1. Introduction
Successful reproductive behavior relies on the ability to identify and approach appropriate mating partners. In many rodent species, including the Syrian hamster, social odors function as the primary signal guiding mate recognition and other reproductive behaviors [1, 2]. Indeed, many rodent species display robust preferences to investigate opposite-sex odors, as well as odor-specific scent marking responses [1, 3, 4].
Substantial evidence suggests that, rather than being fixed across the lifespan, many of these odor-guided behaviors are shaped by prior experience. In particular, olfactory experience during early life appears to play a critical role in the development of odor preferences [5]. Functionally, the olfactory system develops very early in rodents [6, 7] and can process odors within the first few days of life [7–9] or even in utero [10, 11]. By manipulating subjects’ early post-natal olfactory experience, either through cross-fostering techniques [12–17] or exposure to artificial odorants [18–21], several studies have shown that odors associated with the rearing environment are often preferred to unfamiliar odors when tested in adulthood. For example, male Syrian hamsters cross-fostered to Turkish hamster dams display a decreased preference to investigate conspecific odors [22].
Although these studies demonstrate the importance of chemosensory experience in the development of species-specific odor preferences, less is known about how early experience might guide preferences for opposite-sex over same-sex odors. Recent work by Martinez-Garcia and colleagues has provided some initial evidence that the development of sexual odor preferences in female mice may actually require prior chemosensory experience [23]. Specifically, by removing all male siblings from litters early in development (minimizing female subjects’ exposure to male odors) the authors showed that, as adults, these females did not display the typical preference to investigate male over female volatile odors. However, when subjects were allowed to contact the odors sources (providing both volatile and non-volatile components of the odors), these chemically-naïve females did display a strong preference to investigate male odors. The authors hypothesized that the non-volatile components of sexual odors are innately attractive, whereas the volatile components of these odors only acquire attractive qualities after association with non-volatile cues [23, 24].
These results suggest that the development of sexual odor preferences involves some form of early olfactory learning, yet critical questions remain. First, to what extent does the experience-dependent nature of sexual odor preferences observed in female mice generalize to other rodent species, particularly those with divergent ecologies and social structures? Second, does the expression of other forms of odor-guided reproductive behaviors also require prior chemosensory experience? For example, although scent-marking responses to sexual odors are observed in many rodent species and are known to play a critical role in reproductive communication [1, 25, 26], few studies have identified the developmental plasticity of these behaviors.
To address these issues, we tested whether female Syrian hamsters (a solitary species [27]) require early chemosensory experience with male odors in order to show adult proceptive sexual behavior toward male odors. In addition to investigatory preferences for male odors [28, 29], female hamsters also display a sexually motivated scent marking response, referred to as vaginal marking, that is specifically directed toward males or their odors [30–32]. The goal of this study was therefore to measure investigatory preferences (Experiment 1) or vaginal marking responses (Experiment 2) toward sexual odors in female subjects that had restricted exposure to male odors during development [23]. Furthermore, to determine if any experimental effect was specific to processing of the volatile components of sexual odors, these behaviors were tested under two stimulus conditions: (1) volatile odors only; in which contact with the odor source was prevented and (2) volatile and non-volatile odors; in which contact with the odors source was allowed.
2. Materials and methods
2.1. Subjects
All subjects were bred in our laboratory from Syrian hamsters (Mesocricetus auratus) that had been purchased from Charles River Laboratory (Wilmington, MA, USA). Breeding pairs were allowed to mate in a clean cage for thirty minutes and then returned to their home cages. Mated females were housed in an all-female room and monitored daily for the presence of pups. A separate group of singly-housed male and female Syrian hamsters, 3–10 months old, served as odor donors. These hamsters were either bred in our colony or purchased from Charles River Laboratory. Subjects were unrelated to, and had no previous contact with, these odor donors.
Three to five days after birth, subjects were sexed using anogenital distance and female subjects were assigned to one of two experimental groups: naïve or control. Naïve females had all male siblings removed from their litter, whereas control females had an equal number of male and female siblings removed from their litter. Consequently, the handling procedure and total number of pups in the litter were comparable between experimental groups; the primary difference between naïve and control females was the presence or absence of male siblings. A total of 19 litters were used (naïve, 9 litters; control, 10 litters) in Experiment 1; 18 litters were used for Experiment 2 (naïve, 8 litters; control, 10 litters). The final number of pups per litter, after experimental manipulation, ranged from 5 to 6 in the naïve condition and 4 to 7 in the control condition. Subjects remained in the cage with their mother and siblings until 22–24 days of age, when they were singly-housed. In order to prevent exposure to male odors, all subjects were housed in an all-female room and always handled using clean latex gloves.
Prior to behavioral testing (3–4 months of age), estrous cycles of the subjects were determined by monitoring the consistency of vaginal secretion for at least eight consecutive days. Vaginal secretion was collected by gently palpating the vaginal area with a disposable probe; secretion with a stringy consistency indicated animals were in behavioral estrus [33]. Behavioral estrus was also verified by manually stimulating the hind and flank quarters of the female and observing the presence/absence of the lordosis (receptive) posture [34].
All animals were housed in solid-bottom Plexiglas cages (36 cm × 30 cm × 16 cm) and maintained on a reversed 14-h light/10-h dark photoperiod. Food and water were available ad libitum. The Georgia State University Institutional Animal Care and Use Committee approved all animal procedures.
2.2. Experiment 1: Preference to investigate male odors over female odors
2.2.1. Y-maze apparatus
Subjects were tested for their preference to investigate male odors over female odors by presenting these stimuli within a Y-maze apparatus [35]. The Y-maze consisted of a stem arm (61 cm long) and two side arms (68 cm long). All arms of the maze were 10 cm wide, with walls 10 cm high. Each side arm had a stimulus chamber (20 cm long), where odor stimuli (see below) were placed, at its distal end. To measure preference for the volatile components of the odors, perforated doors on the stimulus chambers prevented contact with the odor stimuli, but allowed airflow (Volatile condition). To measure preference for both the volatile and non-volatile components of the odors, stimulus chamber doors were removed, and the subjects were allowed to contact the odor stimuli (Contact condition). A start chamber (20 cm long), with a removable, perforated door, was located at the distal end of the stem arm. An electric fan was located behind the start chamber and pulled air from the stimulus chambers through the entire length of the Y-maze (airflow rate of 2.0 km/hr, measured at the start box). The top of the Y-maze was secured with a clear Plexiglas top to allow for overhead video recording of the subject’s behavior.
2.2.2. Odor stimuli
Male and female odor stimuli were collected from cages that had housed a single odor donor and had not been changed for 12–14 days. Odor stimuli consisted of 12 g of soiled cotton bedding (4 Nestlets, ANCARE, Bellmore, NY); 50 ml of soiled corncob litter; one damp cotton gauze pad that was used to wipe along the inner walls of the odor donor cage; and an additional damp gauze pad that was used to wipe the odor donor’s bilateral flank glands and anogenital region. For female odor stimuli, vaginal secretion was collected onto an additional gauze pad by inducing an estrous donor female into lordosis and gently palpating the vaginal area with a disposable probe. Clean odor stimuli consisted of unsoiled components identical to those of the social odor stimuli. All odor stimuli were stored in plastic bags at 4°C until thirty minutes before use. Odor samples older than three months were discarded, and care was taken to ensure that subjects were not tested with the same individual’s odor more than once. Clean latex gloves were worn while collecting odor samples to prevent contamination of odor cues. For the Volatile conditions, each odor stimulus was used for two consecutive tests. For Contact conditions, each odor stimulus was used for only one test to prevent contamination of the odor sources between subjects.
2.2.3. Behavioral testing
Naïve (n = 22) and control (n = 25) females were tested on consecutive estrous days in a sequence of Y-maze tests: Clean, Volatile-Preference, Contact-Preference. Females were tested twice in each type of Y-maze test, for a total of six tests. To both habituate the subjects to the Y-maze and obtain baseline behavioral data, females were tested with clean odor stimuli in each stimulus chamber (Clean). To measure sexual odor preference under different stimulus conditions (Volatile, Contact), male and female odor stimuli were presented in opposite stimulus chambers. In both the Clean and Volatile-Preference tests, stimulus chamber doors were used to prevent contact with the odor stimuli; In the Contact-Preference tests, the stimulus chamber doors were removed to allow contact with the odor stimuli.
Subjects were placed in the start chamber for one minute, after which the door was removed and subjects were allowed nine minutes to explore the Y-maze. After each test, all surfaces of the Y-maze were thoroughly cleaned with 50% alcohol and allowed to dry. Video recordings of all Y-maze tests were digitized onto a computer and scored using the Observer for Windows, version 5.0 (Noldus Information Technology B.V., Wageningen, The Netherlands). All observers were blind to the condition of the subject and different observers reached at least an 85% inter-observer reliability score prior to coding behavior. Both the time spent investigating the stimulus chambers and the number of entries into each arm of the Y-maze were scored. Arm entry was coded when the front half of the subject’s body crossed into that arm. Investigation of the stimulus chamber was coded when the subject made contact with, or directed its nose within 1 cm of, the stimulus chamber door (Volatile conditions) or the odor source itself (Contact conditions).
2.2.4. Data analysis
For each type of Y-maze test (Clean, Volatile-Preference, Contact-Preference), data were averaged across repeated test days. To establish preference for one stimulus over the other, separate mixed-design ANOVAs, with stimulus (Preference tests: male, female odor; Clean tests: left, right stimulus chamber) as the within-subjects factor and experimental group (naïve, control) as the between-subjects factor, were performed. Interactions for these mixed designs were explained using simple-effects analyses. As a measure of general activity level, a one-way ANOVA was used to compare the total number of arm entries made during the Clean tests between experimental groups.
Finally, in order to determine whether the relative amount of exposure to male odors could affect the development of odor preferences, a Pearson correlation matrix was used to analyze the relationship between the number of male siblings in the litter (prior to experimental manipulation for naïve group) and the strength of the sexual odor preference in the Y-maze (preference score = male odor investigation – female odor investigation). Data were split by experimental condition (naïve, control), and separate correlations were performed for Volatile-and Contact-Preference tests.
2.3. Experiment 2: Vaginal marking in response to male and female odors
2.3.1. Odor stimuli and Apparatus
Females were tested for their vaginal marking responses to male odors or female odors by placing them inside a vacated home cage of a male or female odor donor, respectively. One group of naïve (n = 16) and control (n = 16) females were tested for their vaginal marking responses to only the volatile components of the odor stimuli (Volatile-VM), whereas a separate group of naïve (n = 10) and control (n = 11) females were tested for their vaginal marking responses to both the volatile and non-volatile components of the odor stimuli (Contact-VM). For Volatile-VM tests, a clean inner cage, which had 0.5 cm holes drilled 2.5 cm apart along its walls, was placed inside the stimulus cage. Consequently, subjects were surrounded by, but could not contact, the odor sources. For Contact-VM, females were placed into a stimulus cage that contained a Plexiglas marking plate (40 cm × 19 cm; 0.3 cm holes drilled 2 cm apart) that provided a marking surface but allowed contact with the litter, bedding and walls of the cage. Odor donor cages had not been changed for 13–16 days, and each stimulus cage was used only once.
2.3.2. Behavioral testing
Each female was tested for her vaginal marking response to male odors and female odors on separate and consecutive proestrous days. Vaginal marking by female Syrian hamsters is maximal on proestrus (day before behavioral estrus is observed) [30]. The order of odor stimulus tested (male, female) was counterbalanced across experimental groups. Vaginal marking was measured by placing a female subject into the stimulus cage (see above) for fifteen minutes. To habituate females to the testing condition, females were placed into a clean stimulus cage for five minutes immediately prior to being transferred into a male or female stimulus cage; behavior was not scored during this habituation period. During the testing period, vaginal marking was scored each time the female pressed her genital region into the substrate and moved forward [30]. The total number of vaginal marks made during the test was scored with a hand-held counter, and the latency to make the first vaginal mark was scored with a stopwatch. For subjects that failed to VM during the test, a latency score of 900 seconds (total time of test) was recorded.
2.3.2. Data analysis
Vaginal marking behavior was analyzed with a mixed-design ANOVA, with odor stimulus (male, female) as the within-subjects factor and experimental group (naïve, control) as the between-subjects factor. Separate one-way ANOVAs were performed for Volatile-VM and Contact-VM tests to compare levels of vaginal marking, as well as latencies to make the first vaginal mark, between experimental groups. As in Experiment 1, a Pearson correlation matrix was used to determine the relationship between the number of male siblings in the litter and the differential vaginal marking behavior (VM score = number of VM in response to male odors –number of VM in response to female odors). Data were split by experimental condition (naïve, control), and separate correlations were performed for Volatile- and Contact-Preference tests.
3. Results
3.1. Experiment 1: Preference for male odors over female odors
3.1.1. Clean tests
Both naïve and control females investigated the left and right sides of the Y-maze equally, F(1, 45) = 2.682, p >.05. There was no significant difference in investigation between the experimental groups, F(1, 45) =.106, p >.05, nor was there an interaction between experimental group and stimulus chamber side, F(1, 45) =.986, p >.05. Furthermore, when the investigation times were summed for the left and right arms, naïve and control females did not differ in their total duration of investigation, F(1, 45) =.284, p >.05. Together, these results indicate a lack of side-bias in the Y-maze and equivalent levels of baseline investigation between experimental groups. Levels of activity, as measured by the total number of arm entries, were also not different between naïve and control females, F(1,45) =.033, p >.05. Table 1 summarizes behavioral measures from the Clean tests.
Table 1.
Summary of behavioral measures from Clean Y-maze tests in Experiment 1.
| Total number of arm entries | Investigation time (left) | Investigation time (right) | |
|---|---|---|---|
| Control | 28.1 ± 1.3 | 64.2 ± 5.4 | 60.4 ± 7.5 |
| Naïve | 27.7 ± 1.9 | 67.7 ± 4.1 | 52.5 ± 7.2 |
Values are mean ± SEM, and investigation times are in seconds. Both experimental groups investigated the left and right stimulus sides equally. There were no differences in general activity levels or total investigation levels between experimental groups, all p >.05.
3.1.2. Volatile-Preference tests
Both naïve and control females investigated male odors longer than female odors, F(1, 45) = 20.043, p <.05 (Figure 1a). Importantly, there was no significant effect of experimental group, F(1, 45) = 1.935, p >.05, nor was there a significant interaction between odor stimulus and experimental group, F(1, 45) =.220, p >.05. When investigation times were summed for the two stimulus arms, naïve and control females did not differ in their total duration of odor investigation, F(1, 45) = 1.935, p >.05. There was also no significant relationship between the number of male siblings and the derived preference scores for either naïve females, r =.115, p >.05, or control females, r = −.142, p >.05.
Figure 1.
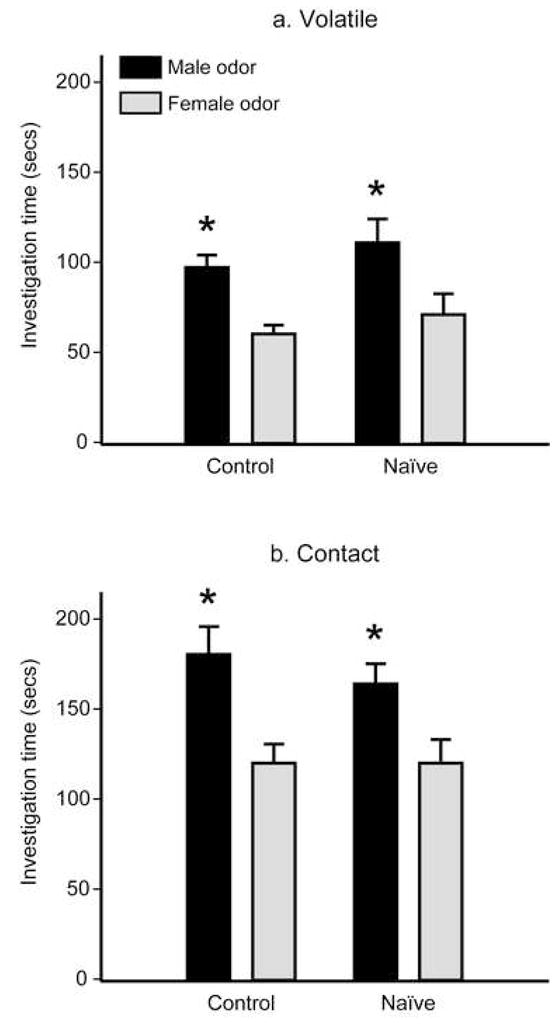
Mean (± SEM) odor investigation times (seconds) from Y-maze preference tests in Experiment 1. All females investigated male odors longer than female odors in tests that either (a) prevented or (b) allowed contact with the odor stimuli. * p <.05 compared to investigation times of female odor within an experimental group.
3.1.3. Contact-Preference tests
As in the Volatile-Preference tests, both naïve and control females investigated male odors longer than female odors when contact with the odor stimuli was allowed, F(1, 45) = 21.022, p <.05 (Figure 1b). There was no significant effect of experimental group, F(1, 45) =.487, p >.05, nor was there a significant interaction between these stimulus and experimental group, F(1, 45) =.700, p >.05. Naïve and control females did not differ in their total duration of odor investigation, F(1, 45) =.487, p >.05. There was also no significant relationship between the number of male siblings and preference scores for either naïve females, r = −.113, p >.05, or control females, r =.016, p >.05.
3.2. Experiment 2: Vaginal marking in response to male and female odors
3.2.1. Volatile vaginal marking tests
There was a significant interaction between experimental group and vaginal marking in response to male and female odors, F(1,30) = 7.267, p <.05. Whereas control females vaginal-marked more in response to male compared to female volatile odors, F(1,15) = 19.947, p <.05, naïve females marked equally in response to the two odor sources, F(1,15) = 1.393, p >.05 (Figure 2a). When levels of vaginal marking were compared directly between groups, however, naïve and control females did not differ in the number of vaginal marks in response to either male odors, F(1,30) = 1.820, p >.05, or female odors, F(1,30) =.952, p >.05. There were no differences in the latencies to vaginal mark in response to male odors, F(1,30) =.957, p >.05, or to female odors, F(1,30) =.001, p >.05 (Table 2). When the variability in vaginal marking behavior was further examined, a significant positive correlation between the number of male siblings in the litter and the derived VM score detected for the naïve condition, r =.573, p <.05 (Figure 3a), but not for the control condition, r = −.195, p >.05 (Figure 3b).
Figure 2.

Mean (± SEM) number of vaginal marks made in response to male or female odors in Experiment 2. (a) When contact with the odor stimuli was prevented, naïve females marked equally in response to male odors and female odors, whereas control females marked more in response to male odors compared to female odors. (b) When contact with the odor stimuli was allowed, all females marked more in response to male odors compared to female odors. * p <.05 compared to the number of vaginal marks made in response to female odor within an experimental group.
Table 2.
Latencies to vaginal mark in Volatile-VM and Contact-VM tests in Experiment 2.
| Volatile-VM | Contact-VM | |||
|---|---|---|---|---|
| Male odor | Female odor | Male odor | Female odor | |
| Control | 137.9 ± 20.3 | 360.7 ± 79.1 | 206.4 ± 52.2 | 600.8 ± 113.0 |
| Naïve | 165.7 ± 20.0 | 357.3 ± 95.3 | 156.6 ± 24.7 | 420.8 ± 112.2 |
Mean (± SEM) latencies (seconds) to make first vaginal mark in response to male or female odor. In either Volatile or Contact tests, there were no differences in vaginal mark latencies between experimental groups, all p >.05.
Figure 3.

Correlations between the number of male siblings in the litter and a derived VM score (number of vaginal marks made in response to male odors – number of vaginal marks made in response to female odors) for Volatile-VM tests in Experiment 2. (a) In the naïve group, the number of male siblings was positively related to the VM score. (b) This relationship was not observed in the control group.
3.3.2. Contact vaginal marking tests
When contact with the odor stimuli was allowed, there were no longer any differences between the vaginal marking responses of naïve and control females. Indeed, both groups vaginal-marked more in response to male odors than to female odors F(1, 19) = 41.587, p <.05 (Figure 2b). There was no effect of experimental group, F(1, 19) =.023, p >.05, nor was there a significant interaction between odor stimulus and experimental group, F(1, 19) = 3.195, p >.05. Furthermore, naïve and control females did not differ in the number of vaginal marks in response to either male odors, F(1,19) =.413, p >.05, or female odors, F(1,19) = 1.332, p >.05. There were no differences between naïve and control females in their latencies to vaginal mark in response to male odors, F(1,19) = 1.790, p >.05, or to female odors, F(1,19) = 1.731, p >.05 (Table 2). Finally, there was no significant relationship between the number of male siblings and VM scores for either naïve females, r = −.113, p >.05, or control females, r =.016, p >.05.
4. Discussion
We show here, for the first time, that female Syrian hamsters require exposure to male odors during early development in order to display appropriate vaginal marking responses to the volatile components of sexual odors. In contrast, the preference to investigate male over female volatile odors does not require this early chemosensory experience. These results suggest that distinct mechanisms regulate the development of vaginal marking and sexual odor preferences in this species.
Vaginal Marking
Females with restricted exposure to male odors throughout development vaginal-marked equally toward male and female volatile odors, whereas control females marked preferentially toward male volatile cues. Thus, previous exposure to male odors was required for the ability to target this form of sexual solicitation behavior toward sexually relevant volatile odors. Importantly, the deficit observed in naïve females was specific to tests that presented only volatile odors; when allowed to contact the odor stimuli, naive females were able to direct their vaginal marking specifically toward male odors. These results suggest that non-volatile odors are sufficient to drive appropriate vaginal marking responses in the absence of any chemosensory experience, whereas volatile cues require previous experience in order to mediate this behavior.
This finding may be due to functional differences and interactions between the two chemosensory systems that process social odors in rodents. Although both the main olfactory system (MOS) and vomeronasal system (VNS) are known to regulate the expression of vaginal marking in female hamsters [29, 36], these systems differ critically in the types of cues to which they respond. In general, sensory neurons of the MOS respond best to volatile chemicals, whereas those of the VNS primarily process non-volatile components of social odors [37, 38]. Thus, the current results indicate that the ability of the MOS to mediate appropriate responses to volatile components of sexual odors may change as a function of previous processing by the VNS. Indeed, further evidence for the experience-dependent plasticity of chemosensory processing has been observed in male hamsters [39, 40] and female mice [23, 24]. Importantly, our results may support the classical conditioning-like model proposed by Martinez-Garcia and colleagues that initially neutral volatile cues acquire attractive properties following association with naturally rewarding non-volatile cues [23, 24].
In addition to the differences in odor stimulus volatility, it is possible that the Volatile- and Contact- tests differed in the absolute concentration of odor stimulus presented. Indeed, allowing contact with the odor stimuli likely presents higher levels of odor stimulus than when contact is prevented. The fact that naïve females displayed similar levels of odor investigation during the Volatile-Preference tests in the Y-maze than compared to control females (Experiment 1) suggests that there are no significant group differences in the ability to detect and respond to these lower concentrations of odors. It remains possible, however, that the deficits in the naïve females’ vaginal marking behavior reflect decreased chemosensory sensitivity to the sexual odors compared to control females. Future experiments that directly compare the detection thresholds for sexual odors in naïve and control females are needed to address this intriguing possibility.
The deficit in vaginal marking behavior observed in naïve females is likely due to their decreased exposure to male odors rather than broad group differences in social experience. Indeed, naïve and control litters received similar handling procedures, therefore minimizing group differences in maternal stress that may affect pup development [41]. It is possible, however, that differences in the sex-ratios of the naïve (all-female) and control (mixed-sex) litters were associated with differences in the quality of maternal care. We did not directly measure maternal behavior in this study. Previous work in rats [42] and mice [43], however, suggests that although all-male litters do receive elevated levels of maternal care, the expression of maternal behavior does not vary between all-female and mixed-sex litters, which is the comparison most relevant to our study. Finally, the absence of male siblings in the naïve litters may indicate that naïve females experienced less play-fighting than compared to control females, as it is a sexually dimorphic behavior [44, 45]. Although this discrepancy remains a possibility, we expect any differences in play-fighting between experimental groups to be minimal because, in Syrian hamsters, the vast majority of play-fighting occurs between same-sex, rather than opposite-sex, siblings [46]. Furthermore, the expression of play-fighting peaks after the age of weaning [47, 48], a time when subjects in the current study were already single-housed and thus no longer under the influence of sibling interactions. We therefore interpret the current results to reflect the critical role of early chemosensory experience in the development of vaginal marking behavior.
Interestingly, we found that the relative amount of exposure to males may be related to the accuracy of vaginal marking in response to volatile odors. Although, as a group, naïve females vaginal-marked equally in response to male and female volatile odors, more male siblings in the litter prior to removal was associated with more sex-typical vaginal marking responses to the volatile odors. Importantly, this relationship was not observed in the control group. These results suggest that, under normal developmental conditions, variability in the number of male siblings has minimal effects on the expression of vaginal marking behavior in adulthood. In the absence of significant exposure to male odors, however, having more male siblings may partially increase the accuracy of vaginal marking responses to volatile odors.
Sexual Odor Preferences
In contrast to vaginal marking behavior, early chemosensory experience does not appear to play a role in the development of investigatory preferences toward opposite-sex odors. When tested in either the Volatile or Contact conditions, naïve females displayed robust preferences to investigate male over female odors that were comparable to control females. Furthermore, the relative amount of exposure to male odors, as indicated by the number of male siblings, had no effect on the strength of sexual odor preferences in either naïve or control females. These results suggest that investigatory attraction to male odor stimuli (volatile or non-volatile) does not require previous exposure to male odors.
These findings differ from previous research showing experience-dependent development of species-specific odor preferences in hamsters. Male Syrian hamsters reared by Turkish hamster dams display decreased preference to investigate anesthetized conspecific females [22], whereas male hamsters cross-fostered into rat litters show increased attraction to investigate rat odors [49]. There are several critical differences between our study and these previous studies that may explain the discrepancy. First, whereas the approach of the current study was to eliminate opposite-sex odors from the early environment, the previous studies [22, 49] employed cross-fostering techniques to alter early experience. This methodological distinction is important, as any behavioral effects that result from cross-fostering are confounded by species differences in parental care or social interactions [50]. Second, the previous studies [22, 49] examined a different type of odor preference (species preference) compared to the current study (sexual preference). It is therefore possible that these different types of odor preferences are regulated by distinct developmental factors.
The observation that sexual odor preferences are not shaped by early chemosensory experience is also in contrast with what has been found in female mice using an experimental paradigm similar to the current study. Chemically naïve female mice are not attracted to the volatile components of male odors, and only display this attraction following contact experience with male odors [23, 24]. These divergent results may reflect critical differences in sociality and ecology between the two species. In contrast to mice, Syrian hamsters are a solitary species that maintain widely dispersed territories [27]. Consequently, there is little chance of incidental contact between individuals after the age of weaning, yet it is critical that animals be able to approach possible mates from a distance. The experience-independent expression of odor preferences in female Syrian hamsters may therefore reflect an evolved mechanism by which to increase contact with mating partners.
If early chemosensory experience is not required for the expression of sexual odor preferences, then what factors do regulate the development of this behavior? There are two broad mechanisms that may allow sexual odor preferences to develop in this species. First, chemosensory experience during an earlier time frame than that targeted by the current study (i.e. prenatal or immediately post-natal) may be sufficient to guide the development of sexual odor preferences. In fact, there is evidence that preferences and aversions for olfactory cues can be conditioned in utero [10, 11], as well as during early postnatal life [51]. Second, the expression of sexual odor preferences may reflect the hormonal organization of brain areas known to regulate this behavior. There is indeed substantial evidence in many rodent species that perinatal hormone exposure organizes the expression of several reproductive behaviors [52], including olfactory preferences [3]. Future studies are needed to determine the role of organizational hormones and perinatal odor environment on the development of sexual odor preferences in female Syrian hamsters.
The results of the current study highlight critical differences in the factors that regulate the development of two odor-guided reproductive behaviors in female Syrian hamsters. Importantly, vaginal marking behavior and sexual odor preferences also differ in their proximate causes. Although both behaviors are modulated by male odors, accurate vaginal marking requires both a functional MOS and VNS [29, 36], whereas the expression of sexual odor preferences involves only MOS processing [29]. Furthermore, the expression of vaginal marking is tightly regulated by changes in levels of circulating gonadal hormones [30, 53], whereas sexual odor preferences do not vary across the estrous cycle or gonadal state [28]. These mechanistic differences may be the outcome of different selective pressures on the evolution of these behaviors and may, therefore, be tied to the distinct developmental factors observed in the current study.
Acknowledgments
We would like to thank Laurie Hale, Larry Burrell and Don Bearden for assistance in behavioral analysis. The current affiliations for P.M.M. and A.P. are Department of Psychology, Georgia State University, and Center for Behavioral Neuroscience (CBN) in Atlanta, GA. This work was supported by NIH grant MH072930-01 and in part from the CBN under STC program of the NSF, under agreement IBN-9876754.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston RE. Chemical signals and reproductive behavior. In: Vandenbergh JG, editor. Pheromones and Reproduction in Mammals. New York: Academic Press; 1983. pp. 3–37. [Google Scholar]
- 2.Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- 3.Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15:615–621. doi: 10.1046/j.1365-2826.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnston RE. Chemical communication in golden hamsters: from behavior to molecules and neural mechanisms. In: Dewsbury DA, editor. Contemporary Issues in Comparative Psychology. Sunderland, MA: Sinauer; 1990. pp. 381–412. [Google Scholar]
- 5.D’udine BAAE. Early experience and sexual preferences in rodents. In: Bateson P, editor. Mate Choice. Cambridge: Cambridge University Press; 1983. pp. 311–327. [Google Scholar]
- 6.Astic L, Saucier D. Ontogenesis of the functional activity of rat olfactory bulb: autoradiographic study with the 2-deoxyglucose method. Brain Res. 1981;254:243–256. doi: 10.1016/0165-3806(81)90035-3. [DOI] [PubMed] [Google Scholar]
- 7.Alberts JR. Olfactory contributions to behavioral development in rodents. In: Doty RL, editor. Mammalian Olfaction, Reproductive Processes and Behavior. New York, San Francisco, London: Academic Press; 1976. pp. 67–91. [Google Scholar]
- 8.Alberts JR, May B. Ontogeny of olfaction: development of the rats’ sensitivity to urine and amyl acetate. Physiol Behav. 1980;24:965–970. doi: 10.1016/0031-9384(80)90157-2. [DOI] [PubMed] [Google Scholar]
- 9.Gregory EH, Bishop A. Development of olfactory-guided behavior in the golden hamster. Physiol Behav. 1975;15:373–376. doi: 10.1016/0031-9384(75)90106-7. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev Psychobiol. 1982;15:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- 11.Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiol Behav. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- 12.Beauchamp GK, Wellington JL. Cross-species rearing influences urine preferences in wild guinea pigs. Physiol Behav. 1981;26:1121–1124. doi: 10.1016/0031-9384(81)90218-3. [DOI] [PubMed] [Google Scholar]
- 13.Denenberg VH, Hudgens GA, Zarrow MX. Mice Reared with Rats: Modification of Behavior by Early Experience with Another Species. Science. 1964;143:380–381. doi: 10.1126/science.143.3604.380. [DOI] [PubMed] [Google Scholar]
- 14.Lagerspetz K, Heino T. Changes in social reactions resulting from early experience with another species. Psychological Reports. 1970;27:255–262. [Google Scholar]
- 15.Mccarty R, Southwick CH. Cross-species fostering: effects on the olfactory preference of Onychomys torridus and Peromyscus leucopus. Behav Biol. 1977;19:255–260. doi: 10.1016/s0091-6773(77)91542-5. [DOI] [PubMed] [Google Scholar]
- 16.Mcdonald DL, Forslund LG. The development of social preferences in the voles Microtus montanus and Microtus canicaudus: effects of cross-fostering. Behav Biol. 1978;22:497–508. doi: 10.1016/s0091-6773(78)92637-8. [DOI] [PubMed] [Google Scholar]
- 17.Quadagno DM, Banks EM. The effect of reciprocal cross fostering on the behaviour of two sepcies of rodents, Mus musculus and Baiomys taylori ater. Anim Behav. 1970;18:379–390. [Google Scholar]
- 18.Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- 19.Mainardi DM, Marsan M, Pasquali A. Causation of sexual preferences of the house mouse: The behaviour of mice reared by parents whose odour was artificially altered. Atti Soc Italiana Nat Milano. 1965;104:325–338. [Google Scholar]
- 20.Marr JN, Gardner LE. Early olfactory experience and later social behavior in the rat: Preference, sexual responsiveness and care of young. J Genetic Psych. 1965;107:167–174. doi: 10.1080/00221325.1965.10532774. [DOI] [PubMed] [Google Scholar]
- 21.Nyby J, Whitney G. Experience affects behavioral responses to sex odors. In: Silverstein RM, editor. Chemical Signals in Vertebrates and Aquatic Invertebrates. New York: Plenum Press; 1980. pp. 173–190. [Google Scholar]
- 22.Murphy MR. Sexual preferences of male hamsters: importance of preweaning and adult experience, vaginal secretion, and olfactory or vomeronasal sensation. Behav Neural Biol. 1980;30:323–340. doi: 10.1016/s0163-1047(80)91210-8. [DOI] [PubMed] [Google Scholar]
- 23.Moncho-Bogani J, Lanuza E, Hernandez A, Novejarque A, Martinez-Garcia F. Attractive properties of sexual pheromones in mice: innate or learned? Physiol Behav. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. [DOI] [PubMed] [Google Scholar]
- 24.Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur J Neurosci. 2005;21:2186–2198. doi: 10.1111/j.1460-9568.2005.04036.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- 26.Thiessen D, Rice M. Mammalian scent gland marking and social behavior. Psychological bulletin. 1976;83:505–539. [PubMed] [Google Scholar]
- 27.Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, Yakti R. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) Journal of Zoology. 2001;254:359–365. [Google Scholar]
- 28.Eidson LN, Maras PM, Epperson E, Petrulis A. Female hamster preference for odors is not regulated by circulating gonadal hormones. Physiol Behav. 2007;91:134–141. doi: 10.1016/j.physbeh.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiol Behav. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- 30.Johnston RE. The causation of two scent-marking behaviour patterns in female hamsters (Mesocricetus auratus) Anim Behav. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- 31.Johnston RE. Olfactory preferences, scent marking, and “proceptivity” in female hamsters. Horm Behav. 1979;13:21–39. doi: 10.1016/0018-506x(79)90032-1. [DOI] [PubMed] [Google Scholar]
- 32.Petrulis A, Johnston RE. Causes of scent marking in female golden hamsters (Mesocricetus auratus): specific signals or classes of information? J Comp Psychol. 1997;111:25–36. doi: 10.1037/0735-7036.111.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Donham RS, Stetson MH. The peripubertal golden hamster and the transition between daily and estrous cycle hormone rhythms. Biol Reprod. 1991;44:1108–1112. doi: 10.1095/biolreprod44.6.1108. [DOI] [PubMed] [Google Scholar]
- 34.Kow LM, Malsbury CW, Pfaff DW. Lordosis in the male golden hamster elicited by manual stimulation: characteristics and hormonal sensitivity. J Comp Physiol Psychol. 1976;90:26–40. doi: 10.1037/h0077260. [DOI] [PubMed] [Google Scholar]
- 35.Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- 36.Johnston RE. Vomeronasal and/or olfactory mediation of ultrasonic calling and scent marking by female golden hamsters. Physiol Behav. 1992;51:437–448. doi: 10.1016/0031-9384(92)90163-v. [DOI] [PubMed] [Google Scholar]
- 37.Meredith M. Sensory processing in the main and accessory olfactory systems: comparisons and contrasts. J Steroid Biochem Mol Biol. 1991;39:601–614. doi: 10.1016/0960-0760(91)90258-7. [DOI] [PubMed] [Google Scholar]
- 38.Kelliher KR. The combined role of the main olfactory and vomeronasal systems in social communication in mammals. Horm Behav. 2007;52:561–570. doi: 10.1016/j.yhbeh.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941:91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- 40.Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- 41.Gutman DA, Nemeroff CB. Neurobiology of early life stress: rodent studies. Seminars in clinical neuropsychiatry. 2002;7:89–95. doi: 10.1053/scnp.2002.31781. [DOI] [PubMed] [Google Scholar]
- 42.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 43.Alleva E, Caprioli A, Laviola G. Litter gender composition affects maternal behavior of the primiparous mouse dam (Mus musculus) J Comp Psychol. 1989;103:83–87. doi: 10.1037/0735-7036.103.1.83. [DOI] [PubMed] [Google Scholar]
- 44.Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- 45.Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- 46.Guerra RF, Vieira ML, Takase E, Gasparetto S. Sex differences in the play fighting activity of golden hamster infants. Physiol Behav. 1992;52:1–5. doi: 10.1016/0031-9384(92)90424-z. [DOI] [PubMed] [Google Scholar]
- 47.Daly M. Behavioral development in three hamster species. Dev Psychobiol. 1976;9:315–323. doi: 10.1002/dev.420090404. [DOI] [PubMed] [Google Scholar]
- 48.Goldman L, Swanson HH. Developmental changes in pre-adult behavior in confined colonies of golden hamsters. Dev Psychobiol. 1975;8:137–150. doi: 10.1002/dev.420080206. [DOI] [PubMed] [Google Scholar]
- 49.Surov AV, Solovieva AV, Minaev AN. The olfactory sexual preferences of golden hamster (Mescricetus auratus): the effects of early social and sexual experience. In: Muller-Schwarze D, editor. Chemical Signals in Vertebrates. New York: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 50.Laviola G, Terranova ML. The developmental psychobiology of behavioural plasticity in mice: the role of social experiences in the family unit. Neurosci Biobehav Rev. 1998;23:197–213. doi: 10.1016/s0149-7634(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 51.Porter RH, Etscorn F. Olfactory imprinting resulting from brief exposure in Acomys cahirinus. Nature. 1974;250:732–733. doi: 10.1038/250732a0. [DOI] [PubMed] [Google Scholar]
- 52.Breedlove SM, Hampson E. Sexual Differentiation of the Brain and Behavior. In: Becker JB, Breedlove SM, Crews D, Mccarthy MM, editors. Behavioral Endocrinology. 2. Cambridge: The MIT Press; 2002. pp. 75–114. [Google Scholar]
- 53.Takahashi LK, Lisk RD. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol Behav. 1983;31:477–482. doi: 10.1016/0031-9384(83)90069-0. [DOI] [PubMed] [Google Scholar]


