Abstract
Exome sequencing and whole genome sequencing (ES/WGS) present patients and research participants with the opportunity to benefit from a broad scope of genetic results of clinical and personal utility. Yet, this potential for benefit also risks disenfranchising populations such as African Americans (AAs) that are already underrepresented in genetic research and utilize genetic tests at lower rates than other populations. Understanding a diverse range of perspectives on consenting for ES/WGS and receiving ES/WGS results is necessary to ensure parity in genomic health care and research. We conducted a series of 13 focus groups (n=76) to investigate if and how attitudes toward participation in ES/WGS research and return of results from ES/WGS differ between self described AAs and non-AAs. The majority of both AAs and non-AAs were willing to participate in WGS studies and receive individual genetic results, but the fraction not interested in either was higher in AAs. This is due in part to different expectations of health benefits from ES/WGS and how results should be managed. Our results underscore the need to develop and test culturally tailored strategies for returning ES/WGS results to AAs.
Keywords: bioethics, exome sequencing, whole genome sequencing, African Americans, return of results
INTRODUCTION
Exome sequencing and whole genome sequencing (ES/WGS) have become important tools for identifying alleles underlying both Mendelian and complex health-related traits [Bamshad et al., 2011; Bamshad et al., 2012; Biesecker, 2010; Gonzaga-Jauregui et al., 2012]. The number of individuals who have undergone ES/WGS over the past few years has steadily increased, and is likely to rapidly accelerate with the advent of commercial CLIA-approved sequencing services that have made ES/WGS widely available to researchers and clinicians.
Use of ES/WGS in both research and clinical settings raises a myriad of ethical, social and legal issues to consider, though most discussions to date have focused on what results should be returned in a clinical setting [Berg et al., 2011; Evans and Rothschild 2012; Green et al., 2012], whether results should be returned at all in a research setting and if so which results [Bredenoord et al., 2011; Fabsitz et al., 2010; Knoppers et al., 2006; Wolf et al., 2012], and what to do about “incidental” – or more appropriately, “secondary” – findings in either setting [Christenhusz et al., 2012; Haga et al., 2012; Kohane et al., 2012; Richardson and Cho 2012; Wolf et al., 2012]. Accordingly, policy makers and professional societies are attempting to develop guidelines for the return of ES/WGS results and attend to concomitant ethical concerns [American College of Medical Genetics and Genomics, 2012; Presidential Commission for the Study of Bioethical Issues, 2012].
Virtually all of the approaches to return of ES/WGS results currently being studied focus on testing in European American (EA) populations. Little effort has been made to consider how the perceived potential benefits and possible harms of ES/WGS results might differ in populations who have been historically at the margins of genomics—despite the fact that ES has been completed on thousands of samples from populations other than EAs. Yet, evidence suggests that preferences for genetic test results, the interpretation of clinical utility, and the impact of receiving results differ among racial and ethnic groups [Butrick et al., 2011; Forman and Hall, 2009; Rahm et al., 2012]. Moreover, there is general consensus that, as a matter of social justice, all populations should have equal access and opportunity to benefit from ES/WGS and explicit efforts should be undertaken to reduce or eliminate barriers to accessing ES/WGS testing and maximize the potential benefits and minimize the harms of ES/WGS [Presidential Commission for the Study of Bioethical Issues, 2012].
African American (AA) populations may be particularly interested in the benefits of genetic information and genetic testing [Lemke et al., 2012; Michie et al., 2011; O'Daniel and Haga, 2011; Singer et al., 2004]. Yet, AAs perhaps face a large number of potential barriers to accessing ES/WGS and benefiting results. Genetic knowledge among some AAs is limited [Akinleye et al., 2011; Goldenberg et al., 2011; Kessler et al., 2007; Suther and Kiros, 2009] and misperceptions of genetic risk, differences in the cultural acceptability of genetic services, and distrust [Eichmeyer et al., 2005] adversely impact test utilization [Susswein et al., 2008] and contribute to underrepresentation of AAs in genetic research. Furthermore, the informativeness of genetic results may differ for AA populations because AAs are more likely to have variants of uncertain significance [Nanda et al., 2005; Tennessen et al., 2012].
The meaning of a genetic test result (i.e., the scientific, medical, and personal understanding of a variant) is likely to vary among cultural contexts [Connell et al., 2009; Long et al., 2011; Vadaparampil et al., 2010]. Studies of AA women at high risk for breast cancer suggest that communalism in the form of group identity, a present-focused temporal orientation, and spirituality may be more important to testing decisions and interpreting results for this population than other populations [Armstrong et al., 2005; Edwards et al., 2008; Hughes et al., 2003]. However, these broad generalizations are based on limited research to date. AAs are a culturally rich and diverse population, and it is likely that perspectives on genetics and return of results differ considerably within and among local and regional communities.
In an effort to understand the attitudes of AAs on return of results from ES/WGS and ultimately empower them to make more informed decisions about whether to undergo ES/WGS and how to manage their results, we performed a qualitative analysis of data obtained from a series of focus groups conducted as part of a larger, ongoing effort to develop innovative and improved strategies and tools for return of results from ES/WGS. The specific questions that we address include: (1) What do AA participants think are the risks and benefits of WGS and why?; (2) What do AA participants think about receiving WGS results for themselves and for their children?; (3) How do AA participants make decisions about whether to receive WGS results, and what to do with them?; and (4) How do AA participants perspectives on WGS differ from those of non-AAs?
MATERIALS AND METHODS
Focus groups were conducted in three strata – AA, non-AA, and both AA/non-AA groups – in order to triangulate the content and themes heard in AA focus groups to AA individuals in the AA/non-AA groups, and vice versa for non-AAs. For this analysis, we focused on the responses of AA participants.
Participants were parents 18 years or older who reside in Seattle/King County, Washington. Participants were recruited in several ways including direct telephone solicitation, from flyers posted at local pediatric clinics and community organizations, and from electronic flyers posted on Seattle area parent electronic lists. Recruitment was conducted on a rolling basis, with the goal of recruiting at least 35 African Americans and at least 35 non-African Americans. As a result, some focus groups were limited to African Americans whereas some were mixed. We conducted 13 focus groups to ensure that we recruited a minimum of 35 AA participants.
Individuals were screened for parental status, age, residence, education, and to determine if their child had a rare genetic condition and therefore had greater than average knowledge of genetics. The overall participant response rate was 40%. The rate for direct telephone solicitation versus flyer-based recruitment was 20% and 59%, respectively. Participants were scheduled to attend focus group sessions at convenient community locations. Materials about the study and informed consent document were mailed to participants. Informed consent was conducted at the beginning of each focus group.
Each focus group was led by a moderator (JY) and a research assistant. Participants were asked to complete an anonymous demographic questionnaire that included questions to ascertain self-described racial and ethnic identity. This data was linked to the participant ID number. The research assistant recorded the speaker for each comment (i.e., talk-turn). Each comment in the transcripts was then assigned a participant ID number and, as a result, linked to self-identified race / ethnicity.
The focus group discussion guide began with a description of a hypothetical WGS study involving parents and children. Participants were asked via a verbal poll at the start and end of the focus group session if they would or would not be willing to participate in such a study and if they would or would not be willing to consent for a child to participate in such a study. Participants’ verbal poll responses were recorded by a research assistant and tabulated. The polling data provides a snapshot of the opinions in the room, however because polling was done verbally there is the possibility that the results may have been influenced by group bias. The moderator then gave a brief overview of genetics and introduced four key concepts about WGS including: (1) the scope of results information that could be available; (2) that knowledge about variants will change over time such that in the future additional results could be available; (3) that the interpretation of results could change over time (e.g., the clinical utility of a variant or lack thereof could become evident); and (4) some results might be surprising or unanticipated. Participants were also asked to consider what types of genetic results they would or would not want to receive, and why, and how they might want to receive those results. The guide was pretested with a group of faculty/staff parents, a pilot focus group was conducted, and minor modifications to the guide were made. Each focus group session was audio-recorded and transcribed. Participants received a $40 grocery gift card and refreshments.
After confirming accuracy, transcripts were uploaded into Atlas.ti (v6). Content analysis was conducted to describe participants’ views about research participation and return of research results [Hsieh and Shannon 2005]. A combination of both deductive and inductive approaches was used to determine themes and codes. A deductive approach was taken in formulating initial codes based on our research questions. For example, comparing themes between AA and non-AA participants, and between participants’ perspectives on themselves and their children were deductive processes. Additional themes and codes were developed following an inductive approach where new codes were allowed to emerge and added. The initial codebook was developed that identified broad themes across all focus groups. Two people independently coded each focus group transcript. Coding differences were reconciled by consensus. The codebook was refined through an iterative process. Coded statements were analyzed for broad themes. For a given theme, all coded statements were reviewed first from the AA-only groups, followed by mixed AA/non-AA groups, and finally non-AA focus groups. All analysis team members reviewed select transcripts and coded passages for thematic content. In this analysis, we focus on AAs perspectives.
This study was approved by the University of Washington Institutional Review Board.
RESULTS
Participants and focus groups
Focus group participants included 41 AAs and 35 non-AAs, the latter consisting largely of EAs (Table I). The mean age (45±11 years AA; 42±9 years non-AA) and age range (28–70 years AA; 26–71 years non-AA) were similar between groups. Twice as many AA men participated than non-AA men. A larger percentage of AA participants identified as Christian (68% AA vs. 26% non-AA) or earned a monthly income <$4,000, while a larger percentage of non-AA participants lived with a partner or were married (74% non-AA vs. 34% AA) and had attained a bachelors degree or higher (60% non-AA vs. 24% AA). A total of 13 focus groups sessions (3–9 participants each) were conducted, of which four included AA only, three included non-AA only, and 6 included both AA and non-AA participants (Supplementary eTable SI – see Supporting Information online).
Table I.
Participants’ Characteristics
| Demographic | AAa (n=41) | Non-AAb (n=35) | Chi-squared significance (p- value)c |
|---|---|---|---|
| Sex | |||
| Female | 29 (71%) | 29 (83%) | |
| Male | 18 (29%) | 6 (17%) | p<0.01 |
| Age range (mean) | 28–70 (45±11) yrs | 26–71 (42±9) yrs | p=0.1951d |
| Marital Status | |||
| Living w/ partner or married | 14 (34%) | 26 (74%) | |
| Not living w/ a partner | 27 (66%) | 9 (26%) | p<0.01 |
| Religion | |||
| Christian | 28 (68%) | 9 (26%) | |
| Other religion | 10 (24%) | 13 (37%) | |
| No religion / no response | 3 (7%) | 13 (37%) | p<0.01 |
| Education | |||
| < Bachelor’s degree | 31 (76%) | 14 (40%) | |
| ≥ Bachelor’s degree | 10 (24%) | 21 (60%) | p<0.01 |
| Monthly household income | |||
| <$4,000 | 29 (71%) | 12 (34%) | |
| ≥$4,000 | 12 (29%) | 23 (66%) | p<0.01 |
refers to African American
refers to non-African African
p-values for Chi-squared tests (Χ2) of variable proportions between AA and non-AA participant groups assuming a confidence level of 0.95.
p-value for t-test for two independent samples comparing mean age between AA and non-AA participant groups assuming a confidence level of 0.95.
Willingness to undergo WGS and receive individual genetic results
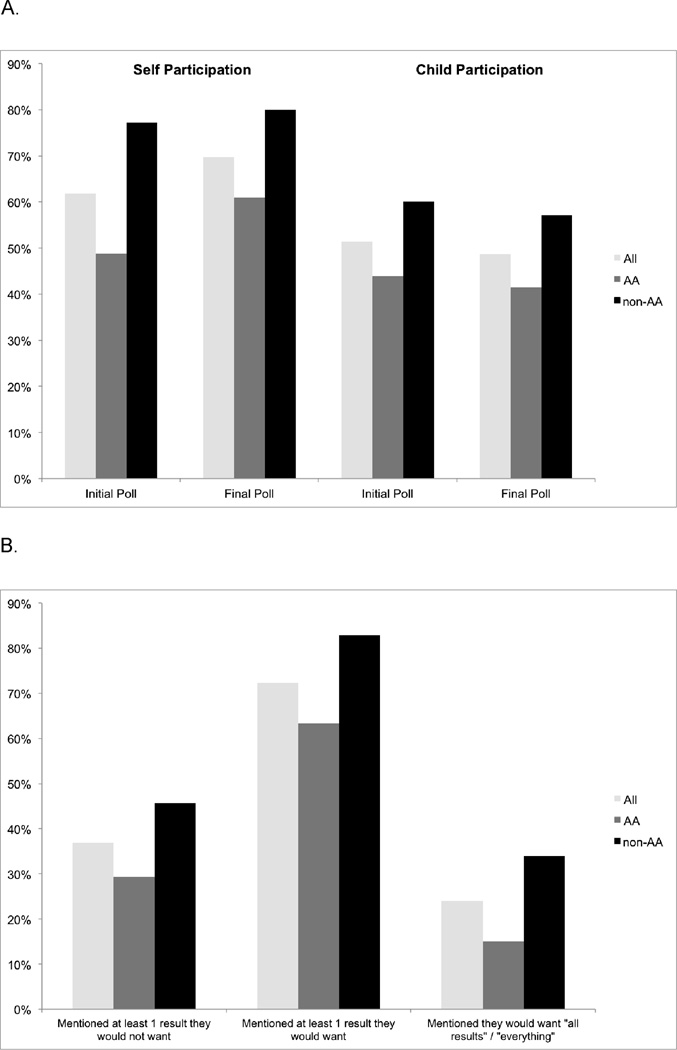
A smaller proportion of AA participants (61%) were willing to participate in a WGS study than non-AA participants (80%) (Fig 1A). This pattern of response was consistent at the start and end of focus group discussions. Parents were slightly less willing to allow their children to participate and the difference in frequency between AAs and non-AAs persisted (41% AA vs. 57% non-AA). Overall, 73% of participants indicated they would want at least one type of individual genetic result, although AAs less frequently wanted WGS results compared to non-AA (64% AA vs. 80% non-AA) (Fig 1B). Participants’ interest in receiving their children’s results were consistent. A minority of participants did not want to receive any results, either their children’s or their own, under any circumstances. Among 18 individuals who indicated that they would want “all results” or “everything” six were AA and 12 were non-AA participants.
Figure 1. Willingness to Participate and Interest in Receiving Results.
A. Participants’ willingness to participate in a WGS study
B. Participants’ interest in receiving individual genetic results
Similarities and Differences between AA and non-AA perspectives
AAs and non-AAs expressed several similar views about WGS and receiving WGS results. Among participants who wanted to receive results, both AAs and non-AAs sought to receive actionable results for common conditions (e.g. cancer, Alzheimer disease) and for conditions for which there was contributing family history. These participants also valued choice around receiving results. Yet, AAs’ perspectives differed from those of non-AAs in several ways. For instance, while non-AAs were concerned about health and long-term care insurance, AAs instead expressed concerns about a lack of access to health care that would limit their ability to follow up on actionable results. Furthermore, while non-AAs were concerned about unauthorized access to their WGS results (e.g., computer hackers), AAs were concerned about law enforcement accessing identifiable genetic information.
AAs expressed several unique perspectives that fall outside the bounds of what has, to this point, constituted the discourse on return of results (Table II). While non-AAs and some AAs expected their health care providers to be involved in receipt of their results and would share their results with their providers, some AAs expressed substantial distrust in the health care system and research such that they preferred not to involve their care providers in their results return. They preferred to review their results independently. Non-AAs focused on how results would shape future actions to improve their health whereas AAs were more concerned about the psychosocial impacts of WGS results. While non-AAs focused on the individual benefits of receiving results, AAs also emphasized racial justice as well as community and societal benefits specific to AAs. Last, AAs perceived the process of making decisions about and interpreting the meaning of WGS results to be communal, involving their social groups and community institutions.
Table II.
| AA | non-AA |
|---|---|
| Distrust of medicine and providers | Trust providers and expect involvement |
| Concerned about specific psycho-social impacts of results | Concerned about what they should do with results |
| Hoped for community and societal benefits specific to AA and racial justice | Focused on individual benefits and contributing to generalizable knowledge through research |
| WGS would be translated through community | Share results with doctors and immediate family |
refers to African American
refers to non-African African
Psychological impact of bad news
AAs had a broader view of the risks and benefits of receiving individual genetic results than non-AAs. About half of AA participants expressed worry about the psychological impact of receiving results. Some AA participants (n=7/41, 17%) were worried about receiving “bad news.” Several AA participants worried that learning about a genetic risk could cause a health condition or symptom. As this mother described, “’Cause the power of suggestion goes a long way with me. Yeah, you can think you're si- if you really wanna be sick, you can seriously think yourself sick” (AA mother, P11-01). One AA participant voiced that the scope of results returned from WGS could overwhelm an already stressful life:
Cause I am in a stressful life. This world is stressful to me, I gotta, you gotta watch everything now. . . . So, no. Just let me live. Thank God for letting me wake up one day at a time. If He take it, He take it. But don’t tell me about it. (AA father, P13-05)
While a few individuals with these concerns did not to want to receive results, several AA participants (n=8/41, 20%) wanted to set boundaries on the scope of “bad news” received. For example, one AA mother stated “[I don’t want to know] what I’m gonna die from. We ain’t going that far. And if you find out whatever it is, you just keep it to yourself (AA mother, P11-03).
In contrast to these concerns, a few AA participants wanted to learn the “good stuff,” meaning predictions that a child was going to be a “genius” or a “musician.” In one focus group, a mother countered participants’ worries about receiving WGS results with the following possibility: “What if you got the results when your baby was 6 months old and you found out that your kid was a genius? (AA mother, P13-01).” While return of such information is not feasible, such comments reflected the hope that genetic results could be reassuring, reflect strengths rather than susceptibilities, and in that way, offset return of results with perceived negative connotations: “Just give me the good stuff. Yeah, because if you got bad genes, you got good genes. . . . But don't just give me the bad” (AA mother, P11-01).
Community and societal benefits
AAs expected a range of benefits from engaging with WGS results including an opportunity for greater communication with their children about family health conditions, and the potential to empower future generations to better manage their health. As this mother explained:
Now there’s distrust for reasons. . . . its always a gamble when we participate in things like this but if we take the dark and the secrecy out and we become a little more transparent, share with our kids, say hey this is what’s happening with you, this is what’s happening in our family, just maybe in the next generation, our child, may be in a better position to take care of themselves. (AA mother, P13-02)
AA participants also wanted to make sure their children and future generations would have access to their (the parents’) results. As one mother explained, “I may not want this information but the generations that come after me, Insha’Allah, God willing, could come back and access it” (AA mother, P09-03). There was also a hope that WGS could contribute to resolving broader societal issues such as the use of common notions of race. As this participant questions, “What does it mean for, you know, hair texture and complexion. . . can it alleviate some of the political ways we look at each other and the impacts of those political ways?” (AA mother, P04-06)
A few participants also stated that they wanted “a copy of their genome” (in addition to or irrespective of specific results). For AAs, justice was the motivating issue and reflected an expectation of reciprocity:
I'm not participating. If I'm just giving you, if I'm giving you me, to get nothing, but somebody else is getting access to, oh, a black women from Africa . . . . No, that doesn't work . . . I'm giving you access to the core of who I am, 'cause that's really what I'm giving you, then you need to give me at the very least, that. Don’t worry about whether I understand or not. Give me the fullest report that you would. . . . So, when that report comes out, it should be just as rich, as robust, and as full as the person [from] Medina’s [a wealthy Seattle suburb] report. Otherwise, I wouldn’t do it. (AA mother, P04-06)
Actionability in the present versus the future
Some AAs (n=6/41, 15%) were interested in receiving results that could provide answers about an illness or health condition with which they were presently affected. For example, this mother described her family’s experience with asthma:
Now with the grandkids, he’s got asthma, the mama’s got asthma, the first one’s got asthma, we don’t know if the 2nd boy or the 3rd boy, but the cousin got it so it skipped, it skipped the babies, the one and the two, then the cousin got it . . . . so I was thinking, so I wonder what’s going on in my family? (AA mother, P13-03)
One AA mother said she would only access potentially actionable WGS results if disease symptoms arose:
…if something starts happening, like I start having seizures and spittin' out and stuff like that, you know, I'd be like oh my gosh, could you please tell me - look in the little box over there [of WGS results]. (AA mother, P11-07)
While interested in results about current existing conditions, some AA participants expressed concern that receiving results about other disease risks could “drive yourself crazy” (AA mother, P07-04) and cause someone to: “pretty much future-trip” (AA mother, P12-07).
In contrast, non-AAs were particularly interested in receiving results that would inform prevention or risk reduction strategies for future illnesses. As this EA mother explained:
I want to know what I’m at risk for. I want to know what my girls are at risk for. I want to see – well I’m a planner . . . . I want to know what I can do to help them prepare. (non-AA mother, P07-02)
Options for return of WGS results
Both AA and non-AA participants wanted options for receiving WGS results and their preferences ranged from face-to-face (n=8/76, 11%), via traditional mail (n=1/76, 1%), by phone (n=5/76, 7%) or use of web-based tools (n=2/76, 3%). Both AAs and non-AAs valued receiving information in “multiple ways,” (non-AA mother, P09-05) recognizing that “everybody receives information differently” (non-AA mother, P10-02).
While participants varied in their opinions about the particular form of results return, we found that among AAs, faith and church community could be a source of support in making decisions about undergoing WGS and managing results. One AA mother described:
I would tell everybody, everybody, everybody, everybody. Like I go to this prayer group and we go there and we pray on Thursdays and I would say, hey all, I found about this study, I signed up, this is what they’re going to do, and then they’d ask 10 questions and then, so then when we come back and we talk about next time, they’d ask me how did that go? (AA mother, P13-01)
She further explained that she would seek help from a prayer group member to read over and help her make sense of her results. Similarly, another participant pointed out “I got people that do understand this, they'll help me” (AA mother, P04-06).
Faith was mentioned in every AA focus group and was one facet of a broader theme of AAs’ preference to engage collectively as a community. In other words, they considered their community as an expert group with which to engage in helping make a decision to undergo WGS:
That’s a community and my people dialogue. So the relationship, it wouldn’t be just with my individual to ask about the research we’re having, but it would be about the collective wisdom and brilliance of my community to think about all those ramifications and then moving forward in that way. (AA mother, P04-02)
DISCUSSION
The majority of both AA and non-AA participants were willing to participate in WGS studies and receive individual genetic results, but the fraction not interested in either was higher in AAs. This is not surprising [O'Daniel and Haga 2011] and, as our results suggest, partly reflects well-known lower levels of trust in biomedical research and access to clinical care in some AA communities [Corbie-Smith et al., 2002; LaVeist et al., 2000]. However, our results also identified several additional factors that may contribute to lower levels of interest.
Perhaps foremost, a present-focused temporal orientation among some AA participants often precluded any consideration of the potential benefits of results that inform risk of future health conditions. Temporal orientation has been reported previously as relevant to genetic test acceptance among AA women [Armstrong et al., 2005; Edwards et al., 2008; Hughes et al., 2003]. When discussing their expectations about the impact of results and preferences for results, AA participants were clearly motivated by expectations that WGS results received would impact the management of their or their family members’ existing health conditions. This was consistent with AAs reported interest in individual medical benefits [Michie et al., 2011]. Yet, in the context of ES/WGS, most results are oriented toward future benefit (e.g., disease risk estimation, family planning, etc.). This misalignment of future benefit and present-oriented expectations is likely to be magnified in the context of offering WGS for return and may contribute to therapeutic misconception about secondary findings [Halverson and Ross, 2012b]. Thus, information about ES/WGS results for late onset conditions must be presented clearly and distinguished from results that inform existing health conditions. This may also directly impact how categories of results are offered and how the offer is made to AAs.
AAs’ worry about “bad news” was a barrier to receiving results. The observation underscores how the pervasive and daily stress of being an AA [Clark et al., 1999], and beliefs about somatization of perceived health risks [De Gucht and Fischler, 2002] may be substantial barriers, indeed rational reasons not to receive genetic results. Further empirical studies are needed to better understand and address population-specific psychosocial impacts of receiving WGS results among AAs and other diverse populations. When coupled with the observed present-focused orientation toward health and illness, these health beliefs may impede even considering the possibility of receiving results, as we found among some of our AA participants. At the same time, this cautious perspective – sometimes characterized by our AA participants as “old school” and rooted in the “deep south” – was countered by the hope that engaging in WGS and receiving results would yield individual, family, community and societal benefits. Among those benefits was a sense of reassurance that might come from receiving “good results.” Currently such results are not being considered for return by most, but participants’ expectations may require researchers and clinicians to consider returning negative results and results for protective alleles [Knoppers et al., 2006], and to develop more effective ways of communicating relative risk information including variants of unknown significance [Lemke et al., 2012]. Moreover, these findings highlight that the risks and benefits of receiving results must be considered using a broader framework, at least in conjunction with discussion about disclosing only clinically actionable results.
Consistent with a broader range of expected risks and benefits, for some AAs, a provider-managed and -driven model for returning results may be unacceptable or less effective [Butrick et al., 2011]. AA participants shared stories about negative health care experiences that stress the importance of maintaining control over the receipt of results. These findings suggest that approaches and tools that allow for participants and patients to have greater autonomy in obtaining and sharing results may be a viable alternative to more conventional approaches to return of results. We found that many AAs expected to manage and share their results in a community context that suggested potential avenues of specifically engaging communities about managing results [Lemke et al., 2012]. These findings highlight that mechanisms to support people as they take their results to their families and communities may be important for returning results.
Consensus is emerging that context matters for returning results. While this is the first study to solicit the perspectives of diverse groups about ES/WGS, attitudes may differ from those who have undergone ES/WGS. There is considerable heterogeneity among AAs in the U.S. along a number of demographic and social axes [Halverson and Ross, 2012a]. Income and education levels differed between AA and non-AA participants and, as a result, this study’s findings may be confounded or applicable to a limited subset of AAs. None of our participants had a rare genetic condition, a context expected to frame future experiences with receiving results. Given that participants resided in the Seattle area, future qualitative and quantitative research across different regions of the U.S. is warranted. While this analysis focuses on response differences between AA and non-AA participants, this is not to imply that there were no similarities in perspectives between AAs and non-AAs groups. Rather, considering the perspectives of AAs that might differ expands how we might want to think about consent for WGS and managing results.
Conclusions
If AAs are to have equal opportunity to make decisions about undergoing ES/WGS and or realize benefits from receiving ES/WGS results, the distrust that results from past experiences of AAs in research and clinical practice, the realities of current health and health care disparities, culturally based health beliefs, and the social structures of AA communities must be considered in building infrastructure for the translation of ES/WGS and results return. This will require a synthesis of innovative strategies for results management and for culturally appropriate communication of health information.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all the parents who participated in this study; staff at Odessa Brown Children’s Clinic, UW Pediatric Care Center, and Meredith Matthews YMCA for supporting recruitment efforts and hosting focus groups; Sue Trinidad and Jacqueline Stock for contributing to the study’s early development; and Amy Lemke and Charmaine Royal for reviewing and providing feedback on drafts of this manuscript. Our work was supported in part by grants from the National Institutes of Health / National Human Genome Research Institute (1U54HG006493 to M.J.B.; 1RC2HG005608 to M.J.B.; 5ROOHG004316 to H.K.T., and 5RO1HG006618 to H.K.T.), and the Life Sciences Discovery Fund (2065508 and 0905001), and the Washington Research Foundation.
Footnotes
We have no conflicts of interest to disclose.
REFERENCES
- Akinleye I, Roberts JS, Royal CD, Linnenbringer E, Obisesan TO, Fasaye GA, Green RC. Differences between African American and White research volunteers in their attitudes, beliefs and knowledge regarding genetic testing for Alzheimer's disease. J Genet Couns. 2011;20(6):650–659. doi: 10.1007/s10897-011-9377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Medical Genetics and Genomics. Policy Statement Points to Consider in the Clinical Application of Genomics Sequencing. 2012 [Google Scholar]
- Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- Bamshad MJ, Shendure JA, Valle D, Hamosh A, Lupski JR, Gibbs RA, Boerwinkle E, Lifton RP, Gerstein M, Gunel M, Mane S, Nickerson DA Centers for Mendelian Genomics. The Centers for Mendelian Genomics: A new large-scale initiative to identify the genes underlying rare Mendelian conditions. Am J Med Genet A. 2012;158A(7):1523–1525. doi: 10.1002/ajmg.a.35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genet Med. 2011;13(6):499–504. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- Biesecker LG. Exome sequencing makes medical genomics a reality. Nat Genet. 2010;42(1):13–14. doi: 10.1038/ng0110-13. [DOI] [PubMed] [Google Scholar]
- Bredenoord AL, Onland-Moret NC, Van Delden JJ. Feedback of individual genetic results to research participants: in favor of a qualified disclosure policy. Hum Mutat. 2011;32(8):861–867. doi: 10.1002/humu.21518. [DOI] [PubMed] [Google Scholar]
- Butrick M, Roter D, Kaphingst K, Erby LH, Haywood C, Jr., Beach MC, Levy HP. Patient reactions to personalized medicine vignettes: An experimental design. Genet Med. 2011;13(5):421–428. doi: 10.1097/GIM.0b013e3182056133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz GM, Devriendt K, Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol. 1999;54(10):805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Connell CM, Scott Roberts J, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(2):110–116. doi: 10.1097/WAD.0b013e318192e94d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162(21):2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- De Gucht V, Fischler B. Somatization: a critical review of conceptual and methodological issues. Psychosomatics. 2002;43(1):1–9. doi: 10.1176/appi.psy.43.1.1. [DOI] [PubMed] [Google Scholar]
- Edwards TA, Thompson HS, Kwate NO, Brown K, McGovern MM, Forman A, Kapil-Pair N, Jandorf L, Bovbjerg DH, Valdimarsdottir HB. Association between temporal orientation and attitudes about BRCA1/2 testing among women of African descent with family histories of breast cancer. Patient Educ Couns. 2008;72(2):276–282. doi: 10.1016/j.pec.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmeyer JN, Northrup H, Assel MA, Goka TJ, Johnston DA, Williams AT. An assessment of risk understanding in Hispanic genetic counseling patients. J Genet Couns. 2005;14(4):319–328. doi: 10.1007/s10897-005-0759-5. [DOI] [PubMed] [Google Scholar]
- Evans JP, Rothschild BB. Return of results: not that complicated? Genet Med. 2012;14(4):358–360. doi: 10.1038/gim.2012.8. [DOI] [PubMed] [Google Scholar]
- Fabsitz RR, McGuire A, Sharp RR, Puggal M, Beskow LM, Biesecker LG, Bookman E, Burke W, Burchard EG, Church G, Clayton EW, Eckfeldt JH, Fernandez CV, Fisher R, Fullerton SM, Gabriel S, Gachupin F, James C, Jarvik GP, Kittles R, Leib JR, O'Donnell C, O'Rourke PP, Rodriguez LL, Schully SD, Shuldiner AR, Sze RK, Thakuria JV, Wolf SM, Burke GL. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Genet. 2010;3(6):574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009;15(Suppl 1):S56–S62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg AJ, Hull SC, Wilfond BS, Sharp RR. Patient perspectives on group benefits and harms in genetic research. Public Health Genomics. 2011;14(3):135–142. doi: 10.1159/000317497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga-Jauregui C, Lupski JR, Gibbs RA. Human genome sequencing in health and disease. Annu Rev Med. 2012;63:35–61. doi: 10.1146/annurev-med-051010-162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Berg JS, Berry GT, Biesecker LG, Dimmock DP, Evans JP, Grody WW, Hegde MR, Kalia S, Korf BR, Krantz I, McGuire AL, Miller DT, Murray MF, Nussbaum RL, Plon SE, Rehm HL, Jacob HJ. Exploring concordance and discordance for return of incidental findings from clinical sequencing. Genet Med. 2012;14(4):405–410. doi: 10.1038/gim.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, Tindall G, O'Daniel JM. Public perspectives about pharmacogenetic testing and managing ancillary findings. Genet Test Mol Biomarkers. 2012;16(3):193–197. doi: 10.1089/gtmb.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson CM, Ross LF. Engaging African-Americans about biobanks and the return of research results. J Community Genet. 2012a;3(4):275–283. doi: 10.1007/s12687-012-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson CM, Ross LF. Incidental findings of therapeutic misconception in biobank-based research. Genet Med. 2012b;14(6):611–615. doi: 10.1038/gim.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Hughes C, Fasaye GA, LaSalle VH, Finch C. Sociocultural influences on participation in genetic risk assessment and testing among African American women. Patient Educ Couns. 2003;51(2):107–114. doi: 10.1016/s0738-3991(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Kessler L, Collier A, Halbert CH. Knowledge about genetics among African Americans. J Genet Couns. 2007;16(2):191–200. doi: 10.1007/s10897-006-9054-3. [DOI] [PubMed] [Google Scholar]
- Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14(11):1170–1178. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
- Kohane IS, Hsing M, Kong SW. Taxonomizing, sizing, and overcoming the incidentalome. Genet Med. 2012;14(4):399–404. doi: 10.1038/gim.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146–161. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- Lemke AA, Halverson C, Ross LF. Biobank participation and returning research results: Perspectives from a deliberative engagement in South Side Chicago. Am J Med Genet A. 2012;158A(5):1029–1037. doi: 10.1002/ajmg.a.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KA, Thomas SB, Grubs RE, Gettig EA, Krishnamurti L. Attitudes and Beliefs of African-Americans Toward Genetics, Genetic Testing, and Sickle Cell Disease Education and Awareness. J Genet Couns. 2011 Dec;20(6):572–592. doi: 10.1007/s10897-011-9388-3. [DOI] [PubMed] [Google Scholar]
- Michie M, Henderson G, Garrett J, Corbie-Smith G. "If I could in a small way help": motivations for and beliefs about sample donation for genetic research. J Empir Res Hum Res Ethics. 2011;6(2):57–70. doi: 10.1525/jer.2011.6.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda R, Schumm LP, Cummings S, Fackenthal JD, Sveen L, Ademuyiwa F, Cobleigh M, Esserman L, Lindor NM, Neuhausen SL, Olopade OI. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925–1933. doi: 10.1001/jama.294.15.1925. [DOI] [PubMed] [Google Scholar]
- O'Daniel J, Haga SB. Public Perspectives on Returning Genetics and Genomics Research Results. Public Health Genomics. 2011;14(6):346–355. doi: 10.1159/000324933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presidential Commission for the Study of Bioethical Issues. Privacy and Progress in Whole Genome Sequencing. 2012 [Google Scholar]
- Rahm AK, Feigelson HS, Wagner N, Le AQ, Halterman E, Cornish N, Dearing JW. Perception of Direct-To-Consumer Genetic Testing and Direct-To-Consumer Advertising of Genetic Tests among Members of a Large Managed Care Organization. J Genet Couns. 2012;21(3):448–461. doi: 10.1007/s10897-011-9477-3. [DOI] [PubMed] [Google Scholar]
- Richardson HS, Cho MK. Secondary researchers' duties to return incidental findings and individual research results: a partial-entrustment account. Genet Med. 2012;14(4):467–472. doi: 10.1038/gim.2012.12. [DOI] [PubMed] [Google Scholar]
- Singer E, Antonucci T, Van Hoewyk J. Racial and ethnic variations in knowledge and attitudes about genetic testing. Genet Test. 2004;8(1):31–43. doi: 10.1089/109065704323016012. [DOI] [PubMed] [Google Scholar]
- Susswein LR, Skrzynia C, Lange LA, Booker JK, Graham ML, 3rd, Evans JP. Increased uptake of BRCA1/2 genetic testing among African American women with a recent diagnosis of breast cancer. J Clin Oncol. 2008;26(1):32–36. doi: 10.1200/JCO.2007.10.6377. [DOI] [PubMed] [Google Scholar]
- Suther S, Kiros GE. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11(9):655–662. doi: 10.1097/GIM.0b013e3181ab22aa. [DOI] [PubMed] [Google Scholar]
- Tennessen JA, Bigham AW, O'Connor TD, Fu W, Kenny EE, Gravel S, McGee S, Do R, Liu X, Jun G, Kang HM, Jordan D, Leal SM, Gabriel S, Rieder MJ, Abecasis G, Altshuler D, Nickerson DA, Boerwinkle E, Sunyaev S, Bustamante CD, Bamshad MJ, Akey JM, Broad GO, Seattle GO NHLBI Exome Sequencing Project. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337(6090):64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadaparampil ST, McIntyre J, Quinn GP. Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genet Couns. 2010;19(6):618–629. doi: 10.1007/s10897-010-9316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Cho MK, Christman MF, Green RC, Hall R, Illes J, Keane M, Knoppers BM, Koenig BA, Kohane IS, Leroy B, Maschke KJ, McGeveran W, Ossorio P, Parker LS, Petersen GM, Richardson HS, Scott JA, Terry SF, Wilfond BS, Wolf WA. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. 2012;14(4):361–384. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.