Abstract
Metallothioneins (MTs), due to the prevalence of reactive thiol groups have previously been shown to quench various DNA alkylating compounds. Here, we present initial results which demonstrate that MTs can also sequester BCNU in simulated physiological conditions. To demonstrate the binding of BCNU to MT, we used an assay that measured the release of the MT-bound divalent cations (Zn+2, Cd+2) upon their displacement by the drug. The rate of release of the cations was higher in pH 7.4 than at pH 7.0, which can be attributed to the fact that BCNU decomposes faster (thus faster release of MT-binding intermediate) at pH 7.4 than at pH 7.0. Our investigation of the possible role of MT in BCNU-resistance arose from our investigation of the mechanisms of BCNU-resistance in the medulloblastoma subline D341 MED (OBR), which has been continuously pulsed with BCNU and O6-benzylguanine (O6-BG). We were able to discount the possibility of raised levels of O6-methylguanine-DNA methyltransferase (MGMT), glutathione, or interstarnd crosslink (ICL) activity as possible modes of BCNU-resistance in the drug-resistant subline. In our earlier publication, transcriptional profiling revealed the upregulation of several MT genes in D341 MED (OBR), relative to its parental line D341 MED.
Keywords: carmustine, BCNU, metallothionein, medulloblastoma, cancer drug resistance
Introduction
BCNU [1,3-bis(2-chloroethyl)-1-nitrosourea] or carmustine, a lipophilic chemotherapeutic drug which can efficiently cross the blood-brain barrier, is widely used in treating various types of brain tumors such as medulloblastoma (Kalifa et al., 1999; Soffietti et al., 2007). However, the effectiveness of this alkylating drug can be moderated by various factors, foremost of which is increased O6-methylguanine-DNA methyltransferase (MGMT) activity in target cells acquired during the course of treatment (Pegg, 1990). MGMT’s repair of the BCNU-derived chloroethyl lesion in the the O6 position of guanine halts the formation of cancer-killing DNA interstrand crosslinks (ICLs) (Tong et al., 1982). We have previously shown that the medulloblastoma subline D341 MED (OBR) which has an acquired resistance to BCNU and the MGMT inhibitor O6-benzylguanine (O6-BG) was lacking MGMT activity (Bacolod et al., 2002). This prompted us to look for other factors which caused these drug-resistant cells to survive in culture despite repeated exposure to BCNU. Ensuing experiments have shown that relative to its parental cell line (D341 MED), D341 MED (OBR) did not exhibit elevated glutathione (GSH) level, as well as glutathione S-transferase (GST) activity, two factors that can also contribute to BCNU detoxification (Berhane et al., 1993). Neither D341 MED (OBR) nor D341 MED had detectable DNA ICL repair activity (Bacolod et al., 2002). However, genome-wide expression analyses revealed the upregulation of a number of metallothionein (MT) genes (MT2A, MT1X, MT1L, and MT1A, MT1E, MT1F, MT1H, MT1B) in the drug-resistant subline (Bacolod et al., 2002). Although, these low molecular weight, thiol rich proteins have been associated with resistance against Pt-based chemotherapy compounds (Doz et al., 1993), studies have shown that overexpression of MTs can also confer resistance against the alkylating agents chlorambucil and melphalan. (Endresen et al., 1983; Kelley et al., 1988). Fenselau and co-workers have demonstrated that specific thiol groups in MT can form covalent linkage with the chloroethyl group of each of the four nitrogen mustard-derived drugs (chlorambucil, melphalan, cyclophosphamide, and mechloroethamine) currently used in clinics (Antoine et al., 1998; Wei et al., 1999; Yu et al., 1995; Zaia et al., 1996). With BCNU and its reactive decomposition products having similarly electrophilic centers, we therefore conducted initial experiments to examine if MTs may indeed sequester BCNU in solutions, and provide rationale to how its upregulation in a medulloblastoma cell line may contribute to the cell line’s BCNU-resistance.
Methodology
Cell Lines and other materials
D341 MED was grown in Improved MEM Zinc Option (Richter’s Modification) Medium (Invitrogen) supplemented with 10% FCS (Invitrogen). The generation, propagation, and maintenance of D341 MED (OBR) has been reported previously (Bacolod et al., 2002). Rabbit liver metallothionein II (MT2) was purchased from Sigma-Aldrich (St. Louis, MO). It was certified to have 1.6% Zn+2 and 6.5% Cd+2 or 3.8 mol and 1.6 mol Zn+2 and Cd+2 per mol protein respectively. BCNU and 4-(2-pyridylazo)resorcinol (PAR) were also purchased from Sigma-Aldrich.
Quantification of metallothioneins in the cell lines
Cellular extracts were prepared by incubating the cells with lysis buffer (50 mM Tris HCl, pH 7.8, 0.15 M NaCl, 1% NP-40, Roche complete mini EDTA-free protease-inhibitor cocktail) at 37°C for 10 min. The supernatant was isolated after centrifugation. Sixty micrograms of protein extracts from the cell lines, and 60 ng of pure rabbit liver MT2 were electrophoresed on NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred onto nylon membrane (Pall). MT2 was then detected using 1:100 dilution of mouse anti-MT monoclonal antibody clone E9 (Dako) followed by 1:1000 dilution of horseradish peroxidase-conjugated anti-mouse IgG (Amersham Biosciences). The presence of MT2 mRNA was verified by Northern blot analysis. The MT2 DNA probe was prepared from a plasmid clone containing the MT2 gene (ATCC). Hybridization was performed using the NorthernMax Ultrasensitive Northern Blotting Kit (Ambion).
Metallotionein-BCNU binding Assay
To test if BCNU binds to MT, we employed the assay originally developed by Laib and co-workers (Laib et al., 1985). A 1-ml solution (either in 0.1 mM Tris at pH 7.0, or 1X PBS at pH 7.4) containing 5 μM MT2, 100 μM PAR, and BCNU (at 0, 0.14, and 0.8 mM) was incubated at 37 °C. Its absorbance at 485 nm was monitored every 10 min for a 150-min period, using the Beckman Coulter DU640 uv/vis spectrophotometer. In addition, solutions without MT2 were also run as controls.
BCNU decomposition Assay
The decomposition of BCNU in the presence or absence of MT2 was monitored using a protocol originally intended to analyze the presence of BCNU in blood of patients undergoing chemotherapy (Krull et al., 1981). Thirty μg/mL (140.2 μM) BCNU or 1X PBS, pH7.4) was incubated at 37 °C, in the absence or presence of 5 μM MT2. After every 30 min, 1 ml of the solution was transferred to a tube containing 1 ml ethyl acetate solution with 5, 5′ diphenylhydantoin (internal standard). The collected samples were subsequently run in reverse phase HPLC to quantitate the amount of intact BCNU in the solution.
Results
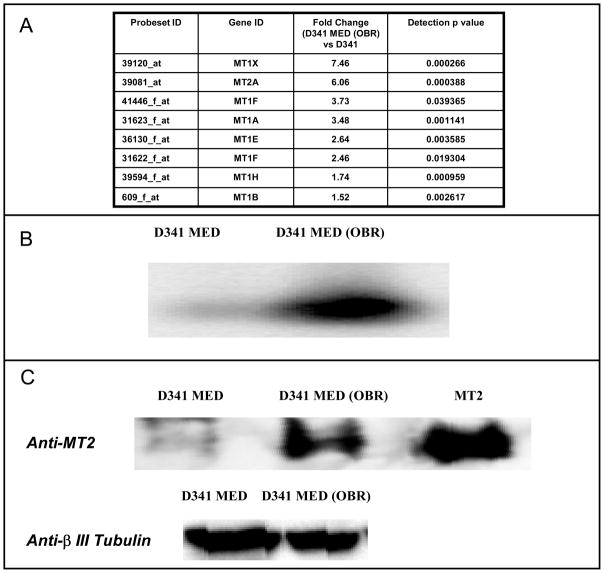
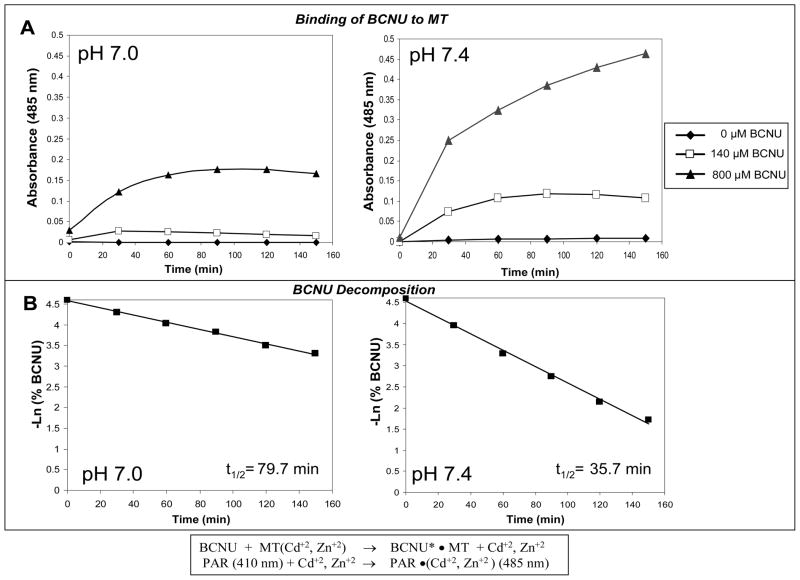
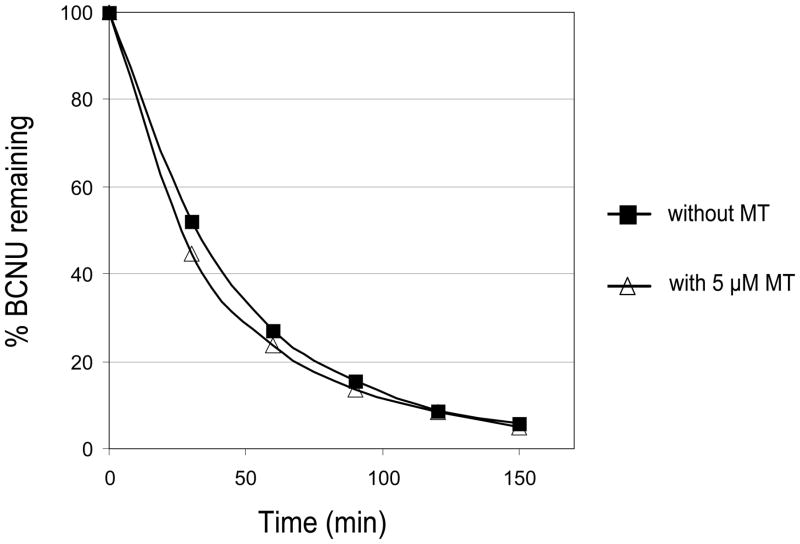
Using Western and Northern blot analyses, we were able to confirm the elevated levels of MTs in the D341 MED (OBR) cell line, relative to the parental cell line as previously shown in our genome-wide transcriptional analysis (Bacolod et al., 2002). In the Northern analysis (Figure 1B), the probe used was a PCR fragment copied from a plasmid carrying the complete MT2A cDNA sequence. However, all the MT1 and MT2 isoforms (the other upregulated MT isoforms in D341 MED (OBR) according to our microarray analysis are MT1X, MT1F, MT1A, MT1E, MT1H, and MT1B ; see Figure 1A) are almost indistinguishable in size and nucleotide sequence. Thus, it is highly likely that the RNA band identified was for mixed population of MT1 and MT2 isoforms. Similarly, the protein band (Figure 1C) detected by anti-MT2 monoclonal Ab may also be for the total MT population due to the Ab’s possible cross-reactivity between the different MT isoforms. The binding of BCNU (or its decomposition product) to MT2 was then monitored by indirect measurement of Cd+2 and Zn+2 displaced by the drug. Upon their release from MT, the cations can bind to PAR causing the compound’s spectral peak to shift from 410 nm to 485 nm (Laib et al., 1985). The increase in the solution’s absorbance at 485 nm over time (Figure 2) clearly indicated the dissociation of the divalent ions from MT2 upon the addition of BCNU. From the graphs, we can see that the rate of cation release was higher in pH 7.4 (PBS buffer) compared to pH 7.0 (0.1 M tris). When we plotted the decomposition of BCNU over time in those two buffer conditions, we can also see that the drug’s decomposition was faster at pH 7.4 (t 1/2=35.7 min) than pH 7.0 (t 1/2=79.7 min). A much earlier study (Loo et al., 1966) has shown that BCNU’s half life at pH 6.8 (0.1 M Tris) and pH 7.4 (0.1 M Phosphate) were 112 and 52 min respectively. Overall, the results from binding and decomposition experiments suggest that a it is BCNU decomposition product which binds to MT. We also tested whether MT itself enhances the decomposition of BCNU. However as shown in Figure 3, MT did not have any effect on the rate of BCNU decomposition.
Figure 1.
Comparison of metallothionein (MT) expression levels in D341 MED (OBR) and its parental line, D341 MED using: (A) Expression array (U95A ver 2) analysis (D341 MED (OBR) vs D341 MED) (Some of these data were also reported in Bacolod et al., 2002), (B) Northern blot, and (C) Western blot.
Figure 2.
(A) The Binding of BCNU to MT2 at pH 7.0 and pH 7.4. Results show that the addition of BCNU to a solution containing PAR and MT2 leads to increase in absorbance at 485 nm, due to the conjugation of PAR to the divalent cations Cd+2 and Zn+2 (depicted in the lower panel in the middle). The binding of BCNU to MT2 is faster at pH 7.4 than at pH 7.0, (B) BCNU also decomposes twice as fast at pH 7.4 than at pH 7.0.
Figure 3.
The lack of effect of MT2 to BCNU decomposition at physiological pH.
Discussion
Metallothioneins (MTs) are present in a variety of central nervous system tissues since these cysteine-rich, low molecular weight proteins are involved in various neuroprotective, regenerative, and cognitive functions [reviewed in (West et al., 2008)]. Among brain tumors (such as gliomas and meningeal tumors), the high expression levels of these proteins have been confirmed immunohistochemically (Maier et al., 1997). The regulation of expression of these proteins (the MT1 and MT2 isoforms in particular) often involves the promoter elements MRE (metal response element) and ARE (antioxidant response element) (ARE), when responding to stimuli by heavy metals and oxidative stress respectively (Haq et al., 2003). The regulatory mechanism which explains how chemotherapeutic alkylating agents induce MT expressions is not yet well understood, but some evidence suggests that it may involve ARE (Desjardins et al., 1998). Nonetheless, it is safe to assume that lengthy exposure to BCNU eventually led to the elevated expression levels of the MT1 and MT2 isoforms in our BCNU-resistant medulloblastoma subline (D341 MED (OBR)) (Bacolod et al., 2002). Interestingly, the D341 MED (OBR) cell line exhibited the upregulation of only the MT1 and MT2 isoforms, while the known brain-specific isoform (MT3), and the other minor isoform (MT4) did not register any change in expression level relative to the parental line. The regulation mechanisms of these two isoforms are yet to be described in literature.
Whether these MTs are expressed transiently (as a response to stimuli), or due to changes in the target cell genome (gene amplification, changes in promoter methylation) (Haq et al., 2003), the elevated levels of these proteins should be a concern in the clinics since it is well documented in literature that MTs can indeed decrease the activities of various alkylating agents. Indeed, there were numerous studies correlating elevated levels of MTs in biopsies to poor prognosis in different types of cancer (Chun et al., 2004; Surowiak et al., 2005; Weinlich et al., 2007; Wulfing et al., 2007). Those include studies which clearly indicated that the patients received chemotherapy regimens which included Pt-based drugs (Surowiak et al., 2005; Wulfing et al., 2007).
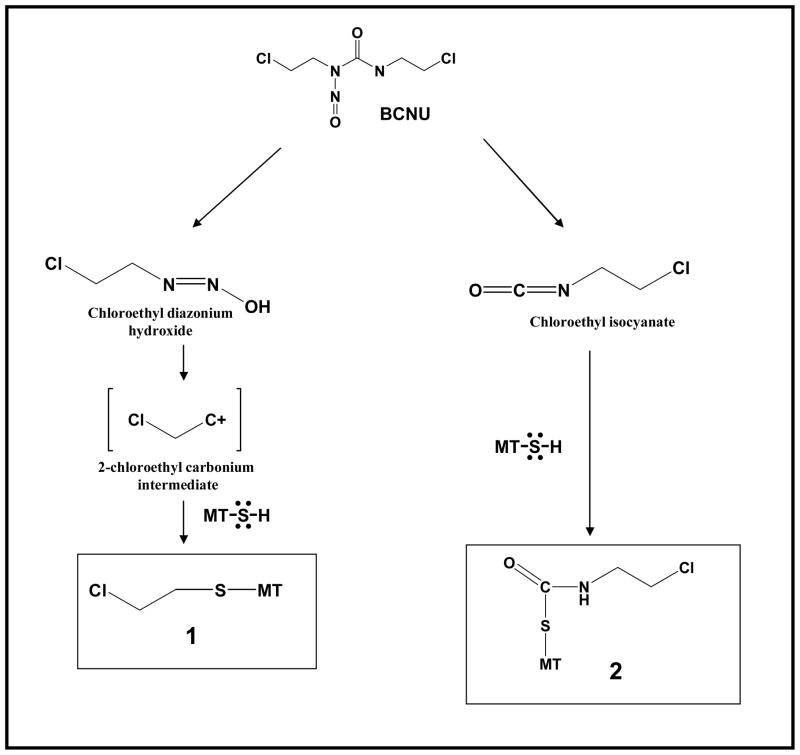
To our knowledge, our report ((Bacolod et al., 2002) and the subsequent evidence offered by this current manuscript) was the first to link elevated MT expression and BCNU resistance in a cancer cell line. We also demonstrated that the rate of binding of MT to BCNU correlated to BCNU decomposition. This suggests that it is the BCNU decomposition product which binds to MT. However, for now (without any supporting structural data) we cannot specifically say which BCNU decomposition product is being sequestered by MT. What we know is that in aqueous solution and physiological pH, BCNU quickly decomposes to chloroethyl isocyanate (CEIC) and chloroethyl carbonium intermediate (Lemoine et al., 1991). In theory, both of these decomposition products have electrophilic carbons capable of reacting to sulfhydryl groups in MTs (See Figure 4). Although, the carbonium intermediate is widely considered as the primary genotoxic (cancer-killing) by-product (since it is the precursor to the genotoxic DNA interstrand crosslink formation) (Tong et al., 1981), various studies have also shown that carbamoylation can also contribute to the overall BCNU cytotoxicity partly explained by its role in inhibiting DNA repair activity (Fornace et al., 1978; Kann et al., 1980). One possible model we can refer to is what we know about glutathiones (GSH). The elevated levels of GHS have been shown to contribute to BCNU resistance in brain tumors (Ali-Osman, 1989; Ali-Osman et al., 1989; Ali-Osman et al., 1990). Initially, it was hypothesized that the chloroethyl carbonium intermediate gets sequestered by GSH. However, subsequent studies have shown that it is mainly the CEIC decomposition product that forms covalent bond with GSH through CEIC’s carbamoylation of the GSH sulfhydryl group (Davis et al., 1993; Stahl et al., 1992). A decapeptide with cysteine in the middle, primarily formed an S-carbamoylation adduct (at cysteine) when reacted with BCNU (Carbone et al., 1997). Thus, there is a possibility that MT contributes to BCNU resistance mainly by inhibiting CEIC.
Figure 4.
Two possible ways a BCNU decomposition product may be sequestered by MT through its numerous thiol groups: thiol capture of the carbonium intermediate to form the chloroethyl adduct (1), or chloroethylisocyanate’s carbamoylation of MT’s thiol group.
We can assume that MT sequestration of BCNU (or its decomposition product) occurs in the cytoplasm (in brain tumors, MTs are located mostly in cytoplasm, but can also be found in nucleus (Maier et al., 1997)). BCNU, on the other hand, can cross a cancer cell’s plasma membrane as an intact molecule (by passive diffusion), although evidence suggests that CEIC can also cross the cell membrane (after its decomposition in the bloodstream) (Begleiter et al., 1977). Once in the cytoplasm, the drug can start to spontaneously decompose into CEIC and the chloroethyl carbonium intermediate, before these reactive species reach the nucleus.
There is an obvious need for further biological investigations to further verify that MTs can contribute to BCNU resistance in medulloblastomas, as well as structural studies that would answer our questions on how exactly MTs sequester BCNU molecules. Overall, the results from our study, as well those by other groups, point to the importance of MTs in resistance against alkylating drugs such as BCNU. If these results can be translated clinically, this suggests that MTs should be a factor to consider in medulloblastoma (or other brain tumor) chemotherapy that is supposed to include BCNU in the regimen. In fact, one earlier study has actually shown that inhibition of MT (by propargylglycine) can help increase the activity of an anti-cancer cocktail which included the alkylating drug melphalan (Satoh et al., 1994).
Contributor Information
Manny D. Bacolod, Department of Surgery, Duke University Medical Center, Durham, NC 27710.
Randy Fedrau, Department of Medicine, Duke University Medical Center, Durham, NC 27710.
Stewart P. Johnson, Department of Surgery, Duke University Medical Center, Durham, NC 27710
Nancy S. Bullock, Department of Surgery, Duke University Medical Center, Durham, NC 27710
Darell D. Bigner, Department of Pathology, Duke University Medical Center, Durham, NC 27710
Michael Colvin, Department of Medicine, Duke University Medical Center, Durham, NC 27710.
Henry S. Friedman, Department of Surgery, Duke University Medical Center, Durham, NC 27710
References
- Ali-Osman F. Quenching of DNA cross-link precursors of chloroethylnitrosoureas and attenuation of DNA interstrand cross-linking by glutathione. Cancer Res. 1989;49:5258–61. [PubMed] [Google Scholar]
- Ali-Osman F, Caughlan J, Gray GS. Decreased DNA interstrand cross-linking and cytotoxicity induced in human brain tumor cells by 1,3-bis(2-chloroethyl)-1-nitrosourea after in vitro reaction with glutathione. Cancer Res. 1989;49:5954–8. [PubMed] [Google Scholar]
- Ali-Osman F, Stein DE, Renwick A. Glutathione content and glutathione-S-transferase expression in 1,3-bis(2-chloroethyl)-1-nitrosourea-resistant human malignant astrocytoma cell lines. Cancer Res. 1990;50:6976–80. [PubMed] [Google Scholar]
- Antoine M, Fabris D, Fenselau C. Covalent sequestration of the nitrogen mustard mechlorethamine by metallothionein. Drug Metab Dispos. 1998;26:921–6. [PubMed] [Google Scholar]
- Bacolod MD, Johnson SP, Ali-Osman F, Modrich P, Bullock NS, Colvin OM, Bigner DD, Friedman HS. Mechanisms of resistance to 1,3-bis(2-chloroethyl)-1-nitrosourea in human medulloblastoma and rhabdomyosarcoma. Mol Cancer Ther. 2002;1:727–36. [PubMed] [Google Scholar]
- Begleiter A, Lam HP, Goldenberg GJ. Mechanism of uptake of nitrosoureas by L5178Y lymphoblasts in vitro. Cancer Res. 1977;37:1022–7. [PubMed] [Google Scholar]
- Berhane K, Hao XY, Egyhazi S, Hansson J, Ringborg U, Mannervik B. Contribution of glutathione transferase M3-3 to 1,3-bis(2-chloroethyl)-1-nitrosourea resistance in a human non-small cell lung cancer cell line. Cancer Res. 1993;53:4257–61. [PubMed] [Google Scholar]
- Carbone V, Salzano A, Pucci P, Fiume I, Pocsfalvi G, Sannolo N, Di Landa G, Malorni A. In vitro reactivity of the antineoplastic drug carmustin and acrolein with model peptides. J Pept Res. 1997;49:586–95. doi: 10.1111/j.1399-3011.1997.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Chun JH, Kim HK, Kim E, Kim IH, Kim JH, Chang HJ, Choi IJ, Lim HS, Kim IJ, Kang HC, Park JH, Bae JM, Park JG. Increased expression of metallothionein is associated with irinotecan resistance in gastric cancer. Cancer Res. 2004;64:4703–6. doi: 10.1158/0008-5472.CAN-04-1063. [DOI] [PubMed] [Google Scholar]
- Davis MR, Kassahun K, Jochheim CM, Brandt KM, Baillie TA. Glutathione and N-acetylcysteine conjugates of 2-chloroethyl isocyanate. Identification as metabolites of N,N′-bis(2-chloroethyl)-N-nitrosourea in the rat and inhibitory properties toward glutathione reductase in vitro. Chem Res Toxicol. 1993;6:376–83. doi: 10.1021/tx00033a020. [DOI] [PubMed] [Google Scholar]
- Desjardins JP, Beard SE, Mapoles JE, Gee P, Thompson JA. Transcriptional activity of quinone methides derived from the tumor promoter butylated hydroxytoluene in HepG2 cells. Cancer Lett. 1998;131:201–7. doi: 10.1016/s0304-3835(98)00153-0. [DOI] [PubMed] [Google Scholar]
- Doz F, Roosen N, Rosenblum ML. Metallothionein and anticancer agents: the role of metallothionein in cancer chemotherapy. J Neurooncol. 1993;17:123–9. doi: 10.1007/BF01050214. [DOI] [PubMed] [Google Scholar]
- Endresen L, Bakka A, Rugstad HE. Increased resistance to chlorambucil in cultured cells with a high concentration of cytoplasmic metallothionein. Cancer Res. 1983;43:2918–26. [PubMed] [Google Scholar]
- Fornace AJ, Jr, Kohn KW, Kann HE., Jr Inhibition of the ligase step of excision repair by 2-chloroethyl isocyanate, a decomposition product of 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1978;38:1064–9. [PubMed] [Google Scholar]
- Haq F, Mahoney M, Koropatnick J. Signaling events for metallothionein induction. Mutat Res. 2003;533:211–26. doi: 10.1016/j.mrfmmm.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Kalifa C, Valteau D, Pizer B, Vassal G, Grill J, Hartmann O. High-dose chemotherapy in childhood brain tumours. Childs Nerv Syst. 1999;15:498–505. doi: 10.1007/s003810050538. [DOI] [PubMed] [Google Scholar]
- Kann HE, Jr, Schott MA, Petkas A. Effects of structure and chemical activity on the ability of nitrosoureas to inhibit DNA repair. Cancer Res. 1980;40:50–5. [PubMed] [Google Scholar]
- Kelley SL, Basu A, Teicher BA, Hacker MP, Hamer DH, Lazo JS. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988;241:1813–5. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Krull IS, Strauss J, Hochberg F, Zervas NT. An improved trace analysis for N-nitrosoureas from biological media. J Anal Toxicol. 1981;5:42–6. doi: 10.1093/jat/5.1.42. [DOI] [PubMed] [Google Scholar]
- Laib JE, Shaw CF, 3rd, Petering DH, Eidsness MK, Elder RC, Garvey JS. Formation and characterization of aurothioneins: Au,Zn,Cd-thionein, Au,Cd-thionein, and (thiomalato-Au)chi-thionein. Biochemistry. 1985;24:1977–86. doi: 10.1021/bi00329a027. [DOI] [PubMed] [Google Scholar]
- Lemoine A, Lucas C, Ings RM. Metabolism of the chloroethylnitrosoureas. Xenobiotica. 1991;21:775–91. doi: 10.3109/00498259109039517. [DOI] [PubMed] [Google Scholar]
- Loo TL, Dion RL, Dixon RL, Rall DP. The Antitumor Agent, 1,3-Bis(2-chloroethyl)-1-nitrosourea. J Pharm Sci. 1966;55:492–497. [Google Scholar]
- Maier H, Jones C, Jasani B, Ofner D, Zelger B, Schmid KW, Budka H. Metallothionein overexpression in human brain tumours. Acta Neuropathol. 1997;94:599–604. doi: 10.1007/s004010050755. [DOI] [PubMed] [Google Scholar]
- Pegg AE. Properties of mammalian O6-alkylguanine-DNA transferases. Mutat Res. 1990;233:165–75. doi: 10.1016/0027-5107(90)90160-6. [DOI] [PubMed] [Google Scholar]
- Satoh M, Cherian MG, Imura N, Shimizu H. Modulation of resistance to anticancer drugs by inhibition of metallothionein synthesis. Cancer Res. 1994;54:5255–7. [PubMed] [Google Scholar]
- Soffietti R, Ruda R, Trevisan E. New chemotherapy options for the treatment of malignant gliomas. Anticancer Drugs. 2007;18:621–32. doi: 10.1097/CAD.0b013e32801476fd. [DOI] [PubMed] [Google Scholar]
- Stahl W, Lenhardt S, Przybylski M, Eisenbrand G. Mechanism of glutathione-mediated DNA damage by the antineoplastic agent 1,3-bis(2-chloroethyl)-N-nitrosourea. Chem Res Toxicol. 1992;5:106–9. doi: 10.1021/tx00025a018. [DOI] [PubMed] [Google Scholar]
- Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M, Lage H, Zabel M. Augmented expression of metallothionein and glutathione S-transferase pi as unfavourable prognostic factors in cisplatin-treated ovarian cancer patients. Virchows Arch. 2005;447:626–33. doi: 10.1007/s00428-005-1228-0. [DOI] [PubMed] [Google Scholar]
- Tong WP, Kirk MC, Ludlum DB. Molecular pharmacology of the haloethyl nitrosoureas: formation of 6-hydroxyethylguanine in DNA treated with BCNU (N,N1-bis[2-chloroethyl]-N-nitrosourea) Biochem Biophys Res Commun. 1981;100:351–7. doi: 10.1016/s0006-291x(81)80103-9. [DOI] [PubMed] [Google Scholar]
- Tong WP, Kirk MC, Ludlum DB. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N′-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1982;42:3102–5. [PubMed] [Google Scholar]
- Wei D, Fabris D, Fenselau C. Covalent sequestration of phosphoramide mustard by metallothionein--an in vitro study. Drug Metab Dispos. 1999;27:786–91. [PubMed] [Google Scholar]
- Weinlich G, Topar G, Eisendle K, Fritsch PO, Zelger B. Comparison of metallothionein-overexpression with sentinel lymph node biopsy as prognostic factors in melanoma. J Eur Acad Dermatol Venereol. 2007;21:669–77. doi: 10.1111/j.1468-3083.2006.02051.x. [DOI] [PubMed] [Google Scholar]
- West AK, Hidalgo J, Eddins D, Levin ED, Aschner M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology. 2008 doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo ES, Dellapiazza D, Wang AS, Lazo JS. Energy-dependent nuclear binding dictates metallothionein localization. J Cell Physiol. 2000;182:69–76. doi: 10.1002/(SICI)1097-4652(200001)182:1<69::AID-JCP8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wulfing C, van Ahlen H, Eltze E, Piechota H, Hertle L, Schmid KW. Metallothionein in bladder cancer: correlation of overexpression with poor outcome after chemotherapy. World J Urol. 2007;25:199–205. doi: 10.1007/s00345-006-0141-8. [DOI] [PubMed] [Google Scholar]
- Yu X, Wu Z, Fenselau C. Covalent sequestration of melphalan by metallothionein and selective alkylation of cysteines. Biochemistry. 1995;34:3377–85. doi: 10.1021/bi00010a029. [DOI] [PubMed] [Google Scholar]
- Zaia J, Jiang L, Han MS, Tabb JR, Wu Z, Fabris D, Fenselau C. A binding site for chlorambucil on metallothionein. Biochemistry. 1996;35:2830–5. doi: 10.1021/bi952243n. [DOI] [PubMed] [Google Scholar]