same experimental conditions. The effect of SIM and increasing frequency of ACTH stimulation tests on free cortisol concentrations (basal, post-ACTH and delta) were identical to that for total cortisol. However, in contrast to total cortisol, free cortisol was unaffected by dexamethasone treatment throughout the 96 h (basal, post-stimulation and delta free cortisol; Figs. 2 and 4).
There were aspects of this study that could conceivably limit the clinical applicability of our results. This was a relatively small study (n=24) performed in healthy, not sick, animals. Nonetheless we used a rigorous design that tested multiple factors in each animal in order to increased power and control for dog-to-dog variability. It was our objective to determine a normal range for HPA testing in healthy animals so that we could identify the effect of intensive care therapies on testing without the confounder of critical illness. In addition, some of the interventions may have been different than standard clinical practice. For example, dexamethasone was administered as an infusion whereas it is commonly given clinically as repeated boluses. Similarly, performance of 7 ACTH stimulation tests within 96 h would be unusual; however, one could imagine performing multiple stimulation tests during a patient’s ICU course, including two within 24 hours, for which the results presented are applicable.
A recent consensus statement from The American College of Critical Care Medicine contains recommendations regarding how to best diagnosis RAI (i.e. delta cortisol < 9 μg/dl or a random total cortisol concentration <10 μg/dl), yet concludes that ACTH stimulation testing should not be used to decide which patients with septic shock or acute respiratory distress syndrome should be treated with glucocorticoids (20). There are two possible explanations as to why no HPA test has prospectively been proven to identify critically ill patients with RAI who would benefit from glucocorticoid therapy: either RAI is not a true entity or the current method of diagnosing RAI is imperfect. In this study, we show that in otherwise healthy animals intensive care substantially impacts many of the current HPA testing modalities. Our results suggest that delta free cortisol measurement--by virtue of the fact that it was not affected by common critical care therapies, dexamethasone treatment or multiple ACTH stimulation tests--may represent the single best test for the evaluation of RAI in subjects in an ICU.
Dexamethasone, whether administered as a high- or low-dose continuous infusion, immediately and persistently lowered basal total serum cortisol and eACTH concentrations. This was consistent with basic science supporting the use of the dexamethasone suppression test used to diagnosis Cushing’s disease whereby dexamethasone suppresses the activity of an ACTH-secreting pituitary tumor and subsequently lowers the serum cortisol level (21, 22). The transient, early increase in delta total cortisol observed in animals treated with dexamethasone has been reported in prior canine studies (23, 24). The mechanism for this effect is not known, although in a study of guinea pigs dexamethasone treatment resulted in increased binding of ACTH by the adrenal glands (25). In contrast, towards the end of the study, dexamethasone infusion caused a blunted total cortisol response to ACTH stimulation, likely a result of glucocorticoid feedback (26) on eACTH secretion and resultant adrenocortical atrophy. The exact timing of the change from hyper- to hypo-responsiveness probably depends on the glucocorticoid dose or possibly route of administration. Experiments using single, small injections of dexamethasone (0.01 or 0.1 mg/kg) have shown no effect on ACTH-stimulated total cortisol concentrations when testing was performed 4–48 h post-dexamethasone treatment (27, 28). However, when an ACTH stimulation test was conducted after administration of a higher dexamethasone dose (1.0 or 5.0 mg/kg), the post-ACTH total cortisol concentration was decreased beginning at 72 and 24 h, respectively (28).
The fact that dexamethasone significantly altered total cortisol measurements but did not affect free cortisol is a clinically relevant finding and may simply be a function of the power of the study--i.e., if we had tested more animals then a significant difference would have been detected. Alternatively, variation in serum cortisol-binding globulin (CBG) concentration or the affinity of CBG for cortisol could account for the lack of concominant changes in free cortisol. Prior in vitro investigations have shown that dexamethasone has varying effects on CBG synthesis and secretion depending on species of the cell studied (29–31). We did not measure CBG concentrations or affinity for cortisol to determine if such changes played a role in the current study. Similarly, we did not analyze plasma albumin levels which can also impact the amount of measured free cortisol and is, in vitro, increased in the setting of dexamethasone (2, 32). Regardless, this experiment highlights the difficulty in interpreting total serum cortisol levels when dexamethasone is administered and brings into question the clinical utility of this test in patients receiving dexamethasone despite earlier guidelines suggesting that dexamethasone could be administered in septic patients prior to performing an ACTH stimulation test (33). In contrast, measurements of free cortisol (basal, post-ACTH and delta) were unaffected by dexamethasone therapy.
The combination of intubation, sedation and mechanical ventilation caused a transient but significant increase in both basal and post-ACTH total and free cortisol concentrations. A prior study in human surgical patients also documented an elevation of the basal cortisol concentration beginning 30 minutes after intubation (34). The normalization of basal and post-ACTH cortisol concentrations over the remainder of the study despite continued mechanical ventilation is potentially due to more pronounced sedation over time, although the relative contribution of the three sedatives used is difficult to determine. For example, it is possible that intensive veterinary care induced stress that activated the HPA axis, but the stress was effectively managed once midazolam and fentanyl levels were optimized. On the other hand, the administration of sedatives (35, 36) have been shown to suppress the HPA axis especially medetomidine (37), and its infusion beginning at 24 h may have lowered cortisol concentrations in this current study. Neither sedation nor any other aspect of intensive care therapies including intubation and mechanical ventilation had an effect on delta total or free cortisol in these otherwise healthy animals. It is also interesting to note that a concomitant increase in endogenous ACTH was not observed at the time that SIM caused a transient increase in cortisol. This would suggest that instead of ACTH, either cytokines or more likely sympathetic innervation of the adrenal gland, is the mediator responsible for the transient increase in cortisol associated with SIM (38–40).
The performance of 7 ACTH stimulation tests vs. 4 over 96 h significantly up-regulated the HPA axis as evidenced by the elevation in basal and ACTH-stimulated total and free cortisol concentrations throughout the study. When one compares the animals at different time points when both groups of animals have received the same number of tests, the animals in the four stimulation group have lower cortisol levels. Thus, the difference in basal and ACTH-stimulated total and free cortisol between the two groups is most likely a function of the fact that the 7 stimulation animals received more frequent testing rather than a greater total of tests. The increase seen with more frequent testing may be due to the trophic effects of ACTH on the cells of the adrenocortical zona fasiculata (41). Alternatively, more frequent ACTH stimulation testing may simulate chronic or repeated stress, and the increased pituitary-adrenal tone may be a function of corticotrophic facilitation (42, 43). Thus, our study suggests that either repeated administration of exogenous ACTH within 24 h or repeated stresses may augment HPA axis activity. As was the case with combined intensive care therapies, the delta total and free cortisol measurement were not statistically different between dogs receiving 7 vs. 4 stimulation tests.
The effects of SIM and the performance of 7 stimulation tests on aldosterone concentrations mirrored those seen with cortisol. SIM caused early and transient increases in aldosterone concentration while completion of 7 stimulation tests resulted in a persistent elevation in aldosterone concentration. Positive pressure ventilation (PEEP) has been shown in human studies (44, 45) to increase aldosterone concentrations, likely secondary to hemodynamic changes occurring both systemically and at the level of the kidneys. Aldosterone concentration may have normalized after 48 h in animals receiving mechanical ventilation due to continued fluid resuscitation. The increase in aldosterone with additional stimulation tests was also expected as ACTH stimulates aldosterone release (46). However, ACTH plays a minor role in control of aldosterone secretion overall, and the lack of an effect of dexamethasone therapy on aldosterone concentrations observed in this study may be due to lack of change in the major stimulants of aldosterone secretion. The feedback effects of glucocorticoids on aldosterone secretion have not been widely studied in dogs, but our results are contrary to a prior study in canines albeit under different conditions (47, 48).
While the reference range of results for HPA testing in healthy adults in the outpatient setting has been previously defined (49, 50), what constitutes a normal HPA test result in a subject receiving intensive medical or veterinary care is less well established. The three variables that we prospectively evaluated—dexamethasone treatment, SIM and frequency of ACTH stimulation testing—all impacted the results of various HPA tests in otherwise healthy animals. Thus, interpretation of traditional ACTH stimulation testing of HPA function becomes extremely complicated when these tests are performed in patients receiving critical care therapies. For example, our data would suggest that performing an ACTH stimulation test in an ICU patient who was intubated in the past 24 h would yield a post-stimulation total cortisol concentration that would be significantly higher than if the test had been performed before intubation or 48 h after intubation. It has been argued that the measurement of serum free cortisol concentration provides better assessment of adrenal gland function in critically ill patients, which may be especially true for patients with septic shock or multi-trauma where corticosteroid-binding globulin (CBG) levels are extremely low (2, 4, 51). The results of this study would further suggest that measurement of the delta free cortisol—the only HPA measurement unaffected by the variables tested—may represent the single best test for the evaluation of the HPA axis in subjects receiving intensive care therapies. Regardless, it is essential that the a normal HPA test range be determined prior to establishing laboratory criteria defining relative adrenal insufficiency in subjects receiving critical care treatment.
Supplementary Material
The mean (± SEM) total cortisol concentrations (nmol/L) for controls, low-dose dexamethasone (0.0138 mg/kg/hr) treated (L) and high-dose dexamethasone (0.275 mg/kg/hr) treated (H) animals both pre- and post-cosyntropin stimulation at each time point are shown. Each animal underwent low (1 μ) and high (5 μ/kg) dose cosyntropin stimulation tests. All time points are in reference to central line placement (time zero). Dexamethasone infusion was started at 6 h. The mean prestimulation values at 4, 24 and 72 h were calculated for all animals including those that did not receive cosyntropin challenges (i.e., including animals in the 4 stimulation group that did not undergo cosyntropin stimulations at these three time points).
Acknowledgments
This study was funded by intramural sources at the Critical Care Medicine Department, Clinical Center at the NIH
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet. 1991;337(8741):582–583. doi: 10.1016/0140-6736(91)91641-7. [DOI] [PubMed] [Google Scholar]
- 2.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350(16):1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 3.Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. The Journal of clinical endocrinology and metabolism. 2006;91(10):3725–3745. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 4.Beishuizen A, Thijs LG, Vermes I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive Care Med. 2001;27(10):1584–1591. doi: 10.1007/s001340101073. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfield RL, Helke J, Lucky AW. Dexamethasone preparation does not alter corticoid and androgen responses to adrenocorticotropin. The Journal of clinical endocrinology and metabolism. 1985;60(3):585–589. doi: 10.1210/jcem-60-3-585. [DOI] [PubMed] [Google Scholar]
- 6.Kintzer PP, Peterson ME. Treatment and long-term follow-up of 205 dogs with hypoadrenocorticism. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 1997;11(2):43–49. doi: 10.1111/j.1939-1676.1997.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Minneci PC, Deans KJ, Banks SM, et al. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141(1):47–56. doi: 10.7326/0003-4819-141-1-200407060-00014. [DOI] [PubMed] [Google Scholar]
- 8.Minneci PC, Deans KJ, Hansen B, et al. A Canine Model of Septic Shock: Balancing Animal Welfare and Scientific Relevance. 2007 doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- 9.Moon PFCK. Mechanical Ventilation. 9. Philadelphia: WB Saunders; 1992. [Google Scholar]
- 10.Gronert GA, Haskins SC, Steffey EP, et al. Plasma electrolyte and metabolite concentrations associated with pentobarbital or pentobarbital-propofol anesthesia during three weeks’ mechanical ventilation and intensive care in dogs. Lab Anim Sci. 1998;48(5):513–519. [PubMed] [Google Scholar]
- 11.Behrend EN, Weigand CM, Whitley EM, et al. Corticosterone- and aldosterone-secreting adrenocortical tumor in a dog. Journal of the American Veterinary Medical Association. 2005;226(10):1662–1666. 1659. doi: 10.2460/javma.2005.226.1662. [DOI] [PubMed] [Google Scholar]
- 12.Hauptman JG, Richter MA, Wood SL, et al. Effects of anesthesia, surgery, and intravenous administration of fluids on plasma antidiuretic hormone concentrations in healthy dogs. Am J Vet Res. 2000;61(10):1273–1276. doi: 10.2460/ajvr.2000.61.1273. [DOI] [PubMed] [Google Scholar]
- 13.Robertson SA, Hauptman JG, Nachreiner RF, et al. Effects of acetylpromazine or morphine on urine production in halothane-anesthetized dogs. Am J Vet Res. 2001;62(12):1922–1927. doi: 10.2460/ajvr.2001.62.1922. [DOI] [PubMed] [Google Scholar]
- 14.Kemppainen RJ, Thompson FN, Lorenz MD. Use of a low dose synthetic ACTH challenge test in normal and prednisone-treated dogs. Res Vet Sci. 1983;35(2):240–242. [PubMed] [Google Scholar]
- 15.Kemppainen RJ, Peterson ME, Sartin JL. Plasma free cortisol concentrations in dogs with hyperadrenocorticism. Am J Vet Res. 1991;52(5):682–686. [PubMed] [Google Scholar]
- 16.Kemppainen RJ, Clark TP, Peterson ME. Preservative effect of aprotinin on canine plasma immunoreactive adrenocorticotropin concentrations. Domest Anim Endocrinol. 1994;11(4):355–362. doi: 10.1016/0739-7240(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 17.Keller-Wood M. Fast feedback control of canine corticotropin by cortisol. Endocrinology. 1990;126(4):1959–1966. doi: 10.1210/endo-126-4-1959. [DOI] [PubMed] [Google Scholar]
- 18.Scheffé H. The analysis of variance. Wiley classics library. New York: Wiley-Interscience Publication; 1999. [Google Scholar]
- 19.Neter J, Wasserman W, Kutner MH. Applied linear regression models. 2. Homewood, Ill: Irwin; 1989. [Google Scholar]
- 20.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical care medicine. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 21.Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. The Journal of clinical endocrinology and metabolism. 1960;20:1539–1560. doi: 10.1210/jcem-20-12-1539. [DOI] [PubMed] [Google Scholar]
- 22.Meijer JC, de Bruijne JJ, Rijnberk A, et al. Biochemical characterization of pituitary-dependent hyperadrenocorticism in the dog. J Endocrinol. 1978;77(1):111–118. doi: 10.1677/joe.0.0770111. [DOI] [PubMed] [Google Scholar]
- 23.Kemppainen RJ, Thompson FN, Lorenz MD. Effects of dexamethasone infusion on the plasma cortisol response to cosyntropin (synthetic ACTH) injection in normal dogs. Res Vet Sci. 1982;32(2):181–183. [PubMed] [Google Scholar]
- 24.Miller RE, Maran JW, Meier GD, et al. Static gain and dynamics of cortisol secretory response to intravenous corticotropin (ACTH) in unanesthetized dogs. Analysis by a linear mean-squared estimator. Ann Biomed Eng. 1976;4(4):364–409. doi: 10.1007/BF02584526. [DOI] [PubMed] [Google Scholar]
- 25.Golder MP, Boyns AR. Selective uptake of radioactivity by the adrenal cortex of dexamethasone-treated guinea-pigs after the administration of 131 I-labelled 1–24 adrenocorticotrophin. J Endocrinol. 1972;53(2):277–287. doi: 10.1677/joe.0.0530277. [DOI] [PubMed] [Google Scholar]
- 26.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Kemppainen RJ, Sartin JL. Effects of single intravenous doses of dexamethasone on baseline plasma cortisol concentrations and responses to synthetic ACTH in healthy dogs. Am J Vet Res. 1984;45(4):742–746. [PubMed] [Google Scholar]
- 28.Kemppainen RJ, Sartin JL, Peterson ME. Effects of single intravenously administered doses of dexamethasone on response to the adrenocorticotropic hormone stimulation test in dogs. Am J Vet Res. 1989;50(11):1914–1917. [PubMed] [Google Scholar]
- 29.Cole TJ, Harris HJ, Hoong I, et al. The glucocorticoid receptor is essential for maintaining basal and dexamethasone-induced repression of the murine corticosteroid-binding globulin gene. Molecular and cellular endocrinology. 1999;154(1–2):29–36. doi: 10.1016/s0303-7207(99)00105-7. [DOI] [PubMed] [Google Scholar]
- 30.Emptoz-Bonneton A, Crave JC, LeJeune H, et al. Corticosteroid-binding globulin synthesis regulation by cytokines and glucocorticoids in human hepatoblastoma-derived (HepG2) cells. The Journal of clinical endocrinology and metabolism. 1997;82(11):3758–3762. doi: 10.1210/jcem.82.11.4362. [DOI] [PubMed] [Google Scholar]
- 31.Smith CL, Hammond GL. Hormonal regulation of corticosteroid-binding globulin biosynthesis in the male rat. Endocrinology. 1992;130(4):2245–2251. doi: 10.1210/endo.130.4.1547738. [DOI] [PubMed] [Google Scholar]
- 32.Hutson SM, Stinson-Fisher C, Shiman R, et al. Regulation of albumin synthesis by hormones and amino acids in primary cultures of rat hepatocytes. The American journal of physiology. 1987;252(3 Pt 1):E291–298. doi: 10.1152/ajpendo.1987.252.3.E291. [DOI] [PubMed] [Google Scholar]
- 33.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30(4):536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 34.Widmer IE, Puder JJ, Konig C, et al. Cortisol response in relation to the severity of stress and illness. The Journal of clinical endocrinology and metabolism. 2005;90(8):4579–4586. doi: 10.1210/jc.2005-0354. [DOI] [PubMed] [Google Scholar]
- 35.Gram LF, Christensen P. Benzodiazepine suppression of cortisol secretion: a measure of anxiolytic activity? Pharmacopsychiatry. 1986;19(1):19–22. doi: 10.1055/s-2007-1017143. [DOI] [PubMed] [Google Scholar]
- 36.Kalogeras KT, Calogero AE, Kuribayiashi T, et al. In vitro and in vivo effects of the triazolobenzodiazepine alprazolam on hypothalamic-pituitary-adrenal function: pharmacological and clinical implications. The Journal of clinical endocrinology and metabolism. 1990;70(5):1462–1471. doi: 10.1210/jcem-70-5-1462. [DOI] [PubMed] [Google Scholar]
- 37.Vaisanen M, Raekallio M, Kuusela E, et al. Evaluation of the perioperative stress response in dogs administered medetomidine or acepromazine as part of the preanesthetic medication (vol 63, pg 969, 2002) American Journal of Veterinary Research. 2002;63(10):1358–1358. doi: 10.2460/ajvr.2002.63.969. [DOI] [PubMed] [Google Scholar]
- 38.Engeland WC, Gann DS. Splanchnic nerve stimulation modulates steroid secretion in hypophysectomized dogs. Neuroendocrinology. 1989;50(2):124–131. doi: 10.1159/000125211. [DOI] [PubMed] [Google Scholar]
- 39.Judd AM, Call GB, Barney M, et al. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Annals of the New York Academy of Sciences. 2000;917:628–637. doi: 10.1111/j.1749-6632.2000.tb05428.x. [DOI] [PubMed] [Google Scholar]
- 40.Pignatelli D, Magalhaes MM, Magalhaes MC. Direct effects of stress on adrenocortical function. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1998;30(6–7):464–474. doi: 10.1055/s-2007-978915. [DOI] [PubMed] [Google Scholar]
- 41.Kemppainen RJ, Thompson FN, Lorenz MD, et al. Effects of continuous alpha (1–24) ACTH infusion in the dog. Hormone and metabolic research. 1985;17(2):58–62. doi: 10.1055/s-2007-1013452. [DOI] [PubMed] [Google Scholar]
- 42.Akana SF, Dallman MF, Bradbury MJ, et al. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131(1):57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 43.Dallman MF, Akana SF, Scribner KA, et al. Stress, Feedback and Facilitation in the Hypothalamopituitary-Adrenal Axis. Journal of Neuroendocrinology. 1992;4(5):517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 44.Annat G, Viale JP, Bui Xuan B, et al. Effect of PEEP ventilation on renal function, plasma renin, aldosterone, neurophysins and urinary ADH, and prostaglandins. Anesthesiology. 1983;58(2):136–141. doi: 10.1097/00000542-198302000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Cox JR, Davies-Jones GA, Leonard PJ, et al. The effect of positive pressure respiration on urinary aldosterone excretion. Clin Sci. 1963;24:1–5. [PubMed] [Google Scholar]
- 46.Arvat E, Di Vito L, Lanfranco F, et al. Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. The Journal of clinical endocrinology and metabolism. 2000;85(9):3141–3146. doi: 10.1210/jcem.85.9.6784. [DOI] [PubMed] [Google Scholar]
- 47.Golden DL, Lothrop CD., Jr A retrospective study of aldosterone secretion in normal and adrenopathic dogs. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 1988;2(3):121–125. doi: 10.1111/j.1939-1676.1988.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 48.Javadi S, Kooistra HS, Mol JA, et al. Plasma aldosterone concentrations and plasma renin activity in healthy dogs and dogs with hyperadrenocorticism. Vet Rec. 2003;153(17):521–525. doi: 10.1136/vr.153.17.521. [DOI] [PubMed] [Google Scholar]
- 49.Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 50.May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med. 1985;79(6):679–684. doi: 10.1016/0002-9343(85)90517-0. [DOI] [PubMed] [Google Scholar]
- 51.Ho JT, Al-Musalhi H, Chapman MJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. The Journal of clinical endocrinology and metabolism. 2006;91(1):105–114. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The mean (± SEM) total cortisol concentrations (nmol/L) for controls, low-dose dexamethasone (0.0138 mg/kg/hr) treated (L) and high-dose dexamethasone (0.275 mg/kg/hr) treated (H) animals both pre- and post-cosyntropin stimulation at each time point are shown. Each animal underwent low (1 μ) and high (5 μ/kg) dose cosyntropin stimulation tests. All time points are in reference to central line placement (time zero). Dexamethasone infusion was started at 6 h. The mean prestimulation values at 4, 24 and 72 h were calculated for all animals including those that did not receive cosyntropin challenges (i.e., including animals in the 4 stimulation group that did not undergo cosyntropin stimulations at these three time points).