Summary
Liver regeneration under normal circumstances proceeds through proliferation of all cellular elements of the liver. Studies with rodent models have shown that when proliferation of hepatocytes is inhibited, progenitor cells arising from the biliary compartment transdifferentiate into “oval/progenitor” cells, which proceed to differentiate into hepatocytes. Recent studies have shown that the same pathways may operate in human liver failure. The growth factor receptors (HGF [hepatocyte growth factor] receptor) and epidermal growth factor receptor are key mitogenic receptors for both hepatocytes and progenitor cells. Our current study used the biliary and progenitor marker EpCAM (epithelial cell adhesion molecule) to detect “regenerative clusters” of mixed cholangiocyte-hepatocyte differentiation. We determined that expression of metabolic equivalent and epidermal growth factor receptor occurs in biliary cells, progenitor cells, and hepatocytes, whereas activation of metabolic equivalent and epidermal growth factor receptor is limited to regenerative cluster hepatocytes. These histologic events are associated with expression of apoptosis-inducing FAS and mitoinhibitory protein glypican 3. Cell proliferation was overall suppressed in regenerative clusters. Transdifferentiation of biliary and progenitor cells appears to be regulated by a complex interaction of signals promoting and arresting growth.
Keywords: Regenerative clusters, MET, EGFR, FAS, Glypican 3, Fulminant Hepatitis
1. Introduction
We have recently shown that, in human liver with fulminant hepatitis, there is a promiscuous expression of hepatocyte- or cholangiocyte-associated transcription factors in either of the 2 cell types [1]. This finding was originally described in experimental models of rodent liver in which hepatocyte proliferation is suppressed and cholangiocytes transdifferentiate to hepatic progenitor cells that, in turn, become small and then mature hepatocytes [2–6]. Similar findings of transdifferentiation of cholangiocytes to progenitor cells and hepatocytes have been previously described for human liver in fulminant hepatic failure by Roskams et al. [7,8]. In rodents, proliferation of either hepatocytes or cholangiocytes is primarily controlled by 2 primary mitogenic receptors: metabolic equivalent (MET) and epidermal growth factor receptor (EGFR) [9–14]. These are the only signaling pathways that induce cell proliferation in chemically defined primary cultures of hepatocytes, and they also induce hepatic enlargement when activated by ligand injection in normal nonhepatectomized animals [9,10]. Inview of the large literature of cholangiocyte progenitor cell hepatocyte transdifferentiation in rodents and in view of the well-defined controlling factors for cell proliferation and differentiation also obtained from experimental models, we wanted to investigate whether external signaling molecules and pathways known to control these processes in rodents also operate in similar conditions in the human liver. In the current study, we used the biomarker EpCAM(epithelial cell adhesion molecule), associated with cholangiocytes and progenitor cells [15–18], to identify areas of mixed hepatocyte and cholangiocyte differentiation in tissues of human livers removed for fulminant liver failure in the course of orthotopic liver transplantation. The purpose of the study was to investigate the expression and stage of activation of the 2 primary mitogenic receptors MET and EGFR in the different epithelial cell subpopulations of the EpCAM-positive clusters. In addition to their mitogenic effects, these receptors are involved in transdifferentiation between hepatocytes and cholangiocytes by inducing very specific patterns of gene expression [19], and they are also expressed in rodent progenitor cells [20–22]. Furthermore, we investigated the expression of the apoptotic receptor FAS, involved in both hepatocyte apoptosis and also in liver regeneration [23]. The protein known as glypican 3 (GPC3) is expressed in progenitor cells in rodents [24], is overexpressed in human liver cancer [25], and was shown by our studies to suppress growth of hepatocytes [26–28]. We extended our study to also include expression of GPC3 as a protein involved in hepatocyte growth regulation and termination of liver regeneration.
2. Materials and methods
2.1. Tissue material submitted to histologic analysis
With approval from the institutional review board of the University of Pittsburgh (institutional review board no. 0501051), paraffin-embedded liver sections were obtained from the archives of the Department of Pathology, University of Pittsburgh Medical Center. The study set included 4 normal livers and 18 cases of fulminant hepatic failure. The cases were obtained from livers explanted for the purpose of orthotopic liver transplantation. The normal adult liver tissue samples were selected from liver specimens resected for metastatic colorectal carcinoma.
2.2. Details on antibodies used for immunohistochemistry
The information for each antibody below is in the following format:
“primary antibody; source and catalog number; retrieval method and buffer details; concentration of antibody applied; secondary antibody used.”
HNF4α: Santa Cruz sc-6556; steam 20 minutes in Dako target retrieval solution; 1:100, 1 hour; donkey antigoat Millipore AP180B.
MET: Santa Cruz sc-161; none; 1:50, 1 hour; goat antirabbit Millipore AP187B.
FAS: Santa Cruz sc-8009; none; 1:50 overnight; donkey antimouse Millipore AP192B.
EpCAM: Santa Cruz sc-66020; steam 20 minutes in Dako target retrieval solution; 1:50, 1 hour; donkey antimouse, Jackson 715-065-150.
GPC3: Santa Cruz 11395; autoclave 15 minutes, 10 mmol/L citrate buffer, pH 6.5; 1:50 overnight; goat antirabbit Millipore AP187B.
EGFR: Cell Signaling 4267; autoclave 15 minutes, 10 mmol/L citrate buffer pH 6.5; 1:40 overnight; goat antirabbit Millipore AP187B.
P-EGFR (Tyrosine [Tyr] 1068): Abcam ab40815; steam 20 minutes, 10 mmol/L citrate buffer pH 6.0; 1:25 overnight; goat antirabbit Millipore AP187B.
P-MET (Tyr 1003): Invitrogen 44-882G; autoclave 15 minutes, 10 mmol/L citrate buffer pH 6.5; 1:50 overnight; goat antirabbit Millipore AP187B.
Ki-67; Thermofisher RM-9106-50; microwave 10 minutes, 10 mmol/L citrate buffer pH 6.0; 1:200, 30 minutes; goat antirabbit Millipore AP187B.
HEPPAR: Dako M7158; none; 1:50, 1 hour; donkey antimouse Millipore AP192B.
NCAM (CD56): Beckman Coulter 6602705; steam 20 minutes in Dako target retrieval solution; 1:10, overnight; donkey antimouse Jackson 715-065-150.
CK19: Dako M0888; microwave 3 minutes in Dako target retrieval solution, high pH; 1:50, 30 minutes; donkey antimouse Jackson 715-065-150.
2.3. Immunohistochemistry
Immunohistochemical localization studies were conducted on formalin-fixed, paraffin-embedded liver sections (4 µm thick). Antigen retrieval was achieved by steaming the slides for 60 minutes in Target Retrieval or Hi pH Target Retrieval solution (Dako, Carpinteria, CA). The slides were bathed in 3% H2O2 solution for 5 minutes to quench endogenous peroxidase. Endogenous avidin and biotin were also blocked using the Avidin-Biotin blocking kit (Vector, Burlingame, CA). Primary antibodies were tested to identify the appropriate concentration and applied uniformly to all tissue sections thereafter. The sections were incubated overnight at 41°C. Nonspecific binding sites were blocked with 10% serum of the appropriate host animal in protein block (Dako) with incubation for 10 minutes, and the biotinylated secondary antibody was applied with incubation for 30 minutes (see Details in 2.2). The sections were then incubated with Vectastain ABC Elite (Vector) at room temperature for 30 minutes. The sections were then visualized with chromogen for 10 minutes, counterstained with aqueous hematoxylin/blue Scott's solution in tap water, crystal mounted, and allowed to dry before a coverslip was placed.
3. Results
The results presented are derived from several cases in which clusters of hepatocytes and biliary cells expressing EpCAM at various levels were the predominant histologic element. All materials were derived from livers resected from cases (total of 18) of fulminant hepatic failure in the course of orthotopic liver transplantation. The cases included 8 cases with acetaminophen overdose, 1 case of hepatitis virus A, 1 case of cirrhosis associated with hepatitis B virus, 1 case of isoniazide toxicity, 1 case of Amanita phalloides toxicity, and 6 cases of cryptogenic cirrhosis of unknown etiology.
3.1. Expression of EpCAM, HEPPAR, and HNF4 in normal liver and fulminant hepatitis
In normal human liver, expression of EpCAM is limited to biliary cells of portal ducts and canals of Hering (Fig. 1A). In cases of moderate hepatic tissue damage (1/8 acetaminophen overdose cases), biliary ducts maintain strong histo-chemical EpCAM reaction, whereas numerous progenitor cells with histologic characteristics intermediate between biliary cells and hepatocytes also exhibit a weak positivity (Fig. 1B). In cases of very severe hepatic damage with loss of more than 80% of the native hepatocytes, clusters of hepatocytes of irregular size and shape and surrounded by or intermingled with biliary cells also appear (Fig. 2). Our study is limited to these histologic areas. Such histologies were seen in 17 of 18 of fulminant hepatitis used in our study. The appearance of such clusters of mixed differentiation was not related to the cause of the fulminant hepatitis but rather to the extent of loss of native hepatocytes. We will refer to clusters of cells with these histologic features as “regenerative clusters.” The cells in the periphery of the regenerative clusters are positive for EpCAM, whereas cells toward the center of the cluster have either absent or weaker EpCAM expression, always with a membranous pattern. In addition to the expression of the progenitor marker EpCAM in hepatocytes, the expression of the hepatocyte marker HEPPAR (hepatocyte paraffin antibody) is seen both in hepatocytes as well as in cells with small hepatocyte features arranged in ductules, as shown in Fig. 3A. The hepatocyte-associated transcription factor HNF4 (hepatocyte nuclear factor 4) is typically not expressed in normal biliary cells [1]. Fig. 3B demonstrates intense expression of HNF4 in cells in the periphery of the regenerative cluster as well as in some adjacent biliary ductules. The intermingled morphology and mixed expression of both hepatocyte and progenitor cell/biliary cell markers suggest an active process of transdifferentiation between hepatocytic and biliary cells in these clusters, comparable with that described in rodents at the beginning of transdifferentiation of cholangiocytes to progenitor cells [3].
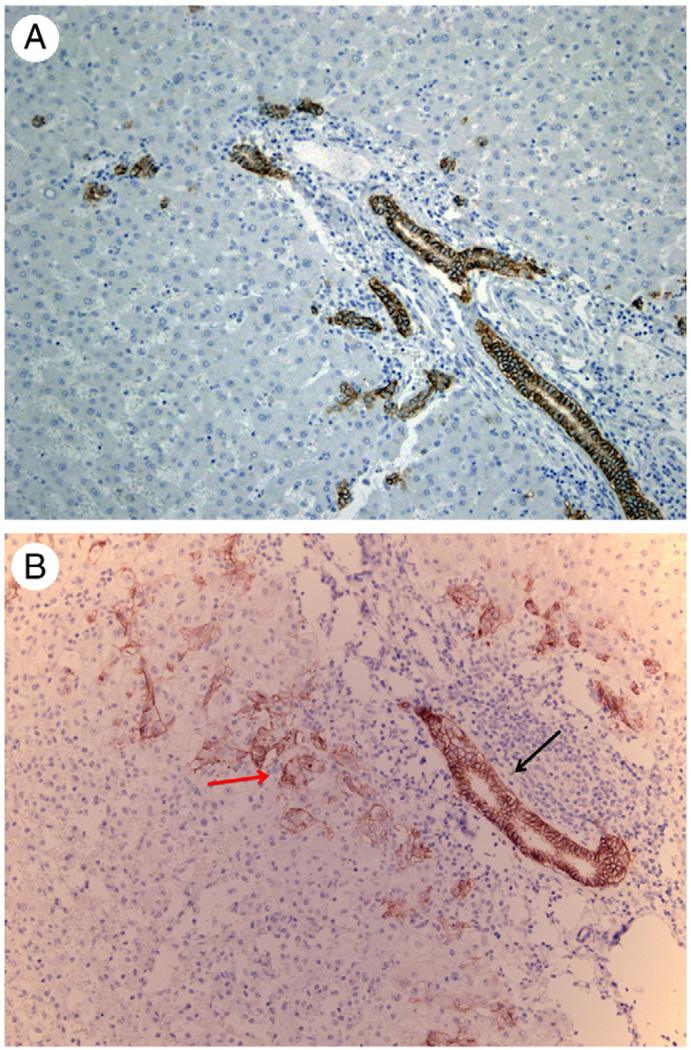
Fig. 1.
A, Immunohistochemistry for EpCAM on section from a normal human liver. Biliary ductules in portal triads and beyond (canals of Hering?) are the only cellular elements positive for EpCAM. Original magnification ×40. B, In a case of moderate hepatic tissue damage, a portal biliary ductule surrounded by inflammatory cells displays the standard strong positivity for EpCAM immunohistochemistry (black arrow). On either side of the portal space, numerous progenitor cells with histologic characteristics intermediate between biliary cells and hepatocytes also exhibit weaker EpCAM positivity (red arrow).
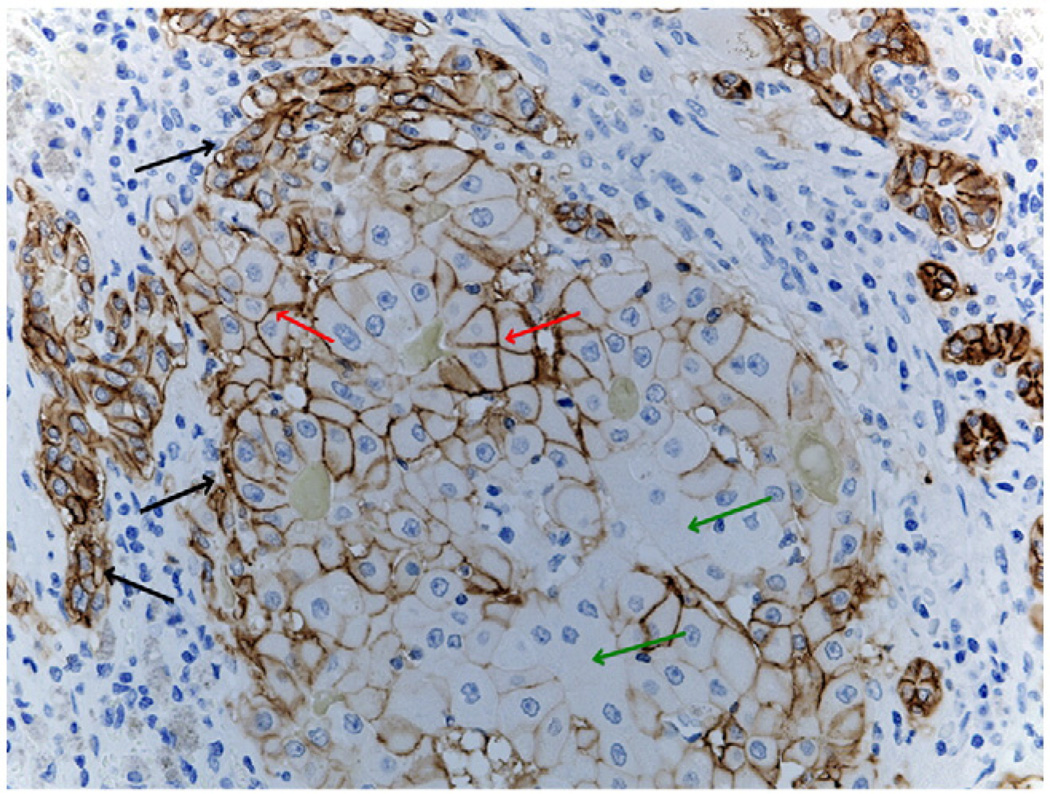
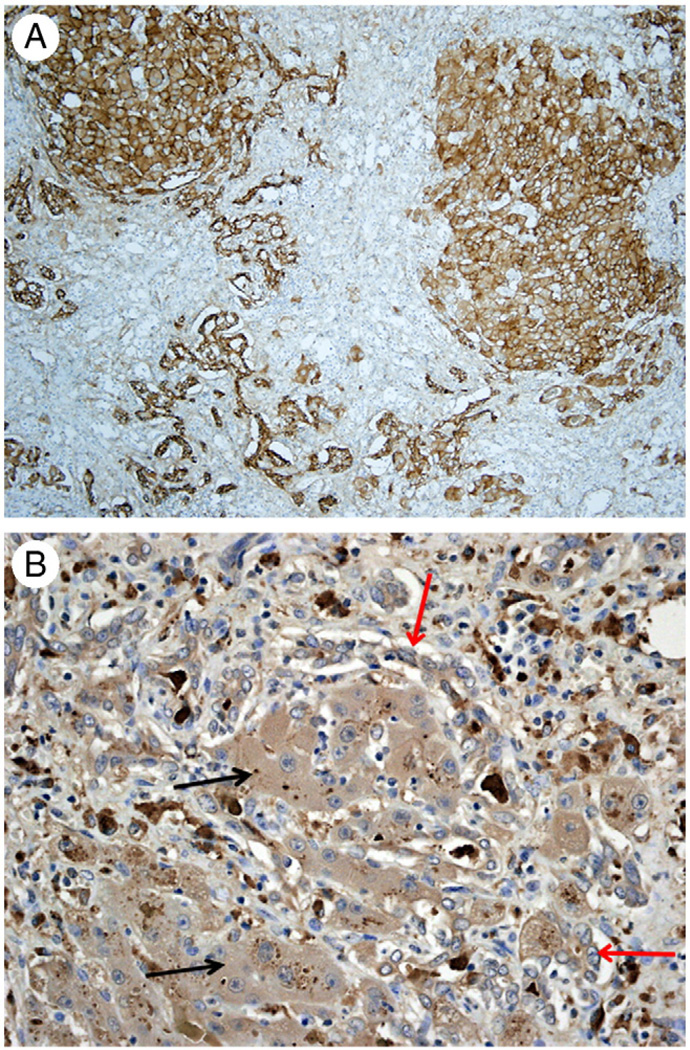
Fig. 2.
EpCAM immunohistochemistry in a regenerative cluster. Hepatobiliary cells in the periphery (black arrows) are strongly positive. Hepatocytes in the center display various degrees of immunoreactivity from strongly positive (red arrows) to completely negative (green arrows). (Original magnification ×100.)
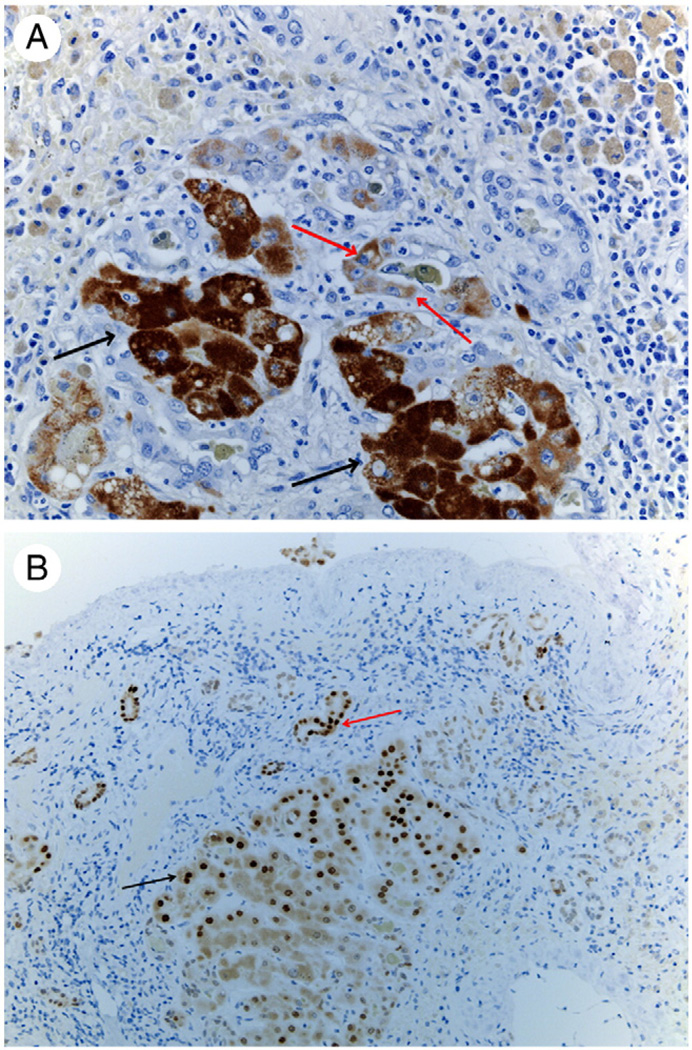
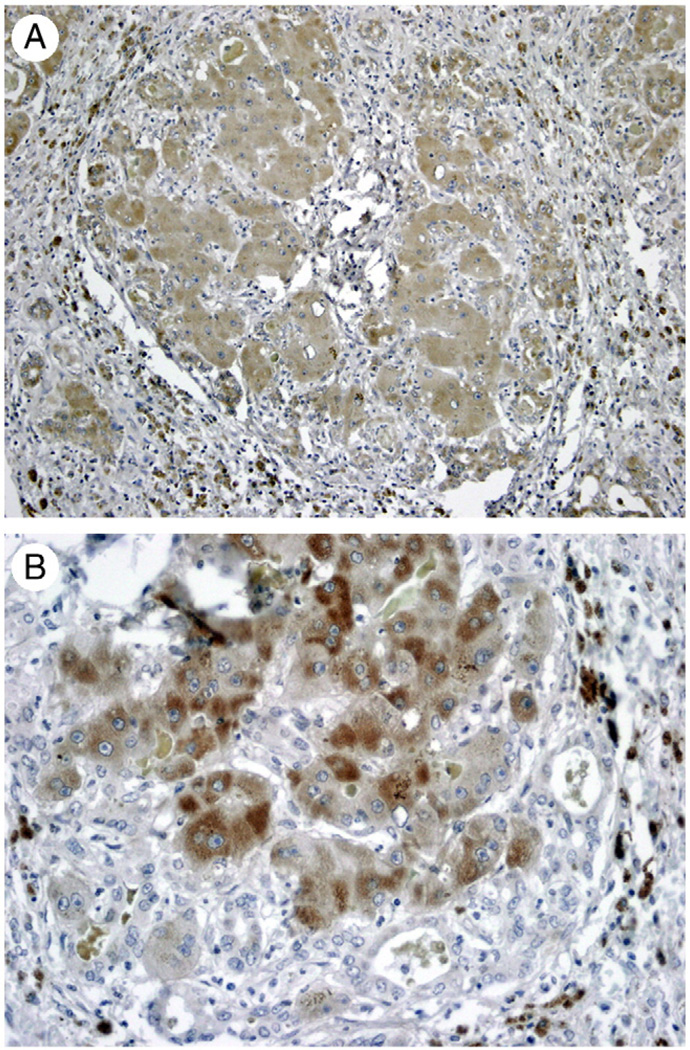
Fig. 3.
A, Immunohistochemistry for hepatocyte-specific biomarker HEPPAR. Hepatocytes in the cluster stain positive (black arrows). HEPPAR-positive cells are also seen in cells embedded in ductules (red arrows). (Original magnification ×100.) B, Immunohistochemistry for hepatocyte-specific transcription factor HNF4α. Nuclei of cells recognizable as hepatocytes in the periphery of the cluster stain positive (black arrow). Positive nuclei are also seen, however, in biliary ductules in the periphery (red arrow). (Original magnification ×100.)
Expression of cytokeratin 7 was not only intense in cholangiocytes but also seen at a lesser intensity in some of the hepatocytes of the regenerative clusters (Fig. 4A). A similar pattern was also seen with cytokeratin 19 (Fig. 4B). Previous studies have described expression of neuroendocrine markers in cholangiocytes and progenitor cells in human liver [7,8,29]. We studied the expression of NCAM1 (neural cell adhesion molecule 1) (also known as CD56). Although cytokeratin expression was seen in occasional hepatocytes, NCAM1 expression was limited to cholangiocytes arranged in ductules and was not present in the hepatocytes of the regenerative clusters (Fig. 4C).
Fig. 4.
A, Immunohistochemistry for cytokeratin 7. Intense immunoreactivity is seen in cholangiocytes (black arrows). Some of the hepatocytes in the regenerative clusters also show weaker immunoreactivity (red arrows). (Original magnification ×100.) B, Immunohistochemistry for cytokeratin 19. Intense immunoreactivity is seen in cholangiocytes (black arrows). Some of the hepatocytes in the regenerative clusters also show weaker immunoreactivity (red arrow). (Original magnification ×100.) C, Immunoreactivity for NCAM1 (CD56) in a regenerative cluster. Positive immunoreactivity is confined to cholangiocytes in ductules (black arrows), whereas cells with hepatocyte morphology are negative (red arrows). (Original magnification ×100.)
3.2. Expression of mitogenic receptors EGFR and MET
We used specific antibodies that distinguish between EGFR and the activated (Tyr 1068 phosphorylated) EGFR. Expression of EGFR is seen both in hepatocytes and biliary cells of the clusters as well as in adjacent biliary ductules (Fig. 5A). The expression of the activated EGFR, however, is much more intense in hepatocytes of the EpCAM-positive transdifferentiation clusters. This is shown in Fig. 5B. Expression of MET was seen with either cholangiocyte or hepatocyte morphology (Fig. 6A). However, expression of the Tyr 1003–phosphorylated activated MET was again seen exclusively in the hepatocytes of the regenerative clusters. This is shown in Fig. 6B.
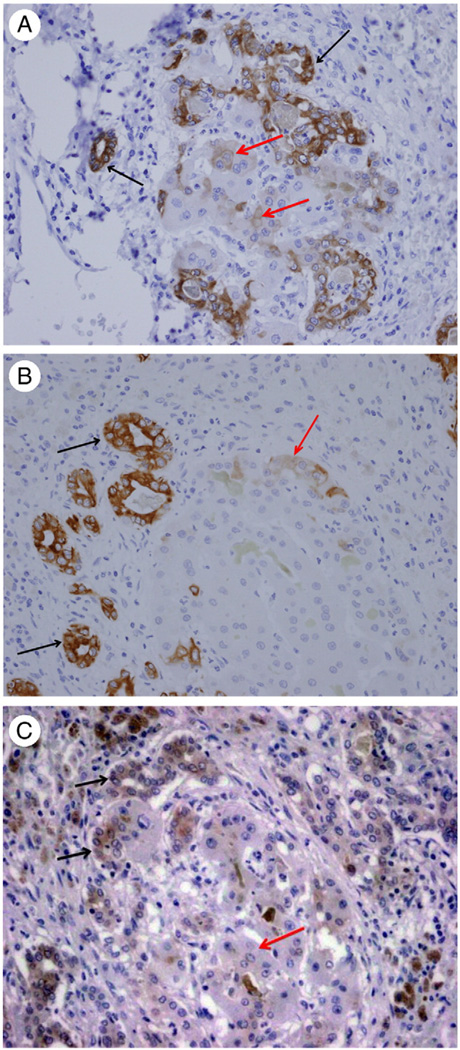
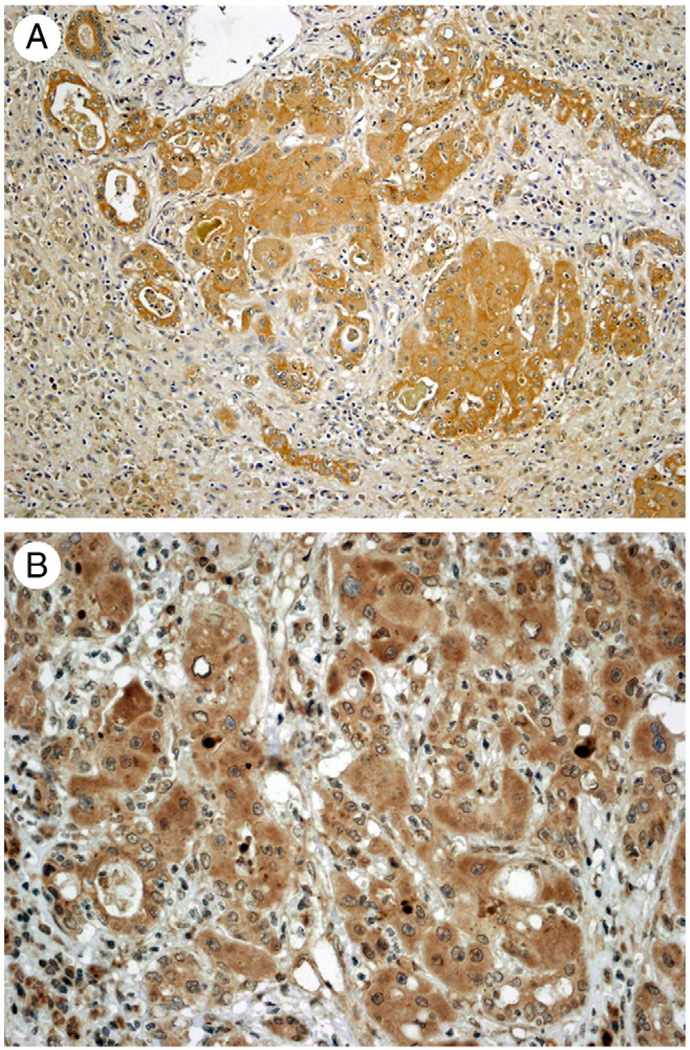
Fig. 5.
A, Immunohistochemistry for EGFR. Both independent biliary ductules and adjacent regenerative clusters stain positive for EGFR. B, Immunohistochemistry for Tyr 1068–phosphorylated EGFR. Positive expression is seen in hepatocytes in the regenerative cluster (black arrows) compared with surrounding biliary ductules that are mostly negative (red arrows). (Original magnification ×100.)
Fig. 6.
A, Immunohistochemistry for HGF receptor MET. Both surrounding biliary ductules and hepatocytes of the regenerative cluster are positive. B, Immunohistochemistry for activated (Tyr 1003 phosphorylated) MET. Positive expression is seen in hepatocytes in the regenerative cluster, whereas surrounding biliary ductules are negative. (Original magnification ×100.)
3.3. Expression of the death receptor FAS and mitoinhibitory protein GPC3
FAS was intensely expressed in both biliary cells and hepatocytes. This was seen in all clusters, and it is shown in Fig. 7A. This was also the case for GPC3, shown in Fig. 7B.
Fig. 7.
Immunohistochemistry for death receptor FAS (A) and GPC3 (B). Both biliary cells and hepatocytes stain positive for these proteins. (Original magnification: A ×100, B ×200.)
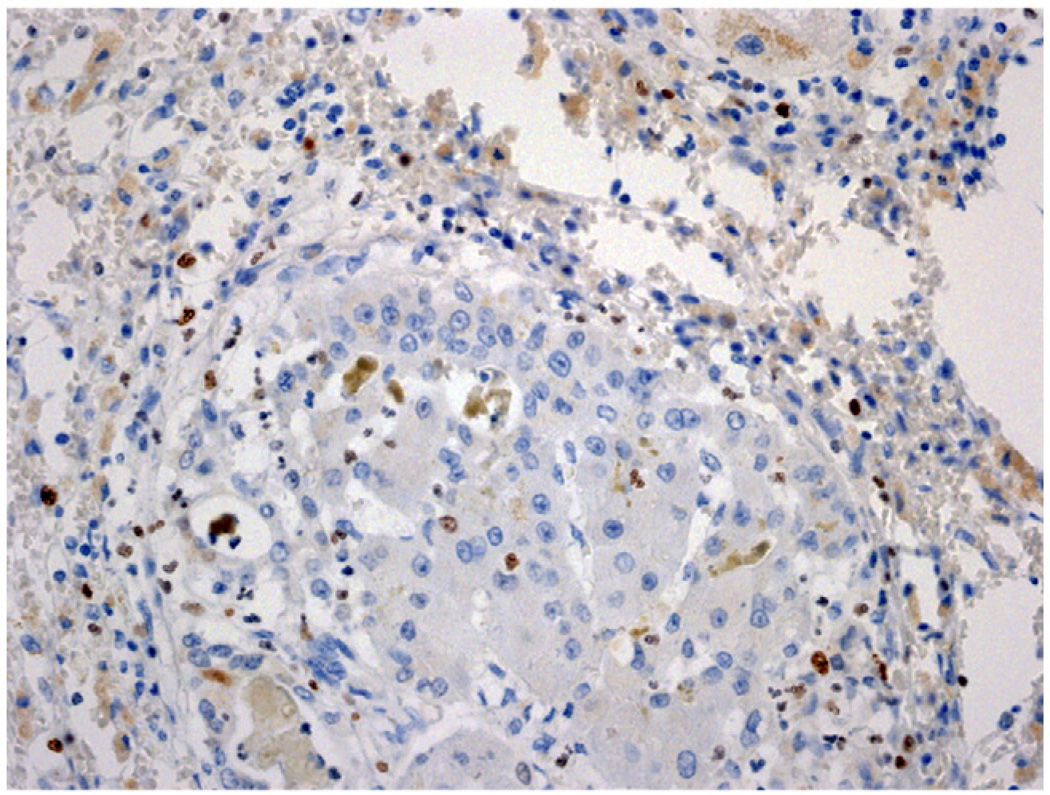
Cell proliferation of both hepatocytes and biliary cells was assessed by expression of marker Ki-67. As shown in Fig. 8, and despite the expression of activated forms of both EGFR and MET in the cells of the cluster, the degree of cell proliferation was minimal and limited primarily to a small number of cells with nonepithelial morphology, situated outside the regenerative clusters.
Fig. 8.
Immunohistochemistry for Ki-67 in a regenerative cluster. Both hepatocytes and biliary cells are mostly negative for Ki-67. Occasional nonepithelial inflammatory cells stain positive (dark brown nuclei). (Original magnification ×100.)
4. Discussion
Our results demonstrate that the gene expression boundaries between hepatocytes and biliary cells are overlapping in EpCAM-positive regenerative clusters seen in fulminant hepatic failure. Biliary and progenitor cell markers such as EpCAM as well hepatocyte specific markers such as HEPPAR and HNF4 are seen intermixed in cells of these clusters that have either hepatocyte or biliary or intermediate morphology. Some of these cells are arranged in ductules, whereas others appear as foci of hepatocytes without clear delineation of hepatic plates (Fig. 2). Given the limits of tests that can be applied to material from resected human liver, one cannot clearly rule out the possibility that biliary cells were arising from hepatocytes or vice versa. In view of the findings from experimental studies in animals [2–6], however, the most likely possibility is that these are foci in which hepatocytes arise from biliary cells. The promiscuous expression of both HNF4 and HEPPAR, consistent with the findings in our previous studies [1], suggest that this is the most likely possibility.
Previous studies have shown that the mitogenic receptors MET and EGFR are uniquely associated with states of transdifferentiation between hepatocytes and biliary cells [19,21]. In a recent study, removal of the HGF activator inhibitor also enhanced transdifferentiation of biliary and progenitor cells to hepatocytes [30]. It is of interest that although EGFR and MET were present in both cholangiocytes and hepatocytes, the activated forms of the mitogenic receptors were present exclusively in the cells with hepatocyte morphology. The effects of these mitogenic receptors are not limited to cell proliferation, however. Examination of the gene expression patterns of hepatic organoids in culture documents unique effects of MET and EGFR specific to transdifferentiation [19]. The absence of cell proliferation as evidenced by the low percentage of cells expressing Ki-67 also suggests that the primary events in these clusters are likely the reorganization of gene expression patterns from one cell to another.
Nonetheless, the lack of demonstrable cell proliferation (as evidenced by expression of Ki-67) in the regenerative clusters is intriguing. We have shown that GPC3, although dramatically overexpressed in hepatocellular carcinomas [25] and rodent liver progenitor cells [24], is associated with cessation of growth of hepatocytes in mice and that transgenic overexpression of GPC3 diminishes liver regeneration and prevents regenerating liver from returning to its original size [26–28]. It is not clear whether FAS expression may also play a similar role. Although the function of FAS is typically that of a receptor-inducing apoptosis, experimental studies have also shown that FAS also plays a role in hepatocyte proliferation [23]. Mice genetically deficient for FAS have defective hepatic regeneration and hepatocyte proliferation [23]. In addition, activation of the HGF/MET signaling pathway abrogates FAS-induced hepatocyte apoptosis [31]. The same has been noted for the EGFR signaling pathway [32]. The rate of apoptosis in the regenerative clusters was extremely low, suggesting that FAS plays a role different than inducing apoptosis in this process. Our results overall suggest that expression and activation of the mitogenic receptors MET and EGFR, combined with potential mitoinhibitory effects of FAS and GPC3, create a set of stimuli in which cell proliferation is limited and the transdifferentiation process is relatively enhanced.
Given the multiplicity of signals involved in these processes, more work needs to be performed to better understand the signaling pathways involved in transdifferentiation of biliary-derived progenitor cells to hepatocytes in human liver. Such understanding may also have therapeutic implications that could determine a positive outcome and limit the need for liver transplantation in cases of human fulminant hepatic failure.
Footnotes
This work was supported by grants from the National Institutes of Health (NIH CA35373 and NIH CA103958) to G.K.M.
References
- 1.Limaye PB, Alarcon G, Walls AL, et al. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab Invest. 2008;88:865–872. doi: 10.1038/labinvest.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- 3.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golding M, Sarraf CE, Lalani EN, et al. Oval cell differentiation into hepatocytes in the acetylaminofluorene-treated regenerating rat liver. Hepatology. 1995;22:1243–1253. doi: 10.1016/0270-9139(95)90635-5. [DOI] [PubMed] [Google Scholar]
- 5.Bisgaard HC, Nagy P, Santoni-Rugiu E, Thorgeirsson SS. Proliferation, apoptosis, and induction of hepatic transcription factors are characteristics of the early response of biliary epithelial (oval) cells to chemical carcinogens. Hepatology. 1996;23:62–70. doi: 10.1002/hep.510230110. [DOI] [PubMed] [Google Scholar]
- 6.Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–352. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 7.Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990;137:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 8.Roskams T, De Vos R, van den Oord JJ, Desmet V. Cells with neuroendocrine features in regenerating human liver. APMIS Suppl. 1991;23:32–39. [PubMed] [Google Scholar]
- 9.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 10.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paranjpe S, Bowen WC, Tseng GC, Luo JH, Orr A, Michalopoulos GK. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am J Pathol. 2010;176:2669–2681. doi: 10.2353/ajpath.2010.090605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–179. doi: 10.1016/j.biocel.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471–1477. doi: 10.1002/hep.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 16.Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 18.Schmelzer E, Reid LM. EpCAM expression in normal, non-pathological tissues. Front Biosci. 2008;13:3096–3100. doi: 10.2741/2911. [DOI] [PubMed] [Google Scholar]
- 19.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Z, Evarts RP, Fujio K, Marsden ER, Thorgeirsson SS. Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat. Am J Pathol. 1993;142:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Z, Evarts RP, Fujio K, et al. Expression of transforming growth factor alpha/epidermal growth factor receptor, hepatocyte growth factor/c-met and acidic fibroblast growth factor/fibroblast growth factor receptors during hepatocarcinogenesis. Carcinogenesis. 1996;17:931–938. doi: 10.1093/carcin/17.5.931. [DOI] [PubMed] [Google Scholar]
- 22.Nagy P, Bisgaard HC, Santoni-Rugiu E, Thorgeirsson SS. In vivo infusion of growth factors enhances the mitogenic response of rat hepatic ductal (oval) cells after administration of 2-acetylaminofluorene. Hepatology. 1996;23:71–79. doi: 10.1002/hep.510230111. [DOI] [PubMed] [Google Scholar]
- 23.Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- 24.Grozdanov PN, Yovchev MI, Dabeva MD. The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Lab Invest. 2006;86:1272–1284. doi: 10.1038/labinvest.3700479. [DOI] [PubMed] [Google Scholar]
- 25.Luo JH, Ren B, Keryanov S, et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology. 2006;44:1012–1024. doi: 10.1002/hep.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Paranjpe S, Bowen WC, et al. Investigation of the role of glypican 3 in liver regeneration and hepatocyte proliferation. Am J Pathol. 2009;175:717–724. doi: 10.2353/ajpath.2009.081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Bell AW, Paranjpe S, et al. Suppression of liver regeneration and hepatocyte proliferation in hepatocyte-targeted glypican 3 transgenic mice. Hepatology. 2010;52:1060–1067. doi: 10.1002/hep.23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CW, Mars WM, Paranjpe S, et al. Hepatocyte proliferation and hepatomegaly induced by phenobarbital and 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene is suppressed in hepatocyte-targeted glypican 3 transgenic mice. Hepatology. 2011;54:620–630. doi: 10.1002/hep.24417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Huang HP, Chang MH, Chen YT, et al. Persistent elevation of hepatocyte growth factor activator inhibitors in cholangiopathies affects liver fibrosis and differentiation. Hepatology. 2012;55:161–172. doi: 10.1002/hep.24657. [DOI] [PubMed] [Google Scholar]
- 31.Kosai K, Matsumoto K, Nagata S, Tsujimoto Y, Nakamura T. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem Biophys Res Commun. 1998;244:683–690. doi: 10.1006/bbrc.1998.8293. [DOI] [PubMed] [Google Scholar]
- 32.Musallam L, Ethier C, Haddad PS, Bilodeau M. Role of EGF receptor tyrosine kinase activity in antiapoptotic effect of EGF on mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1360–G1369. doi: 10.1152/ajpgi.2001.280.6.G1360. [DOI] [PubMed] [Google Scholar]