Abstract
The use of low level laser (light) therapy (LLLT) has recently expanded to cover areas of medicine that were not previously thought of as the usual applications such as wound healing and inflammatory orthopedic conditions. One of these novel application areas is LLLT for muscle fatigue and muscle injury. Since it is becoming agreed that mitochondria are the principal photoacceptors present inside cells, and it is known that muscle cells are exceptionally rich in mitochondria, this suggests that LLLT should be highly beneficial in muscle injuries. The ability of LLLT to stimulate stem cells and progenitor cells means that muscle satellite cells may respond well to LLLT and help muscle repair. Furthermore the ability of LLLT to reduce inflammation and lessen oxidative stress is also beneficial in cases of muscle fatigue and injury. This review covers the literature relating to LLLT and muscles in both preclinical animal experiments and human clinical studies. Athletes, people with injured muscles, and patients with Duchenne muscular dystrophy may all benefit.
Keywords: low level laser therapy, muscle fatigue, muscle injury, mitochondria, ATP, reactive oxygen species, satellite cells
1 Introduction to muscle fatigue
The intense use of muscles during high intensity exercise or during repeated muscle contractions leads to a decrease in muscle performance and appearance of peripheral muscle fatigue [1, 2]. Muscle fatigue is a complex phenomenon with many theories and scientific evidence to explain its process of appearance. Among the scientific evidence, we highlight the depletion of energy sources such as phosphocreatine, glycogen; increased amounts of phosphate inorganic (Pi), adenosine diphosphate (ADP), Ca2+, Mg2+, H+ and lactate; decreased sensitivity of myofibrils to Ca2+ and higher production or accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during exercises [1–3].
Peripheral muscle fatigue can affect one or more of the following events during muscle contraction [1, 2]: (i) action potential generation at the neuromuscular junction, (ii) propagation of action potential along the sarcolemma and also through the T-tubule system, (iii) activation of voltage-dependent sensors at the walls of T-tubules for opening Ca2+ channels in the sarcoplasmic reticulum, (iv) Ca2+ release from the sarcoplasmic reticulum into the sarcoplasm, (v) binding of Ca2+ to troponin C (TnC) and movement of tropomyosin that exposes the binding site of actin with myosin, (vi) formation of cross-bridges and beginning of muscle contraction, (vii) constant pumping of Ca2+ into the sarcoplasmic reticulum and decreased concentration of Ca2+ in the sarcoplasm, and (viii) muscle relaxation.
The energy sources for adenosine tri-phosphate (ATP) synthesis used during muscle contraction may be predominantly aerobic (oxidative) or anaerobic metabolism (lactic and alactic) [1, 2]. The aerobic metabolism obtain energy from the Krebs cycle through of the oxidation of acetyl coenzyme A (acetyl-CoA) and reduction of cofactors as nicotinamide dinucleotide (NAD) and flavin dinucleotide (FAD) to provide protons and electrons to the electron transport chain (ETC) in the mitochondrial cristae. In the ETC oxygen (O2) is the final acceptor of protons and electrons to give syntheses of ATP and producing metabolic water. Considering anaerobic metabolism, O2 does not participate in ATP synthesis: (i) ATP can be produced from hydrolysis of phosphocreatine (anaerobic alactic metabolism) and/or (ii) produced from the oxidation of nicotinamide adenine dinucleotide reduced form (NADH) + H by pyruvate from glycolysis. In addition, during the second process there is production of lactate (anaerobic lactic metabolism) [1, 2].
There are different types of physical exercises, which promote specific adaptations in muscle tissue and biochemical adjustments (energy metabolism) as well as structural changes that lead to better physical performance [2, 4–8].
Strength training or high intensity exercises (i) promotes a greater energy recruitment from anaerobic metabolism (metabolic change), (ii) increases the crosssectional area of skeletal muscle (hypertrophy) through micro-lesions, (iii) modifies the contractile characteristics of the muscle fibers (transition between type I, IIx and IIb fibers to type IIa – structural change); (iv) increases the recruitment, timing and frequency of firing of muscle motor units in activity (neural change) and leads to the development of muscular strength [1, 2, 6–8].
In contrast, endurance or low intensity exercises (i) promotes a greater energy recruitment from aerobic metabolism (metabolic change), (ii) stimulates muscle fibers to develop more mitochondria, increasing size of those already in existence and provides a greater mitochondrial density and oxidative enzymes in the muscle fibers (predominantly type I fibers – structural change), (iii) increases ATP synthesis by mitochondrial pathway and increases resistance to muscle fatigue in exercises [2, 4, 5, 9–11].
Some authors have used low-level laser therapy (LLLT) to accelerate these metabolic and structural changes in muscle with the goal of preventing or reducing muscle fatigue [12–17]. Studies on this issue have used experimental models to identify possible effects of LLLT on muscle tissue subjected to mechanical and metabolic stress from exercise. In particular, resistance to fatigue and improved muscle energy metabolism have been the focus of these pioneering researches [18, 19].
This review select studies involving the use of LLLT for improvement of muscle performance and/or recovery of injuries of muscle tissue acquired from mechanical and metabolic stress that physical exercise can produce. Thereby, studies in experimental models and clinical trials on this topic were included in this revision as well as studies using light emitting-diode therapy (LEDT) for the same purposes. In addition, studies involving mechanisms of action about LLLT, LEDT and its effects on biological tissues were included.
2 Low-level laser therapy and light-emitting diode therapy for improvement of muscle performance
2.1 Low-level laser therapy – Experimental models
Lopes-Martins et al. [18] reported the effects of LLLT (655 nm) on muscle fatigue in rats. Tibialis anterior muscle fatigue was induced by neuromuscular electrical stimulation and measured the reduction of torque and increased muscle damage from blood levels of creatine kinase (CK). LLLT was applied at a single point of the tibialis anterior before fatigue induction. The results showed a reduced fatigue at dose of 0.5 J/cm2 and decreased muscle damage at doses of 1.0 and 2.5 J/cm2.
Vieira et al. [19] verified the effects of LLLT (780 nm) on energy metabolism related to muscle fatigue in rats trained on a treadmill with load corresponding to the anaerobic threshold for 30 consecutive days. After each workout, rats were irradiated on a single point on the femoral quadriceps, tibialis anterior, soleus and gluteus maximus. The results showed a greater inhibition of enzymatic activity of lactate dehydrogenase (LDH), especially the LDHA isoform (pyruvate reductase) in the muscles of trained and irradiated rats, including also heart muscle (not irradiated), suggesting there were systemic effects of LLLT.
The results of these previous studies [18, 19] encouraged other researchers to develop more experimental studies in order to identify other interactions between LLLT and muscle tissue subjected to different physical exercises, as well as the mechanisms of action of LLLT to reduce damage and muscle fatigue [20–24], see Table 1.
Table 1.
LLLT and exercise: experimental models.
| References | LLLT parameters | Muscle(s) | Exercise | Application mode | ||
|---|---|---|---|---|---|---|
| Light source/wavelength | Diode area (cm2) | Performance characteristics | ||||
| [18] | Laser 655 nm | 0.08 |
|
Tibialis anterior | Neuromuscular electric stimulation | Contact, before exercise |
| [19] | Laser 780 nm | 0.039 |
|
Femoral quadriceps, gluteus maximum, tibialis anterior, soleus | Running on treadmill | Contact, after exercise |
| [20] | Laser 904 nm | 0.2 |
|
Tibialis anterior | Neuromuscular electric stimulation | Contact, before exercise |
| [21] | Laser 632 nm | 0.2 | Groups:
|
Gastrocnemius | Downhill running on treadmill | Contact, after exercise |
| [22] | Laser 660 nm | 0.03 |
|
Gastrocnemius | Swimming with load | Contact, after exercise |
| [23] | Laser 904 nm | 0.2 |
|
Tibialis anterior | Neuromuscular electric stimulation | Contact, before exercise |
Liu et al. [21] trained rats on a treadmill in the declined plane (−16 ° slope) at speed of 16 m/min until the animals exhaustion. These authors applied LLLT (632.8 nm) on single point on gastrocnemius muscle and the results showed inhibited inflammation, reduced CK activity in blood serum, reduced muscle levels of malondialdehyde (MDA) 24 h and 48 h after exercise and increased activity of superoxide dismutase (SOD) which is a antioxidant enzyme.
Other studies also verified the effects of LLLT in rats underwent to exercise [22] and induced fatigue by neuromuscular electrical stimulation [20, 25]. Sussai et al. [22] investigated the effects of LLLT (660 nm) on CK levels in blood plasma and muscle cell apoptosis of rats after a swimming protocol for induction of muscle fatigue. The LLLT was applied for 40 s on a single point of the gastrocnemius muscle immediately after fatigue protocol. Compared to the control group, LLLT group had lower levels of CK and apoptosis 24 h and 48 h after induction of muscle fatigue.
Leal Junior et al. [20] developed an experimental model very similar to the study of Lopes-Martins et al. [18]. The authors investigated the effects of LLLT (904 nm) in rats muscle fatigue induced by neuromuscular electrical stimulation. The LLLT was applied on a single point on the tibialis anterior with different treatment times and total energies before muscle fatigue induction. Groups with 1 and 3 J had the highest force peak compared to the control group (without LLLT), and groups with 0.1 J and 0.3 J. The blood lactate levels were lower in all groups irradiated with LLLT. The CK level in blood was also lower in irradiated groups except for group 3 J [20].
Using a similar protocol, de Almeida et al. [23] reproduced the work of Leal Junior et al. [20] and identified that LLLT (904 nm and energy of 1 J) significantly decreased CK level in blood, reduced levels of mRNA expression for protein cyclooxygenase (COX)-2 and increased the expression of COX-1 compared to other groups.
2.2 Low-level laser therapy – Clinical trials: acute responses
The vast majority of papers involving LLLT and exercise in humans investigated the acute effects of this therapy on muscle performance in high-intensity exercises [25–31]. Only three studies have investigated the chronic effects of this therapy [32–34].
One of first published works was a clinical pilot study conducted by Gorgey et al. [26]. The authors applied the LLLT (808 nm) in pulsed mode (pulse repetition of 1–10,000 Hz) for 5 min (low energy) and 10 min (high energy) on the femoral quadriceps before muscle fatigue induction by neuromuscular electrical stimulation. The results showed that LLLT groups had a lower percentage of muscle fatigue compared to the control group, which had statistical significance. There was no significant difference between groups irradiated with LLLT.
Leal Junior et al. [27–29] and de Almeida et al. [25] investigated the effects of LLLT on the biceps brachii performance in double-blind placebo-controlled trial with very similar methodologies. Leal Junior et al. [27] investigated the effects of LLLT (655 nm) applied on the biceps brachii before Scott bench exercise. The exercise was performed with 75 % of the load corresponding to maximum voluntary contraction (MVC) until exhaustion. Five points were irradiated for 100 s on each point. The results showed a significant increase in number of repetitions of the LLLT group compared to placebo group. However, there was no increase in time of exercise and there was no decreasing in blood lactate levels.
With the same experimental design these authors used other LLLT (830 nm) applied on biceps brachii at four points [28]. The radiation time was 50 s at each point before starting exercise in Scott bench. The results were the same previously reported: LLLT group increased the number of repetitions compared to placebo.
De Almeida et al. [25] attempted to identify in a single study which wavelength would be better to increase the biceps brachii performance in high-intensity exercise. The LLLT (660 nm or 830 nm) was applied on biceps brachii at four points for 100 s on each point before starting the Scott bench exercise. The LLLT groups (660 nm or 830 nm) had a greater force and force peak compared to placebo. However, there was no significant difference between irradiated groups.
Another work reporting a double-blind placebo-controlled and randomized trial also involved biceps brachii fatigue [29]. This study investigated the effects of LLLT (cluster with 5 diodes of 810 nm) applied before Scott bench exercise with 75 % of MVC until exhaustion. Two points of the biceps brachii were irradiated for 30 s on each point. There were increases in number of repetitions and time of exercise, decreasing levels of lactate, CK and c-reactive protein after exercise.
Baroni et al. [30] investigated the effects of LLLT (cluster with 5 diodes of 810 nm) on energetic metabolism, muscle damage and delayed onset muscle soreness (DOMS) in young males after 5 sets of 15 eccentric contractions of the femoral quadriceps on the isokinetic dynamometer. There were 6 points of LLLT radiation on the femoral quadriceps during 30 s on each point. The LLLT applied before exercise promoted a smaller increase of lactate dehydrogenase (LDH) activity at 48 h after exercise, a smaller increase of CK in blood at 24 h and 48 h after exercise and less loss of MVC immediately and 24 h after exercise. However, the DOMS measured by visual analog scale (VAS) was equal to the LLLT and placebo groups.
De Marchi et al. [31] verified the effects of LLLT (cluster with 5 diodes of 810 nm) on fatigue, oxidative stress, muscle damage and human physical performance on treadmill. Six points of the femoral quadriceps were irradiated as performed by Baroni et al. [30], 4 points on the hamstrings and two points on gastrocnemius muscles were irradiated before progressive exercise performed on treadmill until exhaustion. The time of radiation was 30 s on each point. LLLT group increased absolute and relative maximal oxygen uptake as well as the time of exercise compared to placebo. The activities of LDH, muscle damage (CK) and lipid damage (thiobarbituric acid reactive substances, TBARS) were all significantly higher only in placebo group after exercise. The activity of SOD, an antioxidant enzyme, was also decreased only in the placebo group after exercise.
2.3 Low-level laser therapy – Clinical trials: chronic responses
Unlike previous researches, Ferraresi et al. [32] and Vieira et al. [33] verified the effects of LLLT on physical training programs in randomized and controlled clinical trials.
Ferraresi et al. [32] investigated the effects of LLLT (cluster with 6 diodes of 808 nm) on a physical training program with load corresponding to 80 % of one repetition maximum (1RM). The training program was performed twice a week for 12 weeks and LLLT was applied immediately after each workout. Seven regions of the femoral quadriceps were irradiated for 10 s per region. The LLLT group had a greater percentage gain of 1RM compared to the trained group without LLLT and the control group after training program. Only the LLLT group significantly increased the mean of peak torque and peak torque of knee extensor muscles in isokinetic dynamometry.
The second study was reported by Vieira et al. [33]. This study verified the effects of LLLT (cluster with 6 diodes of 808 nm) on a physical training program of low intensity (load relative to the anaerobic ventilatory threshold) on stationary bicycle. The physical training was conducted three times a week for 9 consecutive weeks. Five regions of the femoral quadriceps were irradiated with LLLT for 10 s per region. LLLT group was only group to decrease the fatigue index of the knee extensor muscles in isokinetic dynamometry.
Ferraresi et al. [34] conducted a study showing modulations in gene expression of human muscle by LLLT (cluster with 6 diodes of 808 nm). With the same experimental design and parameters of LLLT used in previous study [32], the authors investigated changes of the load at 1RM test and modulation of gene expressions in muscle tissue of the 10 young males allocated into two equal groups: LLLT and without LLLT. Biopsies from the vastus lateralis muscle were performed before and after the training program and analyzed by microarrays to identify modulation of gene expression throughout the whole human genome. Preliminary analysis identified that the LLLT group had more significant increase of load at 1RM test, the genes PPARGC1-α (mitochondrial biogenesis), mTOR (protein synthesis and muscle hypertrophy) and vascular endothelial growth factor (VEGF) (angiogenesis) were significantly upregulated only in the LLLT group compared to the non-LLLT control group. In addition, only in the LLLT group the genes MuRF1 (protein degradation and muscle atrophy) and IL-1β (inflammation) were downregulated [34].
2.4 Light-emitting diode therapy and exercise – Clinical trials: acute responses
Recently LEDT has begun to be investigated for the same previous purpose that LLLT was tested for; i.e., increased performance in human physical exercises [35–38]. LEDT are more accessible light sources and have a larger area compared to the light emitting laser diode.
Despite of the study conducted by Vinck et al. [35] does not have as primary objective an evaluation of muscle performance, this study was the first to report the effects of LEDT on delayed onset muscle soreness and human muscle performance in isokinetic dynamometer. These authors investigated the effects of LEDT (cluster with 32 LEDs of 950 nm) on DOMS after fatigue induction of the biceps brachii using an isokinetic dynamometer. The authors found no significant differences between groups LEDT and placebo for the peak torque and the level of pain measured by algometer and VAS.
Leal Junior et al. [36] compared the effects of a cluster with 69 LEDs (34 LEDs of 660 nm and 35 LEDs of 850 nm) with the single LLLT diode (810 nm) on the physical performance of volleyball players. Three tests of high intensity effort (Wingate test) were conducted on nonconsecutive days, during 30 s each test. The LEDT (cluster with 69 LEDs) or LLLT or placebo treatments were administered on two points on the belly of the rectus femoris muscle for 30 s of radiation on each point before starts the tests. LEDT group had a significant reduction of CK levels in blood after exercise compared to LLLT and placebo group. However, there was no increased performance and significant reduction of lactate levels in LEDT group compared to other groups.
Using the same LED cluster employed in his previous study [36], Leal Junior et al. [37] applied the LEDT for 30 s of radiation on a single point on the belly of biceps brachii muscle before exercise at 75 % MVC at Scott bench. The LEDT group increased the number of repetitions and time of contraction in the proposed exercise. Also reduced CK levels in blood, lactate and c-reactive protein (CRP) after exercise compared to placebo.
Baroni et al. [38] applied LEDT on three points of the femoral quadriceps muscle before starting a protocol of isokinetic dynamometry for induction of muscle fatigue. The radiation time was 30 s on each point. The results showed that the LEDT was effective to prevent the decrease in knee extensor peak torque.
Comparing the effects of LEDT with other modalities of treatment, Leal Junior et al. [39] tested the effects of cryotherapy and LEDT (cluster with 69 LEDs) on muscle recovery in a randomized double-blind placebo-controlled trial. Six athletes performed three Wingate tests on three different days. After each test, the athletes received placebo or LEDT or cryotherapy (5 min of body immersion in water at 5° C). The LEDT was applied on two points of the femoral quadriceps, two points on hamstrings and two points on gastrocnemius. The LEDT significantly decreased CK and lactate levels in blood but did not modulate CRP.
2.5 Parameters employed in LLLT and LEDT studies
LLLT and LEDT have been used in different ways among researchers. These differences comprise the number of radiation points on the muscle group, the parameters used and the timing of radiation: before or after exercise.
The number of irradiation points appears to be an important parameter to effectively cover the muscle group [26, 31–33, 35–39], the irradiation points should be designed to cover the largest area and better distribute the energy applied on muscles [32, 33]. Results of studies using clusters of LLLT or LEDT appear to be better for reducing muscle fatigue and increasing muscle performance compared to the use of a single diode [27, 29, 32, 33, 36]. Figure 1 illustrates some examples of the number and distribution of the irradiation points using LLLT or LEDT on femoral quadriceps.
Figure 1.
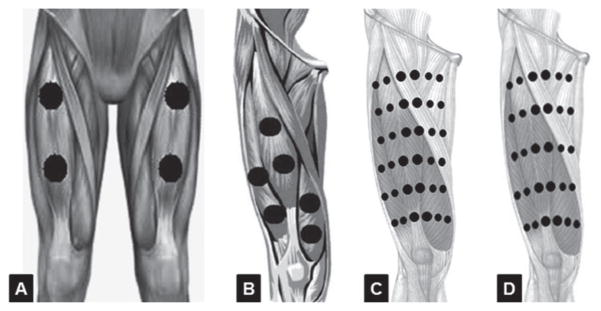
Number of LLLT or LEDT radiation points applied on femoral quadriceps muscle based on references [36] (A), [31] (B), [32] (C) and [33] (D).
The parameters of LLLT and LEDT (particularly wavelength) used in experimental models and in clinical trials vary. However, near infrared (NIR) radiation seems to be the most common wavelength used in clinical trials [26, 28–36], although some studies have used clusters of LEDs with mixed wavelengths (red and near infrared) in the same device [37–39].
The dosimetry and time of radiation of LEDT or LLLT on the muscles are still under investigation in clinical trials [26, 28–39]. There is a wide variation in energy, power, power density and irradiation time; but there is a consensus on how to apply (contact). The clinical trials that targeted the acute effects of LLLT on muscle fatigue increased the total energy (J), power (W) and power density (W/cm2) used at each study [27–31, 36], as reported at Table 2. However, the total energy and power were lower in clinical trials that target chronic effects [32–34].
Table 2.
LLLT and exercise in humans.
| References | LLLT parameters | Muscle(s) | Exercise | Application mode | ||
|---|---|---|---|---|---|---|
| Light source/wavelength | Diode area (cm2) | Performance characteristics | ||||
| [25] | Laser 660 nm versus 830 nm | 0.0028 |
|
Biceps brachii | MVC per 60 s in Scott bench | Contact, before exercise |
| [26] | Cluster with 4 laser diodes 808 nm |
|
Femoral quadriceps | Isokinetic dynamometer | Scanning, before exercise | |
| [27] | Laser 655 nm | 0.01 |
|
Biceps brachii | Scott bench | Contact, before exercise |
| [28] | Laser 830 nm | 0.0028 |
|
Biceps brachii | Scott bench | Contact, before exercise |
| [29] | Cluster with 5 laser diodes 810 nm | 0.0364 |
|
Biceps brachii | Scott bench | Contact, before exercise |
| [30] | Cluster with 5 laser diodes 810 nm | 0.029 |
|
Femoral quadriceps | Isokinetic dynamometer | Contact, before exercise |
| [31] | Cluster with 5 laser diodes 810 nm | 0.0364 |
|
Femoral quadriceps, hamstrings, gastrocnemius | Running on treadmill until exhaustion | Contact, before exercise |
| [32] | Cluster with 6 laser diodes 808 nm | 0.0028 |
|
Femoral quadriceps | Leg press and isokinetic dynamometer | Contact, after exercise |
| [33] | Cluster with 6 laser diodes 808 nm | 0.0028 |
|
Femoral quadriceps | Cicloergometer and isokinetic dynamometer | Contact, after exercise |
| [34] | Cluster with 6 laser diodes 808 nm | 0.0028 |
|
Femoral quadriceps | Leg press and isokinetic dynamometer | Contact, after exercise |
Clinical trials which used LEDT before exercise used total energies much higher to reduce muscle fatigue than those used in studies using LLLT [36–38]. However, power and power density were smaller as might be expected from the larger spot size [36–38], see Table 3. There is no consensus on the best parameters to be used in LLLT or LEDT. So, more studies are needed to investigate the dose-response of these therapies to reduce muscle fatigue and to repair muscle damage [40]. Tables 1–3 show the all parameters of LLLT and LEDT, muscle group irradiated, type of exercise performed and the mode of application in experimental models (Table 1), LLLT in humans (Table 2) and LEDT in humans (Table 3).
Table 3.
LEDT and exercise in humans.
| References | LEDT parameters | Muscle(s) | Exercise | Application mode | ||
|---|---|---|---|---|---|---|
| Light source/wavelength | Diode area (cm2) | Performance characteristics | ||||
| [35] | Cluster with 32 LEDs 850 nm | 18 (Cluster total area) |
|
Biceps brachii | Isokinetic dynamometer | Contact, after exercise |
| [36] | Laser 810 nm versus Cluster with 69 LEDs 660/850 nm |
0.036 versus 0.2 |
versus
|
Rectus femoris | Wingate test | Contact, before exercise |
| [37] | Cluster with 69 LEDs 660/850 nm | 0.2 |
|
Biceps brachii | Scott bench | Contact, before exercise |
| [38] | Cluster with 69 LEDs 660/850 nm | 0.2 |
|
Femoral quadriceps | Isokinetic dynamometer | Contact, before exercise |
| [39] | Cluster with 69 LED 660/850 nm | 0.2 |
versus
|
Femoral quadriceps, hamstrings and gastrocnemius | Wingate test | Contact, after exercise |
3 Mechanisms of action of LLLT and LEDT for performance, repair and to prevent muscle fatigue
Clinical trials using LLLT and LEDT before exercise reported a preventive effect against mitochondrial dysfunction and muscle damage mediated by ROS and RNS, as well as modulation of energetic metabolism [18, 20, 25–31, 36–38]. LLLT and LEDT radiation after the physical exercise aims not only to remedy the mitochondrial and metabolic dysfunction, but also the repair microlesions produced from mechanical and metabolic stress resulting from muscle contraction and ROS/RNS [19, 21, 22, 32–34, 39]. LLLT or LEDT radiation before or after physical exercises has distinct features, but also has common mechanisms of action. The next sub-sections will describe the mechanisms of action of LLLT and LEDT to prevent muscle fatigue and to repair muscle damage.
3.1 Energetic metabolism
3.1.1 Mitochondrial pathway
The infrared radiation emitted by LLLT and LEDT radiation seems to act on cellular energy metabolism, stimulating photochemical and photophysical events in the cell mitochondria [16, 32, 33, 40–44]. Photochemical and photophysical changes may result in increased mitochondrial membrane potential [45] and higher enzyme activity in the respiratory chain [40–43]. The structural change includes the formation of giant mitochondria through the merging of membranes of smaller and neighboring mitochondria [46]. These changes enable mitochondria to provide higher levels of respiration and ATP to cells [32, 33, 41, 43]. Some studies reported an improvement of enzyme activity of the complex IV (cytochrome c oxidase, CCO) [42] in the mitochondrial ETC in skeletal muscle [41]. Silveira et al. [43] showed that all complexes of the mitochondrial ETC (complexes I, II, III and IV) had their enzyme activity improved after NIR radiation.
Considering the effects of LLLT and LEDT on mitochondria, some hypotheses have been proposed to take advantage of the beneficial effects of LLLT and LEDT on endurance (a) and also on strength exercises (b):
Endurance or low intensity exercises: the oxidative capacity of muscle fibers is proportional to its mitochondrial density, since these cellular organelles can completely oxidize energy substrates (glucose, fatty acids and proteins) for ATP synthesis during muscle contraction [2, 4, 5, 9–11]. Endurance or low intensity exercise is a powerful stimulus to promote mitochondrial biogenesis in its own right, favoring aerobic metabolism and reducing muscle fatigue from metabolic origin, as the accumulation of Pi, ADP, H+ and lactate in the sarcoplasm [1, 2, 10, 11]. However, when LLLT and/or LEDT is added to the effects of endurance exercise on the mitochondria, the adaptive process can be increased. Giant and more functional mitochondria (higher enzyme activity) can provide high levels of cellular respiration and ATP synthesis [40, 41, 43] during these exercises, which gives increased oxygen consumption [31] and reduced muscle fatigue [33].
-
Strength or high intensity exercises: these types of exercise have anaerobic metabolism, that can be lactic and alactic [1, 3] and cover exercise performed in most studies involving LLLT [25–32] and LEDT in humans [35–38]. LLLT and LEDT may increase muscle performance and reduce fatigue by three mechanisms suggested by Ferraresi et al. [32] and Vieira et al. [33]:
-
b.1)
Mitochondrial ATP: LLLT and LEDT seem to increase mitochondrial activity, providing higher levels of cell respiration and ATP synthesis [40, 41, 43]. As the muscle fiber recruitment is hierarchical and depends on fiber type (type I, IIa, IIb or IIx, respectively) [47], larger amounts of ATP can be provided by those fibers with oxidative potential (type I fibers and IIa) during strength training or high intensity exercises [32].
-
b.2)
Phosphocreatine re-synthesis: strength or high intensity exercise consumes large amounts of ATP from hydrolysis of phosphocreatine (PCr), which is catalyzed by muscle CK enzyme in the sarcoplasm: PCr + ADP + Cr ↔ ATP. This consumption of ATP is faster than the rate of PCr resynthesis, producing an excess of creatine (Cr), ADP and Pi in the sarcoplasm of muscle fibers (IIa, IIb and IIx especially), contributing to the process of fatigue [1, 2]. However, high concentrations of Cr and ADP stimulate respiration and mitochondrial ATP synthesis in muscles fibers of type I and IIa (high and mean mitochondrial density, respectively) [5], integrating aerobic and anaerobic alactic metabolisms in this type of exercise (Figure 2 A–C) [32]. This metabolic integration is caused by PCr re-synthesis by the mitochondrial Cr shuttle [5, 32, 33]. This shuttle system captures the Cr, ADP and Pi from ATP consumed during muscle contraction and transports it to the mitochondrial matrix, crossing the inner mitochondrial membrane through an adenine nucleotide translocase. The mitochondrial ATP is delivered to muscle using the same pathway, but in the opposite direction, providing energy for the PCr re-synthesis which is catalyzed by CK near the site of muscle contraction. Together with the use of PCr energy to re-synthesize ATP for muscle contraction, there is also renewed production of Cr, ADP and Pi. As both ADP and Pi follow the pathway previously described, the Cr is transported to the inter-membrane space of muscle mitochondria where the mitochondrial CK catalyzes the reaction of PCr re-synthesis using also mitochondrial ATP. Finally, the PCr is transported to the site of muscle contraction and provides the energy necessary for contraction and increasing the ratio of ATP/ADP (Figure 2B). Thus, the PCr re-synthesis that occurs during intervals of exercises [32] as well as during high intensity exercises [33] could provide more ATP re-synthesis and could become the predominant energy source for maximal tests of muscle strength or during short high intensity exercises [32].
-
b.3)
Lactate oxidation by mitochondria: blood lactate has been used to measure muscle fatigue during strength training or high intensity exercise [1, 2]. When the oxygen supply is insufficient and/or delayed for optimal mitochondrial ATP synthesis, pyruvate is reduced to lactate by lactate dehydrogenase in the glycolysis process. This reaction is catalyzed by cytosolic LDH with production of lactate [48]. Lactate is transported to the mitochondrial matrix by monocarboxylate transporters and by means of NAD+ (oxidized nicotinamide adenine dinucleotide) and the enzyme mitochondrial lactate dehydrogenase, lactate is oxidized to pyruvate. Next, the reduced NAD (NADH) is oxidized in the ETC (respiratory chain) and provides electrons and protons required for mitochondrial ATP synthesis. Pyruvate is oxidized to acetyl-CoA in the Krebs cycle and continues to be oxidized for ATP production in the ETC (Figure 2C). This mechanism of lactate oxidation in mitochondria was first proposed by Brooks et al. [48] and recently discussed by Ferraresi et al. [32].
-
b.1)
Figure 2.
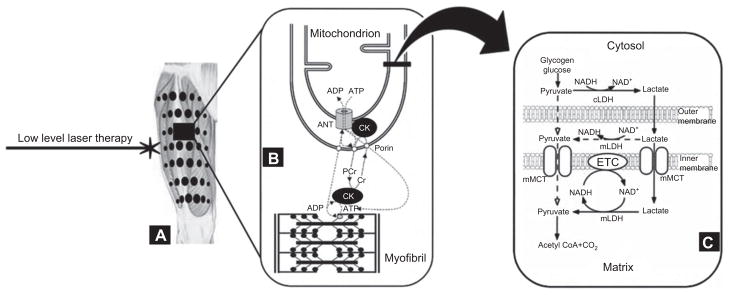
Based on reference [32]: (A) LLLT radiation points on femoral quadriceps muscle. (B) Mitochondrial creatine shuttle mechanism. (C) Lactate oxidation by mitochondrial pathway.
3.1.2 Enzyme modulation
LDH is the enzyme responsible for the reduction of pyruvate to lactate (Pyruvate + NADH + H ↔ H-lactate + NAD+) for ATP synthesis in anaerobic lactic metabolism. The LDH activity is often combined with blood lactate measurements to infer the magnitude of this energy metabolism, as well as the intensity of exercise and how efficient is the buffering of lactic acid to prevent metabolic acidosis (H-lactate + NaHCO3 → Na-CO2 + H2 O + lactate) and to prevent fatigue [1, 2, 33].
LLLT seems to modulate LDH activity in physical exercise, as demonstrated and suggested in previous studies [19, 30, 31, 33]. These studies demonstrated that LDH activity is inhibited by LLLT even in the period in which the supply of O2 is slow or inadequate for mitochondrial ATP synthesis, to enhance muscle performance [19, 30, 31, 33].
The enzymes of aerobic metabolism are also modulated by LLLT and LEDT such as complexes I, II, III and IV of the mitochondrial ETC [40–43] stimulating aerobic metabolism. Other enzymes of aerobic metabolism are possibly modulated by LLLT and LEDT such as citrate synthase and other enzymes of the Krebs cycle because the Krebs cycle provides part of the protons and electrons necessary for ATP synthesis in the ETC.
3.2 Reactive oxygen species and reactive nitrogen species
Superoxide anion (O2•−) is the primary free radical ROS produced mainly by mitochondria and nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase in skeletal muscle [3, 49]. In the mitochondria O2•− is produced as a byproduct of oxidative phosphorylation and therefore its formation is always associated with mitochondrial activity, especially during physical exercise [1, 3, 49]. O2•− is produced mainly at complexes I and III of the ETC which release O2•− in the mitochondrial matrix and at the inter-membrane space, respectively. The production rate of O2•− is approximately 0.15 % of oxygen consumption in mitochondria together with the enzymatic activities of NADPH oxidase, xanthine oxidase and lipoxygenase that also produce O2•− [1, 3, 49]. Beyond O2•−, hydrogen peroxide (H2 O2) and hydroxyl radicals (OH•) are also important ROS [1, 3, 49].
Nitric oxide (NO•) is the primary nitrogen free radical. NO• is synthesized from L-arginine amino acid by nitric oxide synthase (NOS). NO• can be formed from the inorganic anions, nitrate (NO3−) and nitrite (NO2−) and can interact with superoxide anion (O2•−) to form peroxynitrite (ONOO−) [1, 3, 49]. In adult skeletal muscle there is expression of the three isoforms of NOS: neuronal (nNOS or type 1), inducible (iNOS or type 2) and endothelial (eNOS or type 3). However, the predominant isoform is nNOS [1, 3, 49].
ROS and RNS are produced by mitochondria, by NADPH oxidase activity in the sarcoplasmic reticulum and transverse tubules, and also by phospholipase A2 and xanthine oxidase [49]. However, there are defenses against ROS and RNS activity in cells and tissues. These defenses are mainly the antioxidant activity of SOD, glutathione peroxidase (GPX) and catalase (CAT) [49]. There are three isoforms of SOD (SOD1 – 3) and five isoforms of GPX (GPX1 – 5), each with its specific location in the cell and extracellular places. For detailed information see the review by Powers and Jackson [49].
O2•− is rapidly broken down into H2 O2 by antioxidant enzymes (reaction 1) such as SOD-2. H2 O2 is broken down by catalase (reaction 2) and GPX with conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG) (reaction 3) [1, 3, 49]. Moreover, H2 O2 can react with metals such as Fe2+ (reaction 4: Fenton reaction), which produces hydroxyl radicals (OH• and OH−) which are extremely reactive [1, 3, 49]. Fe3+ is present in myoglobin, hemoglobin, CCO, and can be reduced to Fe2+ by superoxide radicals and then produce hydroxyl radicals (reaction 5: Haber – Weiss reaction). Hydroxyl radicals have a short half-life because they react with any organic molecules and can produce damage of proteins, DNA and lipids that are present in the cell membrane and therefore lead to tissue damage [1, 3, 49].
Reaction 1: O2•− + O2•− + 2 H+ → H2 O2 + O2
Reaction 2: 2 H2 O2 → 2 H2 O + O2
Reaction 3: 2 GSH + H2 O2 → GSSG + 2 H2 O
Reaction 4: (Fenton reaction): H2 O2 + Fe2+ → Fe3+ + OH• + OH−
Reaction 5: (Haber–Weiss reaction): O2•− + H2 O2 → OH• + OH− + O2
During physical activity the contraction of skeletal muscle is the main source of ROS and RNS production and produces deleterious effects on muscle fibers, such as cellular and tissue damage, loss of contractile function and exercise performance [1, 3, 49, 50]. ROS and RNS can induce early onset of muscle fatigue in exercise through mechanisms involved in muscle contraction [1, 49] such as:
Decreased ATP synthesis: NO• production in the mitochondria can reduce ATP synthesis by inhibition of CCO, muscle CK and glyceraldehyde-3-phosphate dehydrogenase (glycolytic pathway) thereby reducing ATP production [49].
Regulation of sarcoplasmic reticulum and Ca2+ release: ROS increases Ca2+ content in the sarcoplasm and promotes a slow Ca2+ reuptake [1, 49]. ROS also inhibits the activity of the ATPase enzyme of the Ca2+ pump in the sarcoplasmic reticulum (SERCA) leading to impairment of hydrolysis of ATP used for operating Ca2+ pump [1, 49]. NO• also inhibits the activity of SERCA and results in decreased reuptake of Ca 5 from the sarcoplasm to the sarcoplasmic reticulum [49]. Prolonged exposure to ROS and RNS can induce a lasting release of Ca 5 through changes of the receptor sensitivity of channels for Ca 5 of the sarcoplasmic reticulum [3].
Contractile proteins: ROS and RNS can change the structure of contractile proteins; decrease the sensitivity of myofibrils to Ca2+; oxidize actin, myosin and troponin C impairing the formation of cross bridges, muscle contraction and production of muscle strength [1, 3, 49, 50].
Potential action: ROS seems to reduce the Na+-K+ pump activity, increase amounts of extracellular K+ that reduces the action potential for muscle fiber depolarization during exercise, contributing to the onset of early muscle fatigue [3].
ROS and RNS after muscle fatigue: ROS decreases the sensitivity of myofibrils to Ca2+ by accumulation of H2 O2 (via SOD-2) and/or OH• (via Fenton reaction), impairing contractile function [3, 50]. High levels of O2•− can also inhibit the release of Ca2+ from sarcoplasmic reticulum and/or react with NO• to produce ONOO− which also affects Ca2+ release from the sarcoplasmic reticulum [3, 50]. Furthermore, NO• can also bind to transition metals [49], such as Fe, and impair mitochondrial function [40, 51].
Muscle damage: the lipids that make up the muscle cell membrane (sarcolemma) are attacked by ROS and RNS during process of lipid peroxidation. Disruption of the sarcolemma promotes muscle cell death and all the contents are released into the extracellular environment, causing inflammation (degradation of cellular content), edema, pain and loss of contractile function [1, 49]. In this process, blood levels of muscle CK are increased, making it a useful parameter to measure muscle damage [52].
LLLT and LEDT have been used to combat ROS and RNS produced during physical exercise [20–22, 24, 25, 28–31, 36, 38, 39] for improvement in mitochondrial function that contributes to the reduction of muscle fatigue and the increase in muscle performance. LLLT and LEDT use CCO as the primary photoacceptor and the main effects of this interaction are increased ATP synthesis and increased mitochondrial function [24, 40–43, 51]. The relationship between light, mitochondria, ROS and RNS involves the reduction of ROS and the photodissociation of nitric oxide-cytochrome c oxidase (NO-CCO), contributing to the restoration of oxygen consumption and ATP synthesis in mitochondria [40–51].
NO-CCO photodissociation is based on the hypothesis that NO may compete with oxygen to bind to the iron-sulfur complex (complex I) and to centers of iron and copper (complex IV) in the respiratory chain and inhibit the mitochondrial ATP synthesis [40, 51] mainly in cells metabolically stressed [40], as occurs after muscle contraction [24]. However, the binding between NO-CCO can be broken by visible and NIR light energy [51, 53] restoring mitochondrial function to ATP synthesis [24, 40, 41, 43, 51]. Thus, the use of LLLT or LEDT after physical exercise may be more effective compared to its use before exercises.
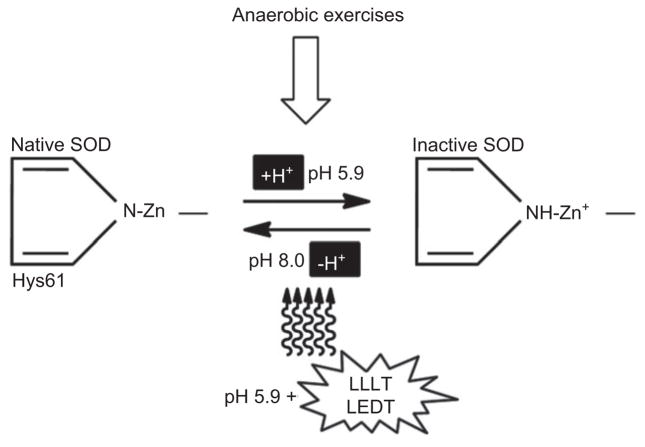
It is known that physical exercise may decrease intracellular pH [1]. An accumulation of H+ can inactivate the Cu-Zn-SOD (SOD-1 and SOD-3) through of a protonation of the residue of histidine 61 (Hys61) in the active center of these enzymes [51]. However, LLLT can reverse this process and re-activate SOD-1 and SOD-3 [51] (Figure 3).
Figure 3.
Based on reference [51]: Deprotonation of histidine and formation of the N-Zn bond to restore the active center structure and activity of the superoxide dismutase enzyme (SOD) by LLLT or LEDT.
De Marchi et al. [31] reported that SOD activity did not change even after intense exercise in LLLT group, thereby increasing physical performance compared to placebo-LLLT group that had decreased SOD activity and had the worst performance. Liu et al. [21] found increases of 44 % and 58 % in SOD activity after 24 h and 48 h after eccentric exercise and irradiation with LLLT.
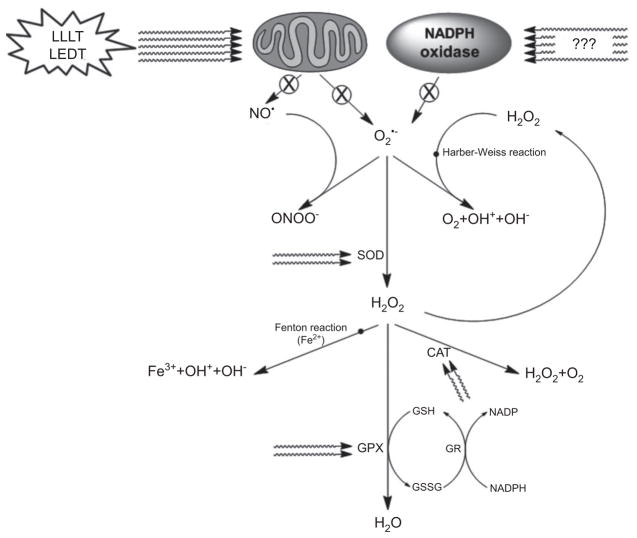
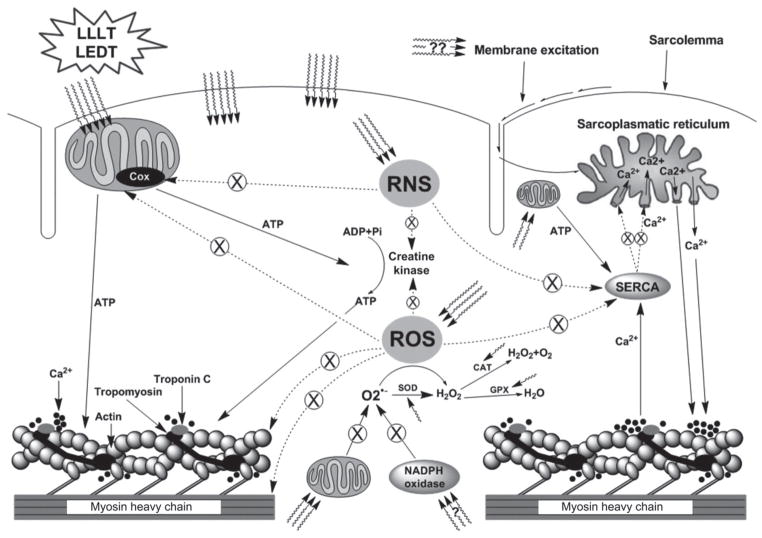
Possibly LLLT and LEDT improves mitochondrial function, dismutation of O2•− via SOD and decreases formation of ONOO− [51]. In addition, LLLT can reduce H2 O2 via CAT and GPX and can reduce the formation of hydroxyl radicals which contribute to the lower damage of the muscle cell membrane, as evidenced by lower lipid peroxidation and lower blood levels of muscle CK reported in previous studies [21, 31]. Also, the activation of calpain and caspase mediated by ROS [49] can be inhibited by LLLT and LEDT, since these therapies can modulate oxidative stress [31, 51]. Figures 4 and 5 illustrate the action of LLLT and LEDT on antioxidant enzymes and ROS and RNS in muscle contraction, respectively.
Figure 4.
Based on reference [1]. Effects of LLLT or LEDT on reactive oxygen species (ROS) and reactive nitric species (RNS) production and antioxidant enzymes.
⊗: decreased or inhibited production.
Figure 5.
Based on references [1, 32, 40, 49]. Effects of LLLT or LEDT on reactive oxygen species (ROS), reactive nitric species (RNS), mitochondria and muscle contraction. SERCA = sarcoplasmatic reticulum Ca2+ pump. Dashed line: inhibited activity and ⊗: decreased or inhibited function.
However, beside increased mitochondrial function and activity of antioxidant enzymes such as SOD, CAT and GPX, other mechanisms of action of LLLT and LEDT may be involved in reduction of muscle fatigue and increase of muscle performance [20–22, 24, 25, 28–33, 36, 38, 39]; these may be: (i) improvement in the sensitivity of myofibrils and Ca2+ channels to the Ca2+ ion, (ii) increase in Ca2+ uptake from sarcoplasm to sarcoplasmic reticulum via Ca2+ pump (ATP dependent), (iii) improvement in the formation of cross-bridges and production of contractile force, (iv) increase in the activity of Na+-K+ pump (ATP-dependent) to reduce the excess of extracellular K+ and ensure the depolarization of muscle fibers to continue the exercise, and (v) lower muscle damage and leakage of muscle contents into the blood, such as muscle CK and myoglobin.
3.3 Repair of muscle damage
Muscle damage is a disruption in the myofibrils which is a process accompanied by inflammation at the site of injury and contractile function losses [54]. The regeneration of muscle tissue after different injurious is a process that usually occurs in six phases taking 21 days overall [54]:
Phase 1 (2 days after injury): necrosis of injured tissue muscle, acute inflammation with swelling, increased collagen deposition, increased connective tissue and scarring formation;
Phase 2 (3 days): satellite cell activation;
Phase 3 (5 days): fusion of myoblasts at the site of injury and increased density of connective tissue;
Phase 4 (7 days): regenerating muscle cells extending outside of the former cylinders of the basal lamina to the injured site and then invading the scar tissue;
Phase 5 (14 days): the scar in the injured site reduces in size and new myofibrils fill the gap at the injured site;
Phase 6 (21 days): interweaving and fusion of the ends of myofibrils and with further reduction in connective and scar tissue.
The regeneration of muscle tissue is limited, but does allow activation, proliferation and differentiation of muscle satellite cells into new myonuclei and/or myofibrils [54–57]. Satellite cells are located at the basal layer of myofibrils and may be quiescent, proliferating or differentiating according to the harmful stimuli [55–57]. These cells are considered reserve cells, because although they are not stem cells such as in embryonic development, these cells are able to renew the myogenesis program in response to a muscle injury [58], such as micro-lesions from strength training or high intensity exercises [55–57, 59–61].
Muscle injuries promote inflammation and higher concentrations of neutrophils and macrophages at the site of injury that produce chemoattractants to attract the satellites cells to the region [56, 57]. Satellite cells are activated, proliferate and differentiate by molecular pathways such as modulation of gene expression related to quiescence (Pax7, c-Met, Myf5), proliferation/activation (MyoD1, Myf5, M-cadherin) and differentiation (desmin, MRF4 and myogenin) [56, 57].
Satellite cells that have already differentiated can be fused to the damaged muscle fibers as new myonuclei, providing gene expression that encodes new contractile proteins or improve the myonuclear domain [62–64]. In addition, these cells can be activated and form new myoblasts that fuse to form myotubes which will produce new myofibrils [56, 57].
Once the importance of satellite cells in the process of muscle repair became known, some authors investigated the effects of LLLT on these cells [65–68]. Studies in experimental models have shown that LLLT increases the formation of new myofibrils that fill the gap in muscle injury and contractile characteristics can return to the site of injury [65, 66]. Bibikova and Oron [67] observed that LLLT (632.8 nm) promoted a greater maturation of young myofibrils compared to those not irradiated. Shefer et al. [68] found an increase of the number and activation of satellite cells around the myofibrils irradiated with LLLT (632.8). Shefer et al. [68] also observed that LLLT (632.8) was effective in increasing levels of B-cell lymphoma 2, an antiapoptotic protein related to cell viability, and decreasing BAX, a pro-apoptotic protein. Nakano et al. [69] reported that LLLT (830 nm) increased satellite cells by 5-Bromo-2′-deoxyuridine (BrdU) incorporation, angiogenesis and maintained the diameter of rat myofibrils submitted to process of muscle atrophy.
Currently there is no study that investigated LLLT or LEDT and satellite cell activity in humans. Only in vitro studies and experimental models are reported in scientific literature, which show a positive influence of LLLT on the cell cycle, proliferation and activation of these cells [66, 68, 70, 71].
In addition to satellite cells, the control of inflammation, proteolysis, ROS and RNS, synthesis and remodeling of collagen and ATP levels are fundamental to the success of muscle damage repair [21, 43, 72–78]. Studies in experimental models have shown that LLLT can modulate amounts of collagen at the site of injury [72, 73, 78]; inhibit inflammation by lower expression of tumor necrosis factor (TNF)-alpha (TNF-α) and COX-2 [21, 74, 77]; decrease ROS by smaller lipid peroxidation (TBARS and lower levels of MDA) [21, 72, 73, 76], reduce RNS by inhibiting synthesis of iNOS [72], decrease the expression of nuclear factor kappa B (NF-κβ) and CK (related to proteolysis) [21, 72, 73]; increase activity of SOD [21, 76], increase the expression of VEGF receptor (VEGFR-1) in the capillaries and in satellite cells [75] and increase activity of respiratory chain for ATP synthesis and repair muscle damage [43].
3.4 Gene expression effects
Several studies report specific molecular pathway signaling for each type of physical training, where the expression and/or suppression of specific genes are essential for better physical performance [10, 11, 79–83].
Strength training or high-intensity exercise have well defined signaling pathways which involve specific genes related to muscle satellite cells at the quiescent state (c-Met genes, Myf5 and Pax7), activated (genes MyoD1, Myf5, M-cadherin) and differentiation to form new myonuclei and/or myofibrils (genes desmin, myogenin and MRF4) in response to micro-lesions [55–57, 59–61]. Also, strength training or high intensity exercise are influenced by genes related to protein synthesis (muscle hypertrophy) as IGF-1, AKT, mTOR and p70 S6K [10, 81]; genes regulating protein degradation (atrophy) as TNF-α, FOXO, FBXO32, TRIM63 or MuRF1, MSTN, E3 ubiquitin ligases and enzymes involved in proteolytic process by ubiquitin-proteasome system [10, 11, 81, 82, 84].
Gene expression changes related to endurance training or low intensity exercises include mitochondrial biogenesis involving upregulation of CCO, nuclear respiratory factor (NRF) 1 and 2, transcription factor A mitochondrial (TFAM) and peroxisome proliferator-activated receptor gamma coactivator 1 α (PPARGC-1α), all related to mitochondrial biogenesis [10, 11, 81, 82, 84]. When PPARGC-1α is upregulated there is an indication of mitochondrial biogenesis and more fatty acids oxidation in the muscles, also relating to the transition between the fiber types as type II to type I, which has greater oxidative potential [10, 79, 80]. This transition provides biomechanical adaptations such as resistance to fatigue in strenuous exercise at low to medium intensity [10, 79, 80].
The identification of the molecular mechanisms modulated by LLLT and LEDT can aid the understanding of the effects of these therapies on the gene expression changes related to different types of exercises [10, 11, 83] as previously suggested [32, 33]. However, the literature has a lack of studies involving gene expression in humans, because it involves invasive procedures like muscle biopsy [83].
With this perspective in mind, Ferraresi et al. [34] investigated the effects of LLLT (cluster with six diodes of 808 nm) on the physical performance of 10 young males undergoing physical strength training. Preliminary results showed that the LLLT group had upregulated the genes PPARGC1-α (mitochondrial biogenesis), mTOR (protein synthesis and muscle hypertrophy) and VEGF (angiogenesis). Furthermore, only the LLLT group downregulated MuRF1 (protein degradation and muscle atrophy) and IL-1 β (inflammation) (Figure 6).
Figure 6.
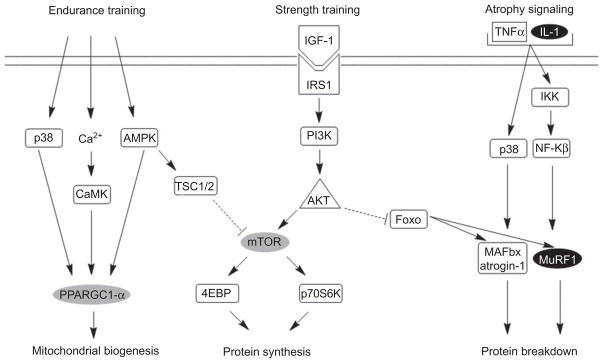
Based on references [10, 11, 84]. Mitochondrial biogenesis, protein synthesis and protein breakdown signaling modulated by LLLT associated to exercise. Gray boxes were upregulated by LLLT and black boxes were downregulated by LLLT.
3.5 Possible mechanisms of action
3.5.1 Changes in relationship between ADP, Pi, Mg2+, Ca2+ and pH
The accumulation of Pi, NAD and Mg2+ in the sarcoplasm may decrease Ca2+ release from the sarcoplasmic reticulum, decrease Ca2+ reuptake by Ca2+ pump, promote a loss of myofibrils sensitivity to Ca2+ and cause a decay in contractile force conducting to muscle fatigue [1]. During tetanic contractions for induction of early fatigue, Pi may reduce the bond between Ca2+ and TnC, inhibiting or even reversing the Ca2+ pump in the sarcoplasmic reticulum, contributing to the higher concentration of Ca2+ into the sarcoplasm. In contrast, in the stages of late fatigue, Pi can inhibit the channels of Ca2+ release, or bind to Ca2+ (Pi-Ca2+) in the sarcoplasmic reticulum, decreasing the available amount of Ca2+ to initiate muscle contraction [1].
The decreased pH in the cell cytosol reduces the affinity of Ca2+ in muscle fibers, probably due to competition between the H+ and Ca2+ to bind to TnC [1]. The LLLT and LEDT may have some modulating effect on the Ca2+ release from sarcoplasmic reticulum and the binding of Ca2+ to TnC through modulation of cellular energy metabolism via mitochondria, PCr re-synthesis and decreased enzyme activity of LDH.
The Ca2+ pump, in particular, may have its function improved by LLLT and LEDT, since it is dependent on ATP [5] and/or LLLT and LEDT may facilitate the dissociation of Pi-Ca2+ and increase the availability of Ca2+ for muscle contraction. These mechanisms involving the Ca2+ ion may perhaps explain the action of LLLT in reducing muscle fatigue during tetanic contractions induced by neuromuscular electrical stimulation [18, 20, 23] and the action of LLLT and LEDT on high intensity exercise [25, 27–33, 37, 38].
3.5.2 Excitability of muscle fiber and electromyography (EMG)
Muscle contraction depends on the electrical excitation of the muscle fibers. When the muscle fiber is excited, the transmission of excitation begins with generation of an action potential which propagates rapidly through the sarcolemma and T-tubules. The rapid spread of the action potential ensures a more uniform muscle contraction. This contraction involves Ca2+ release that is directly linked to Na+ channels (voltage dependent) and K+ (ATP dependent) in the sarcolemma and T-tubules [1].
Repeated muscle contraction has been associated with induction of fatigue because it promotes an increased Na+ influx and K+ efflux from muscle cells. This K+ efflux occurs at every action potential, impairing the depolarization of the muscle fiber by an accumulation of this ion in the T-tubules and their cisterns. Consequently, there is a decrease in the force of muscle contraction. To avoid this imbalance between intra and extracellular Na+ and K+, the Na+-K+ pump works by pumping K+ into the muscle cell (capturing the K+ also in T-tubules) and pumping Na+ to the outside, allowing new action potentials to propagate through muscle fibers and T-tubules to release Ca2+ and promote muscle contraction [1].
LLLT and LEDT can possibly influence the excitation of the muscle fibers and reduce muscle fatigue by indirectly modulating the Na+-K+ pump which is ATP-dependent. Mitochondria surrounding the T-tubules and Ca2+ cisterns can possibly increase their ATP synthesis as a result of LLLT and LEDT. Thus, a greater availability of ATP may improve the function of this pump and prevent an accumulation of extracellular K+ that reduces muscle fatigue [1, 85]. Also, a higher conductance of the Cl− channels in the T-tubules is important to reduce the accumulation of K+ in these tubules and restore the excitability and membrane potential of the muscle fibers [1]. Perhaps the Cl− channels are also modulated by LLLT and LEDT.
Action potentials that reach muscle fibers and cause their depolarization can be identified by EMG during muscle contraction in exercises [1, 86]. This assessment tool may have an important role to elucidate possible effects of LLLT and LEDT on the neuromuscular junction, excitability of muscle fibers, and rate of activation and recruitment of motor units for inferring the magnitude of muscle fatigue.
4 Conclusion
LLLT and LEDT can improve muscle performance, reduce muscle fatigue during exercises and benefit the muscle repair. Despite of many mechanisms of action involving LLLT and LEDT to be clarified in this review, we suggest the need to further studies to investigate others mechanisms of action and others possible applications of the light on muscle tissue. In this context, we have the best perspectives for patients who have neuromuscular diseases such as Duchenne muscular dystrophy [87]. We believe these patients can be benefited with the power of light of LLLT and LEDT, accelerating muscle repair via satellite cells and decreasing oxidative stress of muscle tissue [88]. Then, we encourage researchers to investigate the effects of LLLT and LEDT on patients with Duchenne muscular dystrophy.
Acknowledgments
CF and NAP were funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). MRH was supported by USNIH grant RO1AI050875.
Contributor Information
Cleber Ferraresi, Laboratory of Electro-thermo-phototherapy, Department of Physical Therapy, Federal University of São Carlos, São Carlos, São Paulo 13565-905, Brazil; and Department of Biotechnology, Federal University of São Carlos, São Carlos, São Paulo 13565-905, Brazil.
Michael R. Hamblin, Harvard-MIT Division of Health Science and Technology, Cambridge, MA 02139, USA; and Department of Dermatology, Harvard Medical School, Boston, MA 02115, USA.
Nivaldo A. Parizotto, Laboratory of Electro-thermo-phototherapy, Department of Physical Therapy, Federal University of São Carlos, São Carlos, São Paulo 13565-905, Brazil; Department of Biotechnology, Federal University of São Carlos, São Carlos, São Paulo 13565-905, Brazil; and Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA 02114, USA
References
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Westerblad H, Bruton JD, Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res. 2010;316(18):3093–9. doi: 10.1016/j.yexcr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Westerblad H, Allen DG. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal. 2011;15(9):2487–99. doi: 10.1089/ars.2011.3909. [DOI] [PubMed] [Google Scholar]
- 4.Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol. 2000;528(Pt 2):379–88. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonkonogi M, Sahlin K. Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev. 2002;30(3):129–37. doi: 10.1097/00003677-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Schlumberger A, Wirth K, Schmidtbleicher D, Steinacker JM. Different effects on human skeletal myosin heavy chain isoform expression: strength vs. combination training. J Appl Physiol. 2003;94(6):2282–8. doi: 10.1152/japplphysiol.00830.2002. [DOI] [PubMed] [Google Scholar]
- 7.Aagaard P. Making muscles “stronger”: exercise, nutrition, drugs. J Musculoskelet Neuronal Interact. 2004;4(2):165–74. [PubMed] [Google Scholar]
- 8.Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34(10):663–79. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- 9.Sahlin K, Mogensen M, Bagger M, Fernström M, Pedersen PK. The potential for mitochondrial fat oxidation in human skeletal muscle influences whole body fat oxidation during low-intensity exercise. Am J Physiol Endocrinol Metab. 2007;292(1):E223–30. doi: 10.1152/ajpendo.00266.2006. [DOI] [PubMed] [Google Scholar]
- 10.Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37(9):737–63. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hawley JA. Molecular responses to strength and endurance training: are they incompatible ? Appl Physiol Nutr Metab. 2009;34(3):355–61. doi: 10.1139/H09-023. [DOI] [PubMed] [Google Scholar]
- 12.Enwemeka CS. Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg. 2009;27(3):387–93. doi: 10.1089/pho.2009.2503. [DOI] [PubMed] [Google Scholar]
- 13.Fulop AM, Dhimmer S, Deluca JR, Johanson DD, Lenz RV, Patel KB, Douris PC, Enwemeka CS. A meta-analysis of the efficacy of phototherapy in tissue repair. Photomed Laser Surg. 2009;27(5):695–702. doi: 10.1089/pho.2009.2550. [DOI] [PubMed] [Google Scholar]
- 14.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 15.Lopes-Martins RA, Albertini R, Martins PS, Bjordal JM, Faria Neto HC. Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed Laser Surg. 2005;23(4):377–81. doi: 10.1089/pho.2005.23.377. [DOI] [PubMed] [Google Scholar]
- 16.Bakeeva LE, Manteĭfel’ VM, Rodichev EB, Karu TI. Formation of gigantic mitochondria in human blood lymphocytes under the effect of an He-Ne laser. Mol Biol (Mosk) 1993;27(3):608–17. [PubMed] [Google Scholar]
- 17.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 18.Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC, Jr, Muscará MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol. 2006;101(1):283–8. doi: 10.1152/japplphysiol.01318.2005. [DOI] [PubMed] [Google Scholar]
- 19.Vieira WHB, Goes R, Costa FC, Parizotto NA, Perez SEA, Baldissera V, Munin FS, Schwantes MLB. Adaptation of LDH enzyme in rats undergoing aerobic treadmill training and low intensity laser therapy. RevBras Fisioter. 2006;10(2):205–211. [Google Scholar]
- 20.Leal EC, Junior, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol. 2010;108(6):1083–8. doi: 10.1007/s00421-009-1321-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu XG, Zhou YJ, Liu TC, Yuan JQ. Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg. 2009;27(6):863–9. doi: 10.1089/pho.2008.2443. [DOI] [PubMed] [Google Scholar]
- 22.Sussai DA, de Carvalho PT, Dourado DM, Belchior AC, dos Reis FA, Pereira DM. Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci. 2010;25(1):115–20. doi: 10.1007/s10103-009-0697-9. [DOI] [PubMed] [Google Scholar]
- 23.de Almeida P, Lopes-Martins RÁ, Tomazoni SS, Silva JA, Jr, de Carvalho PT, Bjordal JM, Leal EC., Junior Low-level laser therapy improves skeletal muscle performance, decreases skeletal muscle damage and modulates mRNA expression of COX-1 and COX-2 in a dose-dependent manner. Photochem Photobiol. 2011;87(5):1159–63. doi: 10.1111/j.1751-1097.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Zhao X, Liu TC, Pan H. Low-intensity laser irradiation improves the mitochondrial dysfunction of C2C12 induced by electrical stimulation. Photomed Laser Surg. 2008;26(3):197–202. doi: 10.1089/pho.2007.2125. [DOI] [PubMed] [Google Scholar]
- 25.de Almeida P, Lopes-Martins RA, De Marchi T, Tomazoni SS, Albertini R, Corrêa JC, Rossi RP, Machado GP, da Silva DP, Bjordal JM, Leal EC., Jr Red (660 nm) and infrared (830 nm) low-level laser therapy in skeletal muscle fatigue in humans: what is better ? Lasers Med Sci. 2012;27(2):453–8. doi: 10.1007/s10103-011-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorgey AS, Wadee AN, Sobhi NN. The effect of low-level laser therapy on electrically induced muscle fatigue: a pilot study. Photomed Laser Surg. 2008;26(5):501–6. doi: 10.1089/pho.2007.2161. [DOI] [PubMed] [Google Scholar]
- 27.Leal EC, Junior, Lopes-Martins RA, Dalan F, Ferrari M, Sbabo FM, Generosi RA, Baroni BM, Penna SC, Iversen VV, Bjordal JM. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008;26(5):419–24. doi: 10.1089/pho.2007.2160. [DOI] [PubMed] [Google Scholar]
- 28.Leal EC, Junior, Lopes-Martins RA, Vanin AA, Baroni BM, Grosselli D, De Marchi T, Iversen VV, Bjordal JM. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009;24(3):425–31. doi: 10.1007/s10103-008-0592-9. [DOI] [PubMed] [Google Scholar]
- 29.Leal EC, Junior, Lopes-Martins RA, Frigo L, De Marchi T, Rossi RP, de Godoi V, Tomazoni SS, Silva DP, Basso M, Filho PL, de Valls Corsetti F, Iversen VV, Bjordal JM. Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther. 2010;40(8):524–32. doi: 10.2519/jospt.2010.3294. [DOI] [PubMed] [Google Scholar]
- 30.Baroni BM, Leal EC, Junior, De Marchi T, Lopes AL, Salvador M, Vaz MA. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010;110(4):789–96. doi: 10.1007/s00421-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 31.De Marchi T, Leal EC, Junior, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci. 2012;27(1):231–6. doi: 10.1007/s10103-011-0955-5. [DOI] [PubMed] [Google Scholar]
- 32.Ferraresi C, de Brito Oliveira T, de Oliveira Zafalon L, de Menezes Reiff RB, Baldissera V, de Andrade Perez SE, Matheucci E, Júnior, Parizotto NA. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci. 2011;26(3):349–58. doi: 10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- 33.Vieira WH, Ferraresi C, Perez SE, Baldissera V, Parizotto NA. Effects of low-level laser therapy (808 nm) on isokinetic muscle performance of young women submitted to endurance training: a randomized controlled clinical trial. Lasers Med Sci. 2012;27(2):497–504. doi: 10.1007/s10103-011-0984-0. [DOI] [PubMed] [Google Scholar]
- 34.Ferraresi C, Panepucci R, Reiff R, Júnior E, Bagnato V, Parizotto N. Molecular effects of low-level laser therapy (808 nm) on human muscle performance. Phys Ther Sport. 2012;13(3):e5. [Google Scholar]
- 35.Vinck E, Cagnie B, Coorevits P, Vanderstraeten G, Cambier D. Pain reduction by infrared light-emitting diode irradiation: a pilot study on experimentally induced delayed-onset muscle soreness in humans. Lasers Med Sci. 2006;21(1):11–8. doi: 10.1007/s10103-005-0366-6. [DOI] [PubMed] [Google Scholar]
- 36.Leal EC, Junior, Lopes-Martins RA, Baroni BM, De Marchi T, Rossi RP, Grosselli D, Generosi RA, de Godoi V, Basso M, Mancalossi JL, Bjordal JM. Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg. 2009;27(4):617–23. doi: 10.1089/pho.2008.2350. [DOI] [PubMed] [Google Scholar]
- 37.Leal EC, Junior, Lopes-Martins RA, Rossi RP, De Marchi T, Baroni BM, de Godoi V, Marcos RL, Ramos L, Bjordal JM. Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med. 2009;41(8):572–7. doi: 10.1002/lsm.20810. [DOI] [PubMed] [Google Scholar]
- 38.Baroni BM, Leal EC, Junior, Geremia JM, Diefenthaeler F, Vaz MA. Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed Laser Surg. 2010;28(5):653–8. doi: 10.1089/pho.2009.2688. [DOI] [PubMed] [Google Scholar]
- 39.Leal EC, Junior, de Godoi V, Mancalossi JL, Rossi RP, De Marchi T, Parente M, Grosselli D, Generosi RA, Basso M, Frigo L, Tomazoni SS, Bjordal JM, Lopes-Martins RA. Comparison between cold water immersion therapy (CWIT) and light emitting diode therapy (LEDT) in short-term skeletal muscle recovery after high-intensity exercise in athletes – preliminary results. Lasers Med Sci. 2011;26(4):493–501. doi: 10.1007/s10103-010-0866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–83. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol. 2010;86(3):673–80. doi: 10.1111/j.1751-1097.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- 42.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62(8):607–10. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 43.Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B. 2009;95(2):89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Manteĭfel’ VM, Karu TI. Structure of mitochondria and activity of their respiratory chain in subsequent generations of yeast cells exposed to He-Ne laser light. Izv Akad Nauk Ser Biol. 2005;(6):672–83. [PubMed] [Google Scholar]
- 45.Passarella S, Ostuni A, Atlante A, Quagliariello E. Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem Biophys Res Commun. 1988;156(2):978–86. doi: 10.1016/s0006-291x(88)80940-9. [DOI] [PubMed] [Google Scholar]
- 46.Manteĭfel V, Bakeeva L, Karu T. Ultrastructural changes in chondriome of human lymphocytes after irradiation with He-Ne laser: appearance of giant mitochondria. J Photochem Photobiol B. 1997;38(1):25–30. doi: 10.1016/s1011-1344(96)07426-x. [DOI] [PubMed] [Google Scholar]
- 47.Hodson-Tole EF, Wakeling JM. Motor unit recruitment for dynamic tasks: current understanding and future directions. J Comp Physiol B. 2009;179(1):57–66. doi: 10.1007/s00360-008-0289-1. [DOI] [PubMed] [Google Scholar]
- 48.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA. 1999;96(3):1129–34. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589(Pt 9):2119–27. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vladimirov YA, Osipov AN, Klebanov GI. Photobiological principles of therapeutic applications of laser radiation. Biochemistry (Mosc) 2004;69(1):81–90. doi: 10.1023/b:biry.0000016356.93968.7e. [DOI] [PubMed] [Google Scholar]
- 52.Markert CD, Ambrosio F, Call JA, Grange RW. Exercise and Duchenne muscular dystrophy: toward evidence-based exercise prescription. Muscle Nerve. 2011;43(4):464–78. doi: 10.1002/mus.21987. [DOI] [PubMed] [Google Scholar]
- 53.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36(4):307–14. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 54.Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–64. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 55.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14(2):82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 57.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91(2):534–51. doi: 10.1152/jappl.2001.91.2.534. Erratum in: J Appl Physiol 2001, 91(6), 2414. [DOI] [PubMed] [Google Scholar]
- 58.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin Cell Dev Biol. 2005;16(4–5):575–84. doi: 10.1016/j.semcdb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Chen JC, Goldhamer DJ. Skeletal muscle stem cells. Reprod Biol Endocrinol. 2003;1:101. doi: 10.1186/1477-7827-1-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilborn CD, Taylor LW, Greenwood M, Kreider RB, Willoughby DS. Effects of different intensities of resistance exercise on regulators of myogenesis. J Strength Cond Res. 2009;23(8):2179–87. doi: 10.1519/JSC.0b013e3181bab493. [DOI] [PubMed] [Google Scholar]
- 62.Vierck J, O’Reilly B, Hossner K, Antonio J, Byrne K, Bucci L, Dodson M. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int. 2000;24(5):263–72. doi: 10.1006/cbir.2000.0499. [DOI] [PubMed] [Google Scholar]
- 63.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104(6):1736–42. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 64.Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol. 2007;92(5):783–97. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- 65.Roth D, Oron U. Repair mechanisms involved in muscle regeneration following partial excision of the rat gastrocnemius muscle. Exp Cell Biol. 1985;53(2):107–14. doi: 10.1159/000163302. [DOI] [PubMed] [Google Scholar]
- 66.Weiss N, Oron U. Enhancement of muscle regeneration in the rat gastrocnemius muscle by low energy laser irradiation. Anat Embryol (Berl) 1992;186(5):497–503. doi: 10.1007/BF00185463. [DOI] [PubMed] [Google Scholar]
- 67.Bibikova A, Oron U. Attenuation of the process of muscle regeneration in the toad gastrocnemius muscle by low energy laser irradiation. Lasers Surg Med. 1994;14(4):355–61. doi: 10.1002/lsm.1900140408. [DOI] [PubMed] [Google Scholar]
- 68.Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115(Pt 7):1461–9. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- 69.Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T. Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol. 2009;94(9):1005–15. doi: 10.1113/expphysiol.2009.047738. [DOI] [PubMed] [Google Scholar]
- 70.Ben-Dov N, Shefer G, Irintchev A, Wernig A, Oron U, Halevy O. Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro. Biochim Biophys Acta. 1999;1448(3):372–80. doi: 10.1016/s0167-4889(98)00147-5. Erratum in: Biochim Biophys Acta 1999, 1450(1), 108. [DOI] [PubMed] [Google Scholar]
- 71.Shefer G, Barash I, Oron U, Halevy O. Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta. 2003;1593(2–3):131–9. doi: 10.1016/s0167-4889(02)00350-6. [DOI] [PubMed] [Google Scholar]
- 72.Rizzi CF, Mauriz JL, Freitas Corrêa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, González-Gallego J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38(7):704–13. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 73.Silveira PC, da Silva LA, Pinho CA, De Souza PS, Ronsani MM, da Luz Scheffer D, Pinho RA. Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci. 2012 doi: 10.1007/s10103-012-1075-6. [DOI] [PubMed] [Google Scholar]
- 74.Mesquita-Ferrari RA, Martins MD, Silva JA, Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP. Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci. 2011;26(3):335–40. doi: 10.1007/s10103-010-0850-5. [DOI] [PubMed] [Google Scholar]
- 75.Dourado DM, Fávero S, Matias R, de Carvalho PT, da Cruz-Höfling MA. Low-level laser therapy promotes vascular endothelial growth factor receptor-1 expression in endothelial and nonendothelial cells of mice gastrocnemius exposed to snake venom. Photochem Photobiol. 2011;87(2):418–26. doi: 10.1111/j.1751-1097.2010.00878.x. [DOI] [PubMed] [Google Scholar]
- 76.Luo L, Sun Z, Zhang L, Li X, Dong Y, Liu TC. Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-β 1 in skeletal muscle during the repair process. Lasers Med Sci. 2012 doi: 10.1007/s10103-012-1133-0. [DOI] [PubMed] [Google Scholar]
- 77.Rennó AC, Toma RL, Feitosa SM, Fernandes K, Bossini PS, de Oliveira P, Parizotto N, Ribeiro DA. Comparative effects of low-intensity pulsed ultrasound and low-level laser therapy on injured skeletal muscle. Photomed Laser Surg. 2011;29(1):5–10. doi: 10.1089/pho.2009.2715. [DOI] [PubMed] [Google Scholar]
- 78.de Souza TO, Mesquita DA, Ferrari RA, Dos Santos Pinto D, Jr, Correa L, Bussadori SK, Fernandes KP, Martins MD. Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci. 2011;26(6):803–14. doi: 10.1007/s10103-011-0951-9. [DOI] [PubMed] [Google Scholar]
- 79.Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88(1):99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- 80.Flück M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol. 2006;209(Pt 12):2239–48. doi: 10.1242/jeb.02149. [DOI] [PubMed] [Google Scholar]
- 81.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950–7. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 82.Favier FB, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Arch. 2008;456(3):587–600. doi: 10.1007/s00424-007-0423-z. [DOI] [PubMed] [Google Scholar]
- 83.Stepto NK, Coffey VG, Carey AL, Ponnampalam AP, Canny BJ, Powell D, Hawley JA. Global gene expression in skeletal muscle from well-trained strength and endurance athletes. Med Sci Sports Exerc. 2009;41(3):546–65. doi: 10.1249/MSS.0b013e31818c6be9. [DOI] [PubMed] [Google Scholar]
- 84.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Nielsen OB, de Paoli FV. Regulation of Na +-K + homeostasis and excitability in contracting muscles: implications for fatigue. Appl Physiol Nutr Metab. 2007;32(5):974–84. doi: 10.1139/H07-099. [DOI] [PubMed] [Google Scholar]
- 86.Enoka RM. Muscle fatigue-from motor units to clinical symptoms. J Biomech. 2012;45(3):427–33. doi: 10.1016/j.jbiomech.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 87.Manzur AY, Muntoni F. Diagnosis and new treatments in muscular dystrophies. J Neurol Neurosurg Psychiatry. 2009;80(7):706–14. doi: 10.1136/jnnp.2008.158329. [DOI] [PubMed] [Google Scholar]
- 88.Abdel SE, Abdel-Meguid I, Korraa S. Markers of oxidative stress and aging in Duchene muscular dystrophy patients and the possible ameliorating effect of He:Ne laser. Acta Myol. 2007;26(1):14–21. [PMC free article] [PubMed] [Google Scholar]