Abstract
Cold pre-conditioning reduces subsequent brain injury in small animals but the underlying mechanisms remain undefined. As hypothermia triggers systemic macrophage tumor necrosis factor alpha (TNF-α) production and other neural pre-conditioning stimuli depend on this cytokine, we reasoned that microglia and TNF-α would be similarly involved with cold preconditioning neuroprotection. Also, as slice cultures closely approximate their in vivo counterpart and include quiescent microglia, we used rat hippocampal slice cultures to confirm this hypothesis. Furthermore, inflammatory cytokine gene screening with subsequent PCR and immunostaining confirmation of targeted mRNA and related protein changes showed that cold pre-conditioning triggered a significant rise in TNF-α that localized to microglia and a significant rise in interleukin (IL)-11 that localized mainly to hippocampal pyramidal neurons and, more rarely, astrocytes. Importantly, co-stimulation with cold and IL-11, an anti-inflammatory cytokine that inhibits TNF-α expression, abrogated the otherwise evident protection. Instead, cold pre-conditioning coupled with blockade of IL-11 signaling further enhanced neuroprotection from that seen with cold pre-conditioning alone. Thus, physiological activation of brain pro-inflammatory cytokine signaling, and its amplification by inhibition of coincident anti-inflammatory cytokine signaling, may be opportune targets for the development of novel therapeutics that can mimic the protection seen in cold pre-conditioning.
Keywords: hippocampus, hormesis, innate immunity, interleukin-1β, microglia, slice culture
Cold pre-conditioning effectively reduces brain injury in experimental animals (Nishio et al. 2000; Yunoki et al. 2002). However, no clinical pre-conditioning treatment strategies based on hypothermia have been developed to reduce neurological complications associated with general anesthesia and related surgical procedures (Moller et al. 1998; Bendszus and Stoll 2006; McKhann et al. 2009). This void likely results from the inherent difficulties in administering this form of therapy, which to date has only been applied after the onset of brain disease (for review see Schaller and Graf 2003; Tang and Yenari 2010). In addition, the underlying mechanisms of cold pre-conditioning are unknown. This precludes the development of effective cold pre-conditioning mimetics, although evidence suggests involvement of cytokines.
Fairchild et al. (2000) show that in vitro exposure of monocytes pre-activated by lipopolysaccharide to hypothermia triggers enhanced production of tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β. Microglia, although perhaps not solely derived from monocytes (Simard and Rivest 2004; Chan et al. 2007), are a similar predominant source of cytokines (Hanisch 2002) including TNF-α in uninjured brain (Hulse et al. 2008). Furthermore, microglia are activated by synaptic activity (Ziv et al. 2006), which may act as an adequate pre-activating stimulus for TNF-α production (Kraig et al. 2010) necessary for cold preconditioning to be effective. Second, a wide array of preconditioning stimuli evoke subsequent neuroprotection via mechanisms involving TNF-α and microglia (for review see Hallenbeck 2002; Kraig et al. 2010).
Accordingly, we examined brain cytokine signaling in cold pre-conditioning using hippocampal slice cultures from rats. Slice cultures are ideally suited to this purpose because, while deafferented, they are a mature and functionally intact area of brain that remains viable and stable for weeks in vitro. Importantly, slice culture longevity allows microglia time to become quiescent after 10 days in culture, making the preparation ideal for study of neural immune signaling in vitro, where environmental conditions can be accurately controlled (Ransohoff and Perry 2008). Our results confirmed that cold pre-conditioning neuroprotection involved increased expression of TNF-α from microglia. Considerable evidence points to the involvement of TNF-α in an array of pre-conditioning paradigms. However, IL-11 inhibits TNF-α production. Accordingly, we also focused to the potential involvement of IL-11 in cold pre-conditioning. Our results provide the first evidence that removal of an anti-inflammatory cytokine, namely IL-11, enhances cold pre-conditioning protection. This work has appeared in preliminary form (Kraig et al. 2008; Mitchell et al. 2009).
Materials and methods
Culture preparation and maintenance
We prepared slice cultures and initially maintained them in media containing 23% horse serum (#26050–088; Invitrogen, Carlsbad, CA, USA; Kunkler and Kraig 1997) with transfer to serum-free media after 7 days in vitro and experimental use between 18 and 24 day in vitro. Cultures maintained in serum-free media showed ~90% vitality (Appendix S1).
Experimental manipulations
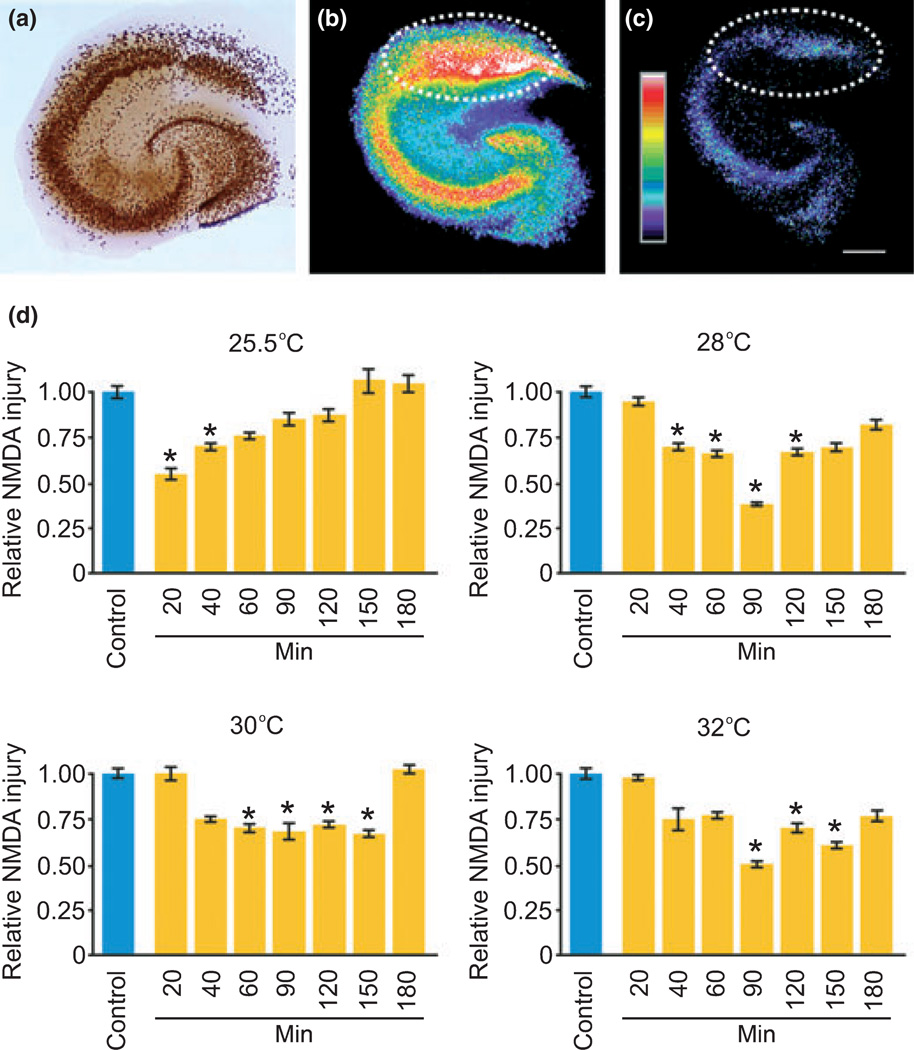
We administered cold pre-conditioning at several temperatures and over various time periods to establish dose–response patterns. Sixwell trays containing serum-free media were allowed to equilibrate to hypothermic temperatures (25.5, 28, 30 and 32°C) for at least 20 min prior to cold pre-conditioning at these temperatures in an incubator (5% CO2 balance air). Slice cultures were transferred from normal incubation conditions to cold pre-conditioning trays for 20, 40, 60, 90, 120, 150, or 180 min. Cultures were then transferred back to media equilibrated at normal incubation conditions for 24 h before excitotoxic injury (described below). With the neuroprotection response pattern established (Fig. 1), we performed all other cold preconditioning at 30°C for 90 min because this temperature showed the broadest effective range and 90 min was about mid-range.
Fig. 1.
Cold pre-conditioning was neuroprotective and followed a U-shaped temperature and time dose–response pattern. (a) Immunostaining for NeuN shows the typical principal neuron cytoarchitecture of a hippocampal slice culture with the CA1 pyramidal neuron area at the top, CA3 area to the left, and dentate gyrus to the lower right. (b–d) We used the fluorescent dead-cell marker Sytox to measure excitotoxic injury from NMDA exposure in the CA1 area (dotted white line area of interest) as a level of injury made relative to control injury cultures. Here, we show an exemplary image of control injury (b) and a significantly reduced level after cold pre-conditioning (25.5°C for 20 min) (c). Scale bar, 250 µm. (d) Cold pre-conditioning produced significant neuroprotective effects that followed a U-shaped or hormetic dose–response pattern over a range of temperatures and durations. For example, cold pre-conditioning at 25.5°C evoked significant (p = 0.002) neuroprotection from 20 (n = 8) and 40 (n = 39) min exposures, but not from 60 (n = 7), 90 (n = 8), 120 (n = 4), 150 (n = 7), or 180 (n = 6) min exposure times versus control (n = 45). Cold pre-conditioning at 28°C evoked significant (p < 0.001) protection from 40 (n = 12), 60 (n = 13), 90 (n = 8), and 120 (n = 17) min exposures, but not from 20 (n = 7), 150 (n = 8), or 180 (n = 7) min exposure times versus control (n = 41). Cold pre-conditioning at 30°C evoked significant (p = 0.01 at 120 min, all other times p < 0.001) protection from 60 (n = 26), 90 (n = 13), 120 (n = 8), and 150 (n = 14) min exposures, but not from 20 (n = 6), 40 (n = 7) or 180 (n = 8) min exposure times versus control (n = 40). Cold pre-conditioning at 32°C evoked significant (p = 0.005 at 120 min, all other times p < 0.001) protection from 90 (n = 17), 120 (n = 8), and 150 (n = 7) min exposures, but not from 20 (n = 8), 40 (n = 8), 60 (n = 8), or 180 (n = 6) min exposure times versus control (n = 15).
Soluble TNF receptor 1 (sTNFR1, 200 ng/mL, #425-R1-050; R&D Systems, Minneapolis, MN, USA) was included during and after cold pre-conditioning to abrogate TNF-α signaling. Slice cultures were initially exposed to sTNFR1 20 min prior to cold preconditioning and continuously exposed to sTNFR1 up until excitotoxic injury.
Slice cultures were exposed to 100 ng/mL recombinant mouse IL-11 (#418-ML; R&D Systems) in serum-free media. To examine effects of cold pre-conditioning with blockade of IL-11, slice cultures were exposed to 100 µg/mL anti-mouse IL-11 neutralizing antibody (#MAB418; R&D Systems). Use of an isotype-specific IgG2A (100 µg/mL) antibody (#02-6200; Invitrogen) served as a sham control. All pharmacological treatments were applied 20 min prior to cold pre-conditioning, and were maintained up until administration of excitotoxic injury.
Excitotoxic injury and quantification
Twenty-four hours after cold pre-conditioning, slice cultures were exposed to NMDA (#454575; Calbiochem, San Diego, CA, USA) and assessment of resultant CA1 pyramidal neuron area injury were performed as previously described (Hulse et al. 2008) with modifications.
We used NMDA-mediated excitotoxicity to model CA1 areaselective neuronal vulnerability, including that seen from ischemia. Our previous work shows that NMDA-induced injury (20–50 µmol/ L for 60 min and normal incubation conditions) produces CA1 area neuronal loss analogous to that seen from oxygen glucose deprivation (Hulse et al. 2008).
Injury severity was registered as a level of injury minus background (i.e. the pre-screen image) via an area of interest selected around the CA1 pyramidal cell layer area for each slice culture. This practice was in contrast to previous work (Hulse et al. 2008) where CA1 area injury was measured as a ratio of injury compared to maximal injury (i.e. created by high doses of or lengthy exposure to NMDA with background subtracted from each image). We often could not maximally injure cultures so that those preconditioned by cold had maximal levels of injury comparable to control cultures. This lapse could obscure otherwise present protective effects. For example, if control injury level was 5 and maximal control injury 10, the injury ratio would be 50%. If cold preconditioned injury was 3 and associated maximal injury 6, a ratio of 50% would again be evident in spite of a 40% protection (i.e. control level of 5 vs. cold-treatment level of 3) (Mitchell et al. 2010).
TNF-α assay
We analyzed slice cultures for TNF-α protein content as previously described using a microsphere-based flow cytometric immunoassay (Kunkler et al. 2004). Total protein was determined using a BCA protein assay kit (#23235; Pierce, Rockford, IL, USA).
Gene expression studies
Techniques for RNA isolation, quantitative PCR, cloning of cDNA for IL-11, and semi-quantitative quantitative PCR array screening to probe for low-level inflammatory mediator expression changes using the RT2 Profiler PCR Array (#PARN-011A) from SABio-sciences (Frederick, MD, USA) followed standard procedures detailed in Appendix S1 and Mitchell et al. (2010).
Immunohistochemistry
Slice cultures were fixed and processed for immunostaining as previously described (Kunkler et al. 2005) either as whole or 20 µm sections (Mitchell et al. 2010) with details described in Appendix S1.
mRNA in situ hybridization
Procedures for cellular localization of TNF-α and IL-11 mRNA are detailed in Appendix S1.
Statistical methods and figure preparation
Data were analyzed using sigmastat (v. 3.5) software (Systat Software, Chicago, IL, USA). All data were subject to normality testing (p-value to reject: 0.05) and equal variance testing (p-value to reject: 0.05). All control values per experiment were standardized to 1.00 with related experimental values adjusted proportionally to allow for interexperimental analyses. anova using Holm-Sidak post hoc testing was performed for multiple comparisons or t-test for comparisons. Significance was defined as p < 0.05.
Images were created using Coreldraw (v. X3) Photoshop (9.0.2; Adobe, San Jose, CA, USA). Confocal images were acquired using a Leica TCS SP2 AOBS laser scanning confocal microscope (University of Chicago Integrated Light Microscopy Core Facility).
Results
Characteristics of cold pre-conditioning neuroprotection
Cold pre-conditioning provided significant neuroprotection against excitotoxic injury (Fig. 1a–c). This neuroprotection followed a U-shaped response pattern for temperature and time (Fig. 1d). Other pre-conditioning stimuli involve TNF-α, a pro-inflammatory cytokine (Hallenbeck 2002; Kraig et al. 2010), and hypothermia triggers prolonged release of TNF-α from macrophages (Fairchild et al. 2000). As microglia, resident macrophages of brain, are the principal physiological source of neural TNF-α, we reasoned that TNF-α may play a similar role in cold pre-conditioning in brain. Indeed, slice cultures exposed to 30°C for 90 min (n = 4) and then harvested 24 h later showed significantly (p = 0.004) increased expression of TNF-α compared with control (n = 7).
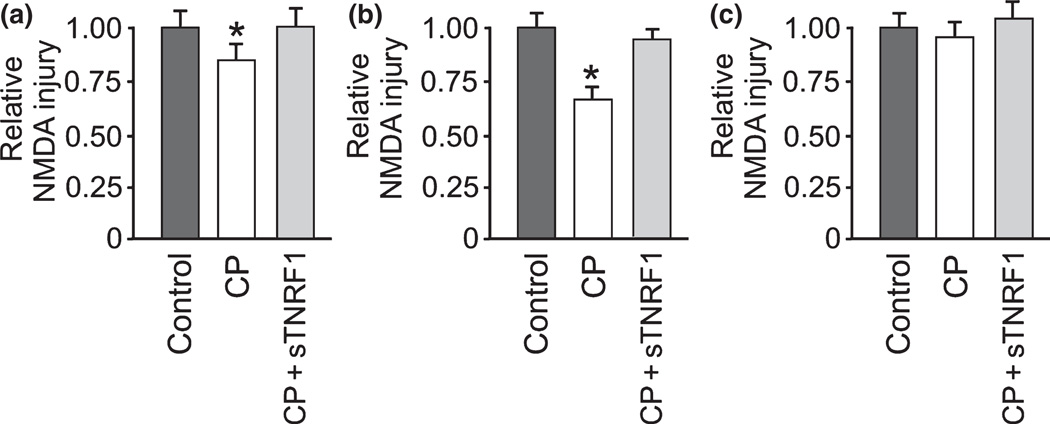
We next explored whether cold pre-conditioning neuroprotection depended on TNF-α. Inclusion of sTNFR1 20 min prior to cold pre-conditioning and continuously thereafter abrogated TNF-α signaling, which removed the significant degree of protection seen in cold pre-conditioning alone (Fig. 2a).
Fig. 2.
Cold pre-conditioning neuroprotection depended on TNF-α and took time to develop. A, Soluble TNF-α receptor 1 (sTNFR1) blocked TNF-α signaling and abrogated the otherwise significant (p = 0.009) neuroprotection from cold pre-conditioning (CP). Cold pre-conditioning evoked neuroprotection at 30°C for 90 min, followed by NMDA-mediated CA1 excitotoxic injury 24 hours later (n = 7–9/group). (b) Cold pre-conditioning neuroprotection (n = 8) remained significantly (p = 0.003) greater than control conditions (n = 8) 3 days after exposure to 30°C for 90 min (n = 8). Inclusion of sTNFR1 (CP + sNTFR1; n = 8) removed this neuroprotection. (c) Finally, cold pre-conditioning at 30°C for 90 min with only 20 min of recovery before excitotoxic injury from NMDA (n = 8) was not protective compared to control (n = 8). Furthermore, inclusion of sTNFR1 (CP + sTNFR1) had no impact (n = 8) on injury levels.
Furthermore, we modified our experimental timeline to investigate the immediate and delayed effects of cold preconditioning. Delayed effects of cold pre-conditioning were examined by performing cold pre-conditioning as described above, but with results quantified using injury levels recorded 3 days after excitotoxic injury. This neuroprotection remained evident 3 days after pre-conditioning and again was completely removed by abrogation of TNF-α signaling by sTNFR1 (Fig. 2b). However, cold pre-conditioning did not produce a protective effect when excitotoxic injury occurred shortly after pre-conditioning (Fig. 2c). These results show that cold pre-conditioning neuroprotection followed a U-shaped dose–response pattern over time and temperature, involved TNF-α, and took time to develop.
PCR array evidence of inflammatory mediator changes from cold pre-conditioning
Although not all mRNAs are translated to proteins (White et al. 1992), cytokines are regulated at the transcriptional level and once transcribed to RNA are faithfully translated to protein (Oppenheim and Feldmann 2001). Therefore, targeted gene analyses via PCR arrays are an efficient means to probe for inflammatory mediator changes of cold preconditioning (Table 1). Furthermore, PCR arrays are recognized to be a highly sensitive, reproducible method for gene screening and were therefore used to begin defining cytokine signaling in cold pre-conditioning (Supporting Information).
Table 1.
Cold pre-conditioning regulates expression of inflammatory mediators
| 2−ΔCt | Fold up- or down-regulation | ||||
|---|---|---|---|---|---|
| Ref Seq | Description | Symbol | CP | Control | CP/Control |
| NM_012675 | Tumor necrosis factor (TNF superfamily, member 2) | Tnf | 1.5E−02 | 5.8E−03 | 2.57 |
| NM_031512 | Interleukin 1 beta | Il1b | 3.7E−03 | 2.4E−03 | 1.58 |
| NM_013123 | Interleukin 1 receptor, type I | Il1r1 | 7.5E−03 | 4.7E−03 | 1.58 |
| NM_019310 | Interleukin 8 receptor, alpha | Il8ra | 2.8E−03 | 4.4E−03 | −1.56 |
| NM_017183 | Interleukin 8 receptor, beta | Il8rb | 1.5E−03 | 7.3E−04 | 2.08 |
| NM_133519 | Interleukin 11 | Il11 | 4.6E−03 | 1.6E−03 | 2.95 |
| NM_012881 | Secreted phosphoprotein 1 | Spp1 | 1.4E−01 | 2.6E−01 | −1.92 |
| XM_213425 | Chemokine (C–C motif) ligand 12 | Ccl12 | 1.0E−01 | 5.4E−02 | 1.95 |
| NM_057151 | Chemokine (C–C motif) ligand 17 | Ccl17 | 1.2E−03 | 7.3E−04 | 1.58 |
| NM_031530 | Chemokine (C–C motif) ligand 2 | Ccl2 | 2.6E−02 | 1.2E−02 | 2.23 |
| NM_013025 | Chemokine (C–C motif) ligand 3 | Ccl3 | 9.1E−02 | 3.1E−02 | 2.95 |
| NM_053858 | Chemokine (C–C motif) ligand 4 | Ccl4 | 5.2E−02 | 1.4E−02 | 3.63 |
| NM_001004202 | Chemokine (C–C motif) ligand 6 | Ccl6 | 6.0E−02 | 3.5E−02 | 1.69 |
| NM_001007612 | Chemokine (C–C motif) ligand 7 | Ccl7 | 3.0E−02 | 1.5E−02 | 1.95 |
| NM_134455 | Chemokine (C–X–C motif) ligand 1 | Cxcl1 | 2.1E−02 | 1.3E−02 | 1.58 |
| NM_182952 | Chemokine (C–X–C motif) ligand 11 | Cxcl11 | 2.6E−03 | 7.7E−03 | −2.91 |
| NM_053647 | Chemokine (C–X–C motif) ligand 2 | Cxcl2 | 1.7E−03 | 1.1E−03 | 1.58 |
| NM_001007604 | Ribosomal protein, large, P1 | Rplp1 | 4.4E+00 | 3.9E+00 | 1.12 |
| NM_012583 | Hypoxanthine guanine phosphoribosyl transferase | Hprt | 1.4E−01 | 1.4E−01 | −1.03 |
| NM_173340 | Ribosomal protein L13A | Rpl13a | 3.2E−01 | 3.0E−01 | 1.04 |
| NM_017025 | Lactate dehydrogenase A | Ldha | 1.3E+00 | 1.3E+00 | −1.03 |
| NM_031144 | Actin, beta | Actb | 4.1E+00 | 4.5E+00 | −1.10 |
Semi-quantitative PCR array analysis from control versus cold pre-conditioning CA1 area of hippocampal slices as detailed in Materials and methods. Fold-up is colored green (i.e. ≥1.5) and fold-down is colored red (i.e. ≥ 1.5).
Cold pre-conditioning increased the expression of a number of chemokines and cytokines. Interleukin-8 receptor expression increased, a chemokine effect that could enhance leukocyte movement (Iizasa and Matsushima 2001). Moreover, the expression of several chemokines that also enhance leukocyte movement (Rollins 2001) was increased, whereas a single chemokine measured (i.e. ligand 11) was reduced. In addition, cold pre-conditioning altered the expression of cytokines. Cold pre-conditioning triggered PCR array evident changes of increased pro-inflammatory innate cytokine mRNA expression for TNF-α, IL-1β, and its receptor IL-1R1. The expression of secreted phosphoprotein 1 (i.e. osteopontin), a cytokine with both pro- and anti-inflammatory effects (Nau 2001), was decreased. However, we focused to IL-11, a cytokine whose expression was maximally (i.e. 2.95-fold) increased by cold pre-conditioning and is directly related to TNF-α homeostasis.
Abrogation of IL-11 signaling, and not IL-11, enhanced neuroprotection
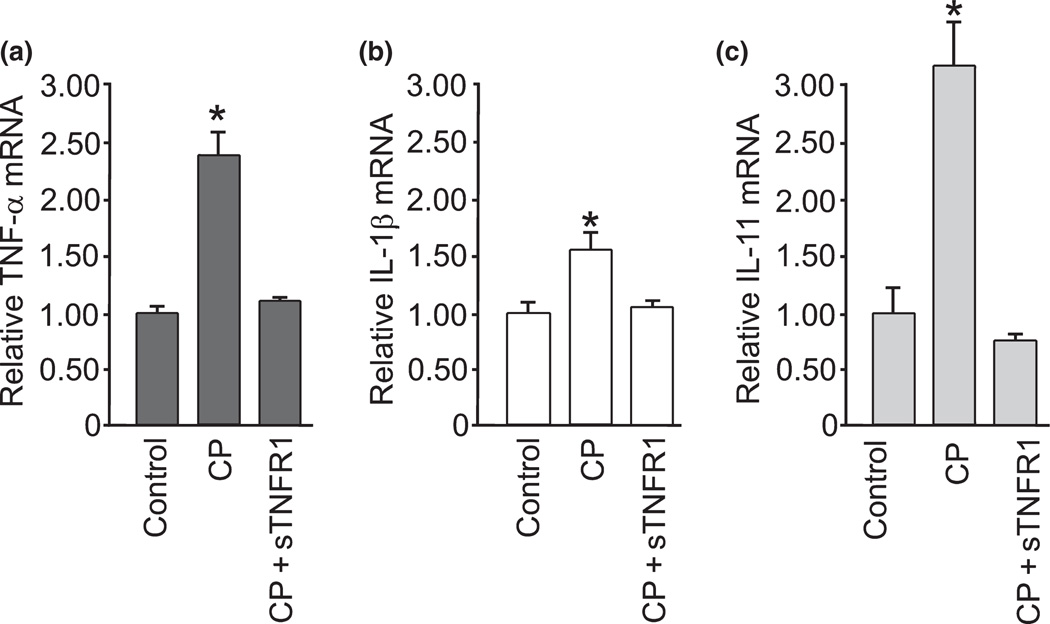
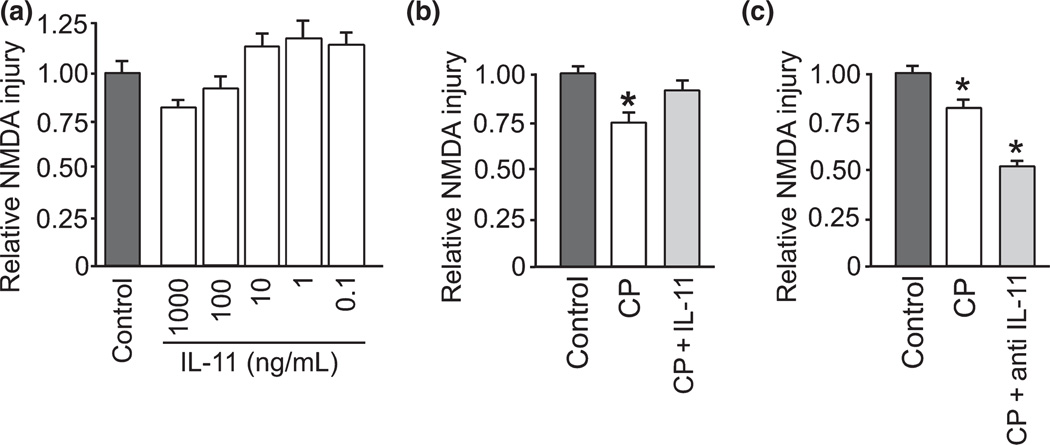
IL-11 is an anti-inflammatory mediator whose expression is stimulated by TNF-α through IL-1β, and IL-11 inhibits expression of TNF-α (Redlich et al. 1996; Trepicchio et al. 1996). We confirmed this signaling interrelation after cold pre-conditioning using real-time RT-PCR for TNF-α, IL-1β, and IL-11 mRNA. Consistent with the PCR array data, probing for specific amplification of these cytokines con- firmed that cold pre-conditioning triggered a significant increase in TNF-α, IL-1β and IL-11 mRNA compared with control conditions (Fig. 3). These PCR results further support the hypothesis that IL-11 may be involved with neuroprotection from cold pre-conditioning. However, when we applied IL-11 to slice cultures to mimic the rise from cold pre-conditioning, no significant protection was seen (Fig. 4a). Furthermore, treatment with IL-11 abrogated neuroprotection otherwise seen with cold pre-conditioning (Fig. 4b). Instead, blockade of IL-11 signaling, by use of a neutralizing antibody, more than doubled the protection from cold pre-conditioning (Fig. 4c).
Fig. 3.
Cytokine mRNA changes further suggest TNF-α involvement in cold pre-conditioning neuroprotective signaling. (a) Cold pre-conditioning (CP) at 30°C for 90 min with 2 h recovery triggered significantly (p < 0.001) increased relative TNF-α mRNA expression compared to control. Cold pre-conditioning plus blockade of TNF-α signaling by inclusion of sTNFR1 (CP + sTNFR1) returned relative TNF-α mRNA expression to a non-significant difference (n = 3/group). (b) Cold preconditioning at 30°C for 90 min with 2 h recovery also triggered a significant (p = 0.01) increase in relative IL-1β mRNA expression compared to control and cold pre-conditioning. In addition, blockade of TNF-α signaling (CP + sTNFR1) returned IL-1β mRNA expression to control levels (n = 3/group). (c) Finally, cold pre-conditioning at 30°C for 90 min with 2 h recovery triggered significantly (p = 0.002) increased relative expression of IL-11 mRNA compared to control whereas cold pre-conditioning with blockade of TNF-α signaling (CP + sTNFR1) triggered a non-significant change in relative IL-11 mRNA expression compared to control (n = 3 per group).
Fig. 4.
Interleukin-11 counteracted TNF-α involvement in cold preconditioning neuroprotection. (a) Pre-conditioning with IL-11, an anti-inflammatory cytokine that inhibits TNF-α, at 1000 (n = 4), 100 (n = 14), 10 (n = 16), 1 (n = 10), and 0.1 (n = 8) ng/mL for 24 h did not significantly protect against subsequent NMDA injury versus control (n = 16). (b) Furthermore, cold pre-conditioning (CP; 30°C for 90 min) with IL-11 (CP + IL-11; 100 ng/mL; n = 21) abrogated the otherwise significant (p = 0.002) neuroprotection from CP (n = 22) versus control (n = 24). (c) In contrast, cold pre-conditioning plus blockade of IL-11 signaling (CP + anti-IL-11; n = 17) by pre-treatment for 24 h with anti-IL-11 neutralizing antibody (100 µg/mL) significantly (p < 0.001) enhanced neuroprotection from cold pre-conditioning alone (n = 13) with both conditions providing significant (p < 0.001) protection over control (n = 25). Finally, the specificity of this IL-11 blocking effect was confirmed by comparison to the effect of cold pre-conditioning plus an isotype, but otherwise non-specific, antibody (CP + IgG2A; 100 µg/ mL; n = 9), which did not significantly (p = 0.401) enhance neuroprotection from cold pre-conditioning alone.
Cellular origin of cold pre-conditioning cytokine signaling variables
To further detail the cold pre-conditioning cytokine signaling, we turned to identifying the cellular sources for the key cytokines involved: TNF-α and IL-11. Treatment with TNFα, IL-1β, or cold pre-conditioning triggered significantly greater IL-11 immunoreactivity in cells with pyramidal neuron morphology (Fig. 5). We confirmed with double-label immunohistochemistry that this increased IL-11 expression was mainly localized to pyramidal neurons (Fig. 6a–f) and, less often, to astrocytes (Fig. 6g). Finally, we used in situ hybridization to further define the cellular origins of TNF-α and IL-11. Consistent with our prior work using laser dissection microscopy with slices examined under physiological conditions (Hulse et al. 2008), we show that TNF-α localized to microglia (Fig. 6h). Furthermore, IL-11 mRNA localized mainly to pyramidal neurons (Fig. 6i).
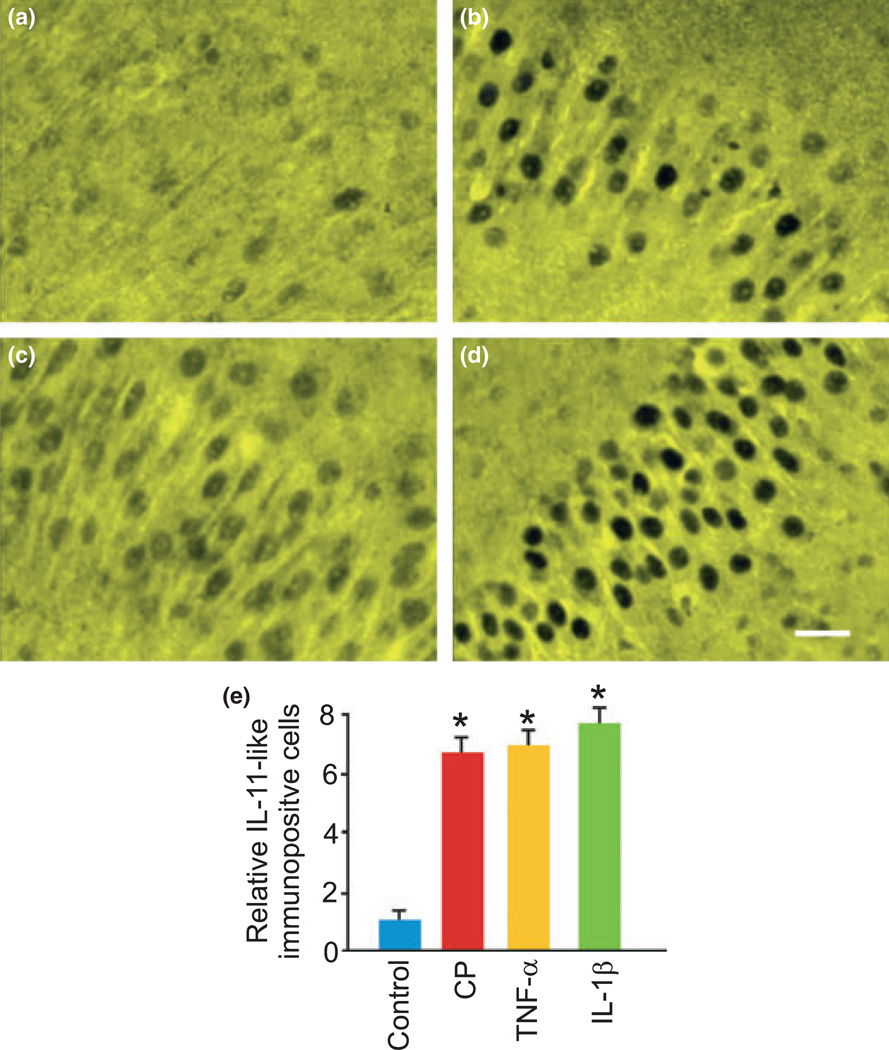
Fig. 5.
Cold pre-conditioning and related effector cytokines increased hippocampal pyramidal layer interleukin-11 immunoreactivity. (a–d) We observed the most IL-11 immunoreactivity at the pyramidal cell layer in all groups. Here, we show faint and sparsely distributed IL-11 immunostaining under control conditions (a) that markedly increased 24 h after cold pre-conditioning CP (30°C for 90 min) (b), TNF-α exposure (TNF-α; 100 ng/mL) (c), and IL-1β exposure (IL-1β; 100 ng/mL) (d). (e) CP, TNF-α, and IL-1β (n = 9 per group) significantly (p < 0.001) increased the number of IL-11- immunopositive cells at the pyramidal cell layer. Scale bar, 50 µm.
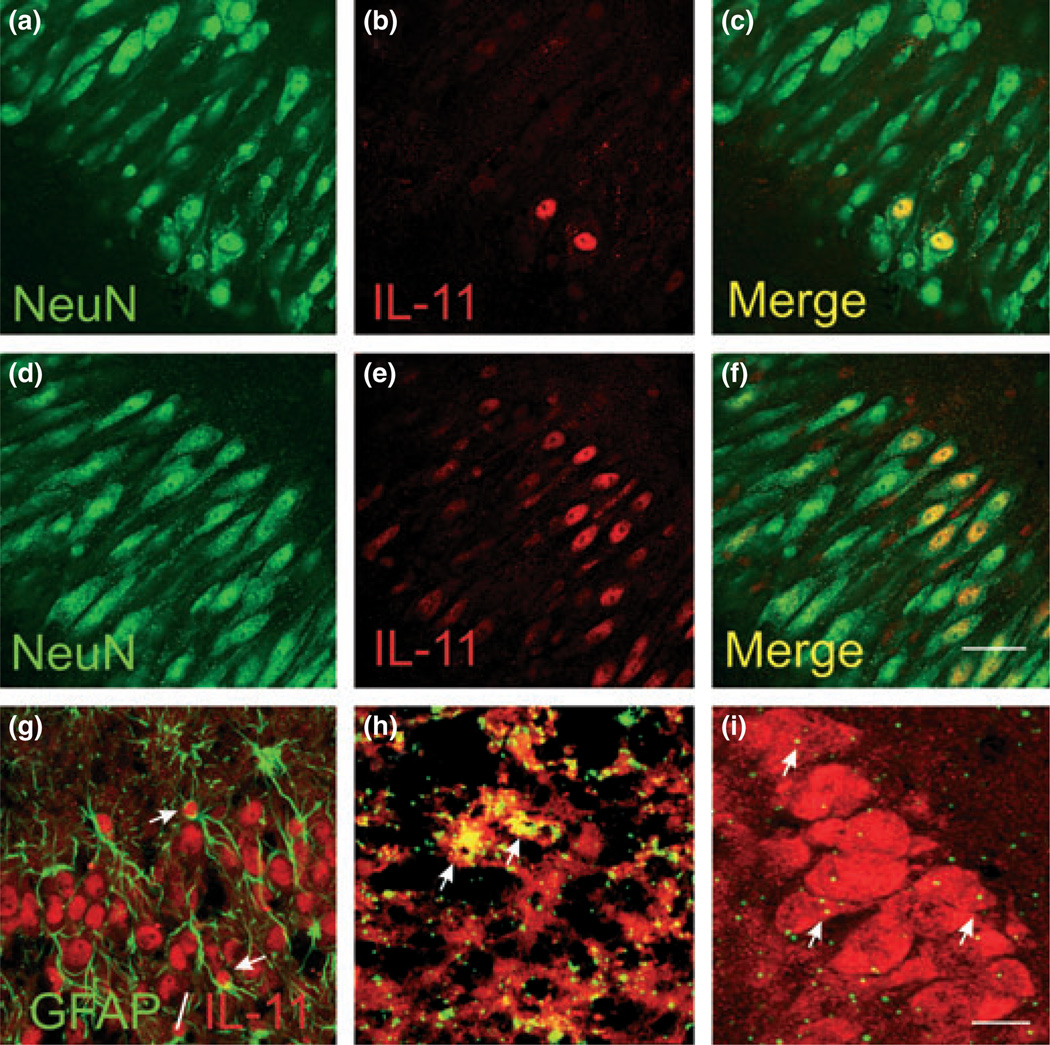
Fig. 6.
Cellular localization of cold pre-conditioning TNF-α and IL-11 changes. (a–c) Control hippocampal slice culture immunostaining with neuronal marker NeuN (a) followed by IL-11 immunostaining (b) showed that the predominant cell type positive for IL-11 immunoreactivity were pyramidal neurons (c). (d–f) Exposure to TNF-α (100 ng/ mL) for 24 h dramatically increased neuron-specific IL-11 immunostaining. Here, we show exemplary confocal photomicrographs (n = 5 per group). Scale bar, 50 µm. (g) Image shows double-labeling with glial fibrillary acidic protein (GFAP) and IL-11 immunostaining at the pyramidal cell layer from an exemplary hippocampal slice culture 24 h after cold pre-conditioning (30°C for 90 min). We observed IL-11-immunopositive astrocytes (arrows) in only a minority of photomicrographs (n = 5). (h) Image shows that mRNA for TNF-α (green) 3 h after cold pre-conditioning (90 min at 30°C) localized to microglia, immunostained with CD11b (red). The resultant exemplary (from n = 5) image shows green spots overlying red cells or the optical conversion of these spots to yellow when both in situ and immunostaining probes are blended at the same plane of focus (arrows). (i) Twelve hours after analogous cold pre-conditioning (n = 5), mRNA for IL-11 (green) localized mainly to pyramidal neurons immunostained with NeuN (red). Arrows point to exemplary in-focus in situ and immunostaining probe markings. Although sparse, these probe markings for IL-11 mRNA concentrate to the pyramidal neuron layer. (g–i) Scale bar, 25 µm (g and h) and 50 µm (i).
Discussion
This study showed that cold pre-conditioning evoked neuroprotection, which involved the pro-inflammatory cytokine TNF-α that emanated from microglia. Furthermore, the protection was dampened by coincident increased expression of IL-11, an anti-inflammatory cytokine predominantly localized to neurons, which if abrogated, significantly enhanced the protective effects of TNF-α.
Evidence points to the involvement of TNF-α in a wide range of stimuli capable of triggering brain pre-conditioning neuroprotection. When TNF-α is elevated to comparatively low levels over time by systemic stimuli applied to brain, this cytokine from the periphery serves as a pre-conditioning stimulus that initiates neuroprotection centrally (Hallenbeck 2002; Dirnagl et al. 2009). Similar protective effects of TNF-α occur after stimuli from within brain that trigger TNF-α production. Increased synaptic activity from environmental enrichment as well as treadmill activity alone (Ding et al. 2005) triggers pre-conditioning neuroprotection and increased brain production of TNF-α (Kraig et al. 2010). For our purposes here, neuronal activity was a priming signal for cold-induced microglial TNF-α production analogous to the lipopolysaccharide used by Fairchild et al. (2000), which, when coupled to reduced temperature exposure resulted in enhanced TNF-α expression from monocytes.
Cold pre-conditioning results here provide further support for the ability of brain itself to employ pro-inflammatory functional and structural changes to trigger adaptive nutritive changes. First, we showed that cold pre-conditioning depended on TNF-α. Second, the cold induced TNF-α change came from activated microglia. Third, the protective effects of cold pre-conditioning were not immediately evident and the resultant neuroprotection showed a U-shaped dose–response pattern. These latter characteristics are consistent with physiological conditioning hormesis (Calabrese et al. 2007), a dose–response pattern seen with all neuroprotective agents reported to date (Calabrese 2008). Importantly, hormesis involves two basic tenets – namely, an initiating irritative (i.e. pro-inflammatory) stimulus must be sufficient to evoke a response and that sufficient time must elapse for a nutritive effect to take place (for review see Kraig et al. 2010). Here, the initiating, irritative stimulus that ultimately provides protection involves the pro-inflammatory cytokine TNF-α. Furthermore, our data show for the first time that reduced anti-inflammatory effects, that is, from IL-11, add to the initiating irritative impact of pro-inflammatory changes needed for adaptive neuroprotection from cold preconditioning.
Anti-inflammatory effects play an important role in the mechanisms by which hypothermia, after the onset of disease, is protective (Schaller and Graf 2003; Tang and Yenari 2010). This likely helps explain helps why IL-11, an anti-inflammatory cytokine, is protective after the onset of disease. For example, treatment with IL-11 enhances survival and reduces TNF-α production after radiation-induced thoracic injury involving macrophages (Redlich et al. 1996). IL-11 also reduces oxidant-mediated injury of endothelial cells (Waxman et al. 2003) and developing lung cells (Chetty et al. 2008). IL-11 reduces ischemia/reperfusion injury in heart, protecting cardiac myocytes against oxidative damage (Kimura et al. 2007). Although less studied in brain, Zhang et al. (2006) show that astrocytes in white matter from patients with multiple sclerosis express IL-11 and this expression supports oligodendrocyte vitality.
In contrast, the current results show that IL-11 has no protective effect before the onset of disease. IL-11 did not improve cold pre-conditioning neuroprotection against subsequent NMDA-mediated excitotoxic injury. Furthermore, IL-11 abrogated the protective effects of cold preconditioning neuroprotection, further supporting the notion that a sufficient, initiating pro-inflammatory stimulus (i.e. TNF-α) is needed for the protection. This suggestion was confirmed by blockade of IL-11 signaling, which significantly enhanced the protective effects of cold pre-conditioning. TNF-α stimulates production of IL-11 via IL-1β and IL-11, in return, inhibits TNF-α expression (Redlich et al. 1996; Trepicchio et al. 1996), effects which are coincident and consistent with cytokine network behavior. We suggest IL-11 serves to mitigate the impact of TNF-α rise. Thus, blocking IL-11 signaling enhances the physiological impact of TNF-α to trigger neuroprotection before the onset of disease.
Although previous work shows IL-11 is expressed in astrocytes within white matter, our work shows that neurons predominantly express IL-11 in gray matter. As noted, IL-11 is expressed in astrocytes from myelinated borders of active and silent multiple sclerosis lesions (Zhang et al. 2006) and in primary astrocytic cultures (Bsibsi et al. 2006). Our work confirms and extends these findings: we found that astrocytes could express IL-11, but neurons were the principal source of this cytokine in gray matter. This neuronal localization is consistent with in situ hybridization work that shows IL-11 mRNA is distributed in the granular layer of the dentate gyrus and the pyramidal cell layers of the hippocampus (Du et al. 1996).
Taken together, our results suggest that physiological activation of brain pro-inflammatory cytokine signaling, and its amplification by inhibition of coincident anti-inflammatory cytokine signaling, may be opportune targets for the development of novel therapeutics that can mimic the protection seen in cold pre-conditioning.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS-19108), the National Institute of Child Health and Human Disorders (5 PO1 HD 09402), the Migraine Research Foundation and the White Foundation. Ms Marcia P. Kraig assisted in the preparation and maintenance of culture systems. We thank Dr Christine Labno of the Integrated Microscopy Core Facility at the University of Chicago for assistance with all confocal imaging. We thank Yelena Y. Grinberg and Aya D. Pusic for reading and commenting on a final version of the manuscript.
Abbreviations used
- IL
interleukin
- TNF-α
tumor necrosis factor alpha
- sTNFR1
soluble TNF receptor 1
Footnotes
The authors have no conflicts or financial interest to disclose.
Supporting information
Additional Supporting information may be found in the online version of this article:
Appendix S1. Supplementary Materials and methods.
Table S1. Complete inflammatory gene expression array for response to cold pre-conditioning.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Bendszus M, Stoll G. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol. 2006;5:364–372. doi: 10.1016/S1474-4422(06)70412-4. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Drug therapies for stroke and traumatic brain injury often display U-shaped dose responses: occurrence, mechanisms, and clinical implications. Crit. Rev. Toxicol. 2008;38:557–577. doi: 10.1080/10408440802014287. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res. Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chetty A, Cao GJ, Manzo N, Nielsen HC, Waxman A. The role of IL-6 and IL-11 in hyperoxic injury in developing lung. Pediatr. Pulmonol. 2008;43:297–304. doi: 10.1002/ppul.20777. [DOI] [PubMed] [Google Scholar]
- Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, McAllister JP, II, Ding Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Everett ET, Wang G, Lee WH, Yang Z, Williams DA. Murine interleukin-11 (IL-11) is expressed at high levels in the hippocampus and expression is developmentally regulated in the testis. J. Cell. Physiol. 1996;168:362–372. doi: 10.1002/(SICI)1097-4652(199608)168:2<362::AID-JCP15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Fairchild KD, Viscardi RM, Hester L, Singh IS, Hasday JD. Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. J. Interferon Cytokine Res. 2000;20:1049–1055. doi: 10.1089/107999000750053708. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat. Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J. Neurosci. 2008;28:12199–12211. doi: 10.1523/JNEUROSCI.3856-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizasa H, Matsushima K. IL-8. In: Oppenheim JJ, Feldman M, editors. Cytokine Reference. Vol. 1. San Diego: Academic Press; 2001. pp. 1061–1067. [Google Scholar]
- Kimura R, Maeda M, Arita A, et al. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine. 2007;38:107–115. doi: 10.1016/j.cyto.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Kraig RP, White DM, Mitchell HM. TNF-α drives cold-preconditioning neuroprotection and reprogramming of inflammatory cytokine cascade reactions. Soc. Neurosci. 2008;34 Prog #151.9. [Google Scholar]
- Kraig RP, Mitchell H, Christie-Pope B, White DM, Kunkler PE, Tang Y-P, Langan G. TNF-α and microglial hormetic involvement in neurological health and migraine. Dose-Response. 2010;8:389–413. doi: 10.2203/dose-response.09-056.Kraig. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Reactive astrocytosis from excitotoxic injury in hippocampal organ culture parallels that seen in vivo. J. Cereb. Blood Flow Metab. 1997;17:26–43. doi: 10.1097/00004647-199701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Kraig RP. Multiplexed cytokine protein expression profiles from spreading depression in hippocampal organotypic cultures. J. Cereb. Blood Flow Metab. 2004;24:829–839. doi: 10.1097/01.WCB.0000126566.34753.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J. Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Selnes OA, Grega MA, Bailey MM, Pham LD, Baumgartner WA, Zeger SL. Subjective memory symptoms in surgical and nonsurgical coronary artery patients: 6-year follow-up. Ann. Thorac. Surg. 2009;87:27–34. doi: 10.1016/j.athoracsur.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HM, White DM, Kraig RP. Cold-preconditioning neuroprotection of hippocampus follows a hormetic dose-response pattern initiated by tumor necrosis factor alpha from microglia and potentially evoked by adaptive interleukin-11 signaling from neurons. Soc. Neurosci. 2009;35 Prog #744.4. [Google Scholar]
- Mitchell HM, White DM, Kraig RP. Strategies for study of neuroprotection from cold-preconditioning. JoVE. 2010;43 doi: 10.3791/2192. Available at: http://www.jove.com/index/Details.stp?ID=2192; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Nau GJ. Osteopontin. In: Oppenheim JJ, Feldmann M, editors. Cytokine Reference. Vol. 1. San Diego: Academic Press; 2001. pp. 659–701. [Google Scholar]
- Nishio S, Yunoki M, Chen ZF, Anzivino MJ, Lee KS. Ischemic tolerance in the rat neocortex following hypothermic preconditioning. J. Neurosurg. 2000;93:845–851. doi: 10.3171/jns.2000.93.5.0845. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Feldmann M. Introduction to the role of cytokines in innate host defense and adaptive immunity. In: Oppenheim JJ, Feldmann M, editors. Cytokine Reference. Vol. 1. San Diego: Academic Press; 2001. pp. 3–20. [Google Scholar]
- Ransohoff RM, Perry VH. Annu. Rev. Immunol. 2008;27:119–415. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Gao X, Rockwell S, Kelley M, Elias JA. IL-11 enhances survival and decreases TNF production after radiation-induced thoracic injury. J. Immunol. 1996;157:1705–1710. [PubMed] [Google Scholar]
- Rollins BJ. MCP-1, MCP-2, MCP-3, MCP-4, and MCP-5. In: Oppenheim JJ, Feldmann M, editors. Cytokine Reference. Vol. 1. San Diego: Academic Press; 2001. pp. 1145–1160. [Google Scholar]
- Schaller B, Graf R. Hypothermia and stroke: the pathophysiological background. Pathophysiology. 2003;10:7–35. doi: 10.1016/j.pathophys.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Tang XN, Yenari MA. Hypothermia as a cytoprotective strategy in ischemic tissue injury. Ageing Res. Rev. 2010;9:61–68. doi: 10.1016/j.arr.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J. Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]
- Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, Elias JA. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am. J. Respir. Cell Mol. Biol. 2003;29:513–522. doi: 10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- White DM, Mikol DD, Espinosa R, Weimer B, Le Beau MM, Stefansson K. Structure and chromosomal localization of the human gene for a brain form of prostaglandin D2 synthase. J. Biol. Chem. 1992;267:23202–23208. [PubMed] [Google Scholar]
- Yunoki M, Nishio S, Ukita N, Anzivino MJ, Lee KS. Characteristics of hypothermic preconditioning influencing the induction of delayed ischemic tolerance. J. Neurosurg. 2002;97:650–657. doi: 10.3171/jns.2002.97.3.0650. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, Brosnan CF, John GR. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J. Neurosci. 2006;26:12174–12185. doi: 10.1523/JNEUROSCI.2289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.