Abstract
Purpose of review
Sexually transmitted infections (STIs) remain a major global public health issue, with more than 448 million incident bacterial infections each year. We review recent advances in STI point-of-care (POC) testing and implications for STI prevention and control.
Recent findings
Accurate immunochromatographic assays to detect HIV, hepatitis C virus (HCV) and syphilis antibodies have made home or supervised self-testing possible. Several studies have demonstrated feasibility and excellent test characteristics for HIV, HCV and syphilis POC tests. Rapid oral HIV tests are now available for purchase at retail sites across the United States. Combined HIV and syphilis tests using a single finger prick blood sample are under evaluation.
Summary
Oral POC STI tests with comparable performance to blood-based POC tests are available for self-testing. POC tests can expand screening, improve syndromic management and reduce loss to follow up. POC STI tests have the potential to facilitate prompt treatment and partner services. POC STI tests create opportunities for new social and financial models of community-based testing services. Increasing equity and access to testing will create challenges in linkage to care, quality assurance, partner services and surveillance. These important developments warrant research to understand appropriate contexts for implementation.
Keywords: HIV, point-of-care, sexually transmitted infection, syphilis, testing
INTRODUCTION
The WHO estimates that worldwide, 448 million new cases of curable sexually transmitted infections (STIs; specifically syphilis, gonorrhoea, chlamydia and trichomoniasis) are diagnosed each year [1]. Chlamydial and gonococcal infections can lead to chronic pelvic pain, ectopic pregnancy and infertility. Approximately 25% of untreated early syphilis infections during pregnancy result in stillbirth and 14% cause neonatal death [1]. These sequelae are entirely preventable if STI testing is utilized.
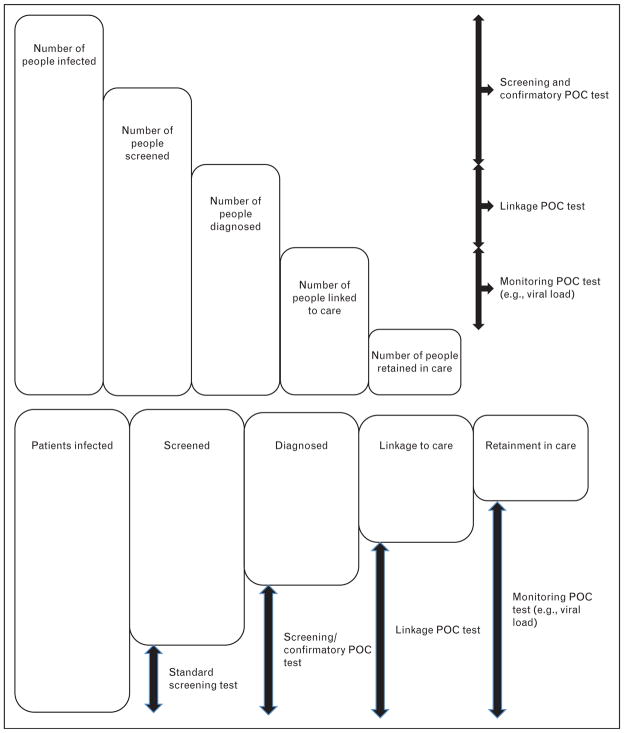
STI tests with high sensitivity and specificity are commercially available, but they are often neither affordable nor accessible to many patients in the developing world where STI burden is greatest. Laboratory-based testing often requires patients to return for their test results, leading to loss to follow-up and delays in treatment and partner services. In recent years, advances in detection technology have made a range of point-of-care (POC) tests available [2,3,4▪▪,5,6]. It is now possible to screen and diagnose STIs at primary healthcare settings using blood from a finger prick or a noninvasive specimen such as oral exudate (Table 1). Molecular assays that can be performed with minimal user input, but provide fast and highly accurate results, are now available for the detection of chlamydia and gonorrhoea. Innovations in the delivery of these POC tests will ensure widespread access to diagnostics so that patients can benefit from evidence-based treatment at the same clinic visit [7]. POC reader technology can securely transmit remote testing results directly to proximate clinical services, decreasing loss to follow-up and improving linkage and retention to care (Fig. 1) [8].
Table 1.
Overview of point-of-care technologies for selected sexually transmitted infections
| Pathogen | Specimen | POC technologies | |||
|---|---|---|---|---|---|
| NAAT | Antigen | Antibody | Multiplex | ||
| HIV | Blood (fingerprick), oral swab | In development | Yes | Yes | Duplex antibody tests with syphilis |
| Syphilis | Blood (fingerprick) | NA | NA | Yes | |
| HCV | Blood (fingerprick), oral swab | No | No | Yes | With HIV tests in development |
| Gonorrhoea | Urethral/vaginal swab | In development | Yes | NA | NAAT with chlamydia |
| Chlamydia | Urethral/vaginal swab | In development | Yes | NA | NAAT with gonorrhoea |
| Trichomonas | Vaginal swab | No | Yes | NA | No |
| Bacterial vaginosis | Vaginal swab | No | Enzyme detection | NA | No |
HCV, hepatitis C virus; NA, not applicable; NAAT, nucleic acid amplification tests; POC, point-of-care.
FIGURE 1.
Overview of the cascade of sexually transmitted infection services and point-of-care testing functions. POC, point-of-care.
The purpose of this review is to describe technological advancements in the diagnosis of STI at the POC in the past 18 months and the implications for linkage to care, partner services, surveillance and disease control. Although there is no universally accepted definition of POC testing, for the purpose of this review, we define POC test as any test that is simple and can provide a rapid result to guide clinical decisions and follow-up during the same encounter [9]. We searched Pubmed, Scopus and Web of Science using the terms ‘rapid testing’, ‘point-of-care testing’, ‘self test’ and ‘home test’ and the names of individual STIs. Our review included STIs for which POCs have been developed. We included hepatitis C virus (HCV) because of the importance of homosexual HCV transmission. We included novel multiplex tests that are currently under evaluation.
HIV POINT-OF-CARE TESTS
With more than 34.2 million people globally living with HIV, and 2.5 million new infections estimated to occur annually, there remains an urgent need to accelerate HIV prevention and control efforts [10]. Rapid and POC diagnostic tests are critical tools to help reduce the prevalence of undiagnosed infection, improve monitoring of clinical outcomes and identify incident infections to target prevention and control efforts. Rapid tests for HIV have been used for almost a decade now to increase access to screening in all levels of the healthcare system and for outreach programmes. These tests have been critical in allowing more individuals to know their HIV status and for monitoring disease trends. But it still requires at-risk individuals to attend a health facility, and as most-at-risk individuals are either marginalized from healthcare or stigmatized, innovations in the delivery of testing services are urgently needed.
HIV self-testing using POC tests introduces new opportunities for expanding screening, although a number of related issues must be addressed for effective implementation. In the first major self-testing project in a low-income setting, Choko et al. [11▪▪] demonstrated the feasibility of HIV self-testing in Blantyre, Malawi, although linkage to care was not investigated. Home HIV POC testing among MSM with high-risk behaviours was acceptable in a small qualitative study in the United States [12]. A randomized controlled trial of HIV POC tests in drug users found that individuals who received POC posttest counselling had no difference in sexual behaviours compared with those who did not [13].
In July 2012, OraSure Technologies, Inc. (Bethlehem, PA) announced that the US Food and Drug Administration (FDA) had approved the OraQuick In-Home HIV test for sale directly to consumers, making it the first and only rapid over-the-counter HIV test approved in the United States. The OraQuick In-Home HIV Test can detect antibodies to both HIV-1 and HIV-2 with an oral swab, providing a confidential in-home testing option with results in as little as 20 min. The OraQuick In-Home HIV Test is a consumer version of a rapid HIV test and is identical to that approved by the FDA for professional testing. In a phase III study, a population of 5662 individuals of unknown HIV status was self-tested under unobserved conditions and reported their results back to clinical sites. Overall specificity was 99.98% [95% confidence interval (CI) 99.90–100.0] and sensitivity was 93.0% (95% CI 86.6–96.9) compared with blood based specimens [14]. A systematic review showed that although OraQuick had a comparable positive predictive value to blood-based specimens in high-prevalence settings (oral 98.7%, 95% CI 85.7–99.9), its positive predictive value decreases to 88.6% (95% CI 77.3–95.9) for blood specimens in low-prevalence settings [15].
The OraQuick In-Home HIV Test is now available for purchase at more than 30 000 retail outlets throughout the United States and online. A consumer support centre provides consumers 24-h support and enables them to talk to highly trained professionals in English or Spanish. These professionals can provide information on HIV/AIDS, how to properly conduct the test and referrals to local organizations for follow-up testing and care. With a guide price of approximately 40 US dollars per kit, the OraQuick In-Home HIV Test will facilitate efforts to diagnose the more than 200 000 Americans who are unaware of their HIV infections. At the same time, concerns have been raised about whether those at greatest risk will be able to afford the test or do so more than once or twice a year to manage ongoing risks. In addition, community discussions have begun to consider whether the technology can assist at-risk individuals to screen potential sexual partners. As more experience with the OraQuick In-Home HIV Test is obtained, additional information on risk compensation, access and the context within which the tests are being used should be monitored and evaluated.
Acute HIV POC tests have been suboptimal to date [16,17,18▪], but a POC test based on the nucleic acid amplification test is being developed [19] and an acute HIV POC test has shown promise in paediatric settings [20▪]. Cell-phone based HIV POCs have also been developed [5], but more operational data are needed in order to understand how best they can be used.
Tests for HIV antiretroviral treatment initiation and monitoring are still severely lacking in resource-constrained settings. The WHO/UNAIDS Treatment 2.0 Initiative emphasizes the essential role that cheaper and simplified diagnostic tools, especially POC technologies, must play in efforts to further expand access to treatment. A number of simplified technologies for CD4 cell count and POC assays for viral load are already available, and for early infant diagnosis are in the pipeline [21]. POC CD4 cell count tests show comparable test sensitivity and specificity to laboratory-based methods and are acceptable to patients [22–24]. A study in Mozambique showed that the introduction of POC CD4 cell count monitoring reduced the proportion of patients lost to follow-up before antiretroviral therapy (ART) initiation from 64 to 33% and the median time to ART initiation from 48 to 20 days [25▪].
SYPHILIS POINT-OF-CARE TESTING
Globally, WHO estimates that 12 million new cases of syphilis occur each year. Of the 1.4 million pregnant women who are infected each year, almost a million women will transmit the infection to their foetus resulting in stillbirth, low birth weight, prematurity and congenital infection [7]. However, prenatal screening for syphilis has been hampered by the lack of access to laboratories that can offer testing. POC tests provide opportunities for increased access to testing of both pregnant women and at-risk populations, thereby reducing disease burden among sexually active adults and accelerating efforts to eliminate congenital syphilis [3]. Integrating POC syphilis screening into Prevention of Mother to Child Transmission (PMTCT) programmes for HIV in Uganda and Zambia showed increased uptake of testing for syphilis [26▪▪]. Integration of HIV and syphilis POC testing in South China increased uptake of both tests compared with lone HIV testing [27▪]. A study showed that treponemal POC tests for syphilis can be introduced in the following contexts: rural antenatal clinics in Tanzania, Uganda and China; both rural and urban clinics in Peru and Zambia; and remote indigenous communities in Brazil. By working with the existing healthcare system to integrate testing, the introduction of POC tests resulted in large numbers of women being tested and treated for syphilis, averting many stillbirths and reducing neonatal mortality [28▪▪]. The study also showed that the introduction of POC testing can strengthen health systems by increasing prenatal screening coverage, improving health outcomes, increasing client and health worker acceptability, and changing policy guidelines to ensure the sustainability of the service offered. In the long term, the development and implementation of an essential POC prenatal package to include essential noncommunicable disease (e.g. anaemia and preeclampsia) testing could be useful.
The persistence of treponemal antibodies mandates the use of a nontreponemal test to identify those who have an active infection and to avoid overtreatment. The availability of treponemal POC tests has fuelled a debate about the merit of screening with a treponemal test followed by confirmation of syphilis with a nontreponemal test. Linkage to clinical care remains a priority in these populations, especially in light of the trend that a substantial portion of syphilis-infected individuals are lost to follow-up prior to confirmatory testing [29▪]. The availability of POC treponemal/nontrepenomal combination tests will hopefully clarify questions about the optimal order of tests and concerns about overtreatment [6]. POC tests that offer multiple tests with a single finger prick specimen for HIV and syphilis are under evaluation [4▪▪]. These will be important tools for the joint elimination of mother to child transmission of HIV and syphilis.
HEPATITIS C VIRUS POINT-OF-CARE TESTING
HCV infection is now recognized to be among the most prevalent chronic bloodborne infections, with more than 150 million people chronically infected and at risk of developing liver cirrhosis and/or liver cancer worldwide, and 3–4 million people infected annually [30]. Although HCV is not efficiently transmitted sexually, except possibly among HIV-positive MSM, persons at risk for infection through injection drug use might seek care in STD treatment facilities, HIV counselling and testing facilities, correctional facilities, drug treatment facilities, and other public health settings where STD and HIV prevention and control services are available. HCV antibody POC tests were recently approved in Europe for bodily and oral fluid [31]. The FDA also approved HCV POC tests for finger stick and venipuncture specimens [32]. OraQuick tests using oral samples or blood have been shown to be comparable to laboratory-based enzyme immunoassays with better specificity and sensitivity compared with four other POC tests [31,33]. US premarket studies in the field and laboratory demonstrated high sensitivities and specificities for the OraQuick fingerstick assay [34,35▪], although two other POC tests were less sensitive and produced false negatives in patients who were coinfected with HIV [34,35▪,36▪]. An OraQuick oral swab POC test implemented in community-based organizations (CBOs) showed similar test characteristics to laboratory-based tests [37▪,35▪].
GONORRHOEA AND CHLAMYDIA POINT-OF-CARE TESTING
Gonorrhoea and chlamydia POC tests have been hampered by low sensitivity and specificity compared with nucleic acid amplification assays in low-prevalence populations and cross-reactivity with nongonococcal Neisseria [38–40]. Given decreasing susceptibility to first-line antibiotic treatment [41], there is also a growing need for POC resistance testing. At the same time, POC tests for the simultaneous detection of chlamydia and gonorrhoea based on isothermal amplification technologies are in final stages of development. They will be more rapid and less expensive than the GeneXpert platform, which is already being sold as a POC test platform [42,43].
OTHER SEXUALLY TRANSMITTED INFECTION POINT-OF-CARE TESTS
Self-testing for trichomoniasis with POC tests has been shown to be acceptable and feasible with training [44]. Bacterial vaginosis POC tests have been available for several years, but no recent studies have examined linkage to care or treatment. Bacterial vaginosis POC tests detect sialidase activity in vaginal fluid, and a recent study confirmed excellent sensitivity and specificity when compared with Gram stain [45].
NEW ORGANIZATIONAL AND FINANCIAL MODELS FOR POIINT-OF-CARE TESTING
POC tests offer unprecedented opportunities for a variety of organizational and financial models for improving health, similar to at-home pregnancy tests and blood sugar tests. STI POC tests accelerate decentralization of testing to remote locations, CBOs, pharmacies and in the home. CBO-based testing provides an opportunity to provide more community-responsive services to most-at-risk populations who are in greatest need of frequent testing. Building on the CBO POC testing model, the Barcelona Checkpoint HIV POC service uncovered a substantial share of new HIV diagnoses in the city [46]. A pilot programme launched by the US Centers for Disease Control in 2012 made HIV POC testing available at 40 pharmacies free of charge [47]. Those who received tests were required to provide identifying information at check-out in order to ensure follow-up. Home testing is another organizational model made possible by simple technology that does not require trained personnel. These new organizational models raise important questions about optimal venues, times, linkage to care follow-up, partner services and quality control. STI POC tests also provide opportunities to create new financial models, giving options outside of the traditional public health service model for most at-risk populations. Revenue generation through the sales of STI POC tests or testing services could be reinvested in CBOs and run as a social enterprise [48]. These models may be especially feasible in middle-income countries among subsets of MSM who are able to pay for POC test services. Pure business models for diagnostic services have also emerged [9,49].
LINKAGE TO CLINICAL CARE
The decentralization of testing services has increased, not decreased, the importance of clinics. Clinical services are critical for diagnostic confirmation, treatment, counselling and follow-up services. This necessity reflects both the limits of decentralized testing and the increasing importance of collaboration between clinic and nonclinical partners (CBOs, pharmacies, businesses) in providing testing and partner services. Local multisectoral networks that incorporate knowledge and expertise from a number of sectors are more likely to be community responsive, sustainable and effective in expanding testing to most at-risk populations [48].
QUALITY ASSURANCE
Although STI POCs are generally simple and easy to perform, ensuring proper implementation that leads to adequate sensitivity and specificity onsite is important. Visually read and manually prepared tests can lead to decreased accuracy of POC tests. Methods for measuring STI prevalence outside of clinics (e.g., dried blood spots) and formalized systems for quality assurance are both needed. Quality assurance on POC testing and case management can help ensure test quality in decentralized settings and identify where remedial training is required [8]. Devices that interpret POC tests can remove the subjectivity of reading and reduce transcription error by automated transmission of testing results to a central database for disease surveillance.
CONCLUSION
Oral POC STI tests with comparable performance to blood-based POC tests are now available for supervised self-testing or home use. POC STI tests can be used to expand screening, improve syndromic management and reduce loss to follow up. Their use can result in accelerated treatment initiation and delays in partner services. They also create opportunities for new social and financial models of community-based testing services. Increasing equity and access to testing creates challenges in linkage to care, quality assurance, partner services and monitoring of disease trends to inform control strategies. These important developments warrant further research to understand appropriate contexts for implementation.
KEY POINTS.
Accurate STI POC tests to detect HIV, HCV and syphilis antibodies now make home or supervised self-testing possible.
Several studies have demonstrated the feasibility of STI POC self-testing, but linkage to care, quality assurance and surveillance remains a concern.
Oral POC HIV tests are available for purchase at retail sites in the United States.
STI POC tests can expand STI screening, improve syndromic management and reduce loss to follow up.
Acknowledgments
J. Tucker is the recipient of an NIH Fogarty Career Development Award (US NIH 1K01TW008200-01A1), the BWH/ASTMH Postdoctoral Fellowship, and C. H. Bien is the recipient of a Doris Duke Medical Foundation International Medical Student Fellowship. Special thanks to Kevin Fenton for his contributions and providing comments on a previous version of this article.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 102).
- 1.WHO. Sexually transmitted infections (Fact Sheet 110) Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Peeling RW. Applying new technologies for diagnosing sexually transmitted infections in resource-poor settings. Sex Transm Infect. 2011;87:ii28–ii30. doi: 10.1136/sti.2010.047647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benzaken AS, Sabidó M, Galban E, et al. Field performance of a rapid point-of-care diagnostic test for antenatal syphilis screening in the Amazon region, Brazil. Int J STD AIDS. 2011;22:15–18. doi: 10.1258/ijsa.2010.010145. [DOI] [PubMed] [Google Scholar]
- 4▪▪.Chin CD, Laksanasopin T, Cheung YK, et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. This Rwandan field study describes a novel microfluidics approach to HIV and syphilis testing. The new platform described can assess multiple pathogens using a single 1-μl finger prick specimen. [DOI] [PubMed] [Google Scholar]
- 5.Mudanyali O, Dimitrov S, Sikora U, et al. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip. 2012;12:2678–2686. doi: 10.1039/c2lc40235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro AR, Esfandiari J, Kumar S, et al. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. Clin Microbiol. 2010;48:4615–4619. doi: 10.1128/JCM.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palamountain KM, Baker J, Cowan EP, et al. Perspectives on introduction and implementation of new point-of-care diagnostic tests. J Infect Dis. 2012;205 (Suppl 2):S181–S190. doi: 10.1093/infdis/jis203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewandrowski K, Gregory K, Macmillan D. Assuring quality in point-of-care testing: evolution of technologies, informatics, and program management. Arch Pathol Lab Med. 2011;135:1405–1414. doi: 10.5858/arpa.2011-0157-RA. [DOI] [PubMed] [Google Scholar]
- 9.Pai NP, Vadnais C, Denkinger C, et al. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012;9:e1001306. doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS. UNAIDS 2011 World AIDS Day report. Geneva: UNAIDS; 2011. [Google Scholar]
- 11▪▪.Choko AT, Desmond N, Webb EL, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8:e1001102. doi: 10.1371/journal.pmed.1001102. The first description of HIV POC self-testing in a low-income country setting, reporting both test characteristics and acceptability among urban Malawians. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carballo-Diéguez A, Frasca T, Balan I, et al. Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS Behav. 2012;16:1–8. doi: 10.1007/s10461-012-0274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metsch LR, Feaster DJ, Gooden L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102:1160–1167. doi: 10.2105/AJPH.2011.300460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration (FDA) Evaluation of the safety and effectiveness of the OraQuick In-Home HIV Test. 102nd Meeting of The Blood Product Advisory Committee (BPAC); 15–16 May 2012; [Accessed 12 October 2012]. Available from FDA: http://tinyurl.com/c9xwaeg. [Google Scholar]
- 15.Pai NP, Balram B, Shivkumar S, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:373–380. doi: 10.1016/S1473-3099(11)70368-1. [DOI] [PubMed] [Google Scholar]
- 16.Kilembe W, Keeling M, Karita E, et al. Failure of a novel, rapid antigen and antibody combination test to detect antigen-positive HIV infection in African adults with early HIV infection. PLoS One. 2012;7:e37154. doi: 10.1371/journal.pone.0037154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel P, Bennett B, Sullivan T, et al. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012;54:42–47. doi: 10.1016/j.jcv.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Rosenberg NE, Kamanga G, Phiri S, et al. Detection of acute HIV infection: a field evaluation of the determine HIV-1/2 Ag/Ab combo test. J Infect Dis. 2012;205:528–534. doi: 10.1093/infdis/jir789. This study of a combined antibody–antigen HIV POC test among 1009 individuals in Malawi demonstrated excellent detection for established HIV infection, but poor performance in detecting acute infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis KA, Rudolph DL, Nejad I, et al. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PLoS One. 2012;7:e31432. doi: 10.1371/journal.pone.0031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Parpia ZA, Elghanian R, Nabatiyan A, et al. p24 antigen rapid test for diagnosis of acute pediatric HIV infection. J Acquir Immune Defic Syndr. 2010;55:413–419. doi: 10.1097/QAI.0b013e3181f1afbc. This study describes a new acute HIV POC test that measures p24 antigen, which demonstrated high sensitivity and specificity in US infant specimens and South African infants. [DOI] [PubMed] [Google Scholar]
- 21.UNITAID. HIV/AIDS diagnostic technology landscape. 2. UNITAID; Geneva: 2012. [Google Scholar]
- 22.Herbert S, Edwards S, Carrick G, et al. Evaluation of PIMA point-of-care CD4 testing in a large UK HIV service. Sex Transm Infect. 2012;88:413–417. doi: 10.1136/sextrans-2012-050507. [DOI] [PubMed] [Google Scholar]
- 23.Sukapirom K. Performance evaluation of the Alere PIMA CD4 test for monitoring HIV-infected individuals in resource-constrained settings. J Acquir Immune Defic Syndr. 2011;58:141–147. doi: 10.1097/QAI.0b013e31822866a2. [DOI] [PubMed] [Google Scholar]
- 24.Manabe YC, Wang Y, Elbireer A, et al. Evaluation of portable point-of-care CD4 counter with high sensitivity for detecting patients eligible for antiretroviral therapy. PLoS One. 2012;7:e34319. doi: 10.1371/journal.pone.0034319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. This study describes the effect of POC CD4 cell count tests on reducing pre-ART loss to follow-up among 929 HIV-positive patients in Mozambique. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Strasser S, Bitarakwate E, Gill M, et al. Introduction of rapid syphilis testing within prevention of mother-to-child transmission of HIV programs in Uganda and Zambia: a field acceptability and feasibility study. J Acquir Immune Defic Syndr. 2012;61:e40–e46. doi: 10.1097/QAI.0b013e318267bc94. This implementation research study among over 15 000 prenatal women in Zambia and Uganda demonstrated how POC syphilis and HIV integration could be successfully achieved. [DOI] [PubMed] [Google Scholar]
- 27▪.Tucker JD, Yang L-G, Yang B, et al. A twin response to twin epidemics: integrated HIV/syphilis testing at STI clinics in South China. J Acquir Immune Defic Syndr. 2011;57:e106–e111. doi: 10.1097/QAI.0b013e31821d3694. This study of 2061 STI patients in South China demonstrated that additional rapid POC testing can be easily integrated into preexisting testing platforms and services, and testing uptake may increase with POC testing integration in comparison with lone STI testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪▪.Mabey DC, Sollis KA, Kelly HA, et al. Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS Med. 2012;9:e1001233. doi: 10.1371/journal.pmed.1001233. This seven-country study examined the introduction of POC syphilis tests, demonstrating a range of health systems benefits and policy changes resulting from the project. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Gupte S, Daly C, Agarwal V, et al. Introduction of rapid tests for large-scale syphilis screening among female, male, and transgender sex workers in Mumbai. India Sex Transm Dis. 2011;38:499–502. doi: 10.1097/OLQ.0b013e318205e45d. This study among a high-risk, high-prevalence cohort in Mumbai, India, demonstrated an increase in syphilis testing uptake among 19 809 sex workers when offered a rapid POC syphilis test, but low acceptance of confirmatory testing. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Hepatitis C (Fact Sheet 164) Geneva: World Health Organization; 2012. [Google Scholar]
- 31.Lee SR, Kardos KW, Schiff E, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods. 2011;172:27–31. doi: 10.1016/j.jviromet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Food and Drug Administration (FDA) [Accessed 12 October 2012];[Internet] OraQuick HCV rapid antibody test – P080027. [Cited 2012 Oct 12]. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P080027.
- 33.O’Connell RJ, Gates RG, Bautista CT, et al. Laboratory evaluation of rapid test kits to detect hepatitis C antibody for use in predonation screening in emergency settings. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03770.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Smith BD, Drobeniuc J, Jewett A, et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011;204:825–831. doi: 10.1093/infdis/jir422. [DOI] [PubMed] [Google Scholar]
- 35▪.Smith BD, Teshale E, Jewett A, et al. Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin Infect Dis. 2011;53:780–786. doi: 10.1093/cid/cir499. This field evaluation of POC HCV testing in four US cities found a high sensitivity and specificity when provided to 1596 IDUs. [DOI] [PubMed] [Google Scholar]
- 36▪.Jewett A, Smith BD, Garfein RS, et al. Field-based performance of three premarket rapid hepatitis C virus antibody assays in STAHR (Study to Assess Hepatitis C Risk) among young adults who inject drugs in San Diego, CA. J Clin Virol. 2012;54:213–217. doi: 10.1016/j.jcv.2012.04.003. This field evaluation of POC HCV testing in four US cities found a high sensitivity and specificity when provided to 1596 IDUs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Drobnik A, Judd C, Banach D, et al. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am J Public Health. 2011;101:2151–2155. doi: 10.2105/AJPH.2011.300251. This field study of a high-risk population in a CBO showed that a POC HCV test using oral specimens had comparable accuracy to enzyme immunoassay methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samarawickrama A, Alexander S, Ison C. A laboratory-based evaluation of the BioStar Optical ImmunoAssay point-of-care test for diagnosing Neisseria gonorrhoeae infection. J Med Microbiol. 2011;60:1779–1781. doi: 10.1099/jmm.0.034116-0. [DOI] [PubMed] [Google Scholar]
- 39.van der Helm JJ, Sabajo LOA, Grunberg AW, et al. Point-of-care test for detection of urogenital chlamydia in women shows low sensitivity. A performance evaluation study in two clinics in Suriname. PLoS One. 2012;7:e32122. doi: 10.1371/journal.pone.0032122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dommelen L, van Tiel FH, Ouburg S, et al. Alarmingly poor performance in Chlamydia trachomatis point-of-care testing. Sex Transm Infect. 2010;86:355–359. doi: 10.1136/sti.2010.042598. [DOI] [PubMed] [Google Scholar]
- 41.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med. 2012;366:485–487. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 42.Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae using residual Aptima samples. J Clin Microbiol. 2012;50:3867–3869. doi: 10.1128/JCM.01930-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor SN, Liesenfeld O, Lillis RA, et al. Evaluation of the Roche cobas(R) CT/NG test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in male urine. Sex Transm Dis. 2012;39:543–549. doi: 10.1097/OLQ.0b013e31824e26ff. [DOI] [PubMed] [Google Scholar]
- 44.Huppert JS, Hesse EA, Bernard MA, et al. Acceptability of self-testing for trichomoniasis increases with experience. Sex Transm Infect. 2011;87:494–500. doi: 10.1136/sextrans-2011-050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kampan NC, Suffian SS, Ithnin NS, et al. Evaluation of BV Blue Test Kit for the diagnosis of bacterial vaginosis. Sex Reprod Healthc. 2011;2:1–5. doi: 10.1016/j.srhc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Pujol F, Meulbroek M, Saz J, et al. BCN Checkpoint: three-year experience (2007–2009) of a community based centre for men who have sex with men in Barcelona shows high effectiveness in HIV detection [abstract]. XVIII International AIDS Conference; 2010; Vienna. 18–23 July 2010; Vienna, Austria: Geneva: IAS Press; 2010. [Google Scholar]
- 47.Kuehn BM. CDC pilot program will offer free rapid HIV tests through pharmacies. JAMA. 2012;308:327–1327. doi: 10.1001/jama.2012.8864. [DOI] [PubMed] [Google Scholar]
- 48.Tucker JD, Fenton KA, Peckham R, Peeling RW. Social Entrepreneurship for Sexual Health (SESH): a new approach for enabling delivery of sexual health services among most-at-risk populations. PLoS Med. 2012;9:e1001266. doi: 10.1371/journal.pmed.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baloch NA, Pai M. Tuberculosis control: business models for the private sector. Lancet Infect Dis. 2012;12:579–580. doi: 10.1016/S1473-3099(12)70122-6. [DOI] [PubMed] [Google Scholar]