Abstract
Background
Patients undergoing coronary artery bypass grafting (CABG) experience a reduction in right ventricular long axis velocities post surgery.
Objectives
We tested whether the phenomenon of right ventricular (RV) long axis velocity decline depends on the chest being opened fully by mid-line sternotomy, pericardial incision, or on the type of operation performed.
Method
By intraoperative transoesophageal echocardiography (TEE) we recorded serial right ventricular (RV) systolic pulse-wave tissue Doppler velocities during 6 types of elective procedure: 53 CABG surgery, 15 robotic-assisted minimally-invasive CABG (RCABG), 28 aortic valve replacement (AVR), 8 minimally-invasive aortic valve replacement (mini-AVR), 5 mediastinal mass excision, and 1 left atrial myxoma excision. Pre and post operative transthoracic echocardiography (TTE) were also conducted.
Results
Surgery without substantial opening of the pericardium did not significantly reduce RV systolic velocities (RCABG 13 ± 1.8 versus 12.4 ± 2.7 cm/s post; mini-AVR 11.9 ± 2.3 versus 11.1 ± 2.3 cm/s; mediastinal mass excision 13.9 ± 3.1 versus 13.8 ± 4 cm/s). In contrast, within 5 min of pericardial incision those whose surgery involved full opening of the pericardium had large reductions in RV velocities: 54 ± 11% decline with CABG (11.3 ± 1.9 to 5.1 ± 1.6 cm/s, p < 0.0001), 54 ± 5% with AVR (12.6 ± 1.4 to 5.7 ± 0.6 cm/s, p < 0.001) and 49% with left atrial myxoma excision (11.3 to 15.8 cm/s). This persisted immediately after pericardial opening to the end of surgery (61 ± 11%, p < 0.0001; 58 ± 7%, p < 0.0001; 59% respectively).
Conclusions
It is full opening of the pericardium, and not cardiac surgery in general, which causes RV long axis decline following cardiac surgery. The impact is immediate (within 5 min) and persistent.
Keywords: RV function, Pericardium, Cardiac surgery, Pulsed-wave tissue Doppler echocardiography
1. Introduction
It is well known that right ventricular (RV) long axis velocities reduce significantly following coronary artery bypass surgery [1–4]. The cause of this phenomenon is disputed, with many hypotheses, including the well-known process of cardioplegia [5], myocardial hypothermia [6] and postoperative adhesions [7]. We previously showed that, in patients undergoing coronary artery bypass surgery the time point at which RV long axis velocities began to decline coincided with full pericardial opening following a full sternotomy [4]. However, it remains unclear whether the onset of RV long axis velocity decline occurs in patients undergoing different cardiothoracic and thoracic procedures, specifically those without significant coronary artery disease. In order to narrow down the range of plausible mechanistic possibilities, this study aimed to assess patients undergoing different cardiothoracic and thoracic procedures, taking intraoperative measurements in a subset of these patients.
By this approach of investigation it would be possible to first ascertain whether the nature of the surgery would affect RV velocity and second, whether the presence of underlying myocardial ischaemia influenced the degree of RV long axis decline.
2. Methods
In this study we set out to examine patients undergoing coronary artery bypass grafting (CABG) via traditional full sternotomy, but also patients undergoing minimally invasive robotic assisted coronary artery bypass grafting (RCABG) via a left-sided mediastinoscopy, patients undergoing isolated aortic valve replacement (AVR) via full sternotomy; patients having minimally-invasive surgery AVR (mini-AVR) via minimal sternotomy; and patients who underwent mediastinal mass excision via left-sided mediastinal sternotomy. In order to determine precisely the time-point at which RV velocity decline occurs we aimed to document carefully the timing of the different stages of the operation using continuous TOE monitoring.
RV long axis velocities were measured using pulsed wave tissue Doppler [8,9] utilising both transthoracic (TTE) [1,10] and transoesophageal (TOE) [11,12] approaches before, during and after cardiac surgery. Conventional TTE was also conducted on all patients before and after surgery where additional long axis annular systolic excursion plane of both the basal RV free wall and septum was measured. Tricuspid and septal annular plane systolic excursion (TAPSE/SAPSE) are popular and easy methods of assessing both left and right ventricular long axis function. Indeed, the estimation of RV function using TAPSE measures has shown to correlate well with angiography derived estimates of RV ejection fraction [13].
Exclusion criteria were reduced concomitant valve or coronary artery surgery or atrial fibrillation. We carried out transthoracic echocardiograms one week before and one month after surgery.
34 patients also agreed to undergo continuous intraoperative monitoring by TOE. These patients underwent recordings of RV tissue Doppler myocardial velocities at frequent intervals from the onset of general anaesthesia to the time of skin suturing. The timing of each stage of surgery was documented for each patient, so that RV velocity data could be mapped to each surgical step.
The study protocol was approved by the hospitals ethics committee (Ref 06/Q0403/163) and the patients gave written informed consent to participate in the study.
3. Measurements
3.1. Pre and post-operative transthoracic echocardiography
Each patient was scanned using conventional 2D, pulsed wave and tissue Doppler imaging techniques one week before and one month after surgery. Patients were placed in the left lateral decubitus position, where using an IE33 Philips Medical System (Andover, Massachusetts, USA) both parasternal and apical imaging windows were imaged using a S5-1, 3.5 MHz transducer at a mean depth of 16 cm.
Left ventricular (LV) ejection fraction (EF) was assessed from the major axis using the Simpson's method of discs. RV dimensions were taken in both minor and major axis positions. RV major axis cavity length, area, and volume measurements were taken and ejection fraction estimates calculated.
Tissue Doppler myocardial velocities were measured by placing the pulsed wave sample volume at the level of the basal RV free wall. Each recorded value was the mean from four consecutive beats. Tricuspid annular plane systolic excursion (TAPSE) [14] and basal septal annular plane systolic excursion (SAPSE) were measured by placing the M-Mode sample volume at the level of the basal RV free wall and basal septum respectively.
To help minimise spectral broadening gain and velocity settings were optimised and pulsed wave sample volume length for each patient was set between 2 and 5 mm. Spectral broadening is the tendency of the pulsed wave Doppler to be spread over a range of velocities rather than show only a single velocity. As the consensus is to measure the top of the velocity trace the effect of spectral broadening is to (alternatively) increase the measured velocity.
Recordings were acquired during normal respiration, and were used to estimate both RV and LV excursion distances (cm) and myocardial velocities (cm/s).
Ventricular filling was assessed using both transmitral and transtricuspid Doppler sampled from the apical window. The Doppler sample volume was placed at the level of the mitral and tricuspid leaflet tips using the guidance of spectral colour Doppler. All Doppler recordings were utilised to calculate the following variables: (a) peak pulsed wave transmitral early diastolic inflow (E-wave) and late diastolic (A-wave) velocities; (b) peak pulsed wave tissue Doppler myocardial velocities marking early diastolic annular motion velocities (E′ wave), late annular diastolic motion velocities (A′ wave) and systolic annular motion (S′) velocities [15]; (c) E:A and E:E′ peak left ventricular compliance ratios; (d) stroke volume (cc); (e) cardiac output (L/min2); (f) tricuspid regurgitation estimates of pulmonary artery pressures (Est PAP mm Hg); and (g) tricuspid regurgitation dP/dt values.
3.2. Intra-operative transoesophageal echocardiography
Intra-operative TOE examinations were to be carried out in 6 types of elective procedure. Imaging was carried out using a multiplane 5-MHz transoesophageal transducer and Vivid I system (GE Healthcare, Waukesha, WI). The probe was placed at a mean depth of 30 cm with a mean angle of 22° where a view of the RV was obtained. Tissue Doppler myocardial velocity estimates were measured by placing the pulse wave sample volume 1 cm from the level of the tricuspid annulus where four consecutive beats were recorded and the mean systolic velocities measured. The tissue Doppler sample volume length for each patient was set between 2 and 5 mm to minimise spectral broadening. The TOE probe was inserted shortly after the onset of general anaesthesia and recording commenced once a working view was located which permitted the operator to replicate the tricuspid annular velocities obtained during the pre-operative TTE.
RV annular velocities were recorded frequently, initially from the onset of general anaesthesia up until the end of the operation when the skin was sutured. Recordings were acquired during standard ventilated respiration.
In patients undergoing mediastinoscopy, mass excision or off pump ‘beating heart’ coronary arterial bypass grafting, either by traditional full thoracotomy or minimally invasive robotic assisted access, velocity recordings were made continuously during the procedure up until the point of skin suturing. In patients undergoing coronary artery bypass grafting, left atrial myxoma removal or aortic valve replacement surgery, by either a traditional full or minimal thoracotomy approach, where the heart needed to be stopped using anterograde crystalloid cardioplegia and the patients placed on a heart and lung bypass machine, recordings were taken up until bypass cannulae insertion. Recording resumed after full weaning from cardiopulmonary bypass and after protamine administration.
3.3. Statistical analysis
Statistical analysis was performed using Statview 5.0 (SAS Institute Inc). Continuous data are expressed as mean ± standard deviation (SD). Distributions were tested for normality using the Shapiro–Wilk test. Comparisons between patients before and after surgery were made using Student's paired t-test. Comparisons between subgroups were made using unpaired t-tests. Because of the relatively large number of statistical tests in this study we have used a threshold p value of 0.01 for statistical significance [16]. However all p values are shown so that the reader may apply any threshold they consider suitable. We have displayed the p values for all the pre-specified comparisons, i.e. not selected solely the significant ones, and therefore the individual p values shown are valid for the individual comparisons. Because all p values are shown in full the reader can re-interpret them however they choose.
4. Results
The TTE measurements of all 110 subjects scanned before and following surgery are shown in Table 2. A list of subjects clinical characteristics are shown in Table 1.
Table 2.
Characteristics of 110 patients who underwent surgery. These measurements were made by transthoracic echocardiography one month before and one month after surgery. Bold values in the table are statistically significant.
| Degree of pericardial opening |
Full pericardial opening |
Minimal pericardial opening |
No pericardial opening |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of surgery |
CABG (n = 53) |
AVR (n = 28) |
LA myxoma excision (n = 1) |
Rob CABG (n = 15) |
Mini AVR (n = 8) |
Med mass excision (n = 5) |
|||||||||||
| Transthoracic echocardiography | Pre | Post | p Value | Pre | Post | p Value | Pre | Post | Pre | Post | p Value | Pre | Post | p Value | Pre | Post | p Value |
| RV EF % | 58.8 ± 12.3 | 53.3 ± 15.3 | 0.05 | 56.5 ± 18.4 | 53.7 ± 13.8 | 0.51 | 56.5 | 65.3 | 57.4 ± 13.9 | 55.6 ± 12.4 | 0.83 | 59.37 ± 11.2 | 48.7 ± 21.9 | 0.53 | 66.9 ± 8.5 | 55.6 ± 15.7 | 0.36 |
| RV FS % | 26.3 ± 7.4 | 23.3 ± 8.2 | 0.06 | 25.6 ± 10.8 | 23.2 ± 8.1 | 0.36 | 24.2 | 29.7 | 25.5 ± 8.8 | 24.4 ± 7.4 | 0.25 | 26.45 ± 6.6 | 21.3 ± 11.3 | 0.6 | 31.3 ± 5.9 | 24.5 ± 9.5 | 0.39 |
| TAPSE (cm) | 2.6 ± 0.5 | 1.1 ± 0.3 | < 0.0001 | 2.5 ± 0.5 | 1.2 ± 0.2 | < 0.0001 | 2.2 | 1.4 | 2.64 ± 0.49 | 2.2 ± 0.3 | 0.02 | 2.45 ± 0.41 | 2.2 ± 0.1 | 0.07 | 2.7 ± 0.7 | 2.7 ± 0.3 | 0.82 |
| RV PW TDI S′ (cm/s) | 13.9 ± 2.6 | 5.8 ± 1.3 | < 0.0001 | 14.1 ± 3.1 | 5.8 ± 1.4 | < 0.0001 | 11.5 | 6.8 | 13 ± 1.8 | 12.4 ± 2.7 | 0.52 | 11.93 ± 2.3 | 11.1 ± 0.7 | 0.55 | 13.9 ± 3.1 | 13.8 ± 3.8 | 0.53 |
| RV PW TDI E′ (cm/s) | 9.6 ± 2.4 | 5.1 ± 2.3 | 0.01 | 9.57 ± 3.3 | 5.4 ± 2.4 | 0.0002 | 9.1 | 5.6 | 8.5 ± 2.8 | 7.4 ± 2.5 | 0.428 | 7.9 ± 2.1 | 7.3 ± 1.8 | 0.61 | 9.9 ± 1.9 | 7.9 ± 1.7 | 0.42 |
| RV end diastolic vol (ml) | 52.9 ± 17.8 | 64.3 ± 29.1 | 0.005 | 49.5 ± 23.7 | 71.5 ± 33.6 | 0.001 | 109.2 | 82.5 | 64.7 ± 36 | 66.3 ± 21 | 0.83 | 73.3 ± 32.2 | 66.3 ± 20.4 | 0.23 | 57.1 ± 33.4 | 52.3 ± 28.4 | 0.925 |
| RV area (cm²) | 17.7 ± 3.9 | 18.9 ± 4.5 | 0.05 | 16.8 ± 4.9 | 20.5 ± 5.2 | 0.001 | 26.2 | 21.5 | 18.3 ± 4.5 | 20.4 ± 3.6 | 0.83 | 20.5 ± 4.6 | 19.6 ± 3.7 | 0.13 | 18.5 ± 6.1 | 17.2 ± 4.5 | 0.76 |
| Right atrial area (cm²) | 16.3 ± 4.3 | 18.2 ± 4.6 | 0.002 | 17.5 ± 5.2 | 20.5 ± 5.8 | 0.048 | 19.7 | 23.4 | 15.3 ± 2.5 | 19.14 ± 4 | 0.007 | 17.3 ± 5.6 | 22.2 ± 9.8 | 0.12 | 18.4 ± 1.7 | 15.7 ± 3.3 | 0.22 |
| Est PAP (mm Hg) | 25.2 ± 8.8 | 22 ± 7.1 | 0.08 | 32.6 ± 13.2 | 20.8 ± 4.6 | 0.04 | 24.2 | 24.7 | 23.4 ± 11 | 22.2 ± 9.8 | 0.58 | 29.2 ± 7.7 | 21.5 ± 7.2 | 0.109 | 22.7 ± 1.2 | 25.8 ± 1.6 | 0.05 |
| TR dt/dP (mm Hg/sec) | 445 ± 139 | 387 ± 129 | 0.02 | 414 ± 104 | 371 ± 113 | 0.64 | 400 | 394 | 366 ± 69 | 368 ± 37 | 0.37 | 364 ± 56 | 350 ± 53 | 0.92 | 633 ± 47 | 429 ± 165 | 0.31 |
| RV circum:length ratio | 3.1 ± 0.3 | 3.2 ± 0.3 | 0.13 | 3.3 ± 0.4 | 3.1 ± 0.3 | 0.86 | 3.81 | 3.7 | 3.4 ± 0.9 | 3.1 ± 0.2 | 0.24 | 3.1 ± 0.5 | 3.2 ± 0.2 | 0.94 | 3.1 ± 0.1 | 3.3 ± 0.3 | 0.05 |
| Stroke volume (cc) | 76 ± 23 | 77 ± 23 | 0.67 | 69 ± 23 | 67 ± 23 | 0.34 | 73.4 | 81.5 | 64 ± 21 | 61 ± 20 | 0.85 | 64 ± 22 | 65 ± 14 | 0.74 | 77 ± 24 | 68 ± 24 | 0.68 |
| CO (L/min²) | 5.1 ± 1.6 | 5.2 ± 1.5 | 0.83 | 4.9 ± 1.8 | 5.3 ± 2.3 | 0.41 | 5.3 | 7 | 4.3 ± 1.7 | 4.1 ± 1.2 | 0.96 | 4.9 ± 2.7 | 4.3 ± 0.8 | 0.9 | 4.8 ± 0.9 | 4.9 ± 1.1 | 0.68 |
| LV EF % | 59.3 ± 6.3 | 57.4 ± 7.4 | 0.62 | 61.4 ± 9.3 | 59.1 ± 8.1 | 0.62 | 58.4 | 57.1 | 56.4 ± 7.9 | 54.9 ± 5.2 | 0.05 | 56.3 ± 4.2 | 63.2 ± 6.3 | 0.8 | 59.3 | 61.2 | 0.32 |
| LV FS % | 25.4 ± 15.1 | 23.8 ± 7.6 | 0.54 | 26.1 ± 10.5 | 23.6 ± 8.1 | 0.84 | 10.9 | 10.4 | 28.4 ± 4.4 | 24.4 ± 6.2 | 0.09 | 22.33 ± 7.3 | 26 ± 6.9 | 0.7 | 17.8 ± 6 | 23.2 ± 6.8 | 0.07 |
| Trans mitral E/A ratio | 0.93 ± 0.46 | 0.97 ± 0.38 | 0.76 | 0.91 ± 0.46 | 1.0 ± 0.6 | 0.96 | 0.75 | 0.87 | 1.04 ± 0.37 | 1.1 ± 0.5.1 | 0.77 | 0.83 ± 0.33 | 1.1 ± 0.5 | 0.45 | 1.1 ± 0.5 | 0.7 ± 0.11 | 0.27 |
| TDI E/E′ ratio | 12.9 ± 5.6 | 14.9 ± 8.6 | 0.1 | 19.1 ± 12.8 | 12.8 ± 7.5 | 0.16 | 14.8 | 14.1 | 11.36 ± 4.2 | 12.4 ± 5.1 | 0.93 | 17.7 ± 11.2 | 16.3 ± 8.4 | 0.84 | 15.7 ± 5.5 | 14.2 ± 8.3 | 0.06 |
| Septal PW TDI E′ (cm/s) | 5.8 ± 1.8 | 5.2 ± 1.6 | 0.02 | 4.9 ± 1.5 | 4.5 ± 1.6 | 0.29 | 4.4 | 4.8 | 7.1 ± 2.3 | 6.1 ± 1.8 | 0.14 | 4.5 ± 1.7 | 5.3 ± 1.4 | 0.61 | 5.2 ± 1 | 5.5 ± 1.1 | 0.11 |
| Septal PW TDI S′ (cm/s) | 6.1 ± 1.4 | 5.1 ± 1.4 | 0.0001 | 5.9 ± 1.9 | 4.4 ± 1.3 | 0.002 | 5.9 | 6.1 | 6.4 ± 1.5 | 5.7 ± 1.5 | 0.38 | 5.18 ± 1.2 | 4.9 ± 0.8 | 0.79 | 5.9 ± 2 | 5.8 ± 2.4 | 0.59 |
| Lateral S′ (cm/s) | 6.7 ± 1.3 | 7.2 ± 1.9 | 0.34 | 7.1 ± 1.7 | 6.9 ± 1.6 | 0.84 | 9.8 | 9.4 | 8.2 ± 2.7 | 8.5 ± 1.4 | 0.42 | 5.6 ± 2 | 6.2 ± 1.6 | 0.83 | 10 ± 2.9 | 9.5 ± 3.2 | 0.5 |
| SAPSE (cm) | 1.2 ± 0.3 | 0.9 ± 0.2 | < 0.0001 | 1.1 ± 0.3 | 0.9 ± 0.2 | 0.002 | 1.23 | 1.28 | 1.4 ± 0.3 | 1.2 ± 0.4 | 0.13 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.64 | 1.3 ± 0.2 | 1.1 ± 0.2 | 0.69 |
| Left atrial area (cm²) | 21.3 ± 5.2 | 21.6 ± 5.5 | 0.64 | 23.2 ± 7.2 | 23.6 ± 6.4 | 0.33 | 26.1 | 25.5 | 20.8 ± 4.5 | 20.3 ± 6.3 | 0.7 | 26.7 ± 7.8 | 23.8 ± 8.14 | 0.2 | 19.7 ± 2.4 | 20.1 ± 4.3 | 0.9 |
LV/RV EF = Left/Right Ventricular ejection fraction; (LV/RV) FS = Left/Right Ventricular fractional shortening; PW TD = Pulsed wave Tissue Doppler; SAPSE = Septal annular plane systolic excursion; TAPSE = Tricuspid annular plane systolic excursion; Est PAP = Estimated Pulmonary artery pressure; TR = Tricuspid regurgitation; CO = Cardiac Output.
Table 1.
Clinical characteristics of 110 patients who underwent surgery.
| Type of surgery | CABG | AVR | LA myxoma excision | Rob CABG | Mini AVR | Med mass excision |
|---|---|---|---|---|---|---|
| (n = 53) | (n = 28) | (n = 1) | (n = 15) | (n = 8) | (n = 5) | |
| Variable | ||||||
| Subjects | 53 (14 females) | 28 (12 females) | 1 | 15 | 8 (3 females) | 5 (1 females) |
| Age | 68 ± 12 | 74 ± 10 | 44 | 65 ± 12 | 65 ± 22 | 63 ± 22 |
| Hypertension | 47 | 11 | 0 | 4 | 3 | 1 |
| Smoking history | 36 | 6 | 0 | 6 | 1 | 2 |
| Family history CHD | 21 | 4 | 0 | 3 | 2 | 0 |
| Hyperlipidaemia | 32 | 11 | 0 | 9 | 4 | 0 |
| Diabetes mellitus | 17 | 1 | 0 | 0 | 0 | 0 |
| Coronary disease | ||||||
| Left anterior descending | 47 | 0 | 0 | 15 | 0 | 0 |
| Circumflex | 49 | 0 | 0 | 0 | 0 | 0 |
| Right coronary artery | 41 | 0 | 0 | 0 | 0 | 0 |
| Pharmacological therapies | ||||||
| Aspirin | 53 | 28 | 1 | 15 | 8 | 1 |
| Statins | 32 | 11 | 0 | 9 | 4 | 2 |
| Calcium channel antagonists | 7 | 3 | 0 | 0 | 1 | 0 |
| Beta-blockers | 43 | 11 | 0 | 11 | 4 | 1 |
| Alpha-blockers | 7 | 3 | 0 | 0 | 1 | 0 |
| A2 blockers | 12 | 2 | 0 | 3 | 2 | 0 |
| ACE inhibitors | 0 | 0 | 0 | 0 | 0 | 0 |
4.1. Subjects
We recruited 110 patients (65 men, mean 69 ± 12 years) undergoing an elective first procedure: CABG (53 patients, 39 men, mean 68 ± 12 years), AVR (28 patients, 16 men, mean 74 ± 10 years), RCABG (15 male patients, mean 65 ± 12 years), mini-AVR (8 patients, 5 men, mean 65 ± 22 years), left atrial myxoma removal (1 male patient, 44 years), and mediastinal mass excision (5 patients, 4 men, mean 63 ± 22 years) surgery between January and September 2009.
All subjects were confirmed by echocardiography to have good left and right ventricular function with no regional wall motion abnormalities and no valve dysfunction of valves not being replaced, other than mild regurgitation.
There was no difference between groups in ages (p = 0.1805 by ANOVA) although there was significant heterogeneity in prevalence between groups for all 17 dichotomous characteristics shown in Table 1 (p < 0.01 for each, by Chi² test).
4.2. Pre and post operative transthoracic findings
4.2.1. Operations that do not preserve pericardial integrity
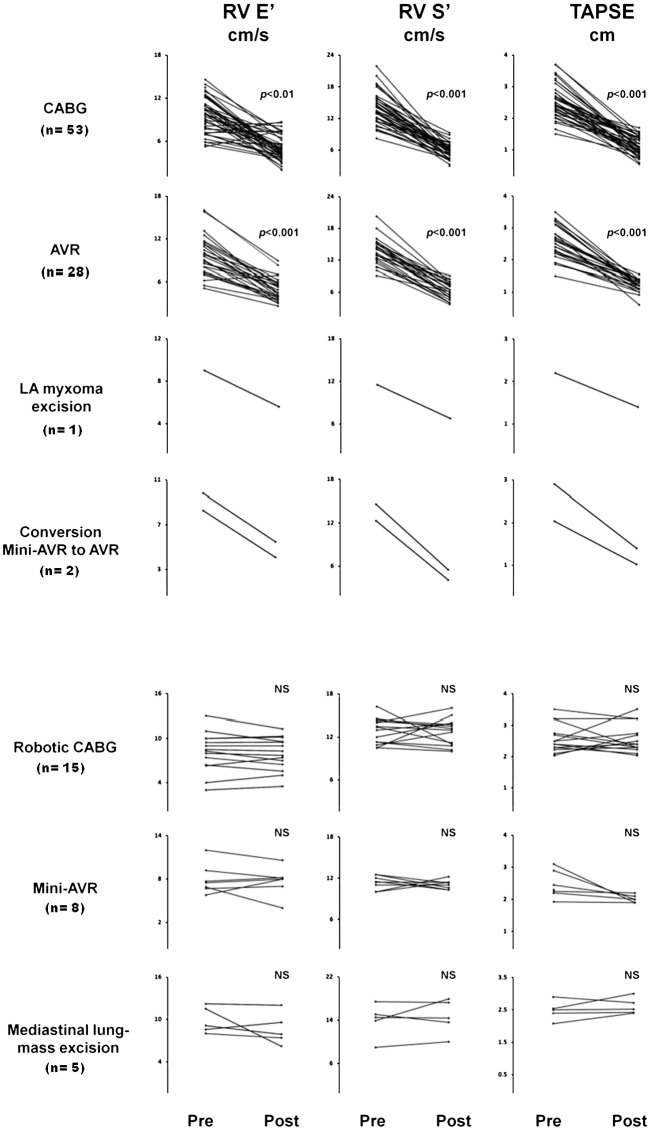
As displayed in Table 2, in those patients undergoing operations where the pericardium was fully opened following a traditional full sternotomy TTE data showed a clear fall in right ventricular long axis S′ velocity. For CABG it fell by 57 ± 11% (13.9 ± 2.6 to 5.8 ± 1.3 cm/s, p < 0.0001). Similarly for AVR it fell by 57 ± 11% (14.1 ± 3.1 to 5.8 ± 1.4 cm/s, p < 0.0001), and left atrial myxoma removal by 41% (11.5 to 6.8 cm/s). TAPSE for CABG fell by 55 ± 13% (2.6 ± 0.5 to 1.1 ± 0.3 cm, p < 0.0001). For AVR it fell by 48 ± 11% (2.53 ± 0.5 to 1.2 ± 0.2 cm, p < 0.0001), and for left atrial myxoma removal it fell by 36% (2.2 to 1.4 cm).
There was also a clear decline in the diastolic long axis E′ velocity. For CABG it fell by 44 ± 27% (9.6 ± 2.4 to 5.1 ± 2 cm/s, p < 0.0002). Similarly for AVR it fell by 44 ± 23% (9.57 ± 3 to 5.4 ± 2.4, p < 0.0001), and for left atrial myxoma excision it fell by 38% (9.1 to 5.6 cm/s, Fig. 1).
Fig. 1.
Changes in RV tissue Doppler S′, E′ and tricuspid annular plane systolic excursion (TAPSE) in the seven surgical procedure groups (110 patients) 1 week before and 1 month after surgery. The top four surgical groups involved full pericardial opening. The bottom three surgical groups required only a small pericardial incision or mediastinal surgery where the pericardium was left intact. The large reduction in RV post operative velocities and excursions was only seen in those surgical procedures where the pericardium was fully opened.
4.2.2. Operations in those patients with coronary artery disease and those without
Between the two groups that allowed for comparison between patients with coronary artery disease and those without – CABG and AVR respectively – there was no significant contrast between the pre-to-post-operative changes in long axis measurements (p values are for contrast between groups by ANOVA). For S′, those undergoing CABG fell by 8.1 ± 2.7, while those undergoing AVR fell by 7.9 ± 2.0 (p = 0.435), for E′, those undergoing CABG fell by 4.5 ± 2.9, while those undergoing AVR fell by 3.8 ± 3.2 (p = 0.194) and for TAPSE, those undergoing CABG fell by 1.4 ± 0.5, while those undergoing AVR fell by 1.3 ± 0.4 (p = 0.114).
While between the two CABG groups that allowed for comparison between patients who had coronary artery disease – CABG and RCABG respectively – there was a significant contrast between the pre-to-post-operative changes in long axis measurements. For S′, those undergoing CABG fell by 8.1 ± 2.7, while those undergoing RCABG fell by 0.8 ± 3.8 (p < 0.00001), for E′, those undergoing CABG fell by 4.5 ± 2.9, while those undergoing RCABG fell by 0.7 ± 3.0 (p = 0.0001) and for TAPSE, those undergoing CABG fell by 1.4 ± 0.5, while those undergoing RCABG fell by 0.4 ± 0.3 (p < 0.00001).
4.2.3. Operation that preserve pericardial integrity
In marked contrast, all 23 patients who underwent minimally invasive surgery (8 mini-AVR, 15 RCABG) showed no significant changes in post operative RV long axis tissue Doppler E′, S′ velocities or TAPSE estimates (Table 2).
Nor did the 5 patients who underwent mediastinal mass excision (which involved opening the sternum but not the pericardium) show any changes in velocities or TAPSE estimates.
4.3. Presence or absence of coronary surgery
In those patients where a traditional full sternotomy approach was used, there was no significant difference in the overall reduction in RV tissue Doppler velocities between those patients with significant coronary artery disease and those without.
Similarly, in those patients who underwent surgery using a minimally invasive approach, there were no significant differences between those that had undergone coronary surgery and those that had not.
4.3.1. Intra-operative findings
Intra-operative TOE examinations were carried out in 6 types of elective procedure in a total of 34 patients: 12 CABG surgery, 7 robotic-assisted minimally-invasive CABG (RCABG), 7 aortic valve replacement (AVR), 6 minimally-invasive aortic valve replacement (mini-AVR), 1 mediastinal mass excision, and 1 left atrial myxoma excision.
Intraoperative monitoring showed that in those patients in whom the pericardium was fully opened RV tissue Doppler velocities reduced immediately following pericardial incision. The time-point at which velocities began to decline was virtually identical in all three operation types. Within the first five minutes of opening the pericardium velocities had reduced: for CABG by 54 ± 11% (11.3 ± 1.9 to 5.1 ± 1.6 cm/s, p < 0.0001), for AVR by 54 ± 5% (12.6 ± 1.4 to 5.7 ± 0.6 cm/s, p < 0.001) and for left atrial myxoma removal by 49% (11.3 to 5.8 cm/s), and remained depressed throughout the operation, with a final intraoperative S′ reduction for CABG of 61 ± 11% (11.3 ± 1.9 to 4.28 ± 1 cm/s, p < 0.0001), for AVR of 58 ± 7% (12.6 ± 1.4 to 5.2 ± 1.1 cm/s, p < 0.0001), and for left atrial myxoma removal 59% (11.3 to 4.6 cm/s).
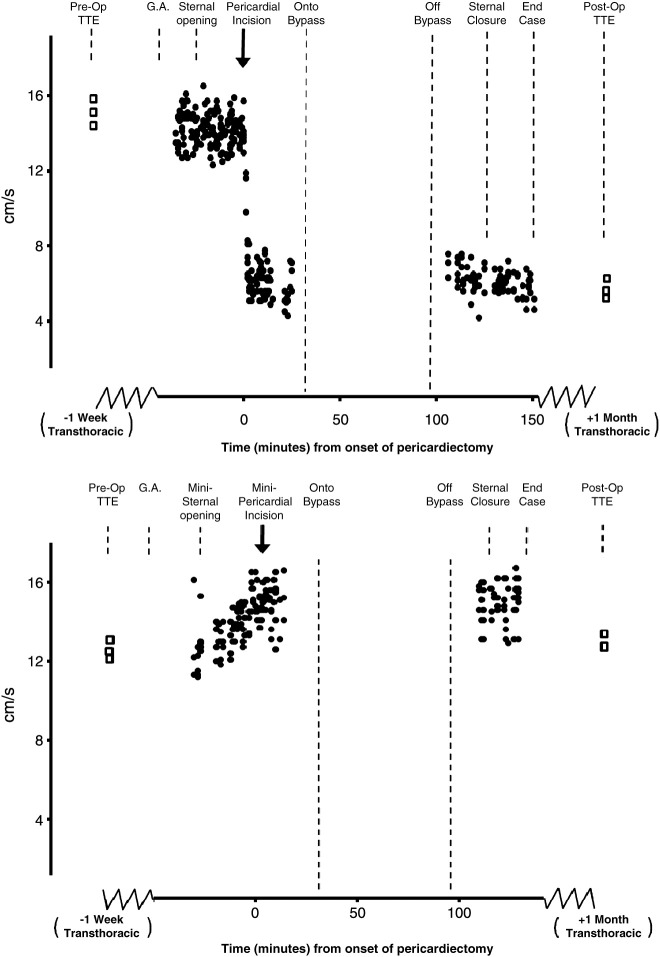
In the mini-AVRs and RCABGs, where the sternum was opened but the smallest possible pericardial incision was made, there were no changes in right ventricular tissue Doppler velocities at the time of minimal pericardial opening (Figs. 2 and 3).
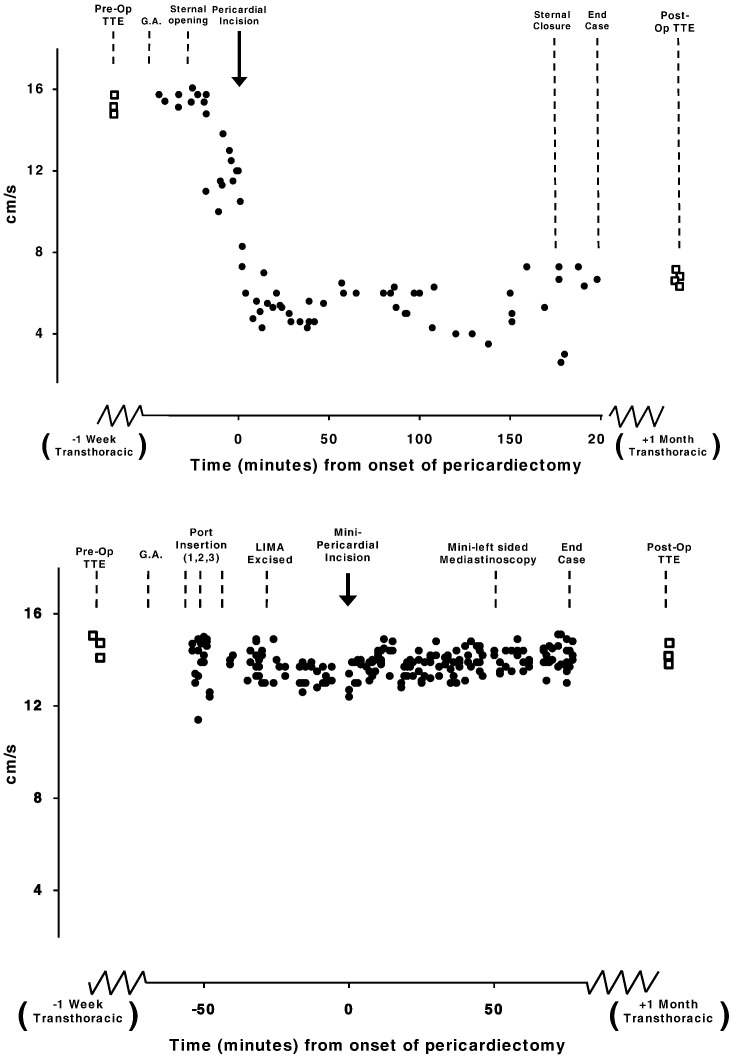
Fig. 2.
Top panel: Time course data from one patient. Intraoperative TOE of RV tissue Doppler S′ velocities recorded during routine on-pump AVR using traditional full sternotomy access. The preoperative TTE velocities are reproduced intraoperatively using TOE and remain unchanged after sternal opening. Following pericardial incision there is an immediate reduction in RV tissue Doppler S′ velocity which remains depressed at the time of closure. Velocities one month later remain depressed. Bottom panel: Time course data from one patient. Intraoperative TOE of RV velocities during minimally invasive AVR. The velocities remain unchanged from the pre operative assessment and do not change throughout the entire operation, even after a small pericardium incision was made. TTE velocities one month later also remain unchanged from pre-operative values.
Fig. 3.
Top panel: Time course data from one patient. Intraoperative TOE of RV tissue Doppler S′ velocities during routine off-pump CABG using traditional full sternotomy access. Bottom graph: Time course data from one patient. Intraoperative TOE of RV tissue Doppler S′ velocities during minimally invasive RCABG using a left-sided mediastinoscopy approach.
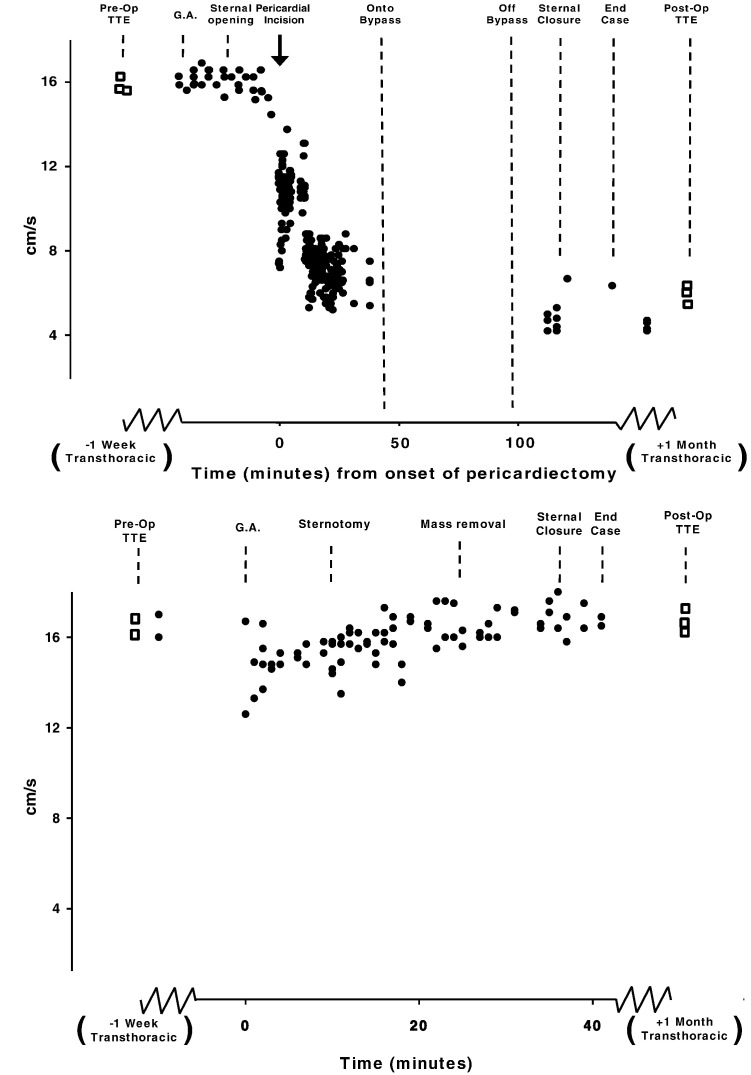
Similarly, intra-operative measurements in those patients who underwent mediastinal mass excision, where the sternum was opened but the pericardium was left intact, also showed no change in RV velocities (Fig. 4).
Fig. 4.
Top panel: Time course data from one patient. Intraoperative TOE of RV tissue Doppler S′ velocities during surgical removal of a left atrial myxoma using a traditional full sternotomy approach. Bottom panel: Time course data from one patient. Intraoperative TOE of RV tissue Doppler S′ velocities during the excision of a left-sided mediastinal mass via thoracotomy only.
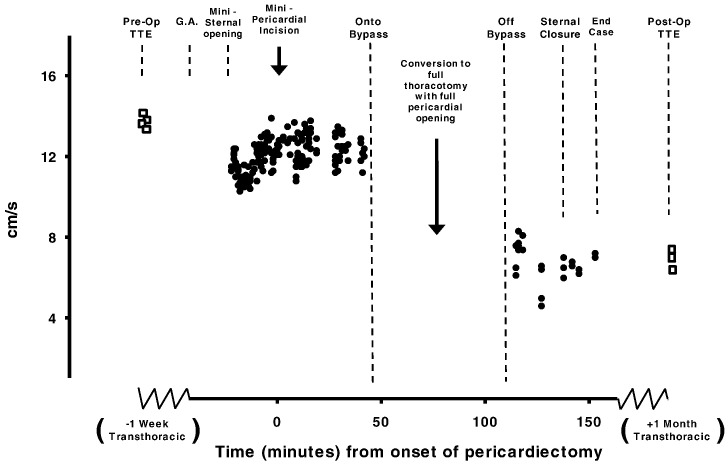
4.4. Conversion from minimally invasive to full sternotomy
Of the 34 patients observed using intraoperative TOE, 2 underwent mini-AVR. The decision to convert from a minimal to a full sternotomy occurred because greater access was needed which was discovered after onset of cardiopulmonary bypass. RV tissue Doppler velocities continued to be recorded in both patients up until the moment the bypass cannulae were inserted, at which point data acquisition had to be suspended. To that stage of the operation no reductions had been seen in the RV velocities of either patient. After sternal conversion and successful aortic valve replacement, both patients were fully weaned from cardiopulmonary bypass. Protamine was administered and data acquisition of RV velocities was resumed.
Post operative findings in these two patients resembled those patients who had undergone surgery full sternotomy from the onset: RV long axis velocities fell, with a final intraoperative S′ reduction by 60% (13.2 ± 0.3 to 5.3 ± 0.9 cm/s). One month post surgery RV long axis S′ velocities remained depressed having fallen by 64% (13.5 ± 1.1 to 4.9 ± 0.9 cm/s), E′ velocities by 48% (9.2 ± 0.8 to 4.9 ± 0.7 cm/s), and TAPSE by 53% (2.64 ± 0.4 to 1.2 ± 0.2 cm). The data of these two patients who had to be converted to full open surgery have not been included in any of the above sections as their classification may be contentious. However we show the full recordings in Fig. 5.
Fig. 5.
Time course data from one patient. Intraoperative TEE of RV tissue Doppler S′ velocities recorded during a routine minimally invasive AVR surgery which required conversion to full sternotomy once after the patient was already on bypass. The preoperative TTE tissue Doppler velocities are reproduced intraoperatively using TEE and remain unchanged following administration of general anaesthesia, minimally-invasive sternal opening, minimally-invasive pericardial incision and even at the point of bypass cannulae insertion, at which point data acquisition was suspended. But once measurements of RV tissue Doppler velocities resumed following sternal conversion, full pericardial opening and successful aortic valve replacement velocities were found to be much lower, and did not improve. One month later, the velocities remain equally depressed.
5. Discussion
Minimally invasive cardiac surgery is an alternative method of conducting routine coronary artery bypass surgery [17,18] and aortic valve replacement [19,20].
In this study we have shown that patients who underwent minimally invasive cardiac surgery, 15 RCABG and 8 mini-AVR, showed no change in either peri- or postoperative RV tissue Doppler velocities. Both RCABG and mini-AVR require a small pericardial incision, at different locations: this limited incision does not affect RV velocities (Figs. 2 and 3).
5.1. Presence or absence of coronary artery disease requiring CABG has no influence on the time-point of RV velocity reduction
The impact of underlying myocardial ischaemia in coronary artery disease patients undergoing CABG is one long-standing hypothesis to explain the reduction in post operative RV velocities [1,9,21]. However, we have found that in the patients with coronary artery disease, specifically those undergoing CABG using a full thoracotomy approach showed a marked decline in RV tissue Doppler S′ velocities, while in contrast those who underwent RCABG, where the pericardium was left almost completely intact, showed no significant decline in RV velocities (Fig. 3).
Meanwhile, patients who were without coronary artery disease and underwent either aortic valve replacement, left atrial myxoma or mediastinal mass excision showed substantial declines in RV velocities where the pericardium was fully incised but did not when it was not fully incised, mini-AVR and mediastinal mass excision. Therefore the link would appear to lie with full pericardial opening and not with coronary artery disease (Fig. 1).
5.2. Temporal isolation of decline in RV velocities
Whether the presence of underlying ischaemia could affect or influence either the time-point at which RV long axis velocities begin to decline, i.e. immediately following full opening of the pericardium, or extent at which RV velocities falls, was until now unknown. A recent study conducted at our centre introduced the concept of sequential intra-operative TEE to identify that virtually all the loss in RV systolic myocardial velocity occurs within the first 3 min following pericardial incision [4].
However that previous study only examined patients undergoing routine coronary bypass surgery with full pericardial opening via a traditional mid-line thoracotomy and therefore could not determine whether pericardial opening or coronary artery disease was necessary or sufficient to cause RV long axis decline [9].
In this present study we observed that this decline did not solely occur in those patients who had isolated coronary artery disease and that there was an instantaneous decline in RV myocardial velocities in all patients when the pericardium was opened fully. Moreover, we also found that in those who had isolated coronary artery disease and underwent minimally invasive cardiac surgery, which does not open the pericardium substantially, this reduction did not occur.
5.3. Sternotomy alone poses no threat to RV long axis velocities
It has been shown that act of performing a sternotomy alone causes an increase in cardiac filling and reduces the effect of mechanical ventilation on ventricular stroke volume and therefore could be classed as another potential mechanism for reducing post operative RV long axis function [22]. Our cohort contained 5 patients undergoing left-sided mediastinal mass excision, in whom the sternum was opened to reveal the pleura but the pericardium was left fully intact. We found no change in RV velocities throughout these operations (Figs. 1 and 4). These findings, together with the timing of the RV velocity decline seen in full thoracotomy cardiac surgery (which occurs at the time-point of pericardial opening and not sternal opening), effectively eliminate the possibility that sternal opening itself is relevant to this phenomenon.
5.4. Clinical impact
The immediate impact of this study is in the clarification of the expectation of standard behaviour after cardiac surgery, so that clinical echocardiographers can be confident in stating the expected behaviour after any particular operation, rather than having only a vague concept that RV velocities sometimes fall after surgery.
It would be premature to make a recommendation to surgeons that the pericardium would be better closed rather than left open at the end of surgery which is the common convention. First, because in our study no patients underwent pericardial closure, and second it is generally not practically possible to close the pericardium completely after surgery to the same level of structural integrity as it was prior to initial opening. This is because the pericardial borders of the ‘T-shaped incision’ are no longer able to be brought together to permit the pericardium to be sutured and closed. Attempts to do so have also been associated with an adverse impact on immediate and short-term haemodynamics [23,24].
Even more fundamentally, it may be too early to speculate that the decline in RV long axis velocities harmfully impacts clinical outcomes. A previous study has shown that the sensitivity and specificity of TAPSE for predicting RVEF dropped significantly in patients receiving CABG and AVR persisting for more than one month after surgery [8] which in combination with the findings of this study raise the possibility that even though it may be a reliable and easy method of assessing RV function in the general population, it may not be usable in this way in those patients who have undergone surgery where the pericardium is fully opened.
This study does not attempt to tell clinicians exactly why RV long axis velocities fall in patients who have undergone cardiac surgery but it does delineate which operations have this effect. Possession of this information in an unambiguous form, separating surgical procedures, is of practical benefit to specialists evaluating RV velocities in patients who have undergone surgery of the heart. For example: those patients with depressed RV long axis velocities after open CABG need not be suspected of having an independent disease process affecting the right heart if their velocities have reduced by half. Conversely, in a patient who has undergone surgery with minimal pericardial opening, a low RV long axis velocity will not be assumed to be an automatic effect of surgery itself and instead another cause will be sought.
5.5. Proposed mechanisms
Although this study excluded beyond all reasonable doubt a large number of possible hypotheses for the mechanism of RV long axis reduction, many possibilities still remain. First, the pericardium may be important for allowing the long axis fibres of the RV to be appropriately aligned to function at full efficiency. Second, changes in intra-cardiac pressures resulting in the release of the pericardium may disrupt normal RV performance. Third, the pattern of RV contraction in a normal state may in fact be enforced by the pressure of the PC and without it the pattern may change.
5.6. Study limitations
RV geometry is complex, and it might be argued that this mode of evaluation is far from comprehensive. However, longitudinal excursion plays the dominant role in RV function [9] and can be monitored intraoperatively with exquisite temporal resolution. It is therefore a good choice of variable to identify the time-point of right ventricular tissue Doppler velocity decline, and to do so in a way that can be interpreted alongside outpatient pre and post operative images [10,25].
We cannot tell if the decline in RV velocities is harmful as this would require a different class of study and many more patients. However, a larger study would not require additional intraoperative measurements as the data collected in this study are sufficient to capture the reduction in RV long axis velocity.
In this study we adopted a convention for quantifying 4 beat averages. In practice it might have been better to use a larger number of beats that were individually tuned to the ratio of the patient heart rate and respiratory rate to minimise the impact of respiration.
However, within our individual patient intra-operative plots (Figs. 2 to 5) each dot represents a 4 beat average so the ability to locate the time point of an underlying velocity, and distinct it from variability is quite good.
Previous studies have established that RV long axis velocity reduction tends to persist for over 12 months after surgery [2,10,11] in contrast to the transient reduction in left ventricular (LV) function which is known to have recovered as soon as 48 h after surgery [26,27].
Given this knowledge, we chose to carry out post-operative transthoracic echocardiograms 1 month following surgery as at this time-point we would still be able to assess a representative cohort of the patients to be recruited. Furthermore, the aim of our study was to identify the types of common cardiac surgery in which RV long axis tissue Doppler velocities decline, and the intra-procedural time-point at which this occurs rather than re-verifying long-term progression. Other studies, where transthoracic echocardiograms were conducted 18 months following surgery, have addressed long-term changes [1].
We found a 15% reduction in TAPSE in the Robotic CABG group and an 18% reduction in the mini-AVR group which did not reach the threshold of statistical significance. We cannot be certain if these differences would reach statistical significance if tested in a larger group. However, the measured declines of 15% and 18% are substantially smaller than the corresponding declines in the open CABG (58%) and AVR (53%) groups.
Because mediastinal mass excision was a less frequently encountered procedure we have less power to detect differences, and indeed one of the five patients did show a sizable decline in one measure. However, that patient did not show a decline in the other measures nor did any of the remaining four patients show a substantial decline in any of the measurements.
(Fig. 1) This is in marked contrast to the operations in the upper 4 sets of panels where almost all the patients showed a substantial decline in almost all measurements. It is on this basis that we believe it is safe to interpret the data showing that mediastinal mass excision does not impact on long axis velocities in the same way that CABG, AVR, myxoma excision or Min-AVR to AVR conversion.
In our experiment we did not identify one single true cause which can explain the post operative reduction in long axis RV velocities. Rather, in one study we have eliminated almost all of the proposed mechanisms and it is therefore a useful contribution to the search for the true cause. Establishing the full chain of events leading to depression of RV long axis velocities may require more advanced techniques, but the present study substantially narrows the range of possible mechanistic sequences that would need to be considered by showing which operations do, and which do not, have this effect.
6. Conclusion
We conclude that it is the act of full pericardial incision which is responsible for the reduction in RV longitudinal velocities, which occurs at the same time-point and to approximately the same extent in all patients in whom the pericardium is fully opened regardless of underlying pathology and reason for surgery (valve disease, coronary artery disease, or other). It does not occur in the patients in whom the pericardium is not fully opened.
Acknowledgements
The authors would like to acknowledge the support of the National Institute for Health Research Biomedical Research Centre and the BHF research excellence award. The BHF supported BU (PG/07/066/22790), RB (PG/07/065/22785), DF (FS/10/038/28268).
The authors also wish to thank the cardiac anaesthetic and cardiothoracic surgical staff at St Mary's Hospital, Paddington.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.
Footnotes
Funding: Extramural funding provided by the British Heart Foundation and Coronary Flow Trust.
References
- 1.Alam M., Hedman A., Norlander R. Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J. 2003;146:520–525. doi: 10.1016/S0002-8703(03)00313-2. [DOI] [PubMed] [Google Scholar]
- 2.Brookes C.I., White P.A., Bishop A.J. Validation of a new intraoperative technique to evaluate load-independent indices of right ventricular performance in patients undergoing cardiac operations. J Thorac Cardiovasc Surg. 1998;116:468–476. doi: 10.1016/S0022-5223(98)70013-3. [DOI] [PubMed] [Google Scholar]
- 3.Michaux I., Filipovic M., Skarvan K., Seeberger M.D. Effects of on-pump versus off-pump coronary artery bypass graft surgery on right ventricular function. J Thorac Cardiovasc Surg. 2006;131:1281–1288. doi: 10.1016/j.jtcvs.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Unsworth B., Casula R.P., Kyriacou A.A., Yadav H., Francis D.P. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. 2010;159:314–322. doi: 10.1016/j.ahj.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christakis G.T., Buth K.J., Weisel R.D., Goldman B.S. Randomised study of right ventricular function with intermittent warm or cold cardioplegia. Ann Thorac Surg. 1996;61:128–134. doi: 10.1016/0003-4975(95)00933-7. [DOI] [PubMed] [Google Scholar]
- 6.Boldt J., Kling D., Dapper F., Hempelmann G. Myocardial temperature during cardiac operations: influence on right ventricular function. J Thorac Cardiovasc Surg. 1990;100(4):562–568. [PubMed] [Google Scholar]
- 7.Wranne B., Pinto F.J., Hammarstrim E. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. 1991;66:435–442. doi: 10.1136/hrt.66.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meluzin J., Spinarova L., Bakala J. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22(4):340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 9.Hammarstrom E., Wranne B., Pinto F.J., Puryear J., Popp R.L. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4(2):131–139. doi: 10.1016/s0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 10.Alam M., Wardell J., Anderson E. Characteristics of mitral and tricuspid annular velocities determined by pulsed was Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–628. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 11.Claude J.D., Tousignant P., Bowry R. Tricuspid annular velocity in patients undergoing cardiac operation using transesophageal echocardiography. J Am Society Cardiol. 2006;19(3):329–334. doi: 10.1016/j.echo.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Derumeaux G., Ovize M., Loufoua J. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation. 1998;97:1970–1977. doi: 10.1161/01.cir.97.19.1970. [DOI] [PubMed] [Google Scholar]
- 13.Ueti O.M. Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuclide angiography. Heart. 2002;88:244–248. doi: 10.1136/heart.88.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaul S., Tei C., Hopkins J., Shah P. Assessment of right ventricular function using two-dimensional echocardiography. American Heart J. 1984;107(3):526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 15.Galiuto L., Ignone G., DeMaria A.N. Contraction and relaxation velocities of the normal left ventricle using pulsed-wave tissue Doppler echocardiography. Am J Cardiol. 1998;81:609–614. doi: 10.1016/s0002-9149(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 16.Perneger T.V. What's wrong with the Bonferroni adjustments. BMJ. 1998;316:1236. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohr F.W., Falk V., Diegler A. Computer-enhanced “robotic” cardiac surgery: experience of 148 patients. J Thorac Cardiovasc Surg. 2001;121:842–853. doi: 10.1067/mtc.2001.112625. [DOI] [PubMed] [Google Scholar]
- 18.Dogan S., Aybek T., Anderben E. Totally endoscopic coronary artery bypass grafting on cardiopulmonary bypass with robotically enhanced telemanipulation: report of forty five cases. J Thorac Cardiovasc Surg. 2002;123:1125–1131. doi: 10.1067/mtc.2002.121305. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Sidiropolous A., Konertz W. Minimally invasive aortic valve replacement (AVR) capered to standard AVR. Euro Journal Cardiothorac Surg. 1999;16:S80–S83. [PubMed] [Google Scholar]
- 20.Cosgrove D.M., Sabik J.F. Minimally invasive approach for aortic valve operations. Ann Thorac Surg. 1996;62:596–597. [PubMed] [Google Scholar]
- 21.Alam M., Wardell J., Andersson E., Samad B.A., Norlander R. Right ventricular function in patients with first inferior myocardial infarction: assessment by tricuspid annular motion and tricuspid annular velocity. Am Heart J. 2000;139:710–715. doi: 10.1016/s0002-8703(00)90053-x. [DOI] [PubMed] [Google Scholar]
- 22.Reuter D.A., Goresch T., Goepfert M.S.G., Goetz A.E. Effects of mid-line thoracotomy on the interaction between ventilation and cardiac filling during cardiac surgery. Br J Anaesth. 2004;92(96):808–813. doi: 10.1093/bja/aeh151. [DOI] [PubMed] [Google Scholar]
- 23.Rao V., Komeda M., Weisel R.D., Cohen G., Borger M.A., David T.E. Should the pericardium be closed routinely after heart operations? Ann Thorac Surg. 1999;67:484–488. doi: 10.1016/s0003-4975(98)01199-0. [DOI] [PubMed] [Google Scholar]
- 24.Hunter S., Smith G.H., Angelini G.D. Adverse hemodynamic effects of pericardial closure soon after open heart operation. Ann Thorac Surg. 1992;53:425–429. doi: 10.1016/0003-4975(92)90262-3. [DOI] [PubMed] [Google Scholar]
- 25.Bleeker G.B., Steendijk P., Holman ER Y.U.C.M. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92(Suppl 1):i19–i26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloner R.A. Clinical evidence for stunned myocardium after CABG. J Card Surg. 1994;9(suppl):397–402. doi: 10.1111/jocs.1994.9.3s.397. [DOI] [PubMed] [Google Scholar]
- 27.Roberts A.J. Serial assessment of left ventricular performance following CABG. J Thorac Cardiovasc Surg. 1981;81:69–84. [PubMed] [Google Scholar]