Abstract
The most prevalent sub-group of abnormal repetitive behaviors among captive animals is that of stereotypies. Previous studies have demonstrated some resemblance between stereotypy in captive animals and in humans, including the involvement of neurological malfunctions that lead to the expression of stereotypies. This malfunction can be evaluated through the use of neuropsychological tasks that assess perseveration as implying a failure of the basal ganglia (BG) to operate properly. Other studies, in contrast, have suggested that stereotypies are the product of neurologically intact individuals reacting to the abnormal nature of their surroundings, and are possibly characterized by an adaptive feature that enables the subject to cope with such adversity. Employing neuropsychological tests and also measuring the levels of fecal corticoids in captive rhesus macaques, we tested the hypothesis that stereotypies are related both to brain pathology and to a coping mechanism with stress, resembling accounts by autistic individuals exhibiting basal ganglia malfunction, and who report a sense of relief when performing stereotypies. Self-directed and fine-motor stereotypies exhibited by the monkeys were positively correlated with perseveration, suggesting BG malfunction; while self-directed stereotypies were also negatively correlated with an increase in fecal corticoids following a stress challenge, suggesting a related coping mechanism. We therefore suggest that not all repetitive, unvarying, and apparently functionless behaviors should be regarded as one homogeneous group of stereotypic behaviors; and that, reflecting reports from autistic individuals, self-directed stereotypies in captive rhesus monkeys are related both to brain pathology, and to an adaptive mechanism that allows those that express them to better cope with acute stressors.
Keywords: Stereotypic behavior, Perseveration, Coping hypothesis, Basal ganglia, Psychological welfare
1. Introduction
1.1. Stereotypic behavior. Background
Captive environments may be considered unnatural for animals as they usually lack essential stimuli or contain aversive factors that limit the appropriate regulation of behavior [1,2]. Consequently, abnormal behavior may develop. The most prevalent sub-group of abnormal repetitive behaviors among captive animals are stereotypies [3,4], which are commonly defined as repetitive, unvarying, and apparently functionless [5]. Recent reports estimate that millions of captive animals world-wide demonstrate stereotypies [6]. Non-human primates (NHP), which are extensively used in scientific research [7], are reported to commonly exhibit this type of abnormal repetitive behavior [8].
1.2. The resemblance of stereotypy among animals to stereotypy in human pathology
Some resemblance exists between stereotypy in captive animals and in humans. For example, the motor output of some stereotypies is very similar in both human and non-human animals (hereafter animals). Thus, pacing is widely expressed in carnivores [9], NHP [8], and in anxious people and prisoners [10]. Equivalent factors are also thought to be involved in the development of stereotypies in humans and animals. For instance, the lack of appropriate parental care and/or lack of sufficient sensory input are thought to contribute to the development of stereotypies in both groups [11,12]. Finally, the expression of stereotypies (among other forms of abnormal repetitive behaviors) is suggested to involve an altered functioning of the parallel interconnecting associations between the cortex and the basal ganglia (BG), in humans as well as in animals [13]. For instance, neuroimaging data show that the motor loop through the putamen is affected in Tourette’s syndrome, while the loops between the orbitofrontal and anterior cingulate cortex with the BG are more relevant in OCD [14]. In rats, the degree of drug-induced motor stereotypies is predicted by the relative activation of two distinct compartments in the striatum: the striosomes and the extrastriosomal matrix, which receive projections from different parts of the cortex. [15]. Thus, different loops connecting the cortex and the BG are involved in the different expressions of abnormal repetitive behaviors in various pathological disorders [16]. These corticostriatal loops act through two pathways with contrasting effects: the direct pathway initiates behavior, while the indirect pathway inhibits behavior [17]. The equilibrium between the two pathways is maintained by the differential action of dopamine on striatal neurons, whereby it increases activity along the direct pathway by binding to D1 receptors, and decreases activity along the indirect pathway by binding to D2 receptors [17]. Behavioral disinhibition (as seen in stereotypies) can result from suppression of the activity of the indirect pathway relative to the direct pathway [18]. The division between cortical and BG areas was suggested earlier in the model of Shallice and Norman, which distinguishes between two brain systems that select and organize behavior: the Contention Scheduling System (CSS), which resides within the basal ganglia, and is responsible for the selection and sequencing of behavioral reactions according to external stimuli; and the Supervisory Attentional System (SAS), which is located in the prefrontal cortex (PFC), and edits the CSS’s selections according to internal non-physiological factors [19,20]. A failure of the CSS to function appropriately may lead to the development of stereotypies, as seen both in animals [21] and in human patients with certain mental disorders [4].
1.3. Utilizing neuropsychological tests to assess BG dysfunction
In order to assess CSS errors non-invasively, neuropsychological procedures have been developed. These procedures measure the subject’s perseveration, which is suggested to be the equivalent expression of stereotypies under experimental procedures, and can be evaluated through the ability of the subject to suppress a previously learnt response when it is no longer appropriate (i.e. in extinction) [2]. Indeed, patients diagnosed with schizophrenia who routinely express various abnormal repetitive behaviors were found to exhibit higher than normal levels of perseverative responses [22]; and similarly, positive correlations between levels of stereotypic behavior and perseveration were reported in a number of animal species [e.g. 23, 24].
1.4. Malfunction, abnormal behaviors, and the coping hypothesis
According to this system-level view of stereotypies, there may be a unifying neurological mechanism to all stereotypies [2,4,18,23]. This approach suggests that stereotypies should be regarded as malfunction behaviors, i.e., behaviors that are a product of CNS pathology. A pathological condition is generally not expected to be beneficial for the subject. However, as suggested by Mills, behaviors that are regarded as abnormal in a certain context, may in fact be adaptive when displayed under particular circumstances [25]. Furthermore, some psychological disorders, such as depression, may increase the individuals’ ability to cope with stressors in their environment [26]. Stereotypies are also hypothesized to result from a shift from cognitive control to stimulus–response learning processes, which may be considered to be adaptive as they free processing capacities, enabling mental processes to be redirected to other tasks [27]. The potential role of abnormal behaviors in promoting the adaptive ability of the subject has led several researchers to test the ability of stereotypic behavior to attenuate stress-induced responses of stereotypers (the coping hypothesis). Support for the coping hypothesis comes from a number of studies reporting, for example, that calves with high levels of oral stereotypies develop fewer stomach wall ulcers than calves with lower levels of that stereotypy [28]; and that blocking stereotypy by dopamine-depleting lesions of the caudate-putamen enhances the corticosterone response in rats [29]. Indeed, the notion that performing stereotypic behavior is rewarding helps to explain the development and sustainability of stereotypies [30]. Other studies, however, did not produce evidence for a stereotypy-related coping mechanism [e.g. 31]. Thus stereotypies tend to be either attributed to neurological pathology, or to an adaptive stress-attenuating response. These two views differ in their therapeutic consequences, and require different measures in regard to management. Notwithstanding, recent reports note positive feelings (e.g. pleasure and relief) accompanying the expression of stereotypies by autistic individuals known to possess an abnormality in the BG [32]. These reports suggest that stereotypies may indeed be related to BG dysfunction while also acting as an adaptive coping mechanism. However, to the best of our knowledge, studies to date have examined the link between stereotypy and either a neurological dysfunction or a coping ability with stress. Here we incorporated both assessments in two sets of experiments, using 15 adult rhesus macaques. In the first experiment, their levels of perseveration were evaluated in order to assess their BG function. In the second experiment the monkeys’ ability to cope with stress was evaluated. The data from both experiments were tested for correlations with the animals’ levels of stereotypy. We predicted that subjects showing higher levels of stereotypic behavior would be more perseverative than individuals expressing lower levels, and at the same time would be better able to cope with acute stressors.
2. Methods
2.1. ARB/ERC
This study was approved by the NICHD Animal Care and Use Committee # ASP-08-13.
2.2. Study animals
Data were collected between September and December 2010. Eight male and seven female, adult, captive-born rhesus macaques (Macaca mulatta) (mean age ± SD 9.1 ± 3.2 years) participated in this study. All were housed in the same room; seven in individual cages (81 cm × 81 cm × 71 cm) due to high levels of aggression that had been observed in prior pairing attempts, and eight were pair-housed in four sets of two connected single cages, at the NIH Animal Center, Poolesville, MD. They were provided with various plastic and metal manipulanda as well as foraging pads. Subjects were fed twice daily with commercial monkey biscuits and seeds immediately after each session. Fresh fruits or nuts were also dispensed daily. Water was provided ad libitum.
2.3. Procedures
2.3.1. Assessment of behavioral stereotypies
The animals were observed between September 2010 and November 2010, using continuous focal animal sampling for 20 min in each observation [33], for a total of eight hours for each animal. Three observation periods (morning, noon, afternoon) were conducted each day of data collection to account for possible daily fluctuations in the exhibition of stereotypies. Observations of each individual were rotated on a set schedule to equalize the number of observations in each daily period. Stereotypic behaviors were recorded on a lap-top computer using JWatcher 1.0 software, and clumped into three distinct behavioral categories (see Table 1) according to their form of expression [34]. Whole body stereotypic behaviors are characterized by gross repetitive motor actions through space and time; self-directed repetitive behaviors comprise behaviors directed at the monkey’s own body; and fine motor stereotypies include more delicate repetitive movements than whole body stereotypies, but which are not self-directed.
Table 1.
A list of the observed stereotypic behaviors divided into three categories: whole-body, self-directed, and fine-motor.
| Whole body stereotypies | |
| Pacing | Repetitive walking same path in cage |
| Swaying | Holding the upper flank of the cage with one arm while swinging |
| Rocking | A back and forth movement of the upper body with feet stationary |
| Bizarre posture | Animal sitting in a distorted manner, the hind limb may hold the trunk of the body |
| Self-directed stereotypies | |
| Biting nails | Clipping the tip of the nails using the teeth |
| Digit sucking | Sucking on a finger or toe |
| Plucking hair | Plucking out hair from the body using a hand |
| Fine motor stereotypies | |
| Licking/biting cage bars | Repetitive licking and/or biting the bars of the cage |
| Head twirling | Circular movement of the head |
| Head tossing | Vertical (up and down) movement of the head |
Following the observations, the animals’ levels of perseveration were determined.
2.3.2. Experiment 1—measurement of perseveration
2.3.2.1. Acquisition stage
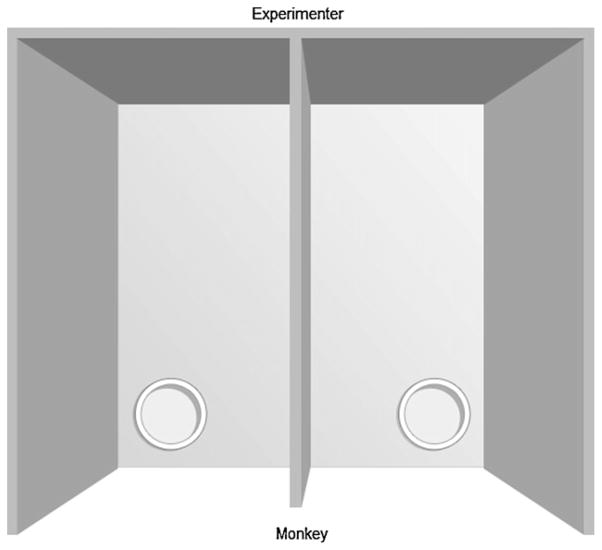
The apparatus was positioned in front of the monkey’s cage, and consisted of a plastic board (40 cm × 40 cm × 2 cm) with two holes (8 cm diameter × 2 cm deep) covered by opaque sliding caps, which the monkey had to push aside in order to reveal the contents of the hole. Holes were positioned 22 cm apart and 1 cm from the front and side edges (see Fig. 1). During the acquisition stage the monkeys were required to discriminate between the two holes such that each individual was rewarded (with a small piece of dried fruit) for choosing its randomly allocated correct hole. A trial was initiated by the experimenter raising the cover of the apparatus and ended either when the animal chose one of the holes, or when no response occurred within 30 s. Inter-trial intervals were 20 s. All animals received 1 session of 20 trials per day until a performance criterion of 90% (18 of 20 trials correct) over 3 consecutive sessions was met, and the monkey was considered to have learned the discrimination.
Fig. 1.
Top view of the apparatus. For details see Section 2.3.2.
2.3.2.2. Extinction stage
Once the performance criterion was met, sessions continued as previously but no rewards were given for responses to either hole. A criterion of 65% (13 of 20) or fewer responses made to the previously ‘correct’ side over 3 consecutive sessions indicated a return to choosing arbitrarily between the two holes: namely, extinction of the acquired response. The total number of responses an animal exhibited during extinction sessions before reaching this criterion was taken as its measure of perseveration. This factor was partialled for the number of trials taken to reach the acquisition criteria in order to control for motivational and general learning differences between the monkeys.
2.3.2.3. Persistent rapid response during extinction
Perseveration can also be assessed by the inability to inhibit rapid responses to the previously correct side. Therefore, the latency to choosing the previously rewarded hole during extinction was also measured, using video analysis. The latency to choose was measured from the point when the board was available to the monkey to the point when contact was made between the monkey and either hole-cap. First, the monkeys’ mean latencies to choosing the ‘correct’ (baited) side during the first and last acquisition session (the last acquisition session was also the last criterion session) were compared in order to test the assumption that their responses would became faster as they learned the task. Next, the monkeys’ mean latencies to choosing the previously ‘correct’ side during extinction was calculated and compared with the mean latencies to choosing the ‘correct’ side during the last acquisition session, in order to test the assumption that their responses during extinction would become slower. Finally, ANOVA with repeated measures was employed, with extinction session number as the within-subject factor and rate of stereotypic behavior as the between-subject factor, in order to examine whether high- and low-stereotypers differ in how their latency to choosing the previously ‘correct’ side changes over time from one extinction session to the next. This analysis was done for the rates of total stereotypy and for each of the three stereotypic behavior categories (whole-body, self-directed, and fine-motor). The cut-off factor (used to separate the monkeys into high- and low stereotypers) for each analysis was the median rate of stereotypy. Thus, high-stereotypers were predicted to have milder increases in mean latencies from one extinction session to the next than low-stereotypers.
2.3.3. Experiment 2—measurement of the reaction to acute stressors
2.3.3.1. Stress challenges
The monkeys were exposed to an acute stressor (a technician entering the room wearing catching gloves, and waving them in front of each cage for approximately 30 s), on three separate occasions throughout the entire experimental period.
2.3.3.2. Fecal corticoids analysis
The next morning fresh samples of feces were collected from the monkeys’ pre-cleaned trays underneath their home cages. Samples were placed in 50 ml tubes (Thomas Scientific) and kept at −20 °C until analysis (RIA). Fecal corticoid analyses were conducted at the Endocrine Laboratory, Smithsonian Conservation Biology Institute, Center for Species Survival, National Zoological Park, Front Royal. Data are presented as nanogram corticoids per gram dry feces.
Three additional fecal samples were collected from each monkey on Mondays in order to provide corticoid baseline levels over the weekend, which is considered to be less stressful due to a lower human activity level [35]. Individual fecal samples of pair-housed macaques were identified by adding a divider to the cage in the evenings preceding sample collection, cleaning the trays underneath each divided cage, and collecting the samples the following morning. We calculated a delta value for each subject by subtracting the average baseline corticoid levels from the average post-stress corticoid levels. Delta values were then tested for correlation with rates of stereotypy.
Rates of stereotypic behaviors, as well as test performance in the evaluation of perseveration, and the reaction to the stress challenges were additionally compared between singly- and pair-housed monkeys.
2.3.4. Statistical analysis
Pearson correlations performed in SPSS 18 were used throughout, following statistical transformations in order to meet parametric assumptions. Independent sample t-tests were used to check for possible differences between singly- and pair housed monkeys, while paired-sample t-tests and ANOVA with repeated measures were employed for the analyses of the latencies to choose a hole. In Experiment 1 tests were two-tailed, and in Experiment 2 tests were one-tailed because the sign of correlation was predicted. Alpha was set at 0.05.
3. Results
3.1. Singly- and pair-housed individuals
There was no difference in any of the measured parameters (rates of stereotypic behavior, number of sessions to reach acquisition and extinction criteria, number of trials to extinction, progressive change in the mean latency to choosing the previously rewarded side during extinction sessions, and in delta corticoid levels) between singly- and pair-housed individuals.
3.2. Rates of stereotypic behaviors
All study animals exhibited at least one type of stereotypic behavior. The most prevalent stereotypy among the 3 behavioral categories was pacing, expressed at different levels among 13 of the 15 study individuals (87% of subjects). Hair plucking was the dominant self-directed stereotypy, presented by 9 monkeys (60% of subjects), while licking or biting the bars of the cage was the most common fine motor stereotypy (12 subjects – 80%). Whole-body stereotypy was expressed at a rate of 0.25 ± 0.22 occurrences per minute (mean ± SD), with a maximum of 0.65 events per minute. The mean rate of self-directed stereotypy was 0.04 ± 0.03 behaviors per minute, with a maximum of 0.10 incidences per minute, while the mean rate of fine-motor stereotypy was 0.11 ± 0.24 stereotypies per minute, with a maximum of 0.93 episodes per minute.
3.3. Number of sessions to reach acquisition and extinction criteria
The monkeys needed 3.8 ± 1.4 (mean ± SD) sessions to acquire the behavior (range of 3–8 sessions), and an additional 3.9 ± 0.9 sessions to reach the extinction criteria (range of 3–6 sessions).
3.4. Experiment 1—stereotypic behavior and perseveration
3.4.1. Pooled categories of stereotypy
No significant correlation was found between the pooled rates of all stereotypic behaviors and the number of trials to extinction of the acquired behavior (p = 0.567, rp = 0.161, n = 15).
3.4.2. Whole-body stereotypy
Whole-body stereotypy was not significantly correlated with the number of trials to extinction of the acquired behavior (p = 0.472, rp = −0.201, n = 15).
3.4.3. Self-directed stereotypy
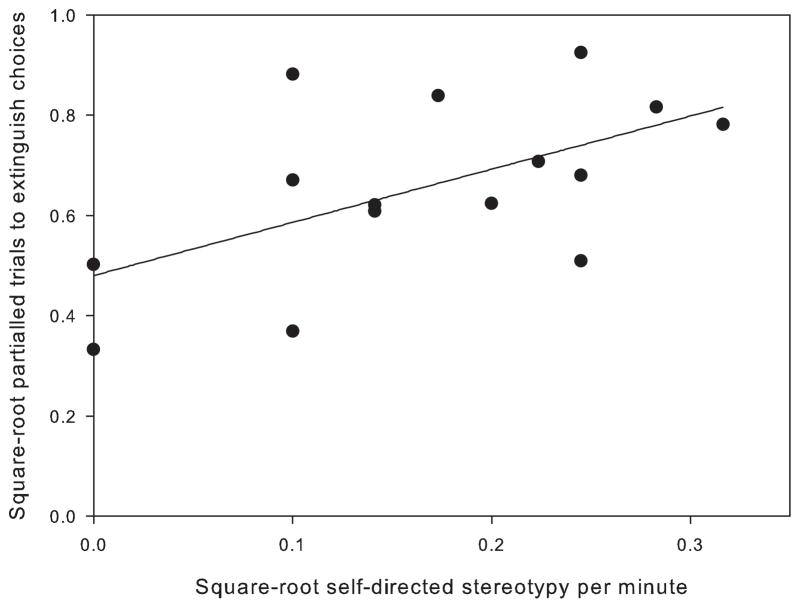
A significant positive correlation was found between rates of self-directed stereotypy and number of trials to extinction and (p = 0.024, rp = 0.577, n = 15) (Fig. 2).
Fig. 2.
Correlation between self-directed stereotypy and the number of trials to extinction of the acquired behavior partialled for (statistically controlled for) the number of trials taken to reach the acquisition criteria, n = 15. Data were square-root transformed to meet parametric assumptions.
3.4.4. Fine-motor stereotypy
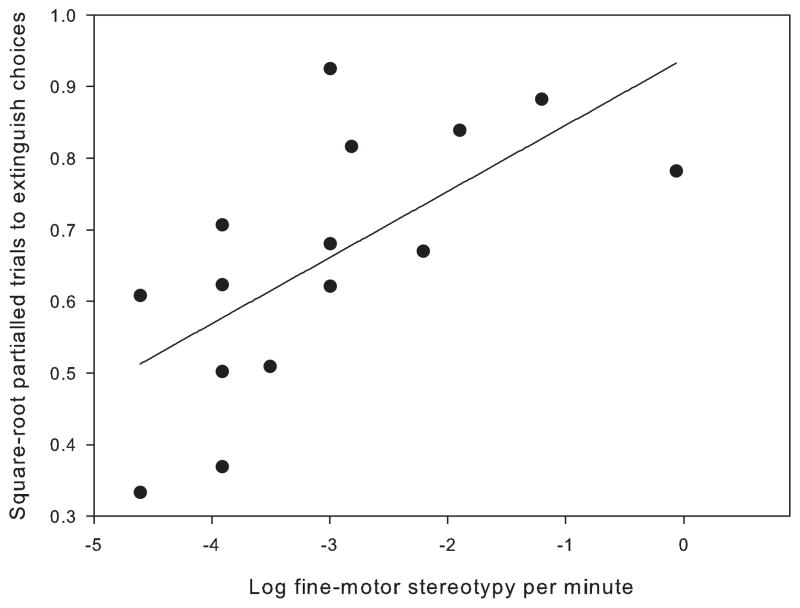
There was a significant positive correlation between rates of fine-motor stereotypic behavior and the number of trials to extinction (p = 0.005, rp = 0.682, n = 15) (Fig. 3).
Fig. 3.
Correlation between fine motor stereotypy and the number of trials to extinction of the acquired behavior partialled for the number of trials taken to reach the acquisition criteria, n = 15. Data were log transformed to meet parametric assumptions.
3.4.5. Latency to choosing the previously rewarded side
The monkeys displayed faster choices during the last session of the acquisition stage (which was also the last session of the learning criterion) compared to the first session (mean ± SD 1.3 s ± 0.8 and 3.0 s ± 1.7 respectively, t(14) = 4.123, p < 0.01). In the extinction stage, the mean latency to choosing the previously rewarded side was longer than in the last session of the learning criterion (7.2 s ± 3.7 and 1.3 s ± 0.8 respectively, t(14) = −6.574, p < 0.01). However, there was no difference between high- and low stereotypers in their progressive change in the latency to choosing the previously baited hole from one extinction session to the next. This similarity was evident in the lack of significant interaction between extinction session number and levels of stereotypic behavior, both for the total mean rate of stereotypic behavior (p = 0.532), and for the three behavioral categories (whole-body – p = 0.369, self-directed – p = 0.591, fine-motor – p = 0.407).
3.5. Experiment 2—stereotypic behavior and response to acute stressors
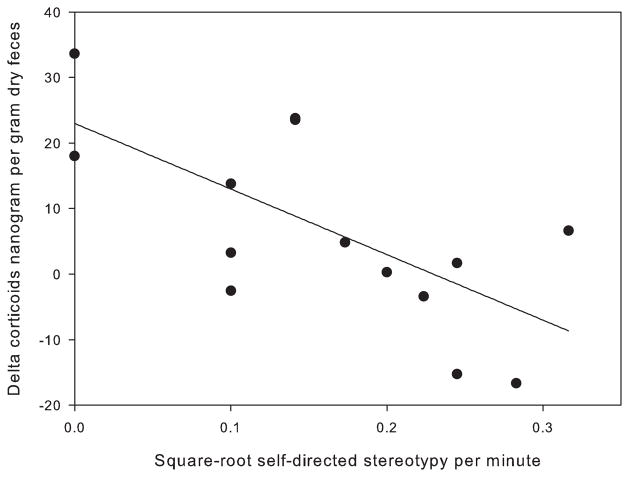
Self-directed stereotypy was the only category that was significantly negatively correlated with delta corticoid levels (ng/g fecal corticoids post-stress challenge minus baseline levels) (p = 0.003, rp = −0.673, n = 15) (Fig. 4).
Fig. 4.
Correlation between self-directed stereotypy and delta fecal corticoids (stress challenge levels minus baseline levels), n = 15. Stereotypy data were square-root transformed to meet parametric assumptions.
4. Discussion
4.1. Stereotypies and perseveration
4.1.1. The number of trials needed to extinguish the previously acquired behavior
The results of the present study suggest that certain stereotypies are related to perseveration, as evidenced by the larger number of trials needed to extinguish the previously acquired behavior in high-stereotypers compared to low-stereotypers. This finding is in accordance with previous studies involving both humans [22] and animals [23,24] that reported such a link. The use of neuropsychological tests to assess perseveration allows deduction of the neurological function of the subject, in particular of the indirect pathway of the BG [18]. Accordingly, both stereotypies and their experimental manifestations (i.e. perseveration) result from a failure of the CSS, located within the BG, to inhibit a response [18]. Additional support is found in MRI studies reporting various abnormalities in the BG among trichotillomania [36], and Tourette’s syndrome patients [37]. It is therefore suggested that the high levels of self-directed and fine-motor stereotypies expressed by rhesus macaques may reflect brain pathology, and these behaviors can thus be considered malfunctional. Categorizing stereotypies as malfunctional rather than maladaptive behaviors has several implications: first, as Mills [25] suggested, the classification of problem behaviors is required for their diagnosis and appropriate treatment plan. Maladaptive behaviors that reflect a normally functional animal reacting to the abnormalities within its environment require a fundamentally different intervention (e.g. environmental enrichment) to that of malfunctional behaviors that mirror a physiological pathology (e.g. pharmacological treatment). Second, using abnormal animals in scientific experiments may introduce unaccounted for factors into the results, thereby impairing validity, reliability, and replicability of the findings [2]. Moreover, suggesting that certain stereotypies are pathological in nature despite no previous neurological intervention, emphasizes the potentially destructive influence that captive environments have on animals, and should encourage scientists and decision-makers to seek ways by which to increase the psychological welfare of the animals. Indeed, unsuitable housing conditions are known to have deleterious effects on both brain development and behavior in both animals [38] and humans, such as the Post-Institutional Autistic Syndrome that was reported in orphans who were severely neglected prior to their adoption [11]. Finally, some authors [16,18] claim that certain self-directed behaviors that have been classified here as stereotypies should instead be categorized as compulsive behaviors. Compulsive behaviors differ from stereotypies in that they involve more flexible motor patterns, but occupy more rigid repetition of a goal [2], and involve other brain areas (PFC and BG respectively) [18]. Furthermore, stereotypies and compulsive behaviors should manifest in two distinct types of perseveration (recurrent or continuous, and ‘stuck-in-set’, respectively) [18]. However, despite the apparent disagreement between this view and the results of this work, we maintain that the behaviors that were classified here as self-directed are indeed ‘true’ stereotypies (like all the other abnormal repetitive behaviors that were observed in this research) since they were all rigid in motoric output each time they were displayed. Thus, for example, when subjects plucked their hair, they always used the same hand to pull hairs from the same part of the body in a highly rhythmical fashion. The significant correlation found between self-directed behaviors and perseveration is thus not surprising, and also does not contradict the classification of analogous forms of self-directed repetitive behaviors such as barbering in mice, which is more flexible in form, and goal-directed [2].
In contrast to the correlations between self-directed and fine-motor stereotypies and the ability to suppress a previously acquired behavior, no such finding was attained with whole-body stereotypy. The most prominent stereotypy in this group was pacing, which was also reported to be the most common among rhesus macaques in much larger samples than the current one [8]. Interestingly, results from another recent study suggest that in capuchin monkeys, stereotypic head twirls are a reliable indicator of a negative affective state whereas pacing is not [51]; and a different study showed that the use of serotonergic agents (fluoxetine, and buspirone) had no impact on levels of pacing among captive rhesus monkeys [39]. The role and meaning of stereotypic pacing among NHP thus remain elusive and open to interpretation. In zoo-housed carnivores, levels of pacing were found to be correlated with the habitat-range size, and the daily distance traveled in the wild [40]. Therefore, it can be suggested that whole-body stereotypy such as pacing reflects the motivation of the animal to expend energy, and perhaps relieve the boredom associated with the impoverished environment.
Although using the animals’ performances in extinction as a measure of perseveration has been reported in a number of species [e.g. 23, 24, 41], additional techniques have been developed in order to better replicate human neuropsychological tasks, and to exclude other factors such as motivation or hedonic differences that may influence extinction learning (e.g. the bias-corrected gambling task [4]). In the current study, however, we chose to employ extinction learning as a measure of perseveration, since our analyses included internal controls that address the concerns that may be related to this paradigm by partialling for spatial discrimination performance [41], and because it has been used successfully in several different species, including in rhesus macaques [42].
4.1.2. The latency to choose the previously rewarded side
Stereotypies observed in human mental disorders, as well as those artificially induced by drugs and brain lesions, appear to be related to an inability to suppress rapid responses when they are no longer appropriate (i.e. in extinction) [30]. The current analysis shows that, as expected, the monkeys’ responses became faster as they learned the task, and then slower as extinction progressed. However, individuals with higher rates of stereotypy did not differ from individuals with lower levels in their ability to suppress rapid responses when they were no longer appropriate. It can be suggested that while measuring the number of trials that are needed to extinguish the previously acquired behavior is more resistant to external disturbances (such as stimuli unconnected to the experiment), other unrelated features, such as distractions within the subject’s immediate environment, may also have influenced the latency to choose. Therefore, in future work subjects should be tested in an isolated environment, and trials in which unrelated interruptions were observed should be excluded from the analysis.
It may also be suggested that despite the difficulty shown by stereotyping macaques in inhibiting their response when it was no longer rewarded (as seen in the number of trials to extinction), their perception that the response was no longer suitable nonetheless remained unaffected (as seen in the similar progressive change in latencies to choosing the previously rewarded side during extinction displayed by high- and low-stereotypers). This phenomenon is somewhat similar to the knowledge-action dissociation observed in stereotypic humans that are unable to withhold the inappropriate repetition of a previous response despite knowing that it is not the correct one [43]; as well as in bank voles, in which the most stereotypic voles presented the smallest increases in their latencies to choose the previously rewarded side (i.e. were unable to suppress rapid response), and at the same time returned to randomly choosing between the two sides (i.e. learned the extinction task) [23]. The inability to inhibit a behavior despite knowing it is no longer appropriate was suggested to cause frustration in the animals, and thereby impair their psychological welfare [18].
4.1.3. Stereotypic behavior and the coping hypothesis
The notion that stereotypies involve stress-reducing effects has been examined in several studies, but a single firm conclusion has not been reached, as some have indicated a stress-alleviating effect of stereotypic behaviors (in humans; e.g. [44,45], in animals; e.g. [46]), while others have not [31,47].
The results of the second experiment suggest that expressing self-directed stereotypy may involve an adaptive mechanism that enables the animals to better cope with stress. Monkeys with high levels of self-directed stereotypy had milder increases in their fecal corticoids following an acute stress challenge than subjects with lower rates of that stereotypy. Indeed, self-directed stereotypy such as digit-sucking were previously reported to have a calming effect on the individual [48], and this rewarding effect is a potential contributor to the repetitive nature of the behavior [49]. If self-directed stereotypy is indeed adaptive, then preventing animals from expressing it is likely to exacerbate the animals’ psychological wellbeing, and should be avoided. Nevertheless, it is emphasized that the appearance of self-directed stereotypy on its own is not a sign of good welfare; only that, within an aversive environment, those individuals that express those behaviors are likely to suffer the least [6]. Finally, although we did not assess the levels of stereotypic behaviors immediately after the stress challenges, it is predicted that their expression will increase at this time due to their potential stress-alleviating effect. This prediction should be the subject of future research.
4.1.4. Interpreting the combined results of Experiments 1 and 2
Among the three stereotypic categories, one (whole-body) was not correlated with either perseveration or with stress-alleviating effects; another (fine-motor) was correlated with perseveration; and the third (self-directed) was correlated with both perseveration and better coping abilities with stress. These results suggest that viewing all the repetitive, unvarying, and apparently functionless behaviors as one homogeneous group of stereotypic behaviors is misleading, since they are probably related to different causes: some are malfunctional behaviors, some maladaptive, and some cannot be termed non-functional at all. For example, the finding that whole-body stereotypies do not correlate with perseveration suggests that they are not linked to decreased dorsal striatal inhibitory behavioral control, and therefore do not fit to the ‘Unifying Theory’ proposed by Garner [18]. In addition, the apparent adaptive value of expressing self-directed abnormal repetitive behaviors in coping with stress contradicts the notion that stereotypic behaviors are functionless. Thus, the discrepancy found between the classical definition of stereotypic behaviors and the results of the current research emphasizes the need for a more accurate classification of all abnormal repetitive behaviors in order to better understand their causal factors, their functions, and their relevance to the welfare of those that express them. Mason’s [50] suggestion to redefine stereotypic behavior as a ‘repetitive behavior induced by frustration, repeated attempts to cope, and/or CNS dysfunction’ emphasizes the environmental and mechanistic causes of these abnormal behaviors, rather than focusing on their phenotypic expressions, and may indeed offer a more suitable means by which to classify these behaviors.
To the best of our knowledge, this study reports for the first time a dual relation of stereotypies to both an indication of brain pathology and to an adaptive quality of the expression of that pathology. This idea is in concert with reports from autistic individuals that are known to suffer from BG dysfunction [32], while also reporting a sense of relief when expressing stereotypies [6]. Although the present study points to a relationship between the expression of stereotypy and a malfunction in the underlying neurological mechanism on the one hand, and at a coping mechanism with stress on the other, further research is needed to determine whether these two stereotypy related factors affect one another.
Acknowledgments
We wish to thank Stephen J. Suomi, Pam Noble and Ernest Davis for their assistance in arranging this study; Daphna Joel and two anonymous reviewers for insightful remarks on previous drafts of the paper; and Naomi Paz for her help in preparing and editing this manuscript.
Footnotes
This work is dedicated to the memory of James Winslow.
References
- 1.Anna I, Olsson S, Dahlborn K. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment’. Laboratory Animals. 2002;36:243–70. doi: 10.1258/002367702320162379. [DOI] [PubMed] [Google Scholar]
- 2.Garner JP. Stereotypies and other abnormal repetitive behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J. 2005;46:106–17. doi: 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R. Symposium on indices to measure animal well-being: behavioral, physiological and functional aspects of stereotyped behavior: a review and a re-interpretation. Journal of Animal Science. 1986;62:1776–86. doi: 10.2527/jas1986.6261776x. [DOI] [PubMed] [Google Scholar]
- 4.Garner JP, Meehan CL, Mench JA. Stereotypies in caged parrots, schizophrenia and autism: evidence for a common mechanism. Behavioural Brain Research. 2003;145:125–34. doi: 10.1016/s0166-4328(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 5.Mason G. Stereotypies and suffering. Behavioural Processes. 1991;25:103–15. doi: 10.1016/0376-6357(91)90013-P. [DOI] [PubMed] [Google Scholar]
- 6.Mason GJ, Latham NR. Can’t stop, won’t stop: is stereotypy a reliable animal welfare indicator. Animal Welfare. 2004;13:57–69. [Google Scholar]
- 7.Conlee KMH, Erika H, Stephens, Martin L. A demographic analysis of primate research in the United States. Alternatives to Laboratory Animals. 2004;32:314–22. doi: 10.1177/026119290403201s52. [DOI] [PubMed] [Google Scholar]
- 8.Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- 9.Mason GJ, Cooper J, Clarebrough C. Frustrations of fur-farmed mink. Nature. 2001;410:35–6. doi: 10.1038/35065157. [DOI] [PubMed] [Google Scholar]
- 10.Ridley RM, Baker HF. Stereotypy in monkeys and humans. Psychological Medicine. 1982;12:61–72. doi: 10.1017/s0033291700043294. [DOI] [PubMed] [Google Scholar]
- 11.Hoksbergen R, Laak J, Rijk K, van Dijkum C, Stoutjesdijk F. Post-institutional autistic syndrome in romanian adoptees. Journal of Autism and Developmental Disorders. 2005;35:615–23. doi: 10.1007/s10803-005-0005-x. [DOI] [PubMed] [Google Scholar]
- 12.Latham NR, Mason GJ. Maternal deprivation and the development of stereotypic behaviour. Applied Animal Behaviour Science. 2008;110:84–108. [Google Scholar]
- 13.Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Research Reviews. 1998;28:235–85. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 14.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive–compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 15.Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereo-typy. Nature Neuroscience. 2000;3:377–83. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 16.Garner JP, Thogerson CM, Dufour BD, Wurbel H, Murray JD, Mench JA. Reverse-translational biomarker validation of abnormal repetitive behaviors in mice: an illustration of the 4P’s modeling approach. Behavioural Brain Research. 2011;219:189–96. doi: 10.1016/j.bbr.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of Neurology. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Garner JP. Perseveration and stereotypy—system-level insights from clinical psychology. In: Mason GJ, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications to welfare. CABI; 2006. pp. 121–52. [Google Scholar]
- 19.Norman DA, Shallice T. Attention to action: willed and automatic control of behaviour. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation: advances in research and theory. New-York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- 20.Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 21.Tanimura Y, King MA, Williams DK, Lewis MH. Development of repetitive behavior in a mouse model: roles of indirect and striosomal basal ganglia pathways. International Journal of Developmental Neuroscience. 2011;29:461–7. doi: 10.1016/j.ijdevneu.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crider A. Perseveration in schizophrenia. Schizophrenia Bulletin. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- 23.Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behavioural Brain Research. 2002;136:83–92. doi: 10.1016/s0166-4328(02)00111-0. [DOI] [PubMed] [Google Scholar]
- 24.Vickery SS, Mason GJ. Stereotypy and perseverative responding in caged bears: further data and analyses. Applied Animal Behaviour Science. 2005;91:247–60. [Google Scholar]
- 25.Mills DS. Medical paradigms for the study of problem behaviour: a critical review. Applied Animal Behaviour Science. 2003;81:265–77. [Google Scholar]
- 26.Price J, Sloman L, Gardner R, Gilbert P, Rohde P. The social competition hypothesis of depression. In: Baron-Cohen S, editor. The maladaptive mind; classic readings in evolutionary psychopathology. Hove: Psychology Press; 1997. pp. 241–53. [Google Scholar]
- 27.Toates F. The interaction of cognitive and stimulus–response processes in the control of behaviour. Neuroscience & Biobehavioral Reviews. 1997;22:59–83. doi: 10.1016/s0149-7634(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 28.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience & Biobehavioral Reviews. 1999;23:925–35. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 29.Jones GH, Mittleman G, Robbins TW. Attenuation of amphetamine-stereotypy by mesostriatal dopamine depletion enhances plasma corticosterone: implications for stereotypy as a coping response. Behavioral and Neural Biology. 1989;51:80–91. doi: 10.1016/s0163-1047(89)90686-9. [DOI] [PubMed] [Google Scholar]
- 30.Wurbel H. The motivational basis of caged rodents’ stereotypies. In: Mason G, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications to welfare. CABI; 2006. pp. 86–120. [Google Scholar]
- 31.Wurbel H, Freire R, Nicol CJ. Prevention of stereotypic wire-gnawing in laboratory mice: effects on behaviour and implications for stereotypy as a coping response. Behavioural Processes. 1998;42:61–72. doi: 10.1016/s0376-6357(97)00062-4. [DOI] [PubMed] [Google Scholar]
- 32.Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23:613–24. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 33.Martin P, Bateson P. Measuring behaviour. 2. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 34.Novak MA, Meyer JS, Lutz C, Tiefenbacher S. Deprived environments: developmental insights from primatology. In: Mason G, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications to welfare. CABI; 2006. pp. 153–89. [Google Scholar]
- 35.Lambeth SP, Bloomsmith MA, Alford PL. Effects of human activity on chimpanzee wounding. Zoo Biology. 1997;16:327–33. [Google Scholar]
- 36.O’Sullivan RL, Rauch SL, Breiter HC, Grachev ID, Baer L, Kennedy DN, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biological Psychiatry. 1997;42:39–45. doi: 10.1016/S0006-3223(96)00297-1. [DOI] [PubMed] [Google Scholar]
- 37.Berardelli A, Curr A, Fabbrini G, Gilio F, Manfredi M. Pathophysiology of tics and Tourette syndrome. Journal of Neurology. 2003;250:781–7. doi: 10.1007/s00415-003-1102-4. [DOI] [PubMed] [Google Scholar]
- 38.Wurbel H. Ideal homes? Housing effects on rodent brain and behaviour Trends in Neurosciences. 2001;24:207–11. doi: 10.1016/s0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- 39.Fontenot MB, Padgett EE, III, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comparative Medicine. 2005;55:67–74. [PubMed] [Google Scholar]
- 40.Clubb R, Mason G. Animal welfare captivity effects on wide-ranging carnivores. Nature. 2003;425:473–4. doi: 10.1038/425473a. [DOI] [PubMed] [Google Scholar]
- 41.Garner JP, Mason GJ, Smith R. Stereotypic route-tracing in experimentally caged songbirds correlates with general behavioural disinhibition. Animal Behaviour. 2003;66:711–27. [Google Scholar]
- 42.Beauchamp AJ, Gluck JP. Associative processes in differentially reared monkeys (Macaca mulatta): sensory preconditioning. Developmental Psychobiology. 1988;21:355–64. doi: 10.1002/dev.420210406. [DOI] [PubMed] [Google Scholar]
- 43.Turner M. Towards an executive dysfunction account of repetitive behaviour in autism. In: Russell J, editor. Autism as an executive disorder. Oxford: Oxford University Press; 1997. pp. 57–100. [Google Scholar]
- 44.Fox MW. Psychopathology in man and lower animals. Journal of the American Veterinary Medical Association. 1971;159:66–77. [PubMed] [Google Scholar]
- 45.Soussignan R, Koch P. Rhythmical stereotypies (leg-swinging) associated with reductions in heart-rate in normal school children. Biological Psychology. 1985;21:161–7. doi: 10.1016/0301-0511(85)90027-4. [DOI] [PubMed] [Google Scholar]
- 46.Novak MA. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- 47.Terlouw EMC, Lawrence AB, Ladewig J, De Passille AM, Rushen J, Schouten WGP. Relationship between plasma cortisol and stereotypic activities in pigs. Behavioural Processes. 1991;25:133–53. doi: 10.1016/0376-6357(91)90016-S. [DOI] [PubMed] [Google Scholar]
- 48.Wolff PH, Simmons MA. Nonnutritive sucking and response thresholds in young infants. Child Development. 1967;38:631–8. doi: 10.1111/j.1467-8624.1967.tb04584.x. [DOI] [PubMed] [Google Scholar]
- 49.Wurbel H. The coping hypothesis of stereotypic behavior. In: Mason G, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications to welfare. CABI; 2006. pp. 14–5. [Google Scholar]
- 50.Mason G. Stereotypic behaviour in captive animals: fundamental and implications for welfare and beyond. In: Mason G, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications to welfare. CABI; 2006. pp. 325–51. [Google Scholar]
- 51.Pomerantz O, Terkel J, Suomi JS, Paukner A. Stereotypic head-twirls, but not pacing, are related to a ‘pessimistic’-like judgment-bias among captive tufted capuchins (Cebus apella) 2012 doi: 10.1007/s10071-012-0497-7. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]