Abstract
Monkeypox virus (MPXV) was discovered in 1958 during an outbreak in an animal facility in Copenhagen, Denmark. Since its discovery, MPXV has revealed a propensity to infect and induce disease in a large number of animals within the mammalia class from pan-geographical locations. This finding has impeded the elucidation of the natural host, although the strongest candidates are African squirrels and/or other rodents. Experimentally, MPXV can infect animals via a variety of multiple different inoculation routes; however, the natural route of transmission is unknown and is likely to be somewhat species specific. In this review we have attempted to compile and discuss all published articles that describe experimental or natural infections with MPXV, dating from the initial discovery of the virus through to the year 2012. We further discuss the comparative disease courses and pathologies of the host species.
Keywords: aerosol, animals, infection, intrabronchial, intradermal, intramuscular, intranasal, intratracheal, intravenous, outbreak, primates, subcutaneous
Orthopoxviruses (OPVs) have host specificities ranging from narrow (e.g., ectromelia and variola [VARV]) to broad (e.g., cowpox and vaccinia [VACV]). Monkeypox virus (MPXV) has a broad host-range and is capable of infecting many species from across the globe. In nature, the major environs of MPXV are restricted to the Congo Basin (CB) and West Africa (WA). The MPXV virion is a brick-shaped enveloped virus of 200–250 nm, characterized by surface tubules and a dumbbell-shaped core. Humans and highly susceptible nonhuman primates (NHPs) infected with MPXV have near identical clinical manifestations compared to humans infected with VARV. For humans, the only obvious difference in clinical signs is the absence of lymphadenopathy in smallpox patients [1,2]. Despite the similarity, MPXV remained primarily of academic interest throughout the 1960s; however, this attitude changed when the scientific community realized that MPXV could lethally infect humans in known smallpox-free locales.
Although there are clinical similarities in humans, MPXV is not considered to be the direct ancestor to VARV; instead, both viruses are believed to have evolved from progenitor poxviruses most similar to the cowpox virus (CPXV) lineage [3,4]. Genomic differences amongst MPXV isolates have been mapped using restriction fragment length polymorphisms [5] and DNA sequencing techniques [6,7]. Like other OPVs, MPXV strains demonstrate gene variation towards the terminal regions, with conservation towards the center of the genome.
In this review, records pertaining to natural or experimental infections of animals with MPXV have been extensively researched. The authors have given the origin of the isolate (either CB or WA) and the specific strain name where known; if the strain and origin are unknown, it has simply been referred to as ‘MPXV’. Doses and routes of inoculation are given where known. The authors have taken effort to focus on ‘confirmed’ cases of MPXV, therefore, cases of likely MPXV infections in animals may only be discussed transiently where appropriate; for example, several OPV-specific serological surveys of animals in various regions have been conducted in an attempt to elucidate MPXV host species [8-11], and some literature report on natural ‘smallpox’ in various NHPs (reviewed in [12]).
Natural history
Field studies have revealed that MPXV infects many species that inhabit all strata of the lowland tropical forest within central and west Africa [13-20]. The infected species have some similar and dissimilar traits based on diet and habitat preferences; approximately 40% are arboreal, 40% are semiterrestrial and 20% are terrestrial [13].
Several studies suggest that there may be no one reservoir of MPXV; rather, several animal species may support MPXV. The only reported case of MPXV being isolated from a wild animal consisted of MPXV being isolated from a diseased squirrel, Funisciurus anerythrus [17]. In the 2003 shipment of African rodents that introduced MPXV into the USA, cell culture demonstrated MPXV in three out of six Funisciurus sp. (rope squirrel), one out of 15 Cricetomys sp. (Gambian-pouched rat) and eight out of ten Graphiurus sp. (African dormouse) [21] (as well as several other animals that were found to be positive by PCR; see below). The lack of MPXV antibodies in sera from certain animal species is also informative. Sera from terrestrial rodents of the genera Lophuromys, Lemniscomys, Oenomys, Thamnomys and Praomys were negative (579 sera samples) [16]. One study tested the transmission of MPXV from infected squirrel tissue to other naive squirrels via ants – no transmission was reported [16]. However, Petrodromus tetradactylus (four-toed elephant-shrew), an insectivore that consumes minimal plant material and yet is seropositive for OPV antibodies, suggests that the role of insects in the natural lifecycle of MPXV may be worth evaluating. The presence of MPXV antibodies in so many distinct species and virus detection in specimens from Funisciurus sp., Cricetomys sp. and Graphiurus sp. suggest that the natural lifecycle is a complex interaction of reservoir hosts and incidental species.
Virulence differences between WA & CB strains
Sequence analysis of the genomes of several CB and WA MPXV isolates revealed approximately 95% identity amongst all MPXV isolates. This value approaches 99% when comparing between CB or WA isolates, allowing separation into two clades based on geographical origin, sequence homology and disease severity [6,7]. Consistent with the aforementioned restriction fragment length polymorphism analysis, the greatest DNA sequence diversity between the two clades is localized to the terminal regions that encode for predicted host-response modifier proteins. Several animal studies have shown that CB isolates of MPXV have increased virulence compared with WA isolates. In the intranasal (in.) CAST/EiJ mouse strain, disease severity (which is a commonly accepted biomarker of morbidity in OPV-induced disease [22-26]) is similar between viral strains at doses of 105–106 PFU, and both strains cause 100% mortality; however, at 104 PFU the mortality rate of the WA-infected mice decreases to 50%, while it remains at 100% in CB-infected animals. Further more, at a 103 PFU dose, 12% of WA-infected animals die compared with 60% of CB-infected animals, and the maximum weight loss is 10% in WA animals compared with 22% in CB animals. These studies revealed that the median lethal dose (LD50) for the WA strain in CAST/EiJ mice is 7600 PFU, approximately 1 log higher than that of the CB strain [27]. Following a footpad (FP) infection of BALB/c and C57BL/6 mice, it was observed that edema occurred following infection with both the CB and WA strains; however, the level of edema was less and resolved quicker in WA infected animals. Furthermore, following an in. infection of these mice, it was reported that CB animals experienced weight loss whereas WA animals did not; and in the case of the in. infected BALB/c strain, ruffled fur was seen on CB-infected but not on WA-infected animals [28]. No animals died in these mouse experiments. In squirrels, the intradermal (id.) LD50 values for both strains are approximately the same (~0.5 PFU); however, the disease courses are different, with more severe clinical signs, earlier symptom onset and earlier mortality characterizing the CB-infected animals [29]. For prairie dogs, a mortality rate of 25 and 50% was recorded in animals infected with a CB strain via the in. or subcutaneous (sc.) route, compared with 0% mortality in WA-infected animals. A higher temperature and increased weight loss was also recorded in CB-infected animals, again indicating increased virulence of CB strains [30]. These results were confirmed by a study that calculated that the WA in. LD50 value is approximately 100-times higher than that of the CB strain [31]. In NHPs, it was found that disease severity and mortality was higher in cynomolgus macaques (Macaca fascicularis) infected with 106 PFU of a CB strain (ZR-599) via the sc. route compared with animals challenged with a WA strain (Liberia) [32].
The CB strain has also been suggested to be more virulent in humans. Virulence differences between WA and CB MPXV are supported by epidemiological analyses that observed a similar prevalence of antibodies in non-vaccinated humans in both regions [16]; however, 90% of reported cases and 100% of fatalities occurred in the Congo Basin, compared with 0% of fatalities in the WA cases [33].
Discovery of MPXV & natural outbreaks
Initial outbreak of MPXV in Denmark (1958)
MPXV was first discovered during a nonfatal outbreak at an animal facility in Copenhagen, Denmark, in 1958. The facility received a continual supply of Asian monkeys (mostly M. fascicularis) and rhesus macaques (Macaca mulatta), which were used for polio vaccine research. The first outbreak occurred 2 months after the monkeys had been received and the second outbreak occurred 4 months after the initial outbreak. The outbreaks occurred in M. fascicularis that had arrived from Singapore. Upon arrival, monkeys were treated with antibiotics and appeared in satisfactory health. The outbreak manifested itself with vesiculopustular skin eruptions that were observed over the entire trunk, tail, face, limbs, palms of the hands and soles of the feet. Despite the disease, the general health of the animals appeared relatively normal and the lesions formed crusts, healed and fell off, leaving a scar. No lesions were observed in tissues upon autopsy. The outbreak lasted approximately 2 weeks and involved six out of 32 animals. The second outbreak occurred in another shipment of animals from Singapore. The initial health of the animals appeared normal; however, the pox disease was observed in 11 out of 120 animals. Animals were examined and scattered healed lesions were observed in a further 12 monkeys 1 month later. Several weeks later, a further two animals developed the disease [34]. MPXV was isolated from the pustules of diseased animals by incubation in eggs. After incubation, the pocks were reported to resemble those of VARV, but were distinctly different to those of VACV and CPXV. Neutralization experiments with VACV antisera from humans and rabbits were used to confirm that the virus belonged to the OPV genus. Further experiments (complement-fixation, hemagglutination inhibition (HAI), electron microscopy and diffuse-precipitation) also suggested that the virus belonged to the OPV genus. Interestingly, MPXV was reported to be isolated from the kidneys of healthy animals that had been sacrificed for other reasons. The origin of the isolate is thought to be WA, based on genome sequence comparisons [6]. Further studies in other animals were performed and are described below [34].
Outbreak of MPXV in the USA (1959)
A second MPXV outbreak was also reported the same year as the Von Magnus et al. [34] report in a colony of captive monkeys in Philadelphia, USA. Small numbers of animals of all ages and both sexes were infected; however, because of the large numbers of animals in a given cage and the inability to examine all animals, it was impossible to determine the exact number of diseased animals [35]. This outbreak predominantly affected the M. fascicularis species; however, unlike the case reported by Von Magnus et al. [34], M. mulatta was also affected, although less severely. Subsequent serological findings revealed that a large number of M. mulatta had been infected without showing clinical evidence. In the outbreak, two distinct types of disease occurred; the first consisted of an acute disease in M. fascicularis animals only. The disease was characterized by facial edema that extended to the cervical region. Severe difficulty in breathing was also observed, which apparently led to death by asphyxiation. At the same time, papular eruptions were present over various parts of the body, with ulcerative lesions in the oral mucosa and generalized lymphadenopathy. The second form of the disease, which presented in M. fascicularis and M. mulatta, was more common and resulted in cutaneous eruptions with no other obvious clinical signs. Initially, the lesions formed a single crop of papules which became pustular and then crusted over after 7–10 days, leaving small scars. Hemorrhagic lesions were particularly associated with fatal cases. All parts of the body surface were involved, with the most commonly affected areas being the buttocks, hands, feet, face and hind limbs. Histopathology of the skin revealed focal proliferation of the epidermis, followed by necrosis and a recruitment of inflammatory cells, with the thickness of the epidermis increasing from three to four layers to 25–30. MPXV was isolated from naturally infected animals using embryonated chicken eggs and tissue culture. Similar to the findings by Von Magnus et al. [34], in chorioallantoic membranes the lesions typically spread along the blood vessels, were smaller than those produced by VACV, and were not typical of those associated with CPXV infection.
Outbreak of MPXV in the USA (1962)
A second outbreak of MPXV was reported in the USA in two M. fascicularis monkeys approximately 45 days after exposure to whole-body irradiation [36]. Lesions were clinically similar to those described by Von Magnus et al. and Prier et al. [34,37], that is: pox-like eruptions, severe facial and cervical edema, hemorrhagic ulcerations, dyspnea and bloody diarrhea. Both infected monkeys died after 12 days. One nonirradiated monkey also became sick with cervical edema and ulcerated areas on the arms and forehead; however, this animal survived. HAI titers from animals housed in the same room as those exhibiting disease revealed that 89% of animals were positive; in contrast, only 11% of animals held in separate rooms were positive [36].
Outbreak of MPXV at Rotterdam Zoo (1964)
One of the first indications of the broad host-range of MPXV, and its propensity to also infect larger species in pan-geographical locations, came from an outbreak at Rotterdam Zoo. MPXV was apparently introduced by Central/South American giant anteaters (Myrmecophaga tridactyla), which became ill approximately 12 days after arrival; however, it is suspected that the anteaters contracted the virus from previous contact with monkeys elsewhere. Following a pox-like illness, characterized by multiple skin lesions, the animals were sacrificed. The anteaters had been housed close to the Asian orangutan (Pongo pygmaeus) enclosure and these orangutans (n=10) also became severely ill with erythema and had purulent nasal discharge on the mucous membranes. Lesions were also observed on the face, legs and body. Six out of ten animals died after a few days of clinical signs and the remainder survived following a long period of convalescence. It is not clear if the virus was transmitted by aerosol or fomites. Several other species also became infected, with various degrees of mortality and morbidity, including both African gorillas (Gorilla gorilla) and most (n unknown) chimpanzees (Pan troglodytes), which became ill and presented with pox lesions; the only Asian gibbon (Hylobates lar) in the park, which died after 18 days of severe illness and presented with vesicles on the face, trunk and limbs; three (total n unknown) South American squirrel monkeys (Saimiri sciureus) died, with one out of three presenting with pox lesions; four (total n unknown) African owl-faced monkeys (Cercopithecus hamlyni), which became sick and presented with lesions on the lips that resolved within a few days; and a South American common marmoset (Hapale jacchus), which became sick and died following reddening and swelling of the area around the nose and eyes with lesions on the face and belly. Some other Hapale also presented with similar swelling, but had no further clinical signs [38,39].
Outbreak of MPXV in the USA (2003)
A third outbreak of MPXV in the USA occurred via the importation of rodents from Ghana, WA (see below). Infected animals went on to infect native prairie dogs, which were housed in close proximity to the African rodents. The prairie dogs proved to be highly susceptible to the virus and transmitted MPXV to approximately 40 humans – the first known case of human monkeypox (MPX) outside of Africa [21,40]. Interestingly, humans were only infected by the prairie dogs – which acted as amplifying hosts – and not by any of the rodents imported from WA. More detailed descriptions of animal infections are discussed below.
Nonhuman primates as models of MPXV
Evidence of MPXV infection of NHPs has been reported in several African serological surveys. In a 1986 survey in north Zaire, 39 primates were tested for MPXV specific antibodies; of which, two out of 22 crowned monkeys (Cercopithecus ascanius) and one out of three red-tailed monkeys (Cercopithecus pogonias) were positive. The remaining animals, all of different species, were negative [19]. During serological surveys in WA, two lesser white-nosed monkeys (Cercopithecus petaurista) were found to be positive for MPXV-specific antibodies, as well as one western colobus monkey (Colobus badius) [14]. A second serological survey tested 13 wild-caught monkeys for MPXV-specific antibodies, two Chlorocebus aethiops and one Cercopithecus petaurista were positive [15]. This could indicate that monkeys of the Cercopithecus genus have a propensity for MPXV infection.
MPXV has only been detected in NHPs in nature in Africa, and many African primates appear to have been exposed at some point. It is therefore unfortunate that, due to lack of availability, experimental studies have not been performed in African primates; rather, the bulk of studies have been performed in cynomolgus and rhesus macaques, which are native to various parts of Asia where MPXV has never been reported. This choice of primate was likely made for several reasons: because these animals had been observed to be susceptible to the disease in previous outbreaks; because these animals are readily available; because these primates are well studied; and because they thrive well in a research vivarium.
Early experimental studies on MPXV pathogenesis in M. fascicularis & M. mulatta
The original report by Von Magnus et al. included an id. inoculation of MPXV (dose unreported) in the palm of the hand of two (presumably seronegative) M. mulatta monkeys which resulted in no signs of illness [34]; however, similar inoculation in an M. fascicularis monkey resulted in a local pustule surrounded by edema 7 days post-infection (p.i.) and a slightly elevated temperature between days 5 and 9 p.i. [34]. These id. findings were confirmed by Prier, who also found that an intravenous (iv.) challenge resulted in generalized eruptions and virus recovery from skin lesions [35,37,41].
One of the earliest studies on experimental infection of M. mulatta investigated the protective capability of vaccination (by traditional scarification [SCR]) with VACV against an iv. challenge with 105.5 median tissue culture infective dose (TCID50) of MPXV isolated from the second US outbreak [36]. Challenge at 35 days postvaccination resulted in no disease manifestations in five out of six monkeys; however, one animal developed bloody diarrhea and died 9 days p.i.; but necropsy did not reveal any patho logical manifestations of MPX. In unvaccinated animals, severe MPX disease was reported, with characteristic vesicles, pustules, and necrotic lesions on the dermis of the face, arms, legs and tail – one out of five monkeys died on day 9 p.i. [42].
Gispen et al. challenged M. fascicularis via SCR or via the in. route with MPXV (Utrecht 64-7255) [38] isolated from animals from the Rotterdam Zoo outbreak [39]. The scarified monkey showed a temperature rise on day 6 and a local eruption of pox lesions 1 day later that had crusted over and fallen off by day 20. A second temperature rise was also reported on day 11 followed by secondary eruption of a few discrete vesicles on the inner side of the thigh, arms, chest and belly. The in. inoculated animal had a febrile reaction on day 7 and 8, followed by the appearance of discrete vesicles on the neck, chest, belly, inner-thigh and the hollow of the knees. Both animals survived [38].
By far the most comprehensive early studies on MPXV pathogenesis were conducted in a series of experiments by Wenner et al. in the late 1960s. Challenge (with a strain of MPXV originally recovered from a pustule of M. fascicularis) of M. fascicularis with 105 PFU via the iv., intramuscular (im.) and sc. routes revealed strikingly similar disease patterns marked by an abrupt temperature increase from day 2–4 that lasted only 1–2 days followed by a second temperature increase on day 6 p.i. that lasted for approximately 5 days [34,43]. Eleven out of 12 animals developed a rash at this time with a generalized exanthema on day 7–11 characterized by the typical papule, vesicle, pustule and scab appearance over a period of 3–7 days. Lesions were also observed on the soles of the feet, palms, buccal mucosa and soft palate. No animals died. A second experiment using the same dose of MPXV repeated the im. study but also included im. inoculations of M. mulatta. Generally, the disease progression in M. mulatta followed that of M. fascicularis, but with reduced severity and a delay in disease biomarker appearance by approximately 2 days: that is, seven out of nine and nine out of nine animals developed a rash for M. mulatta and M. fascicularis from day 9–16 and 8–20, respectively, and lesions were far less pronounced in the former. Virus could not be detected in tissues before day 6 and could rarely be found in visceral organs after rash onset. HAI and neutralizing antibodies (NA) developed and reached maximum levels at 3 weeks p.i., and all animals subsequently challenged with VACV via the im. route were resistant, whereas control animals developed typical VACV lesions and generalized vaccinia [1,2].
Further studies by Wenner et al. investigated the effect of increasing the virus dose on MPX disease in M. fascicularis inoculated via the im. route with a strain derived previously from blood obtained on the fifth day from a monkey that developed MPX (strain not identified). Generally, disease progression mirrored those of the previous studies [1,2]. Animals regularly developed MPX from 10−1–10−5 virus dilutions (from tissue culture-prepared virus). Emergent clinical features at each dilution were almost indistinguishable; however, the variability in viremia was high and was not dose-dependent. By day 6 there was a high concentration of MPXV in the tonsil, spleen, lymph nodes, bone marrow and skin lesions. Ten out of 34 (doses not given) animals died at day 10–15, characterized by a rash more intense than in survivors and marked by a more severe illness from days 4–11, with progressive dehydration and weight loss. Sentinel monkeys remained healthy until days 18–20, when three out of four developed fever followed by MPX disease (no indication of the route of infection was provided). Three out of four sentinel animals developed rash; however, all sentinels were HAI positive by day 23 following clinical signs of illness [44,45].
The im. model was also used to evaluate the drug methisazone for the treatment of smallpox in the M. fascicularis model. Infected controls presented with a disease course similar to that reported above [44,45]. The infected and drug-treated group had a mortality rate of 20% (one out of five) with death on day 18 p.i. The infected and untreated group had a mortality rate of 66% (two out of three) with death on days 15 and 23 p.i. The drug failed to prevent or modify the disease course [46].
These early studies revealed that M. fascicularis is highly susceptible to MPXV, and that inoculation via several routes of infection (iv., SCR, in., im. and sc.) elicit a similar disease course that typically results in little or no mortality. Challenge of M. mulatta via the iv. route resulted in low mortality (one out of five) and again, a disease course similar to that produced by im. challenge. M. mulatta appears to be more resistant to an im. challenge compared with M. fascicularis, as indicated by disease severity; however, both species are infected, as evidenced by antibodies. The low mortality rates in these studies compared with more recent studies (see below) indicates that a lower inoculum was used and/or a less-virulent strain of MPXV was administered.
Experimental infections of M. fascicularis & M. mulatta via the iv. route
With the realization that the Soviet Union had weaponized VARV [47], the USA initiated a research program to license modern smallpox vaccines, produce additional stocks of vaccinia immunoglobulin and license at least two antiviral drugs targeted at different stages of the replication cycle. The most thoroughly utilized model for these developments is the iv. challenge of M. fascicularis and M. mulatta.
The iv. route
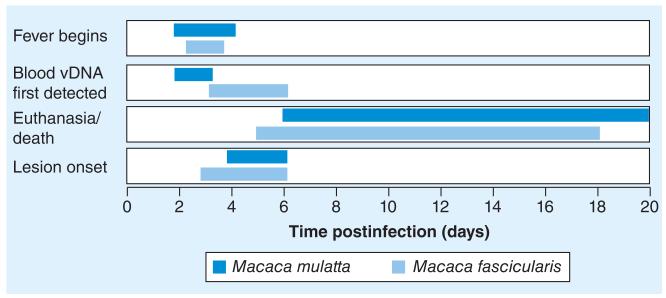
MPXV iv. inoculations in NHPs have been extensively evaluated as a model for smallpox and human monkeypox. The major disadvantage to this route is that the initial infection of respiratory tissue, incubation and prodromal phases are completely bypassed, and thus it does not accurately model the natural route of transmission. The iv. route is primarily used to test medical countermeasures against the most rigorous challenge route. Indeed, the majority of vaccination and antiviral development studies have utilized the iv. route of exposure in M. fascicularis and M. mulatta. Figure 1 shows the development of four key disease biomarkers in M. fascicularis and M. mulatta challenged with 107 PFU of CB (Z79). At high challenge doses the disease patterns were similar between both NHP species.
Figure 1. Four disease biomarkers are shown following an intravenous 107 PFU Congo Basin (Z79) challenge in Macaca mulatta and Macaca fascicularis.
Initial detection of fever, vDNA in blood and lesion appearance are shown. The ranges of euthanasia/death are also shown (see Tables 1 & 3 for more details).
vDNA: Viral DNA.
iv. inoculation of M. fascicularis
Details of iv. challenges are found in Table 1 and Figure 1. Similar responses following an iv. challenge with 107 PFU of CB (Z79) have been reported by several groups: viral load increases rapidly from approximately day 4, fever occurs at approximately day 3 p.i., with lymphadenopathy at day 3–4. A vesiculopustular rash develops from approximately day 4, initially on the oral mucosa followed by axillary and inguinal regions. Weight loss and a typical skin lesion count of >1000 lesions is frequently reported, followed by euthanasia or death between days 9 and 15 [23,48,49]. Goff et al. used a slightly attenuated CB (Z79-GFP) recombinant to further analyze the disease progression and found that they could detect lesions 1–2 days earlier under fluorescence [50]. Fluorescence was brightest in lesions of the nonkeratinized lining of the oral cavity and least prominent in the thickly keratinized skin of the soles and palms. Necropsy revealed lesions in the skin, mouth and upper airway, the lining of the GI tract, enlargement of lymph nodes, the spleen and pulmonary congestion. Immunohistochemistry (IHC) on euthanized animals using anti-VACV or anti-GFP antibodies detected antigens in numerous sites, including the pox lesions of the skin, mouth, nose, mandibular and axillary lymph nodes, esophagus, lung, liver, spleen and testis [50]. Multiple studies have examined the efficacy of vaccines and antivirals in the iv. model and are summarized in Table 2.
Table 1. Intravenous Macaca fascicularis challenges with Congo Basin strain (Zaire 79).
| Dose | Fever onset (duration) |
Weight loss (%) |
Lesion onset (maximum lesion count) |
Mortality/ euthanasia (days of death) |
Blood vDNA detected† (duration of detection) |
Infectious virus from swabs (duration of detection) |
Ref. |
|---|---|---|---|---|---|---|---|
| 107 | Day 3 | 4–10 | Day 3–6 (>1000) | 2/6 (by day 18) | Day 6 (to day 27) | [23] | |
| 107 | 2–6 | Day 5–6 (>2500) | 4/4 (5, 7, 7, 7) | Day 5–7 (to day 7) | [48] | ||
| 106 | 4–8 | Day 6 (>1200) | 4/6 (12, 12, 12, 14) | Day 6–9 (to day 14) | [48] | ||
| 107 | Day 3 (to day 7) | 1–10 | (>250) | 8/8 (by day 9) | Throat from day 2–6 Blood from day 5 |
[102] | |
| 107 | 6–11 | Day 4 (>1000) | 4/6 (9, 9, 11, 14) | Day 4 (to day 28) | [103] | ||
| 107 | Day 3–4 (>1500) | 3/3 (11, 12, 13) | Day 4 (to day 13) | [104] | |||
| 107 | Day 5–8 (>1400) | 3/3 (9, 13, 13) | Day 4 (to day 12) | [105] | |||
| 107 | Day 3 (to day 9) | 7–10 | By day 6 (>750) | 3/4 by day 15 | Day 4 (to day 15) | [106] | |
| 107 | By day 6 (TNTC) | 5/5 (11, 11, 11, 13, 15) |
Day 4 (to day 15) | [107] | |||
| 107 | Day 3 (to day 12) |
>4 | Day 6 (>200) | 3/3 (9, 11, 15) | Day 3 (to day 15) | [49] |
Does not include detection at day 0 following intravenous challenge.
TNTC: Too numerous to count; vDNA: Viral DNA.
Table 2. Evaluation of therapeutics and prophylactics against intravenous inoculation of Macaca fascicularis and Macaca mulatta†.
| Vaccine/treatment/therapy (administration days prechallenge) |
MPXV strain (PFU) |
End point | Outcome | Ref. | |
|---|---|---|---|---|---|
| Primary | Secondary | ||||
| M. fascicularis vaccination | |||||
| MVA (120 and 60) | CB Z79 (107) | Death | Blood vDNA/lesions (onset, description)/ weight change |
Survival/low vDNA levels/1–36 lesions (day 9–15, small atypical lesions)/no weight loss |
[23] |
| MVA (120) + Dryvax (60) | Survival/low vDNA levels/no lesions/no weight loss | ||||
| Dryvax (60) | Survival/low vDNA levels/no lesions/no weight loss | ||||
| MVA (30) or MVA (10) | CB Z79 (107) | Death | Blood vDNA/lesions (onset, description)/ weight change |
Survival/reduced vDNA/2 to >996 lesions (day 6–9)/no weight loss | [48] |
| Dryvax (30) or Dryvax (10) | Survival/low-level vDNA/no lesions at day 30 vaccination; 0–29 lesions at day 10 vaccination (day 6–9)/no weight loss |
||||
| MVA (10) or Dryvax (10) | CB Z79 (106) | Death | Blood vDNA/lesions (onset, description)/ weight change |
Survival/reduced vDNA/MVA lesions (day 6–9); Dryvax no lesions/ no weight loss |
[48] |
| MVA (6) | 1 of 4 mortality/reduced vDNA/lesions (day 6–9)/6% weight loss in nonsurvivor |
||||
| Dryvax (6) | Survival/reduced vDNA/lesions (day 6)/no weight loss | ||||
| MVA (4) | Survival/reduced vDNA/lesions (day 6)/no weight loss | ||||
| Dryvax (4) | 3 of 4 mortality/reduced vDNA/lesions (day 6)/15% weight loss | ||||
| ACAM2000 (60) or Dryvax (60) | CB Z79 (107) | Death | Temperature/virus in blood and throat swabs/ lesions/weight change |
Survival/normal temperature/virus by day 5/no lesions/1% weight gain |
[102] |
| NYCBH (49 and 28) | CB Z79 (107) | Death | Temperature/weight change/blood vDNA |
Survival/normal temperature/weight gain/low vDNA | [108] |
| NYCBH with E3L deletion (49 and 28) | 2 of 8 mortality/slight temperature increase/4% weight loss/ increased vDNA |
||||
| Dryvax (119) | CB Z79 (107) | Death | Lesions/blood vDNA | Survival/0–7 lesions/low vDNA levels | [107] |
| A33+B5+L1+A27 subunit proteins (119, 91, 35) | Survival/0–32 lesions/reduced vDNA | ||||
| A27+F9+H3+L1+A33+A56+B5 DNA vaccine (0, 28, 56)‡ |
CB NR-523 (107) |
Death | Lesions/blood vDNA/ temperature/weight change |
Survival/133–175 lesions/reduced vDNA/normal temperature/no weight change |
[106] |
| Wyeth§ or Wyeth-IL15§,¶ or Wyeth-IL12§,¶ or MVA§ or MVA- IL15§,¶ (all at 1000) |
CB Z79 (107) | Death | Lesions (onset)/blood vDNA/temperature/ weight |
Survival/>200 lesions (day 6)/low vDNA levels/normal temperature/ some weight loss >4% |
[49] |
| MVA-IL2§,¶ (1000) | 2 of 3 survival/>200 lesions (day 6)/low vDNA levels/normal temperature/some weight loss >4% |
||||
| M. fascicularis vaccination + antiviral therapy | |||||
| Dryvax (59) | CB Z79 (107) | Death | Lesions (onset)/weight change |
Similar to above Dryvax outcomes | [103] |
| Dryvax (59) + cidofovir (59) | 1 of 6 survival/600–1800 lesions (day 7)/4–11% weight loss | ||||
| ST-246# (day 0 or 3 p.i.) | CB Z79 (107) | Death | Lesions/blood vDNA | Survival/no lesions/low vDNA levels | [104,105] |
| M. mulatta vaccination | |||||
| Dryvax (>365) | CB Z79 (107) | Death | Lesions (onset)/blood vDNA/throat swab/ temperature |
Survival/no clinical changes | [51] |
| L1R+A27L+A33R+B5R DNA vaccine (multiple over ~365)†† |
Survival/<50 lesions (day 6)/low vDNA levels/throat swab from day 4/mild temperature increase |
||||
| Dryvax (26)/control AB | CB Z79 (107) | Death | Lesions/blood vDNA | Survival/no lesions/no vDNA | [109] |
| Dryvax (26)/antibody CD8 depletion (1, 0, +1, +2, +6) | |||||
| Dryvax (29)/antibody CD4 depletion (4, 0, +5, +10) | |||||
| Dryvax (26)/antibody CD20 depletion (34, 27, 26, 19, 11, 1, 0, +1, +3, +6) |
3 of 4 mortality/lesions TNTC/high vDNA levels | ||||
| VIG (3, 0) | Survival/12–330 lesions/ reduced vDNA levels | ||||
| L1R+A27L+A33R+B5R DNA (35, 31, 25)‡‡ | CB Z79 (107) | Death | Lesions (onset)/blood vDNA |
2 of 3 mortality/lesions TNTC/high vDNA levels | [110] |
| L1R+A27L+A33R+B5R DNA (35, 31, 25) ‡‡ followed by L1R+A27L+A33R+B5R proteins (16, 12, 5)§§ |
Survival/3–15 lesions (day 7)/low vDNA levels | ||||
| Dryvax (896) | CB Z79 (107) | Death | Lesions/vDNA in blood/ temperature |
Survival/0–200 lesions/low vDNA levels/no temperature change | [111] |
| MVA-HIV recombinant (1092) | Survival/0–200 lesions/reduced vDNA levels/no temperature change |
||||
| MVA-HIV recombinant (912)¶¶ | CB Z79 (107) | Death | Lesions (onset)/blood vDNA |
Survival/7–38 lesions (days 7–12)/low vDNA levels | [112] |
| Vaccination of immunodeficient M. mulatta ## | |||||
| MVA (388, 360)/Dryvax (180) | CB Z79 (107) | Death | Lesions/throat swab/ blood swab/blood vDNA |
3 of 4 mortality/lesions TNTC/high levels blood swab/high levels blood vDNA |
[113] |
| NYVAC (388, 360)/Dryvax (180) | |||||
| Dryvax (180) | 2 of 4 mortality/lesions TNTC/high levels blood swab/high levels blood vDNA |
||||
Vaccines administered by the following routes: MVA (intramuscular); ACAM2000 (scarification); Dryvax (scarification).
Administered intramuscularly or intradermally with similar results.
Administered intradermally.
Recombinant viruses.
Administered for 14 days.
Administered via gene gun.
Administered intramuscularly and intradermally.
Administered intramuscularly.
Administered intramuscularly or intradermally using a needle-free Biojectior or in the palatine tonsils via a SyriJet MkII; with similar results regardless of route.
Immunodeficent due to infection with SIV-induced AIDS.
CB: Congo Basin; MPXV: Monkeypox virus; p.i.: Postinfection; TNTC: Too numerous to count; vDNA: Viral DNA; VIG: Vaccinia immunoglobulin.
iv. inoculation of M. mulatta
Several studies have utilized the iv. model in M. mulatta (Figure 1 & Table 3). Hooper et al. showed that animals infected with 108 PFU of CB (Z79) caused organ-hemorrhagic MPX with death on day 6 [51]. The likely cause of death was cardiovascular collapse secondary to multiple organ failure. It was subsequently determined that this dose did not accurately model smallpox or natural infections with MPXV. A lower dose of 106 PFU caused an eruptive rash on day 6, which progressed to disseminated exanthema with >100 lesions per animal; however, all animals survived challenge. A 107-PFU dose resulted in the development of grave MPX, and animals succumbed on days 7, 10 and 14 p.i., with associated fever commencing on days 2–4 and viral DNA (vDNA) being detected from days 2–6. The cause of death in the day-7 monkey was likely hemorrhage of the lymph nodes, heart, lungs, urinary bladder, uterus and digestive tract. In addition, there was hepatopathy, splenomegaly, lymphadenopathy, diffuse pulmonary edema, and degeneration and necrosis of the bone marrow. Animals that died on days 10 and 14 presented with a disseminated exanthematous rash, marked lymphadenopathy, mild splenomegaly, mild pulmonary edema and a notable absence of remarkable pathology in other organs. The most noticeable manifestation was the generalized vesiculopustular rash that became evident on day 6 that followed typical macule, papule, vesicle, pustule progression until crusts at day 10. Lesions were primarily distributed on the hands and face, but rarely on the abdomen [51]. Multiple studies have examined the efficacy of vaccines and antivirals in the iv. model and are summarized in Table 2.
Table 3. Intravenous Macaca mulatta challenges with Congo Basin strain (Z79).
| Dose | Fever onset (duration) |
Lesion onset (maximum lesion count) |
Mortality/euthanasia (days of death) |
Blood vDNA detected† (duration of detection) |
Infectious virus from swabs (duration of detection) |
Ref. |
|---|---|---|---|---|---|---|
| 107 | Day 2 (to day 8) | 2/2 by day 13 | Day 2 (to day 8) | [111] | ||
| 107 | Day 6 (734) | 0/0 (but severe disease) | By day 3 (to day 15) | [112] | ||
| 107 | Day 4 (TNTC) | 3/3 (11, 17, 21) | By day 3 (to day 9‡) | [110] | ||
| 107 | Day 2–4 (to day 14) | Day 6 (>100) | 3/3 (7, 10, 14) | Day 2–6 (to day 14) | Throat from day 6 (to day 14) |
[51] |
| 108 | Only on day 2 in 1/2 | No lesions | 2/2 (6, 6) | [51] | ||
| 106 | Day 2 (to day 8) | Day 6 (>100) | 0/2 (but severe disease) | [51] |
Does not include detection at day 0 following intravenous challenge.
No samples taken from day 9.
TNTC: Too numerous to count; vDNA: Viral DNA.
In summary, both M. mulatta and M. fascicularis are highly susc eptible to MPXV administered via the iv. route at ≥106 PFU and respond well to vaccines and antivirals. Fever develops at approximately the same time (~day 2–4), but skin lesions and death are slightly delayed in the M. mulatta model (Figure 1). Blood vDNA is detected earlier in the M. mulatta model compared with M. fascicularis, which has a delay of approximately 2 days with significant variability in detection. One key observation is the interspecies variability between animals – particularly in mortality and lesion onset – which makes direct comparisons between M. fascicularis and M. mulatta difficult; moreover, only four studies have evaluated the iv. M. mulatta model compared with nine for M. fascicularis.
Experimental infections of M. fascicularis & M. mulatta via respiratory routes
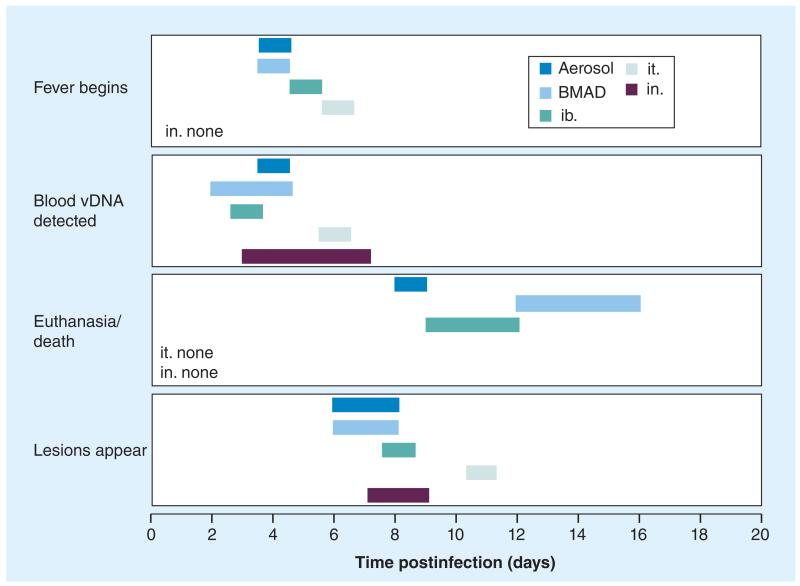
Experimental challenges via respiratory routes are outlined below. Figure 2 presents a schematic of the presentation of fever, vDNA detection in blood, euthanasia/death and the appearance of skin lesions in M. fascicularis. Table 4 summarizes the key findings from respiratory challenges.
Figure 2. Four disease biomarkers are shown following five different respiratory challenges with 106 PFU of monkeypox virus (Congo Basin) in Macaca fascicularis.
Initial detection of fever, vDNA in blood and the lesion appearance are shown. The ranges of euthanasia/death are also shown. Data is taken from Table 4.
BMAD: Bronchoscope-microsprayer aerosol delivery; ib.: Intrabronchial; in.: Intranasal; it.: Intratracheal; vDNA: Viral DNA.
Table 4. Respiratory challenges with Congo Basin strains in Macaca fascicularis and Macaca mulatta.
| Route | Species | Dose (PFU) |
Fever onset (duration) |
Weight loss |
Lesion onset (maximum lesion count) |
Mortality (days of death or euthanasia) |
Blood vDNA detected (duration of detection) |
Oral vDNA detected (duration of detection) |
Infectious virus from oral swabs (duration of detection) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Aerosol | MF | 105 EIU | Day 5–8 | Day 9–10 | 2/4 | [52] | ||||
| Aerosol MF | 104–105 | Day 6–7 | Day 6–7 | 15/15 (9–17) | [53] | |||||
| Aerosol | MF | 104 | Day 5 (4 days) | No sig† | Day 6–8 | 2/3 (10, 11) | Day 6 (to day 22) | Day 6 (to day 23) | [54] | |
| Aerosol | MF | 105 | Day 4 (5 days) | No sig† | Day 6–8 | 4/6 (8, 9, 10, 10) | Day 6 (to day 22) | Day 8 (to >day 26) | [54] | |
| Aerosol | MF | 4 × 105 | Day 3 (5 days) | No sig† | Day 6–8 | 5/6 (11, 10, 10, 9, 8) | Day 4 (to day 20) | Day 6 (to >day 26) | [54] | |
| Aerosol | MF | 106 | Day 4 (5 days) | No sig† | Day 6–8 | 2/3 (8, 9) | Day 4 (to day 14) | Day 6 (to day 18) | [54] | |
| BMAD | MF | 3 × 106 | Day 4 (4–8 days) |
10–15% | Day 8 (304) | 1/3 (12) | Day 4 (to day 20) | [24] | ||
| BMAD | MF | 8 × 106 | Day 4 (until death) |
>10% | Day 6 (550) | 2/2 (12, 16‡) | Day 2 (until death) | [24] | ||
| BMAD | MF | 3 × 107 | Day 4–6 (<2 days) |
~10% | Day 4–10 (350) | 2/3 (8, 8) | Day 2–4 (8–17 days) | [24] | ||
| ib. | MF | 104 | Day 6 (10 days) | Day 8 (170) | 0/3 | Day 5 (17 days) | [55] | |||
| MF | 105 | Day 7 (7 days) | Day 9 (237) | 1/6 (9) | Day 5 (12 days) | Day 7 | [55] | |||
| MF | 106 | Day 5 (9 days) | Day 8 (288) | 2/3 (9, 12) | Day 3 (17 days) | Day 7 | [55] | |||
| ib. | MM | 105 | Day 7–12 | 0/4 | Day 4–14 (~10 days) | [56] | ||||
| it. | MF | 106 | Day 6 (5 days) | Day 11 (>100) | 0/3 | Day 6 (13 days) | Day 4 (23 days) | Day 4 (23 days) | [58] | |
| it. | 107 | Day 5 (until death) |
Day 8–10 (>100) | 3/3 (15, 19, 19) | Day 4 (until death) | Day 4 (until death) | Day 4 (until death) | [58] | ||
| it. | MF | 107 | Day 9 (>100) | 6/6 (9, 9, 11, 11, 15, 15) |
Day 5 (until death) | [59] | ||||
| in. | MF | 106 | None | ~10% | Day 7–9 (178)§ | 0/2 | Day 3–7 (10–20 days) | [32] |
No significant changes over time; however, survivors were noted to be approximately 20% heavier than nonsurvivors at the start of the study.
A third animal died on day 22 but is thought to have died from secondary bacterial infection and has therefore been excluded.
One animal had 178 lesions and the other had one lesion.
BMAD: Bronchoscope-microsprayer aerosol delivery; EIU: Egg infectious unit; ib.: Intrabronchial; in: Intranasal; it: Intratracheal; MF: Macaca fascicularis; MM: Macaca mulatta; Sig: Significant; vDNA: Viral DNA.
Aerosol
Aerosol exposure of M. fascicularis was first evaluated in 1961. Administration of virus (dose unspecified) resulted in 50% mortality (day of death not specified) and the development of a typical rash by day 9–10 p.i. An elevation in temperature was noted between day 5–8 p.i. as well as bronchiolitis, bronchitis and peri bronchitis. Fibrinous necrosis was found in the bronchial walls, peri bronchial lymph oid tissues and bronchopulmonary lymph nodes [52].
Exposing M. fascicularis to a ~6.5 × 104 dose of CB (Z79) resulted in death or euthanasia as a result of fibrinonecrotic bronchopneumonia [53]. Exanthema, enanthema, anorexia, fever, cough and nasal discharge began to present on days 6–7 p.i. By days 9–10 p.i. all animals had exanthema, enanthema, depression and weakness which worsened until death. The lower airway epithelium served as the principal target for primary infection and the tonsil, mediastinal and mandibular lymph nodes were also infected early in the course of infection. Lesions affecting lymph nodes, thymus, spleen, skin, oral mucosa, the GI tract and the reproductive system were caused by a monocytic cell-associated viremia; however, there was no cell-free viremia detected at any time. Detectable poxvirus antigen was limited to sites exhibiting obvious morphological involvement and was most prominent in epithelial, macrophage, dendritic and fibroblast cells of affected tissues. The presence of antigen as determined by IHC correlated with virus-infected tissues as observed by ultrastructural examination. Necropsy findings revealed that the lungs were congested, failed to collapse and presented with edema atelectasis and necrosis throughout the lobes. The oral cavity of most animals presented with glossitis and stomatitis, with gingivitis in 50% of animals; generally, the dorsal surface of the tongue and hard palate were involved and presented with depressed foci of necrosis, erosion or ulceration. Infectious virus was isolated from the buffy coats of samples taken from most animals at day 9 p.i. [53].
A separate study also evaluated aerosol exposure of M. fascicularis with CB (Z79) at doses ranging from 104–106 PFU [54]. Unlike the Zaucha et al. study [53], which focused primarily on histopathological and IHC findings, Nalca et al. focused on disease biomarkers; however, overlapping pathological and disease progression/presentation studies revealed strikingly similar results, with fibrinonecrotic bronchopneumonia being the most distinctive lesion observed and being the attributed cause of death [54]. Interestingly, lesion numbers were not dose-dependent but were generally higher in survivors compared with nonsurvivors, and it was noted that heavier animals had increased protection. Similar to the study by Hahon and McGavran, fever occurred from day 5; however, fever was significantly longer, with conclusion not being reached until 13–15 days later [52]. Interestingly, groups receiving lower-dose inoculums had lower temperature elevations and fevers of shorter duration. Serum chemistry was evaluated and it was found that total protein, albumin levels, LDH levels and CRP were altered with disease progression. vDNA levels, which were slightly higher in nonsurvivors compared with survivors, could be detected in the blood and throat-swabs from day 4 and peaked at day 10 p.i., with levels typically returning to normal over the following 4–12 days. In tissue, DNA from the lung and pox lesions had the highest viral loads; high loads were also reported in the spleen, gonads, axillary lymph nodes and inguinal lymph nodes [54].
Bronchoscope-microsprayer aerosol delivery
The main disadvantage of typical aerosol delivery is that the inhaled dose of virus for each monkey varies considerably. The bronchoscope-microsprayer aerosol delivery (BMAD) method results in a dose-dependent incubation period before disease onset, the development of a disease that resembles smallpox with systemic dissemination, and results in pathology that is consistent with inhalation MPX. Use of the microsprayer reduces the incidence of lobar pneumonia and allows for the production of a clinical disease with similar pathology to that produced using aerosol challenges, albeit with a fraction of the required virus volume. BMAD allows for a more precise administration of virus inoculum. Goff et al. delivered MPXV CB (Z79) via the BMAD method to M. fascicularis (Figure 2 & Table 4); 10–100-fold (106–107) increases in dose only doubled mortality, indicating a poor dose response [24]. Those animals in the high-dose group followed a similar but accelerated disease course compared with the lower-dose animals, and two out of three animals succumbed to infection by day 8. Fever developed in two animals on day 4 and on day 6 in the third animal. Temperature remained elevated for 40 h; in the surviving animal, temperature returned to baseline, whereas the other two animals had temperature drops below baseline at the terminal stages. All animals lost approximately 10% body weight. Lymphadenopathy was first observed on day 6 and lesions developed from day 4, with total lesion counts being 350, 85 and 22. A typical pox lesion pattern was observed and higher lesion counts were observed in animals infected at the lower inoculums. In the lowest inoculum groups, vDNA was detected in the blood from day 4 and increased steadily until day 12. Animals that succumbed or were euthanized had similar gross pathological findings. Fibrinonecrotic bronchopneumonia was a consistent finding; the lungs were edematous and red and failed to collapse. Often, multiple necrotic foci were present. The trachea contained bloody froth and had multifocal or coalescing necrotic, dark-red mucosal lesions. Other gross lesions observed included the typical vesiculopustular, umbilicated and scabbed skin lesions, oral ulcers, enlarged peripheral lymph nodes, and proliferative and necrotizing or ulcerative lesions in the esophagus, stomach, and urinary bladder. Histological analysis revealed lesions consistent with fibrinonecrotic bronchopneumonia, necrosis of the trachea and surrounding mediastinal tissues, pulmonary edema, and pleuritis. Other lesions were noted in a large variety of tissues including stomach, intestines, liver, testes, bone marrow, skeletal muscle, thymus and spleen. IHC largely confirmed pathological findings.
Intrabronchial
The intrabronchial (ib.) route of infection delivers virus in a similar fashion to aerosol delivery; however, it has the advantage that it is easier to perform the inoculation and does not require the specialized equipment and facilities inherent to aerosol experiments. Furthermore, a more accurate inoculum dose can be delivered. Johnson et al. evaluated the disease course in M. fascicularis infected by the ib. route with 104–106 PFU of CB (Z79). End point criteria/death was observed in 66 and 15% of the NHPs challenged at the 106–105-PFU doses, respectively. In the 106 PFU-infected animals, the mean time to death was 20 days. Oxygen saturation (SpO2; in circulating blood and the peripheral tissue) and respiratory rate experiments revealed elevated respiratory rates from day 5 p.i. – that did not return to normal levels in animals that succumbed to disease – and SpO2 levels that dropped from day 14. Fever developed at day 6.3 and lasted until day 14, and lesions developed at approximately day 7.6. vDNA in the blood was present from days 3–20 with peak levels at day 13.7 and virus detection in oral and nasal swabs occurred at days 5–9 and 7–9, respectively, with peak levels at days 11–14 and 9–12, respectively, and NA could be detected from days 7–8. The overall cytokine/chemokine response to infection was proinflammatory; however, the levels within groups were highly variable [55]. Generally, animals receiving the lower-dose inoculums (105 and 104 PFU) presented with biomarker manifestations at similar times to those in the higher dose groups; however, clinical signs were less severe, with few lesions reported (Table 4).
In an ib. study utilizing a sublethal infection (105 PFU of CB [Z79]) of M. mulatta, it was found that pustular lesions developed by day 7–12 on the skin and oral mucosa. These numerous and widespread lesions progressed from the pustular to the crusting stage at day 12–14 before finally scabbing and healing at day 18–28. Animals also developed coughs and labored breathing between day 7 and 14. All infected animals developed fever, with a peak temperature recorded between days 7 and 14; interestingly, temperature remained elevated until week 3 or 4 p.i. vDNA levels were measured in whole blood, peripheral blood mononuclear cells and bronchoalveolar lavage (BAL) fluids, and generally it was found that elevated viral loads paralleled the appearance of skin lesions and the development of fever. A direct correlation was observed between viral load and clinical signs. Detection of vDNA in blood was highly variable at approximately days 4–14 and lasted approximately 10 days before becoming undetectable. In BAL fluid, vDNA was detected between days 7 and 10 and remained elevated until day 42, when levels were undetectable [56]. Interestingly, the proteome from the BAL fluid was also characterized, and it was found that elevated levels of proteins indicative of inflammation were produced, along with a dramatic decrease of structural and metabolic proteins [57].
Intratracheal
Infection of M. fascicularis with 106 PFU of MPXV (CB) via the intratracheal (it.) route is summarized in Figure 2 [52]. Infection of M. fascicularis via the it. route with 107 PFU of CB (MSF#6) resulted in death/euthanasia by day 15-19 p.i. (Table 4). Lesions developed by day 8–10 p.i. and were accompanied by anorexia and dyspnea. By day 14 the lesions were characterized as pustules and clinical signs of illness became more severe. Similar to other studies, histopathological examination of the lungs of the dead animals revealed macroscopic lesions characterized as fibrinonecrotic bronchopneumonia and cutaneous lesions characterized by acanthosis, necrosis and intracytoplasmic amphophilic inclusion bodies. Other changes to tissues were reported: tracheitis, necrotizing glossitis, lymphadenitis and splenitis with lymphoid depletion. vDNA could be detected in the blood and saliva from day 4 and increased rapidly until death, with peak levels reported at days 11–15. Fever was recorded from day 5 until death.
The it. challenge route has been used to demonstrate the efficacy of vaccination with MVA (IMVAMUNE®) and the classical VACV-based vaccines (Elstree-RIVM and Elstree-BN) in various combinations. All vaccinated animals presented with a brief elevation in body temperature between days 5 and 8 and slight elevations in blood and throat viral loads. Only one animal developed pocks; all other animals had no clinical signs of disease other than the temperature elevation [58]. In another study, M. fascicularis was infected via the it. route (107 PFU of CB [MSF#6]) and several different treatment regimens were evaluated: vaccination with RIVM; treatment with 5 mg/kg of CDV; and treatment with 5 mg/kg HPMPO-DAPy. Animals in the control group died by day 15 and presented with similar clinical signs to those in Stittelaar et al.’s studies [58]. These experiments revealed that antiviral treatment is more efficacious than post-exposure vaccination following MPXV infection of M. fascicularis (Table 4) [59].
Intranasal
An in. infection (Utrecht strain) of M. fascicularis was first evaluated in 1967 by Gispen et al. who observed a febrile reaction on days 7–8, followed by the appearance of vesicles on the neck, chest, belly, inner-thigh and the hollow of the knees [38]. Both animals survived. A second study by Noble (also using M. fascicularis) reported different results; two monkeys were inoculated with 108 PFU of MPXV (Utrecht 65–32), but failed to demonstrate skin lesions, facial edema or death; however, animals did seroconvert by 3 weeks p.i. [60]. More recently, Saijo et al. evaluated disease in M. fascicularis challenged with either 108 PFU of WA (Liberia) or CB (Zr-599) [32]. In Liberia strain-infected animals, body-weight decreased by approximately 10% following challenge, and clinical signs included loss of appetite, rhinorrhea and conjunctival discharge, diarrhea, irritability and a typical skin rash. The structures of the mucous membranes in the nasal cavity were damaged due to necrosis and inflammatory cell accumulation. vDNA could be detected in the blood from day 4 and peaked by day 9. In monkeys infected with 106 PFU of CB (Zr-599), one out of two presented with severe clinical signs and the other had very mild clinical signs; however, both survived. In the more severe cases, 178 skin lesions were recorded; these ulcerative lesions were still exudative on day 18 and were more severe compared with Liberia-infected animals, whose lesions had dried and were covered in scar tissue by day 18. vDNA levels peaked between days 5–10 and were 2 logs higher than those recorded for Liberia animals. Inappetence was also increased in these animals. Gross MPX-associated lesions were observed in the lymphoreticular system (tonsil, spleen, thymus and radial, inguinal, axillary, and submandibular lymph nodes), in the liver, pancreas and ileum [32].
The efficacy of vaccination with VACV strain Lister and LC16m8 was evaluated in M. fascicularis challenged in. with 108 PFU of WA (Liberia). Nonvaccinated animals presented with typical clinical signs (as above), whereas vaccinated animals presented with no clinical signs, no histopathological changes and no changes to viral load [61].
Experimental infections of M. fascicularis & M. mulatta via other routes
Subcutaneous
Prier and Sauer were the first to demonstrate that a sc. infection of M. fascicularis and M. mulatta, with a virus isolated from the 1959 US outbreak, caused local lesions without spread to other body parts [37]. Olsen et al. found that, following a 104.6 PFU WA (Cop) sc. challenge, a M. mulatta (n = 1) became ill with generalized MPX disease from day 7; this animal also survived [62]. Unfortunately, these findings are not consistent with more contemporary studies. Recently, M. fascicularis was challenged with 106 PFU of CB (Zr-599) via the sc. route [61]. Infection with MPXV was lethal and monkeys lost approximately 15% of their body weight. Papulovesicular skin lesions appeared on day 7 and animals presented with 390–1150 lesions. vDNA was detected by day 4 and reached a peak by day 12–15. Clinical signs were so severe that monkeys were euthanized on day 18. Lesions were detected in the lymphoid system, lung, trachea, stomach, small intestine, colon, rectum, liver, urinary bladder and uterus. CRP was measured as an indicator of inflammation and was significantly increased. Lymphopenia and thrombocytopenia were also detected. IFN-γ was elevated from day 4 and reached a peak at day 7 [61].
In a second study, Saijo et al. compared the disease of M. fascicularis inoculated sc. with 106 PFU of WA (Liberia) or CB (ZR-599) [32]. Generally, the sc. challenge caused milder disease, with findings similar to those of earlier studies [62]. Infection of three out of four ZR-599 animals was fatal and body mass decreased by 10–20% without any sign of recovery (except in the animal which survived). One out of three Liberia-infected animals died. The typical papulovesicular rash appeared on days 7–9 with higher lesion numbers in ZR-599-infected animals (390–1150 in fatal cases and 95 in surviving animal) compared with Liberia-infected animals (881 in fatal case and 29–196 in surviving animals); however, the morphologies of the lesions were similar. The most noticeable clinical signs in both groups were anorexia and diarrhea. The lymphoreticular system (radial, inguinal, axillar and submandibular lymph nodes, tonsil, thymus, spleen and pharynx) of both groups presented with the most significant lesions. The most significant differences between the two groups were the appearance of lesions with granulomatous inflammation in the stomach, small intestine and colon in ZR-599-infected animals. Antigen detection revealed that ZR-599 was present in organs that presented with lesions, as well as in the genitourinary tract, respiratory tract and gastrointestinal organs. Liberia infection was generally restricted to the skin, lymphoid and reticuloendothelial systems. The lungs of ZR-599-infected animals were entirely and diffusely affected by the infection but were unaffected in Liberia-infected animals.
Saijo et al. also evaluated the protection provided by vaccination with VACV Lister and LC16m8 in a M. fascicularis 106 sc. challenge with CB (Zr-599) [61]. Following vaccination a ‘take’ was reported in both groups. Following challenge (5 weeks post vaccination), animals vaccinated with either vaccine presented with no indications of disease except for local cutaneous lesions at the site of infection in LC16m8 animals; however, these lesions were much milder than those observed in the nonvaccinated group. Animals generally presented with no clinical signs of MPX. vDNA could be detected in the blood of vaccinated animals; however, the levels were lower than those of naive animals and persisted for a shorter time. Nonvaccinated animals (two out of two) all died with typical disease.
Comparison of inoculation routes
It is difficult to determine which inoculation route best recapitulates natural transmission of MPXV in M. fascicularis and M. mulatta. Early reports of natural outbreaks are rather vague and the viral strains are usually unknown; this presents a problem because many studies have indicated markedly different pathologies between CB and WA strains. Furthermore, it is likely that the inoculum in natural outbreaks is far lower than those administered experimentally. It is interesting that the 1958 outbreak in Denmark occurred 2 months after the animals were received. This could indicate that MPXV had been transmitted via fomites that had been in the vicinity for some time, or it is possible that MPXV had been amplifying in the colony for several months before the outbreak – although this does not explain why so few of the animals presented with clinical signs. id. inoculation from the Denmark outbreak and sc./id. from 1959 US outbreak caused few or no clinical signs, which indicates that infection did not occur via the skin. It is interesting that the 1959 US outbreak indicated that many M. mulatta did not present with disease but were seropositive. This is consistent with other findings that found that M. mulatta is less susceptible to the virus than M. fascicularis.
Since OPVs utilize various transmission mechanisms, one cannot assume that MPXV favors one mechanism over another; however, human infections in the US 2003 outbreak suggest that the virus gains access through the skin and likely through the respiratory tract too. The majority of M. fascicularis and M. mulatta studies have been geared toward modeling smallpox and therefore have utilized respiratory infection routes including aerosol, BMAD, ib., in. and it. It is difficult to evaluate which of these routes is the best method for inoculation because the transmission route for MPXV is unknown and, to some extent, may be species-specific. None of the investigated routes adequately mimic natural infection with MPXV and VARV. The main feature of most of the respiratory tract routes (except in.) was an infection with a high level of mortality with the presentation of a fibrinonecrotic bronchopneumonia; interestingly, a high incidence of fibrinonecrotic bronchopneumonia and interstitial pneumonitis was reported in fatal cases of human MPX [16,63,64]. Consideration should be given to the fact that the mechanical activity of inserting the delivery-apparatus (except for in aerosol challenges) could also scratch the tissue and initiate an inflammatory response at the inoculation site. The advantage of aerosol delivery over it. delivery is that it. deposits virus directly in the airways without regard to particle size and the physiological deposition that occurs during the process of inhalation. That said, the it. route led to 100% mortality in both studies, whereas mortality was variable in the aerosol studies. The drawbacks of aerosol infection are the very high dose of virus required for the nebulizing application and the variability of the exposure dose [24]. The aerosol route deposits virus to the distal airways and alveoli, which may more closely mimic exposure from biowarfare compared with natural transmission, which likely deposits virus in the upper respiratory tract. The ib. route delivers virus in a similar fashion to aerosol delivery and disease clinical signs are also similar. Inoculation is based on an anatomical landmark and the quantity of inoculums can be readily measured and completely administered. The procedure delivers the inoculum into the left tertiary bronchus using a pediatric bronchoscope, which is also inexpensive compared with aerosol delivery and likely makes the ib. route more appealing; it also gives similar mortality values as aerosol at similar doses. Two studies have made a direct comparison between lethal ib. and iv. challenges, which found that the maximum tolerated dose was double that experienced by iv.-challenged animals, and fever developed significantly later (2.6 days compared with 6.3) in ib. animals [55,65]. Furthermore, the number of lesions in the ib. model was significantly lower than those of the iv. model (range 194–644 and 60–1552 for ib. and iv., respectively). vDNA in the blood was present from days 3–20 in the ib. group and from days 2–11 in the iv. group; peak vDNA levels were at days 13.7 and 8.2, respectively. It should be noted from this data that lesions developed in the iv. and ib. models at approximately 2 days and approximately 4 days following the onset of viremia, respectively. In the iv. model, MPXV from oral and nasal swabs was detected at days 2–7 and 4–6, respectively; which was approximately 3 days earlier than in the ib. model. Cytokine/chemokine responses in the ib. model were also delayed by several days (~6). In general the distribution and level of virus replication in tissue was comparable between both routes, although levels were higher in the lymphoid and reticuloendothelial tissues from the iv. group. Notable differences were observed in the axillary, popliteal and cervical lymph nodes, where titers were 100-fold higher in the iv. groups. Other tissues with detectable virus included the liver, testes, ovaries, uterus, kidney and spinal cord. In summary, several key events were delayed in the ib. model compared with iv.: fever, lesion development, peak viremia, viral shedding in oral and nasal excretions, peak cytokine levels and end-point criteria. These data indicate that disease progression in ib. animals is delayed, which is understandable as the iv. route bypasses many early components of respiratory disease. iv. animals also experienced no changes in respiratory rates compared with ib., which was elevated [55].
When comparing the ib. to the it. route, it was found that it. animals had 100% mortality and a shorter time to death (16.3 vs 20 days in ib.); however, histopathology indicated similar lung pathology with fibrinonecrotic pneumonia reported in both it. and ib., thus, it would seem that the it. and ib. routes are comparable. BMAD allows for delivery directly above the tracheal carina of a large-particle aerosol via a microsprayer attached to a bronchoscope, resulting in a similar disease course to that of an aerosol-inoculated monkey. It also offers advantages over the aerosol route, that is: a dose-dependent incubation period before disease onset, the ability to accurately deliver a fixed dose of virus, and the fact that the equipment required for inoculation is inexpensive and easy to use. The disadvantage to BMAD is that it only has 30–60% mortality at a 106–107 PFU dose compared with it., which has 100% mortality at a similar dose. The in. route is difficult to evaluate because dose volume can affect the degree of infection of the respiratory tract; with low volumes it is localized to the upper respiratory tract, whereas larger volumes result in greater involvement of the lower respiratory tract. Past and recent studies have not demonstrated mortality even at doses of 108 PFU [32,38,60,61]. Therefore, it would appear that for lower respiratory tract inoculation each route has some advantage over the other. Only the it. route so far has demonstrated 100% lethality, although this level of lethality is not a feature of human MPX or smallpox.
By far the most pathogenic route appears to be sc., which causes 100% mortality (in CB strain) at a relatively low dose of 106 PFU. Given that the natural route of transmission of MPXV is possibly via the skin – as is the case with other poxviruses – consideration should be given to this route for antiviral and vaccine studies instead of the less physiologically relevant iv. route. That said, if the overall goal of a MPXV animal model is to develop a better human/variola model, then respiratory challenges would be more appropriate.
Experimental & natural MPX in small animals
Prairie dogs: early finding
Prairie dogs (Cynomys ludovicianus), native to the USA, were infected with MPXV when housed in close contact with various rodent species imported from Africa [40,66,67]. Approximately 110 prairie dogs were sold and 15 became ill, of which ten died rapidly. Analysis of animals from the initial outbreak revealed ocular and nasal mucoid discharge, swollen eyes, anorexia, tongue ulcers and red-brown consolidations involving 50% of the pulmonary parenchyma [68,69]. Two animals presented with livers that were red with scattered with mottled areas. OPV antigens were identified in areas with grossly and microscopically identified lesions. The lungs had concentric, coalescing bronchoalveolar pneumonia and inflammation extending to the bronchiolar walls and alveoli. Active viral replication was demonstrated in the lungs and tongue, and viral antigen was abundant in the lung bronchial epithelial cells [68]. In a third animal, a systemic MPXV infection with extensive and severe lesions in numerous organs was reported. Ulcers were noted on the tongue and hard palate, the cranial and middle lobes of the lungs were red, depressed and firm. Remaining parenchyma was pale tan and failed to collapse. Multiple white plaques were observed in the visceral pleura. Cervical and thoracic lymph nodes were swollen and small. White, firm foci with umbilicated necrotic centers were sparsely distributed throughout the glandular portion of the stomach and small intestine [69]. Multifocal, necrotizing lesions were reported in a diverse range of organs, such as thymus, brown fat, colon, liver, uterus and vagina, among others [69].
Prairie dogs: in. & intraperitoneal challenges
The observation of disease in the US MPX outbreak provided the impetus to establish a prairie dog model. Following intraperitoneal (ip.) inoculation with 105 PFU of WA (US-2003) most animals became lethargic and anorexic. All infected animals died 8–11 days p.i. and did not develop skin lesions. MPXV could be detected in the blood and throat from day 5–6 p.i. with levels increasing until death. Highest titers were reported in the livers and spleens; low levels were detected in the kidneys and lungs. Hematoxylin and eosin staining at necropsy revealed that spleens showed moderate–severe necrosis, the livers showed centrilobular necrosis and hepatocellular inclusion bodies, and the lungs exhibited mild-to-moderate thickening of the interstitium with infiltration by mononuclear inflammatory cells. The spleen and liver inclusion bodies were positive for OPV antigens by IHC [70].
Three studies have evaluated the in. route of infection. Xiao et al. evaluated a 105.1 PFU WA (US-2003) challenge and found that three out of five infected animals died by day 11–14 p.i. Surviving animals developed vesicular lesions on the lips and tongue and had nasal congestion and discharge. In the three fatal infections, MPXV appeared in the throat from day 3 p.i. and in the blood from days 8–9 p.i. At death, animals had the highest levels of virus in the lungs and lower levels in the liver, spleen, kidney and heart. No virus was detected in the brain. No hepatic lesions or splenic necrosis was observed. The lungs of dead animals showed marked edema, hemorrhage and necrosis. Surviving animals were in apparent good health but with some histological foci of inflammation in the skin. By IHC, viral antigen could be detected in the liver, lungs, mediastinum, bronchus and mediastinal lymph nodes [70]. The pattern of experimental infection in the in. group concurs with the clinical and pathological observations made during the 2003 US MPXV outbreak in prairie dogs, with similar mortality rates and similar clinical signs [68]. Hutson et al. compared the response to disease following in. or id. challenges with WA (US-2003) and CB (358) viruses at 104.5 PFU [30]. in. and id. WA-infected animals all survived and had similar disease presentation and clinical signs, with lesions developing at 9–12 days p.i.; however, fewer lesions were observed in in.-infected animals and id.-infected animals developed a lesion at the inoculation site by 6–9 days p.i. The id. CB-infected animals also developed a lesion at the inoculation site and again the lesion count was slightly fewer for in.-infected animals compared with id. infected animals. Lesions again occurred at days 9–12, with mortality rates of 50% for id.-infected and 25% for in.-infected animals. MPXV DNA could be detected in the blood from days 3–15 and 6–15 for id.- and in.-infected animals, respectively. Detection of DNA by qPCR, showed similar onset, kinetics and cessation in CB- and WA-infected animals.
Animals challenged in in. dose-escalation (102–105 PFU) experiments with WA and CB strains again experienced the development of lesions that were higher in number at higher doses. An earlier disease-onset and an increase in mortality, morbidity and viral shedding was observed as doses were increased, with 25–75% and 50–100% mortality at 103–105 dose for WA and CB, respectively. Dose-escalation studies revealed that the CB and WA strains had LD50 values of approximately 103 and 105, respectively [31]. These data reveal that the CB strain is more virulent than the WA strain in the prairie dog model, as evidenced by a higher lesion count, a greater trend towards weight loss, a significantly higher percent increase in temperature and higher mortality. Furthermore, the id. route appeared to be more pathogenic than the respiratory (in.) challenges, as evidenced by a slightly more severe disease with increased mortality (in CB-infected animals) [28,31].
Prairie dogs: transmission studies
Hutson et al. evaluated the transmission of the WA strain (US-2003-044) between prairie dogs via three different experimental routes: contaminated bedding (fomites); cohousing of animals; and respiratory/nasal discharge [25]. Primary animals were inoculated with a sublethal dose of 103 PFU via the in. route and developed clinical signs similar to those reported previously. To evaluate transmission via bedding (fomites), the primary animal was infected and placed in a trough until day 16 p.i. Upon removal of the challenged animal, 3 naive animals were cohoused in the vacated trough. These contact animals all developed ten to 14 lesions from days 11–18, lost 8–14% body weight and had a mortality rate of one out of three. Contact animals in the cohoused studies and in the respiratory/nasal-discharge studies also had mortality rates of one out of three and one out of two, respectively. In general, contact animals developed similar, but often more severe, clinical signs as in. challenged animals, indicating that virus was transmitted to the naive animals and produced a more virulent disease than in the in. challenged animals. These observations are supported by the finding that contact-infected animals often shed larger quantities of virus in oral secretions and in blood – which is in agreement with the findings of the US 2003 MPXV outbreak.
Prairie dogs: vaccination studies
A study by Keckler et al. evaluated the protective capability of Dryvax, ACAM2000 and IMVAMUNE against an in. CB (Congo-23) challenge [71]. A ‘take’ was observed after vaccination with Dryvax and ACAM2000 on days 2–7 and scab detachment on day 19–25. All vaccinated animals developed antibodies, and it was shown that following MPXV challenge, the vaccinated animals developed antibodies faster than nonvaccinated controls. Following a 106-PFU dose, the challenged animals fared poorly, with one out of two animals dying on day 11 p.i. and the second experiencing severe illness, multiple lesions, severe nasal involvement and no recovery from weight-loss; this animal was euthanized. Both Dryvax and ACAM2000 protected against death and rash, and a single dose of IMVAMUNE also protected against death, but animals developed a modified rash (n = 8 was used in each vaccination group).
Prairie dogs: antiviral studies
The antiviral drug, ST-246 has also been evaluated using the in. CB strain (ROC-2003-358) model. Groups of animals were treated orally with 30 mg/kg of the antiviral drug ST-246 daily, for 14 days, to evaluate its efficacy in a lethal (105 PFU) in. model [72]. Treatment began at day 0 p.i., 3 p.i. or when clinical signs (rash) were observed. At day 8 p.i., three out of four vehicle-treated animals developed clinical signs similar to those previously observed [71]. At day 6–8 p.i., animals began to lose weight (2–10%), one animal developed a typical papular or pustular rash 2 days before death (on day 12 p.i.), whereas one out of four only developed a petechial rash just before death; a third animal did not develop a rash at all. Three out of four infected animals had died by day 10–12 p.i. Drug treatments at day 0 (prophylactic treatment) protected all animals, which lost minimal weight, and infectious virus could not be detected from swabs (except for oral swabs on day 12 p.i.). Animals treated with ST-246 on day 3 p.i. (postexposure treatment) also remained visibly asymptomatic and lost minimal weight. Following rash-onset, three out of four animals were treated with ST-246 from day 10–12 (therapeutic treatment) when a rash of five to 50 lesions appearing as macules developed on the chest, abdomen, back, groin and tongue. The fourth animal did not develop a rash until day 24 (2 weeks after the other animals in the group). Animals that developed a pustular rash (two out of four) experienced the most weight-loss, but ultimately all animals survived infection. These findings suggest that rash onset is a suitable trigger for the initiation of antiviral therapy, and this is neatly aligned with the US FDA requirement that the trigger for treatment of smallpox or human monkeypox be based on a visual manifestation [73].
Prairie dogs: summary
Findings from in. prairie dog challenges appear to be relatively consistent to what was found in the US 2003 strain outbreak [68,69]. One drawback to the in. model is that some animals fail to develop rash and that the time of rash onset is inconsistent; also the type of rash that the animals present with appears to be variable. Furthermore, mortality rates from various studies often fail to produce a 100% lethal model, although it seems clear that the CB strain is more pathogenic than WA strains [28,30,31,70,71]. That said, the prairie dog model appears to be an excellent system to study MPXV pathogenesis as well as the efficacy of antivirals and vaccines, and this is only diminished by the lack of prairie dog reagents and the inability to breed the animals in a vivarium.
Squirrels
A 1979 survey of monkeys, arboreal and terrestrial rodents, and bats in Zaire revealed MPXV-specific antibodies in the Thomas’s rope squirrel (F. anerythrus). A second survey in 1985 focused on collecting samples from animals in the areas surrounding villages with human MPXV infections. A total of 172 terrestrial rodents, 22 squirrels, 120 sheep and goats and 67 domestic cats were sampled. One squirrel (F. anerythrus) in this group (found 50 m from a village) had round/oval, flat lesions of 2–3 mm in diameter on the abdomen and inguinal areas. MPXV was isolated from the skin, lungs, spleen and kidney and MPXV-specific antibodies were detected in the blood. This is the first and only isolation of MPXV from a wild animal in nature [17]. Two out of a total of 18 F. anerythrus (18 out of 22 of the squirrels captured were F. anerythrus) appeared healthy, but also had MPXV antibodies [17,74].
A third survey in Zaire in 1986 examined the surrounding areas of villages that had experienced human MPXV infections suspected of coming from infected squirrels. A total of 233 rodents (including small terrestrial rodents and Gambian rats) were negative for MPXV-specific antibodies. A total of 25% (79 out of 320) of F. anerythrus and 16% (six out of 37) of red-legged sun squirrels (H. rufobrachium) were found to have MPXV-specific antibodies [19]. These squirrels commonly inhabit the forests around human settlements in the rural areas of Zaire [18]. Further west in the country, a survey was conducted where no human MPXV cases had been reported; MPXV-specific antibodies were reported in 49% (58 out of 119) of ribboned rope squirrels (F. lemniscatus) and 19% (six out of 31) of Gambian sun squirrels (H. gambianus) [16]. These findings over four widely separated areas indicate that MPXV is circulating in squirrels of the genera Funisciurus and Heliosciurus. The results of the CDC testing of 800 small animals imported prior to the 2003 US MPXV outbreak were supportive of these findings. These animals consisted of nine different species and six genera of African rodents. Testing revealed that three out of six Funisciurus sp. were positive by MXPV-specific PCR and virus isolation; however, H. gambianus squirrels were not positive by PCR (n = 27) and were not tested for virus isolation [21]. Testing of two field sites where the squirrels imported to the USA were captured revealed that 40 and 14% of Funisciurus and Heliosciurus, respectively, were positive for OPV-specific antibodies; however, the studies did not evaluate if these were MPXV-specific antibodies [75].
MPXV isolated from an infected F. anerythrus squirrel (from the African survey described above) was used at 106 PFU to infect Eurasian red squirrels (Sciurus vulgaris) via the in., per os (PO) and SCR routes. At day 1 p.i. an elevated temperature was observed and at day 3–5 p.i. animals became limp and stopped moving; dyspnea followed in in.- and PO-infected animals. Disease was noted to develop most rapidly in animals infected by PO or in. routes. Skin lesions were not observed and death occurred by days 7–8 p.i. Virus titers of 106 PFU/ml (on chorioallantoic membranes) were reported in lungs, livers, kidneys, spleens and the esophagus of dead animals, regardless of route. Nasal discharge was also reported for all animals, regardless of route [76].
Shelukhina et al. evaluated the response of Funisciurus sp., Protoxerus sp., and Heliosciurus sp. to in., sc. and PO challenges with a CB strain. Squirrels were infected with 105–106 PFU and developed an acute, generalized infection with 100% lethality. Susceptibility varied between species at lower challenge doses. sc. challenge resulted in a thick, red papule at the inoculation site. Skin lesions (in non-fur areas, or the borders of the skin and mucous membranes) occurred in only a few animals challenged in. or PO with nonlethal doses. Transmission studies found that infected animals were able to transmit the disease to naive animals by airborne or direct contact [Shelukhina EM et al., Unpublished Data].
The 13-lined ground squirrel (Spermophilus tridecemlineatus) has been extensively evaluated as a model for MPXV infections. Following a 105.1-PFU challenge via the in. and ip. routes with a WA strain (US03), a fulminant disease with lethargy and anorexia was reported. No skin lesions, respiratory distress or other obvious clinical signs were reported. Death occurred at days 6–7 p.i. for ip.-infected animals and at days 8–9 p.i. for in.-infected animals. Virus cultures were positive from throat swabs at day 4 p.i. for both routes; however, cultures for blood were positive at days 5 and 3 p.i. for in. and ip., respectively. High levels of virus were detected in the kidney, lungs, heart and brain, but the highest levels were in the liver and spleen. Virus levels in organs appeared not to be related to route of infection. At death, the ip.-infected animals presented with liver centrilobular hepatocellular necrosis, steatosis and basophilic inclusion bodies, and the spleen had necrosis characterized by lymphocytic karyorrhexis in the white pulp, and fibrinoid necrosis, congestion and endothelial cell swelling in the red pulp. The lungs had thickening of the alveolar septa by inflammation with focal consolidation. in.-infected animals exhibited lesions in the liver, with multifocal steatosis and diffuse hepatocellular necrosis. Lungs of the in.-infected animals also had variable consolidations and interstitial inflammation with some necrosis in the peribronchial lymphoid tissue. Cytoplasmic inclusion bodies were present in all livers. Splenic necrosis was similar to that observed in ip.-infected animals, but in.-infected animals also had necrosis in the lymph nodes [77].
S. tridecemlineatus was also used in the development of a sc. model using CB (Z79) and WA (US03) strains. Both viruses produced a similar fulminant disease with 100% lethality at a 100 PFU dose (LD50 = 0.35 and 0.46 for Z79 and US03, respectively) by days 6–11 for both strains. Respiratory distress was uniform and rapid, with bleeding diatheses, anorexia and lethargy from day 3 in CB, and days 4–5 in WA-infected animals. Clinical signs were more severe for CB-infected animals, animals experienced epistaxis and tissue damage in the lung was increased in CB-infected animals, and pulmonary edema and hemorrhage occurred earlier in the disease course. From day 5, CB-infected animals experienced respiratory distress, labored breathing and wheezing; however, similar clinical signs were only observed in WA-infected animals within the 24 h preceding death. Viral loads in the blood, lungs, liver, spleen and skin lesions increased until death, with loads 1–2 logs higher in CB-infected animals. MPXV antigen was detected in the lungs, liver, lymphoid organs, esophagus and intestines, again with higher intensity and diffusion of IHC staining occurring in the respiratory tract of CB-infected animals [29]. Clinical signs and disease course in the sc. WA-challenged animals appeared to be very similar to those reported for in. and ip. WA-challenged animals [77]. Although both viral strains produced lethal disease, an earlier day of death, more severe clinical signs, and higher viral and tissue loads indicate the increased virulence of the CB strain.
The antiviral drug, ST-246, has been tested in the sc. S. tridecemlineatus model. Squirrels were infected with 100 PFU of CB (Zaire 1979–005) and treated with 100 mg/kg/day of ST-246 for 14 days, with treatment commencing at 0, 24, 48, 72 and 96 h p.i. Placebo treated animals developed signs of illness by day 4 and experienced 100% mortality by days 6–9 p.i. Groups treated with ST-246 at 0, 24, 48 and 72 h p.i. had 100% survival, and the 96-h groups had 33% mortality, with two out of six animals euthanized on day 7 p.i. No weight change was observed in any surviving animals. Animals with treatment initiated at 0, 24, 48, and 72 h p.i. developed no detectable viremia or viral titers in the liver, spleen and lung at day 7. Interestingly, these groups did not develop an antibody response, suggesting that insufficient antigen was generated in the presence of ST-246 to trigger an immune response [78].
The serological surveys, and experiments performed by Shelukhina are strongly supportive of the hypothesis that Funisciurus and Heliosciurus are a major reservoir of MPXV. Consideration should be given to developing these species as models to further understand the biology of the virus. Experiments in the 13-lined ground squirrel were also informative with mortality rates of 100% following low-dose sc. challenges; thus, highlighting the sensitivity of squirrels to MPXV. The only drawback of the 13-lined ground squirrel model is the lack of rash that is observed in Funisciurus and Heliosciurus in an experimental setting and was observed in Funisciurus in nature. In summary, the squirrel is a good model of MPX and should be considered for further evaluation.
Rabbits
Rabbits were evaluated in several older (circa 1970s) reports and generally adults were resistant to challenge (except intracranial [ic.] and SCR in albinos); however, animals less than 10 days old were sensitive to SCR, ic., PO and in. challenges – presumably the disparity exists due to the absence of a fully functioning immune system. Two reports give differing accounts of the outcome following an id. challenge; one reports a severe hemorrhagic reaction followed by pustular lesion development at various sites on the body, but no deaths [34,79-81]. Adult albino rabbits used in the second study developed swelling at the inoculation site, a rash over the body on day 7 and death by days 9–12 [82]. ic. inoculation was fatal to adults and 2-day-old rabbits; in adults, a fatal meningoencephalitis was reported, with death at 6–9 days p.i. [38]. SCR resulted in fever, and on approximately day 3 the development of a localized papulopustular lesion in adults and albino adults was followed by a full recovery [81,82]; however, it was fatal to 2-day-old animals [34]. Interestingly, SCR and id. serial passages were successful in many animals [36,38,41,79] and generated red, indurated lesions with black-brown necrotic centers [79]. sc. administration resulted in a generalized infection with widespread pox eruptions followed by a full recovery in adults [34,41]. iv. challenge of adults was reportedly followed by acute disease, an explosive rash (papules that later developed into pustules followed by scabbing) on the skin and mucous membranes observed on days 5–6 p.i. and full recovery [34,37]. PO challenge resulted in generalized disease and pox eruptions on the skin, ears and lips and death by days 4–14 in 10-day-old animals; however, no reaction was observed in adults [81]. in. challenge was particularly effective in 10-day-old rabbits and caused weight-loss and death by days 4–5 with no rash development [81]. More recently, following the 2003 US MPX outbreak, a rabbit was reported to have become ill after exposure to an ill prairie dog at a veterinary clinic [40]; however, no rabbits could be identified that were MPXV-specific PCR positive or positive for virus isolation [21]. The failure to observe severe disease in adult animals infected by these routes of infection limits the utility of this model.
Mice
Mice have been evaluated as models since the discovery of MPXV. Adult non immunocompromised mice are generally resistant to challenge (except via ic. route and sometimes ip. route), with adult BALB/c and C57BL/6 mice experiencing some weight-loss when infected via the in. or FP routes with 105 PFU of the CB (Z79) strain but not with the WA (US03) strain [28].
ic. challenge of adults results in death in all mouse strains and ages tested [36-38,41,46]. in. inoculation results in 100% mortality by day 15 and by day 17 in adult SCID mice [26,34,37,41,81]. Following a 102–103 PFU CB (Z79) challenge, 129 stat1−/− and C57BL/6 stat1−/− mutants also experienced weight loss with 40 and ≥90% mortality by day 10, respectively; however, the antiviral drugs, CMX001 and ST-246, were shown to protect the stat1−/− mutants from lethal challenges [26]. White mice at 8-days-old are partially sensitive to PO and id. inoculations (40 and 50%, respectively) and fully susceptible to in. inoculations. Mice at 12-days-old experience 24% mortality following a PO challenge (dose not given) [37,81].
Following an ip. challenge, adult BALB/c mice became sick but survived [83]; other adult strains have been reported to be both resistant and sensitive to ip. challenge [36,41], with SCID (~102 PFU) and newborn Swiss Webster (dose not given) mice all becoming sick and experiencing 100% mortality by days 11, 8–16 and 4, respectively [26,46].
Robust disease in adult nonimmuno compromised mice has only been achieved in the CAST/EiJ strain, which experienced 25% mortality following a 104-PFU FP CB (Z79) challenge with swelling and edema presenting on the foot, but no mortality when virus was administered by SCR in. challenges resulted in 100% mortality by day 8 for both CB (Z79; LD50 = 680 PFU) and WA (US-C1) challenges (LD50 = 7.6 × 103 PFU); however, these mice could be protected by Dryvax vaccination or treatment with CDV. Following a 104-PFU CB (Z79) ip. challenge, the CAST/EiJ strain experienced 100% mortality by day 8 with an LD50 of 12 PFU; in contrast to the other challenge routes, these mice experienced little to no weight loss. Two other nonimmunocompromised strains are also sensitive to MPXV, but to a lesser extent than the CAST/EiJ strain; following in. challenges the MOLF/EiJ and PERA/EiJ strains experience 75 and 40% mortality, respectively [27,84].
Other small animals
The multistate US MPX outbreak revealed that multiple species of animals had been infected with MPXV from approximately 800 small animals imported from Ghana: two out of 21 Gambian-pouched rats (Cricetomys sp.) tested positive for MPXV-specific PCR and one out of 15 tested positive by virus isolation [66]. One out of six southern opossum (Didelphis marsupialis) tested positive by PCR and virus isolation. Several other species also tested positive for MPXV-specific PCR: one out of 22 African hedgehogs (Atelerix sp.), one out of four Jerboas (Jaculus sp.), one out of four gray short-tailed opossums (Monodelphis domestica) and one out of four woodchucks (Marmota monax) [21]. In addition, a 1986 survey in Zaire found that one out of four porcupines (Atherurus africanus) were seropositive for MPXV-specific antibodies [16].
Various small animals have been experimentally tested for their susceptibility to MPXV. Guinea pigs failed to show signs of disease following iv., ip., id., sc., PO or ic. inoculation, although animals inoculated via the in. and ic. routes developed antibody titers by day 14 p.i. FP-infected guinea pigs also presented with no systemic disease, but did experience swelling and edema at the injection site after 7–10 days, development of a granulomatous lesion/edema confined to the injection site, and antibody titers by day 14; however, the lung, liver and spleen of these animals had no viral titers on days 6, 11, 16 and 22 p.i. [82]. SCR resulted in a slight inflammatory reaction on day 2 and the development of discrete papules on day 4, followed by scabbing and healing [34,37,41,81,82]. The multimammate mouse (Mastomys natalensis) was reported to be highly susceptible to ip. and in. challenge; however, details of the disease have not been forthcoming [16]. Adult chickens (Gallus gallus) failed to present with clinical signs following inoculation via iv., ip., id., and sc. routes [34,82]; however, when inoculated id., 3–4-day-olds and 4–8-week-olds developed vesicles that dried-up without leaving a scar by day 10 [82]. White rats (Rattus sp.) had no clinical signs following in., iv. or SCR. Newborn rats (1–3 days old) experienced 100% mortality by days 5–6, with virus replication in the lungs and liver following an in. challenge [81]. Adult hamsters (genus not reported) were resistant when infected via the PO, in., ic. or SCR routes; however, during the first 7 days p.i., virus was detected in the lungs, liver and spleen of animals infected by the ic. route [81]. In another study, hamsters were infected with 106-107 PFU via intracardiac injection. No visible disease was observed. Serial sacrifices were performed and virus was detected in blood and internal organs (not specified) during the first 7 days p.i. After 7 days the virus was no longer detected in most animals. At 14 days p.i. NA were found; however, seroconversion only occurred in 50% of animals. A generalized pathology was noted primarily in the liver, kidney and brain; all of which had focal lesions. The main manifestations were perivascular lymphohistiocytic infiltrate and damage to the endothelial structure of blood vessels [81,85].
Two animal species have been shown to be highly susceptible to severe disease: in. inoculation of cotton rats (Sigmodon sp.) induced an acute generalized illness with labored breathing, coughing, rhinitis, conjunctivitis, progressive emaciation and 50% mortality (time to death not given). High concentrations of virus were detected in the blood and internal organs (unspecified) and antibodies were produced in survivors. iv. inoculation caused a more severe disease course with 100% mortality (time to death not given) [86]. Following the US MPXV outbreak, nine African dormice tested positive by PCR and virus isolation [21,66]. Vivarium-bred Graphiurus kelleni has subsequently been challenged with the CB (Z79) strain and experienced 100% mortality following a 104-PFU FP infection, and was observed to have an in. LD50 of 12 PFU. The average day of death at a 200-PFU in. inoculum was 9 days, with 14% weight loss (similar mortality was observed with the COP-58 strain following in. challenges). Following a 104-PFU in. challenge, virus was detected in nasal lavages by day 2, the spleen by day 3 and in the liver, lungs and blood by day 4 p.i. The highest viral titers were observed in the liver at day 8. From day 3–4 p.i., dehydration, conjunctivitis, upper GI tract hemorrhage, hepatomegaly, and lymphadenopathy was reported. By day 4–5 p.i., rhinitis was observed, along with lymphoid necrosis in the submandibular lymph nodes, spleen and thymus. Hepatocellular necrosis and hemorrhage in the lungs, stomach and small intestine were observed. Necrosis and hemorrhage was noted to affect many organs [87]. Vaccination with Dryvax 4 weeks prior to a 104-PFU in. challenge resulted in 100% protection. Treatment with cidofovir at 4 h p.i. with a 103-PFU in. challenge resulted in 81% protection [87].
In summary, many small animals appear to have the basic characteristics that could allow them to be developed into useful models, excluding guinea pigs, rats, chickens and hamsters, which do not develop robust disease and should not be developed further. Of the animals involved in the US 2003 outbreak, the Gambian-pouched rat and southern opossum were positive for both vDNA and virus isolation. A subsequent serological survey in the area of Ghana where the US 2003 rodents were imported from, found that approximately 5 and 2% of Gambian-pouched rats were positive for OPV DNA and antibodies, respectively [75]; one drawback to the Gambian pouched rat is that it is a relatively unused model, although some rudimentary studies have been performed [88,89]. The southern opossum, although native to South America and therefore not a natural host of MPXV, could also be utilized for studies, and has previously been used as a model of other viral infections [90]. The multimammate mouse, which has been utilized in at least one virus study (minute virus), was also reported to be highly susceptible to MPXV, and this species should also be evaluated further [91]. The main drawbacks to all of the potential models are the lack of available reagents to monitor immune responses, disease progression, pathology and transmission. Furthermore, the already-available models of MPXV disease are fairly comprehensive, thus making the development of more models somewhat counterintuitive. The disease presentation of cotton rats is reminiscent of that of prairie dogs (although it is not clear if cotton rats develop a rash). Cotton rats should be considered for further development – particularly in light of the fact that this species is relatively well used in various other disease models [92]. The dormouse proved susceptible to low-dose inoculums of MPXV and responded well to both vaccine and antiviral therapy. Lack of reagents for cotton rats and dormice limit their use as small-animal models of MPXV.
Experimental & natural MPX in large animals other than M. fascicularis & M. mulatta
During the Rotterdam Zoo outbreak of 1964, chimpanzees were noted to present with pox lesions on the skin, but experienced no other signs of general illness [39]. Chimpanzees were challenged with MPXV (U65–32) via the iv. route (106.8 TCID50) with an isolate derived from one of the infected orangutans from the 1964 Rotterdam zoo outbreak [38,39]. An animal previously vaccinated with the smallpox vaccine developed no signs of disease; however, a nonvaccinated animal developed a mild infection with a few scattered eruptions on the face and hands. Previously splenectomized and nonvaccinated chimpanzees were also challenged via the iv. route and two out of three animals initially developed a few lesions, but lesion development continued until most of the body was covered. Exanthema was severe, with confluent lesions on the face, arms, legs and upper-body. Crusts formed and appeared to be healing at the time of necropsy (day = 22). One out of three animals developed severe lymphadenopathy and CNS depression; however, only a few lesions were reported at the time of death (day 10) [93].
Two adults baboons (Papio cynocephalus) were inoculated im. with 107.5 TCID50 (COP strain [34]) and were housed with six immature (6–7 months) sentinels. By day 8, inflammation was observed at the injection site and by day 11 pox lesions appeared on the face and extremities of both animals. One animal also had truncal lesions. Lesions increased in number and developed into pustules, which crusted over and healed by day 18. HAI antibodies were detected by day 8, and peaked by day 14 p.i. Both animals fully recovered. Two out of six sentinel animals developed lesions on the face and extremities at day 28; however, fewer lesions were observed compared with the inoculated animals, although the disease course was similar and the animals were resistant to re-challenge with 108.5 TCID50. The remaining four out of six sentinels did not develop illness and were HAI antibody-negative when challenged at 91 days. Inflammation was observed at the inoculation site and exanthema was observed by day 7 p.i. Vesicular lesions were observed on the face and extremities by day 10 p.i. One animal also developed exanthema on the trunk, face, mucous membranes, buccal cavity and nasopharyngeal area and died on day 12 p.i. Maximum HAI antibody levels were reported at day 16–24 p.i. and MPXV could be isolated from the throat and stool of all animals during the second week after appearance of lesions [94].
In a second baboon experiment, two gr oups of ani mals were inoculated with 107 TCID50 via the SCR route (WA, COP strain). Animals in the first group were approximately 1 year old and those in the second group were approximately 3 months old. Initially, one animal was inoculated with virus and subsequent animals were inoculated with serial passages from lesions from the previous animal. The animals from the first group experienced erythematous and pustular lesions at the SCR site and an increase in rectal temperature until day 6 p.i and enlargement of the axillary and inguinal lymph nodes. Secondary lesions appeared on the trunk, extremities, face and mouth. Lesions became vesicular, pustular and hemorrhagic. Subsequent animals infected by serial passage experienced similar clinical signs with decreasing levels of severity. No animals died. The initially infected animal and the first two serial-passage-infected animals in the second (younger) group followed a disease course similar to those in the first group; however, with each passage the pustular lesions at the site of SCR contained increasing amounts of fluid that became increasingly hemorrhagic, with subcutaneously edematous SCR sites extending to the hips, base of the tail, abdomen and chest. All baboons from passage 4 died by day 17 and virus was constantly isolated from the throat, stools and blood samples as well as the heart, lung, thymus, adrenal and lymph nodes from dead animals. These data suggest that younger baboons are more susceptible to MPXV compared with older animals and/or that MPXV was adapting to the host [95].
Experimental inoculations via the in. routes have been performed on tufted capuchins (Cebus apella) and red-faced spider monkeys (Ateles paniscus), which failed to become sick; however, in the case of the capuchins, a coinfection via the ip. and ic. route did result in the development of skin lesions and HAI antibodies [60]. Inoculation of pigs (Sus sp.) by rubbing virus into the skin did not result in disease; however, MPXV could be detected in the skin from days 0–5 with gradual lowering of titers. NA were not detected in heat-inactivated sera, but there were significant levels of heat-labile neutralizing antibody activity [96].
Conclusion
The broad host-range of MPXV is a cause for concern, as it may facilitate the adaptation of MPXV to new hosts in new regions. Inspection of the host-range reveals that MPXV can infect animals from pan-geographical locales; namely, North America, South America, Asia, and Africa. Data from the Rotterdam Zoo outbreak indicates that MPXV is a highly transmissible disease and that there is no correlation between severity and geographical location of the diseased host [39]. It is interesting that MPXV has not been reported in nature outside of Africa, suggesting that species from other continents either cannot support the long-term lifecycle of MPXV, or that the virus has not been introduced to those locations. The broad host-range of MPXV suggests that it would have the capacity to expand out of Africa if given the opportunity, much like VACV becoming established in water buffalo in India and in cattle in Brazil [97-99]. Introduction of MPXV to locales outside of Africa could be disastrous. Of note is that nearly all recent NHP experiments have utilized the highly sensitive M. fascicularis and M. mulatta – both of which are restricted to Asia and are not natural hosts of MPXV, as serological surveys of these, and other primates, in India and the Malaysian peninsula were all negative [11,100]. The origins of the monkeys that caused outbreaks in various institutions were mostly of Asian origin [12]; this suggests that the animals were either cohoused at some point with infected African animals, or that specialized shipping containers contained fomites from previously transported, infected African animals; or, that MPXV does circulate in Asia and has thus far been undetected. Interestingly, the 1959 US outbreak of M. fascicularis and M. mulatta could be attributed to the cohousing of these animals with African C. aethiops, which made up approximately 5% of the population; however, these monkeys apparently presented with no observable clinical signs of disease [35,37]. Assuming that MPXV does not circulate in Asia, that specialized shipping containers were used to import animals from Africa or Asia, and that squirrels – the proposed reservoir – and/or other potential reservoir rodents were unlikely to be cotransported, MPXV could have more than one reservoir host in Africa, which is likely to be a NHP. Supporting this argument is the finding of MPXV-specific antibodies in Cercopithecus sp. and Colobus sp. captured in Africa [14,15,19]. African primates, such as Cercopithecus, inhabit a similar strata of the rainforest to squirrels, and it has been observed that these monkeys mix with various rodents [14], giving a basis for interspecies transmission.
Future perspective
MPXV was first discovered over 53 years ago in a disease outbreak in an animal facility in Denmark [34]. Subsequent outbreaks, field surveys and experimental studies have revealed that the virus has the capacity to infect and cause high levels of mortality and morbidity in a broad spectrum of hosts from across the globe. Despite these experiences, definitive proof as to the natural host of MPXV remains elusive – although prime candidates are squirrels. Indeed, a natural MPXV infection has still only been recorded in one animal – the squirrel F. anerythrus [17]. Future field studies utilizing modern experimental assays should be considered in Central and Western Africa. The CDC is likely to be the only US-based organization with the facilities and infrastructure to complete such a task.
Respiratory (in., ib., aerosol and BMAD) NHP challenge models of MPXV have been developed with high levels of success as determined by the presentation of disease; however, these routes of challenge do not induce the reproducible level of 100% mortality that is characteristic of the iv. models [23,24,51,53,55,58,61]. This in itself would not be a problem, since in nature MPXV is likely to not cause 100% mortality; however, the primary purpose of NHP studies is to develop models of smallpox and human monkeypox for the development of disease countermeasures, and to date the FDA encourages lethal models for these studies; thus, the iv. route is the obvious choice. Two alternatives should be considered to the iv. route: the it. route has been shown to also induce 100% mortality with a longer disease course and later symptom onset than that of the iv. route. The later disease onset and the respiratory challenge recapitulate classical smallpox [55]; the sc. route also leads to high mortality and morbidity levels and should be considered as a nonrespiratory model of human OPV infections. Several studies have found that MPXV may transmit to humans via routes other than respiratory; namely, by handling infected animals and/or by consuming infected meats. For these reasons, a nonrespiratory challenge model may be required in the future, once the exact mechanism of human-to-human spread is elucidated – the sc. model would be an obvious choice [32].
One drawback to the NHP studies is the high virus inoculum that is required for mortality to be achieved. The prairie dog model has the advantage of being a small animal model that has an extended disease course, presents with skin lesions and has lesion onset that has been used as a trigger for therapeutic intervention. It has also been extensively tested in vaccine and antiviral studies with good success [71,72]. One drawback to the model is that, like the NHP models, an artificially high inoculum is required for mortality [30,70]. This finding is not always observed in small animal models; the 13-lined ground squirrel, dormouse and CAST/EiJ mice all have relatively low LD50 values. The two former species are not well-described and limited reagents are available; however, antiviral efficacy has been demonstrated in both [78,87]. The CAST/EiJ strain is arguably the best small animal model of MPXV for the following reasons: the strain is sensitive to a low-dose respiratory (in.) challenge; the strain is immunocompetent; the strain can be easily bred in a research vivarium, facilitating the execution of studies requiring a large number of animals; a multitude of reagents, assays and kits are available for the mouse; and many vaccines and antivirals have already shown efficacy against OPV challenges in the mouse model [27,84]. For these reasons, it is likely that the CAST/EiJ strain will be the primary small animal choice for the future, with the prairie dog also providing useful data. Both models should also be considered for synergy studies with the two lead anti-OPV antivirals, CXM001 and ST-246 [101].
The next 10 years will reveal interesting developments in our understanding of MPXV in many animal systems. The majority of these findings will be driven by the need to develop and approve countermeasures to protect humans against MPXV from both natural outbreaks and from the potential release of the virus as a biological weapon.
Executive summary.
Monkeypox virus: basic facts
-
■
First isolated from cynomolgus monkeys in Denmark, 1958.
-
■
Responsible for several outbreaks in monkey colonies and zoos.
-
■
Monkeypox virus (MPXV) belongs to the family Poxviridae subfamily Chordopoxvirinae and genus Orthopoxvirus. Other members include variola (smallpox virus), vaccinia, cowpox and ectromelia (mousepox virus).
-
■
The virion is enveloped, brick-shaped and 200–250 nm in size.
-
■
Orthopoxvirus have varying host specificities, ranging from narrow to broad. MPXV has a broad host-range.
-
■
Highly susceptible monkeys experimentally infected with MPXV present with almost identical clinical signs to humans infected with variola.
-
■
The natural lifecycle probably involves a complex interaction between reservoir and incidental hosts.
MPXV can be separated into distinct groups
-
■
At least two separate groups, or ‘clades’, of MPXV exist: West African and Congo basin.
-
■
MPXV isolates from Central Africa (Congo basin) are more virulent than those from West Africa.
-
■
Congo basin and West African isolates are approximately 95% identical. This value approaches 99% when comparing isolates from within the Congo basin or West African regions.
MPXV host-range
-
■
MPXV likely circulates in many species in Africa; however, squirrels appear to be the most likely reservoir of the virus.
-
■
Monkeys of the Cercopithecus genus also appear to have a propensity for MPXV infection.
-
■
Over 40 species have been documented to have been naturally or experimentally infected with MPXV.
-
■
Infected species have originated from North America, South America, Africa and Asia.
-
■
Cynomolgus monkeys (M. fascicularis) and Rhesus monkeys (M. mulatta) have been utilized for the majority of experimental infections in NHPs. These species are susceptible to intravenous, aerosol, intradermal, intramuscular, intrabronchial, intratracheal, intranasal and subcutaneous routes of infection. The disease appears more severe in M. fascicularis than in M. mulatta.
-
■
Various small-animal models have been developed, of which the prairie dog, squirrel, dormouse, and CAST/EiJ mouse are the most robust.
Use of MPXV models in evaluation of therapeutics & prophylactics
-
■
Traditional, attenuated, subunit and DNA vaccines have been shown to protect many species against MPXV challenge. Some studies have shown that post-exposure vaccination has also provided protection. Attenuated vaccines were safe in immunocompromised M. mulatta but did not provide protection against subsequent challenge.
-
■
The antiviral drug ST-246 protected M. fascicularis, 13-lined ground squirrels and prairie dogs against a lethal MPXV challenge. The antiviral drug CMX001 protected stat1−/− mice against a lethal MPXV challenge and cidofovir treatment protected better than post-exposure vaccination in an intratracheal M. fascicularis model.
Acknowledgements
The authors thank J Esposito and P Jahrling for their input and useful discussions, and R Crump and L Camilo de Oliveira for critically reviewing the manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Wenner HA, Kamitsuka P, Macasaet F, Kidd P. Pathogenesis of monkey pox. Antimicrob. Agents Chemother. (Bethesda) 1968;7:40–44. [PubMed] [Google Scholar]
- 2.Wenner HA, Macasaet FD, Kamitsuka PS, Kidd P. Monkey pox. I. Clinical, virologic and immunologic studies. Am. J. Epidemiol. 1968;87(3):551–566. doi: 10.1093/oxfordjournals.aje.a120846. [DOI] [PubMed] [Google Scholar]
- 3.Shchelkunov SN, Totmenin AV, Babkin IV, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll DS, Emerson GL, Li Y, et al. Chasing Jenner’s vaccine: revisiting cowpox virus classification. PLoS One. 2011;6(8):e23086. doi: 10.1371/journal.pone.0023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito JJ, Knight JC. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143(1):230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo Basin. Virology. 2005;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86(Pt 10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 8.Breman JG, Nakano JH, Coffi E, Godfrey H, Gautun JC. Human poxvirus disease after smallpox eradication. Am. J. Trop. Med. Hyg. 1977;26(2):273–281. doi: 10.4269/ajtmh.1977.26.273. [DOI] [PubMed] [Google Scholar]
- 9.Marennikova SS, Ladnyj ID, Ogorodinikova ZI, Shelukhina EM, Maltseva NN. Identification and study of a poxvirus isolated from wild rodents in Turkmenia. Arch. Virol. 1978;56(1–2):7–14. doi: 10.1007/BF01317279. [DOI] [PubMed] [Google Scholar]
- 10.Marennikova SS, Seluhina EM, Mal’ceva NN, Ladnyj ID. Poxviruses isolated from clinically ill and asymptomatically infected monkeys and a chimpanzee. Bull. World Health Organ. 1972;46(5):613–620. [PMC free article] [PubMed] [Google Scholar]
- 11.Sehgal CL, Ray SN. Survery of Rhesus monkeys (Macaca Mulatta) for haemagglutination-inhibition antibody against vaccinia/variola and monkey pox viruses. J. Comm. Dis. 1974;6(3):233–235. [Google Scholar]
- 12.Arita I, Henderson DA. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968;39(2):277–283. [PMC free article] [PubMed] [Google Scholar]
- 13.Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2(1):17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- 14■.Breman JG, Bernadou J, Nakano JH. Poxvirus in West African nonhuman primates: serological survey results. Bull. World Health Organ. 1977;55(5):605–612. Serological surveys revealing several species of nonhuman primates (NHPs) that presented with evidence of infection.
- 15.Gispen R, Brand-Saathof BB, Hekker AC. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull. World Health Organ. 1976;53(4):355–360. [PMC free article] [PubMed] [Google Scholar]
- 16.Jezek Z, Fenner F. Human Monkeypox. Karger, Basel, Switzerland: 1988. [Google Scholar]
- 17■■.Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1(8472):98–99. doi: 10.1016/S0140-6736(86)90748-8. First and only isolation of monkeypox virus from a wild animal.
- 18.Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull. World Health Organ. 1988;66(6):747–752. [PMC free article] [PubMed] [Google Scholar]
- 19.Khodakevich L, Szczeniowski M, Manbu-Ma-Disu, et al. The role of squirrels in sustaining monkeypox virus transmission. Trop. Geogr. Med. 1987;39(2):115–122. [PubMed] [Google Scholar]
- 20.Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7(3):434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21■.Hutson CL, Lee KN, Abel J, et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 2007;76(4):757–768. Details of animals infected in the 2003 US outbreak.
- 22.Parker S, Chen NG, Foster S, et al. Evaluation of disease and viral biomarkers as triggers for therapeutic intervention in respiratory mousepox – an animal model of smallpox. Antiviral Res. 2012;94(1):44–53. doi: 10.1016/j.antiviral.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 24■.Goff AJ, Chapman J, Foster C, et al. A novel respiratory model of infection with monkeypox virus in cynomolgus macaques. J. Virol. 2011;85(10):4898–4909. doi: 10.1128/JVI.02525-10. Bronchoscope-microsprayer aerosol delivery challenge of NHPs.
- 25.Hutson CL, Carroll DS, Gallardo-Romero N, et al. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS One. 2011;6(12):e28295. doi: 10.1371/journal.pone.0028295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stabenow J, Buller RM, Schriewer J, West C, Sagartz JE, Parker S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against monkeypox virus. J. Virol. 2010;84(8):3909–3920. doi: 10.1128/JVI.02012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27■.Americo JL, Moss B, Earl PL. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 2010;84(16):8172–8180. doi: 10.1128/JVI.00621-10. Intranasal comparison challenges of NHPs with West Africa and Congo Basin strains.
- 28.Hutson CL, Abel JA, Carroll DS, et al. Comparison of West African and Congo Basin monkeypox viruses in BALB/c and C57BL/6 mice. PLoS One. 2010;5(1):e8912. doi: 10.1371/journal.pone.0008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbrana E, Xiao SY, Newman PC, Tesh RB. Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med. Hyg. 2007;76(1):155–164. [PubMed] [Google Scholar]
- 30.Hutson CL, Olson VA, Carroll DS, et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 2009;90(Pt 2):323–333. doi: 10.1099/vir.0.005108-0. [DOI] [PubMed] [Google Scholar]
- 31.Hutson CL, Carroll DS, Self J, et al. Dosage comparison of Congo Basin and West African strains of monkeypox virus using a prairie dog animal model of systemic orthopoxvirus disease. Virology. 2010;402(1):72–82. doi: 10.1016/j.virol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saijo M, Ami Y, Suzaki Y, et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 2009;90(Pt 9):2266–2271. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
- 33.Esposito JJ, Fenner F, Poxvirus . Fields Virology. Lippincott Williams and Wilkins; NY, USA: 2001. pp. 2885–2921. [Google Scholar]
- 34■■.Von Magnus P, Andersen EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Path. Micro. Scand. 1959;46(2):156–176. Discovery of monkeypox virus.
- 35.Sauer RM, Prier JE, Buchanan RS, Creamer AA, Fegley HC. Studies on a pox disease of monkeys. I. Pathology. Am. J. Vet. Res. 1960;21:377–380. [PubMed] [Google Scholar]
- 36.Mcconnell SJ, Herman YF, Mattson DE, Erickson L. Monkey pox disease in irradiated cynomologous monkeys. Nature. 1962;195:1128–1129. [Google Scholar]
- 37.Prier JE, Sauer RM. A pox disease of monkeys. Ann. NY Acad. Sci. 1960;85:951–959. doi: 10.1111/j.1749-6632.1960.tb50015.x. [DOI] [PubMed] [Google Scholar]
- 38.Gispen R, Verlinde JD, Zwart P. Histopathological and virological studies on monkeypox. Arch. Gesamte Virusforsch. 1967;21(2):205–216. doi: 10.1007/BF01241445. [DOI] [PubMed] [Google Scholar]
- 39.Peters JC. An epizootic of monkeypox at Rotterdam Zoo. Int. Zoo Yearbook. 1966;6(1):274–275. [Google Scholar]
- 40.CDC Update: multistate outbreak of monkeypox – Illinois, Indiana, and Wisconsin, 2003. Mort. Morb. Wkly Rep. 2003;52(23):537–540. [PubMed] [Google Scholar]
- 41.Prier JE, Sauer RM, Malsberger RG, Sillaman JM. Studies on a pox disease of monkeys. II. Isolation of the etiologic agent. Am. J. Vet. Res. 1960;21:381–384. [PubMed] [Google Scholar]
- 42.Mcconnell S, Herman YF, Mattson DE, Huxsoll DL, Lang CM, Yager RH. Protection of rhesus monkeys against monkeypox by vaccinia virus immunization. Am. J. Vet. Res. 1964;25:192–195. [PubMed] [Google Scholar]
- 43.Rouhandeh H, Engler R, Taher M, Fouad A, Sells LL. Properties of monkey pox virus. Arch. Gesamte Virusforsch. 1967;20(3):363–373. doi: 10.1007/BF01241954. [DOI] [PubMed] [Google Scholar]
- 44.Wenner HA, Bolano CR, Cho CT, Kamitsuka PS. Studies on the pathogenesis of monkey pox. 3 Histopathological lesions and sites of immunofluorescence. Arch. Gesamte Virusforsch. 1969;27(2):179–197. doi: 10.1007/BF01249642. [DOI] [PubMed] [Google Scholar]
- 45.Wenner HA, Cho CT, Bolano CR, Kamitsuka PS. Studies on the pathogenesis of monkey pox. II. Dose-response and virus dispersion. Arch. Gesamte Virusforsch. 1969;27(2):166–178. doi: 10.1007/BF01249641. [DOI] [PubMed] [Google Scholar]
- 46.Cho CT, Bolano CR, Kamitsuka PS, Wenner HA. Methisazone and monkey pox virus: studies in cell cultures, chick embryos, mice and monkeys. Am. J. Epidemiol. 1970;92(2):137–144. doi: 10.1093/oxfordjournals.aje.a121186. [DOI] [PubMed] [Google Scholar]
- 47.Alibek K. Biohazard: The Chilling True Story Of The Largest Covert Biological Weapons Program In The World – Told From The Inside By The Man Who Ran It. Dell Publishing; NY, USA: 1999. [Google Scholar]
- 48.Earl PL, Americo JL, Wyatt LS, et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl Acad. Sci. USA. 2008;105(31):10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zielinski RJ, Smedley JV, Perera PY, et al. Smallpox vaccine with integrated IL-15 demonstrates enhanced in vivo viral clearance in immunodeficient mice and confers long term protection against a lethal monkeypox challenge in cynomolgus monkeys. Vaccine. 2010;28(43):7081–7091. doi: 10.1016/j.vaccine.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goff A, Mucker E, Raymond J, et al. Infection of cynomolgus macaques with a recombinant monkeypox virus encoding green fluorescent protein. Arch. Virol. 2011;156(10):1877–1881. doi: 10.1007/s00705-011-1065-1. [DOI] [PubMed] [Google Scholar]
- 51■.Hooper JW, Thompson E, Wilhelmsen C, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. Intravenous challenge of NHPs.
- 52.Hahon N, McGavran MH. Air-borne infectivity of the variola-vaccinia group of poxviruses for the cynomolgus monkey, Macaca irus. J. Infect. Dis. 1961;109:294–298. doi: 10.1093/infdis/109.3.294. [DOI] [PubMed] [Google Scholar]
- 53■.Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81(12):1581–1600. doi: 10.1038/labinvest.3780373. Aerosol challenge of NHPs.
- 54.Nalca A, Livingston VA, Garza NL, et al. Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS One. 2010;5(9):e12880. doi: 10.1371/journal.pone.0012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55■.Johnson RF, Dyall J, Ragland DR, et al. Comparative analysis of monkeypox virus infection of cynomolgus macaques by the intravenous or intrabronchial inoculation route. J. Virol. 2011;85(5):2112–2125. doi: 10.1128/JVI.01931-10. Intravenous and intrabronchial comparison challenges in NHPs.
- 56.Estep RD, Messaoudi I, O’Connor MA, et al. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 2011;85(18):9527–9542. doi: 10.1128/JVI.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown JN, Estep RD, Lopez-Ferrer D, et al. Characterization of macaque pulmonary fluid proteome during monkeypox infection: dynamics of host response. Mol. Cell. Proteomics. 2010;9(12):2760–2771. doi: 10.1074/mcp.M110.001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58■.Stittelaar KJ, Van Amerongen G, Kondova I, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. Intratracheal challenge of NHPs.
- 59.Stittelaar KJ, Neyts J, Naesens L, et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439(7077):745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 60.Noble J., Jr. A study of new and old world monkeys to determine the likelihood of a simian reservoir of smallpox. Bull. World Health Organ. 1970;42(4):509–514. [PMC free article] [PubMed] [Google Scholar]
- 61.Saijo M, Ami Y, Suzaki Y, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J. Virol. 2006;80(11):5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olsen RG, Blakeslee JR, Mathes L, Nakano JH. Preparation and evaluation of a noninfectious monkey pox virus vaccine. J. Clin. Microbiol. 1977;6(1):50–54. doi: 10.1128/jcm.6.1.50-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janseghers L, Matamba M, Colaert J, Vandepitte J, Desmyter J. Fatal monkeypox in a child in Kikwit, Zaire. Ann. Soc. Belg. Med. Trop. 1984;64(3):295–298. [PubMed] [Google Scholar]
- 64.Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull. World Health Organ. 1988;66(4):459–464. [PMC free article] [PubMed] [Google Scholar]
- 65.Dyall J, Johnson RF, Chen DY, et al. Evaluation of monkeypox disease progression by molecular imaging. J. Infect. Dis. 2011;204(12):1902–1911. doi: 10.1093/infdis/jir663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CDC Update: multistate outbreak of monkeypox – Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morb. Mortal. Wkly Rep. 2003;52(27):642–646. [PubMed] [Google Scholar]
- 67.CCD Update: multistate outbreak of monkeypox – Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morb. Mortal. Wkly Rep. 2003;52(24):561–564. [PubMed] [Google Scholar]
- 68■■.Guarner J, Johnson BJ, Paddock CD, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 2004;10(3):426–431. doi: 10.3201/eid1003.030878. Establishment of the prairie dog model.
- 69.Langohr IM, Stevenson GW, Thacker HL, Regnery RL. Extensive lesions of monkeypox in a prairie dog (Cynomys sp) Vet. Pathol. 2004;41(6):702–707. doi: 10.1354/vp.41-6-702. [DOI] [PubMed] [Google Scholar]
- 70.Xiao SY, Sbrana E, Watts DM, Siirin M, Da Rosa AP, Tesh RB. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 2005;11(4):539–545. doi: 10.3201/eid1104.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keckler MS, Carroll DS, Gallardo-Romero NF, et al. Establishment of the black-tailed prairie dog (Cynomys ludovicianus) as a novel animal model for comparing smallpox vaccines administered preexposure in both high- and low-dose monkeypox virus challenges. J. Virol. 2011;85(15):7683–7698. doi: 10.1128/JVI.02174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith SK, Self J, Weiss S, et al. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J. Virol. 2011;85(17):9176–9187. doi: 10.1128/JVI.02173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.US FDA . Guidance For Industry. Animal Models – Essential Elements To Address Efficacy Under The Animal Rule. MD, USA: 2009. [Google Scholar]
- 74.Khodakevich L, Szczeniowski M, Nambu Ma D, et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop. Geogr. Med. 1987;39(1):56–63. [PubMed] [Google Scholar]
- 75.Reynolds MG, Carroll DS, Olson VA, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multispecies involvement in the absence of widespread human disease. Am. J. Trop. Med. Hyg. 2010;82(4):746–754. doi: 10.4269/ajtmh.2010.09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76■.Marennikova SS, Shelukhina EM, Zhukova OA. Experimental infection of squirrels Sciurus vulgaris by monkey pox virus. Acta Virol. 1989;33(4):399. First experimental infections of squirrels.
- 77■.Tesh RB, Watts DM, Sbrana E, Siirin M, Popov VL, Xiao SY. Experimental infection of ground squirrels (Spermophilus tridecemlineatus) with monkeypox virus. Emerg. Infect. Dis. 2004;10(9):1563–1567. doi: 10.3201/eid1009.040310. Establishment of a squirrel model.
- 78.Sbrana E, Jordan R, Hruby DE, et al. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 2007;76(4):768–773. [PubMed] [Google Scholar]
- 79.Gispen R, Brand-Saathof B. ‘White’ poxvirus strains from monkeys. Bull. World Health Organ. 1972;46(5):585–592. [PMC free article] [PubMed] [Google Scholar]
- 80.Marennikova SS, Gurvich EB, Shelukhina EM. Comparison of the properties of five pox virus strains isolated from monkeys. Arch. Gesamte Virusforsch. 1971;33(3):201–210. doi: 10.1007/BF01254676. [DOI] [PubMed] [Google Scholar]
- 81.Marennikova SS, Seluhina EM. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull. World Health Organ. 1976;53(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- 82.Sehgal CL, Ray SN. Laboratory studies on monkeypox virus. J. Commun. Dis. 1982;14(1):26–35. [PubMed] [Google Scholar]
- 83.Osorio JE, Iams KP, Meteyer CU, Rocke TE. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One. 2009;4(8):e6592. doi: 10.1371/journal.pone.0006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Earl PL, Americo JL, Moss B. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient gamma interferon response. J. Virol. 2012;86(17):9105–9112. doi: 10.1128/JVI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shchelukhina EM, Marennikova SS. Generalized monkeypox in orally infected rabbits and white mice. Vopr. Virusol. 1975;(6):703–705. [PubMed] [Google Scholar]
- 86.Marennikova S, Shelukhina EM, Shenkman LS. Cotton rats (Sigmodon hispidus) as an experimental model of monkeypox infection; Presented at: Twelfth International Poxvirus Symposium; St. Thomas, US Virgin Islands.Jun 6–10, 1998. [Google Scholar]
- 87.Schultz DA, Sagartz JE, Huso DL, Buller RM. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology. 2009;383(1):86–92. doi: 10.1016/j.virol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dzenda T, Ayo JO, Lakpini CA, Adelaiye AB. Seasonal, sex and live weight variations in feed and water consumptions of adult captive African giant rats (Cricetomys gambianus, Waterhouse – 1840) kept individually in cages. J. Anim. Physiol. Anim. Nutr. (Berl.) doi: 10.1111/j.1439-0396.2012.01287.x. doi:10.1111/j.1439-0396.2012.01287.x (2012) (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 89.Mgode GF, Weetjens BJ, Cox C, et al. Ability of Cricetomys rats to detect Mycobacterium tuberculosis and discriminate it from other microorganisms. Tuberculosis (Edinb.) 2012;92(2):182–186. doi: 10.1016/j.tube.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Trujillo CM, Rodriguez L, Rodas JD, Arboleda JJ. Experimental infection of Didelphis marsupialis with vesicular stomatitis New Jersey virus. J. Wildl. Dis. 2010;46(1):209–217. doi: 10.7589/0090-3558-46.1.209. [DOI] [PubMed] [Google Scholar]
- 91.Haag A, Wayss K, Rommelaere J, Cornelis JJ. Experimentally induced infection with autonomous parvoviruses, minute virus of mice and H-1, in the African multimammate mouse (Mastomys coucha) Comp. Med. 2000;50(6):613–621. [PubMed] [Google Scholar]
- 92.Niewiesk S. Current animal models: cotton rat animal model. Curr. Top. Microbiol. Immunol. 2009;330:89–110. doi: 10.1007/978-3-540-70617-5_5. [DOI] [PubMed] [Google Scholar]
- 93.Mcconnell S, Hickman RL, Wooding WL, Jr., Huxsoll DL. Monkeypox: experimental infection in chimpanzee (Pan satyrus) and immunization with vaccinia virus. Am. J. Vet. Res. 1968;29(8):1675–1680. [PubMed] [Google Scholar]
- 94.Heberling RL, Kalter SS. Induction, course, and transmissibility of monkeypox in the baboon (Papio cynocephalus) J. Infect. Dis. 1971;124(1):33–38. doi: 10.1093/infdis/124.1.33. [DOI] [PubMed] [Google Scholar]
- 95.Heberling RL, Kalter SS, Rodriguez AR. Poxvirus infection of the baboon (Papio cynocephalus) Bull. World Health Organ. 1976;54(3):285–294. [PMC free article] [PubMed] [Google Scholar]
- 96.Soekawa M, Moriguchi R, Morita C, Kitamura T, Tanaka Y. Electron-microscopical observations on the development of vaccinia, cowpox and monkeypox viruses in pig skin. Zentralbl. Bakteriol. Orig. A. 1977;237(4):425–443. [PubMed] [Google Scholar]
- 97.Kolhapure RM, Deolankar RP, Tupe CD, et al. Investigation of buffalopox outbreaks in Maharashtra State during 1992–1996. Indian J. Med. Res. 1997;106:441–446. [PubMed] [Google Scholar]
- 98.Kroon EG, Mota BE, Abrahao JS, Da Fonseca FG, de Souza Trindade G. Zoonotic Brazilian Vaccinia virus: from field to therapy. Antiviral Res. 2011;92(2):150–163. doi: 10.1016/j.antiviral.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 99.Singh RK, Hosamani M, Balamurugan V, Bhanuprakash V, Rasool TJ, Yadav MP. Buffalopox: an emerging and re-emerging zoonosis. Anim. Health Res. Rev. 2007;8(1):105–114. doi: 10.1017/S1466252307001259. [DOI] [PubMed] [Google Scholar]
- 100■■.Arita I, Gispen R, Kalter SS, et al. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull. World Health Organ. 1972;46(5):625–631. NHP serological surveys.
- 101.Parker S, Handley L, Buller RM. Therapeutic and prophylactic drugs to treat orthopoxvirus infections. Future Virol. 2008;3(6):595–612. doi: 10.2217/17460794.3.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marriott KA, Parkinson CV, Morefield SI, Davenport R, Nichols R, Monath TP. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei H, Huang D, Fortman J, Wang R, Shao L, Chen ZW. Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox. J. Virol. 2009;83(2):1115–1125. doi: 10.1128/JVI.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jordan R, Goff A, Frimm A, et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 2009;53(5):1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 2009;53(6):2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hirao LA, Draghia-Akli R, Prigge JT, et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011;203(1):95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buchman GW, Cohen ME, Xiao Y, et al. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine. 2010;28(40):6627–6636. doi: 10.1016/j.vaccine.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denzler KL, Babas T, Rippeon A, et al. Attenuated NYCBH vaccinia virus deleted for the E3L gene confers partial protection against lethal monkeypox virus disease in cynomolgus macaques. Vaccine. 2011;29(52):9684–9690. doi: 10.1016/j.vaccine.2011.09.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 110.Heraud JM, Edghill-Smith Y, Ayala V, et al. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 111.Nigam P, Earl PL, Americo JL, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Earl PL, Americo JL, Wyatt LS, et al. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366(1):84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edghill-Smith Y, Bray M, Whitehouse CA, et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 2005;191(3):372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]